Abstract
Background
Vascular remodeling relies upon extracellular matrix restructuring by the matrix metalloproteinases (MMPs). Induction of MMP-2 and MMP-9 by biological signaling molecules has been defined, but whether a mechanical stimulus such as elevated wall tension may generate MMP promoter activation remains unknown. Accordingly, this study examined whether MMP promoter activation would occur as a function of wall tension.
Materials and Methods
The MMP-2 or MMP-9 promoter sequences were fused to the reporter gene lacZ and inserted into the mouse genome. Thoracic aortic rings were harvested (6 preparations/construct) and maintained under physiological conditions at predetermined tension values corresponding to 0, 70, 85 and 100 mmHg for 3 hours. Relative gene expression of lacZ, directly reflecting MMP promoter activity, was then quantified by QPCR.
Results
MMP-2 promoter activity decreased to 0.42±0.11 at 0 mmHg and increased to 1.57±0.24 fold at 100 mmHg (p<0.05), whereas MMP-9 was unaffected.
Conclusions
Using unique transgenic constructs with homology to human MMP promoters, this study demonstrated that a physiologically relevant mechanical stimulus was sufficient to differentially induce MMP promoter activation.
Keywords: vascular remodeling, wall tension, thoracic aorta, matrix metalloproteinase
Introduction
Vascular remodeling in response to physiologic or pathologic stressors involves structural adaptation of the vessel wall extracellular matrix (ECM).(1) Hemodynamic forces, vessel injury, inflammation, and oxidative stress have all been shown to promote vascular remodeling by stimulating production of matrix metalloproteinases (MMPs), the major effectors of ECM degradation and reorganization.(2) The MMPs are a family of extracellular proteases capable of degrading all ECM proteins. The gelatinase subclass (MMP-2 and MMP-9), utilize elastin; gelatin; types IV, V, and VII collagen; and other ECM components as substrates.(3) MMP-2 also possesses interstitial collagenase capabilities and can degrade type I collagen.(4) Elevated MMP proteolytic activity within the vascular wall may be regulated by alterations in gene transcription or translation, as well as propeptide activation or endogenous inhibition of these proteases.
The influence of hemodynamic force on vessel wall restructuring can be attributed to two mechanical components, shear stress and wall tension.(5) Based upon the Laplace’s Law, the wall tension of any cylinder is the product of the internal pressure and the vessel radius.(6) This theorem is often used to describe the amplified and potentially deadly forces acting upon the wall of large aortic aneurysms,(7) but it also pertains to other vascular beds and may provide a foundation from which the initial driving force underlying pathological vascular remodeling may be extrapolated. Experimental manipulation of wall tension to simulate a hypertensive state may provide insight into alterations in cell signaling and gene expression that drive matrix remodeling. However, no study has demonstrated whether and to what degree this purely mechanical stimulus can influence type-specific MMP promoter activity. Accordingly, we utilized unique transgenic mouse lines carrying MMP promoter/reporter gene constructs to test the hypothesis that elevated wall tension will differentially induce MMP-2 and MMP-9 promoter activation.
Materials and Methods
MMP reporter strains
Promoter activity was measured in CD-1 wild-type mice as well as MMP-2 and MMP-9 reporter mouse strains utilizing both male and female mice. The transgenic CD-1[MMP-2:β-gal] reporter mice were generated with a 5-kb fragment of the rat MMP-2 gene extending from -1686 to the second exon,(8) while a rabbit MMP-9 gene segment from base pairs -522 to +19 was isolated to create the 3445[MMP-9:β-gal]/CD-1 reporter mouse line.(9) In both cases, the MMP promoter region was linked to the β-galactosidase gene lacZ, and transgene expression was confirmed by reaction of tail tissue with the β-galactosidase substrate X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) as well as by PCR amplification of tail-clip DNA with primers specific for lacZ.
Experimental Design
In this study, wall tension was directly applied to segments of murine descending thoracic aorta utilizing an ex vivo vascular ring apparatus as described below and previously.(10, 11) Aortas excised from reporter mice were stretched at wall tension values concordant with mean arterial pressures corresponding to mild hypertensive states, and MMP promoter activity was assessed by measuring lacZ gene expression through quantitative real-time polymerase chain reaction (QPCR). All mice were maintained according to the National Institutes of Health Guide to the Care and Use of Laboratory Animals (National Research Council, Washington, DC, 1996) and this animal protocol was approved by the MUSC Institutional Animal Care and Use Committee.
Preparation of aortic rings
After inducing general anesthesia with 2% isoflurane, mice were intubated and underwent a left posterolateral thoracotomy. The descending thoracic aorta was harvested and immediately placed in cold Krebs-Hanseleit buffer (118mM NaCl, 4.6mM KCl, 1.2mM KH2PO4, 25mM NaHCO3, 2.5mM CaCl2, 0.5mM Na2-EDTA, 11mM Glucose, 1.2mM MgSO4, pH 7.4). The endothelium and extraneous connective tissue were removed and the vessel was divided into 3 mm long segments (typically 3 per mouse) which were each mounted on parallel wires in a water jacketed tissue bath system (Radnoti, Monrovia, CA; 25ml) maintained at 37°C and connected to an isometric force transducer (Radnoti, Monrovia, CA). The vessels were allowed to equilibrate on the ring apparatus for 30 minutes in the absence of tension and washed every 15 minutes with warm Krebs-Hanseleit solution aerated with 95% O2/5% CO2. Micrometer measurements at zero tension and calculations set forth by He et al.,(12) were used to derive the mean internal circumference and cross-sectional area of the vessels. Breaking stress was quantified in three rings by slowly increasing ring tension over a 3-5 minute period until evidence of tearing was visualized. All studies of aortic ring tension were digitally measured and graphically recorded (BioBench; National Instruments, Austin, TX).
Defining optimal tension and experimental pressure gradients
To accurately examine the effect of elevated tension on promoter activity, the native material properties of the murine thoracic aorta required definition. The elastic recoil of the medial ECM generates the passive tension of the vessel, and this was quantified by sequentially stretching vessel segments (n=8 for each strain) in 0.1 g increments and allowing 3 minutes for equilibration. To prevent stretch-induced vessel contraction during this process, the calcium chelator EGTA (5 mM) was added to the Krebs-Hanseleit solution. During the 3 minute equilibration period, the ECM microfibrils would adapt and relax, demonstrating a decay in the transduced vessel tension. When the difference between the initial applied tension and the value recorded at the end of the equilibration period was significantly less than 10%, all of the elastic recoil was considered overcome, and the applied tension value was equivalent to the passive tension of the vessel. Mean percent decline at each level of applied tension was compared to a standard of 10% using a one-sample t-test.
The optimal tension of the aortic ring is an equilibration point where all passive tension has been overcome and maximum active (contractile) tension can be generated by the medial smooth muscle cells. Having defined the passive tension, fresh aortic rings (n=7 for MMP-2, n=8 for MMP-9) were stabilized for 30 minutes in standard Krebs-Hanseleit buffer at a range of tension values encompassing the passive tension value derived above. Contraction was stimulated by adding 100 mM KCl to the bath and the peak tension generated over the ensuing 8 minutes was recorded. The rings were washed with Krebs-Hanseleit solution every 10 minutes for the following 30 minutes. These steps were repeated at 0.1 g increments from 0.4 to 0.8 g of applied tension. Comparisons among active tension values were conducted by one-way ANOVA with a post-hoc Tukey’s multiple comparison test.
Because the experimentally derived optimal tension value alone does not account for differences in the physical parameters of a given aortic ring (i.e. cross-sectional area, ring surface area…etc.), the optimal tension value was converted to a more physiologically relevant pressure equivalent. The rings were then subjected to pressure equivalents mimicking mild hypertension. Calculations were performed according to Lalli, et al., and He, et al., (Appendix A)(12, 13) The cross-sectional area of aortic rings from MMP-2 reporter mice was calculated at 0.31±0.01mm2 and the ring surface area was 7.55±0.07mm2. In MMP-9 reporter mice, the cross-sectional area was 0.33±0.02mm2 and the ring surface area was 7.49±0.10mm2. Using these values, optimal tension was converted to a pressure equivalent of 70 mmHg in each strain. To compare tension-induced responses between mouse lines, rings derived from the MMP-2 and MMP-9 mice were subsequently stretched at a mean arterial pressure (0, 70, 85, and 100 mmHg) which corresponded to 0.0, 0.7, 0.85, and 1.0 g of tension, respectively. For both strains, the amount of applied tension was far below the measured breaking tension of greater than 4 g.
Inducing MMP promoter activity
After fresh rings were equilibrated at zero tension for 30 minutes, they were set at optimal tension (70 mmHg) and allowed to stabilize for an additional 30 minutes. Aortic rings were then subjected to 3 hours at approximately 0, 70, 85, or 100 mmHg with buffer exchange every 30 minutes (n=6 for each strain). Tissue viability was confirmed at the end of the experimental stretch period by stimulating the aortic ring with 100 mM KCl and documenting the continued ability to contract. Each aortic ring was subsequently snap frozen in Buffer RLT (QIAGEN, Valencia, CA), and stored at -80°C.
QPCR amplification of lacZ
Individual aortic rings underwent rotor-stator homogenization in Buffer RLT (QIAGEN, Valencia, CA), total RNA was extracted using the RNeasy Fibrous Tissue MiniKit (QIAGEN, Valencia, CA), and reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA). QPCR was performed using TaqMan chemistry with primers for the reporter gene lacZ (TaqMan Gene Expression Assays on Demand catalog # 185757894 – 1,2,3; Applied Biosystems, Foster City, CA). Primers for 18s rRNA were used as the internal standard (TaqMan Gene Expression Assays on Demand catalog # 4333760 F; Applied Biosystems, Foster City, CA).
Reactions were run in the BioRad MyiQ Single Color Real Time PCR Detection System (BioRad, Hercules, CA) set to detect 6-FAM labeled probes. The initial denaturing cycle consisted of 10 minutes at 95°C, and was followed by 45 cycles of 30 seconds at 95°C and 1 minute at 60°C. Threshold cycle (Ct) values were recorded and fold gene expression of lacZ as compared to optimal tension was calculated by the ΔΔCt method.
QPCR amplification of MMP-2 and MMP-9
To evaluate native MMP-2 and MMP-9 promoter activity, aortic rings from CD1 wild-type mice were processed similar to described above. Briefly, total RNA extracted from three wild-type aortic rings at each pressure gradient was pooled, converted to cDNA, and underwent QPCR with primers for murine MMP-2 and MMP-9 gene transcripts (TaqMan Gene Expression Assays, catalog # Mm00439508_m1 and Mm00442991_m1, respectively; Applied Biosystems, Foster City, CA).
Statistical Analysis
Fold expression of MMP2, MMP9, and lacZ gene transcription, as compared to optimal tension (70 mmHg), was evaluated using a two-sided one-sample t-test versus a fixed value of one. All values are reported as a mean +/- SEM. Stata 8 statistical software (Intercooled, College Station, TX) was utilized for all calculations and values were considered significant at p<0.05.
Results
Optimal tension is defined as the point where all passive tension is overcome and maximal active (contractile) tension can be generated. In the MMP-2 and MMP-9 reporter mouse strains, these criteria were met at 0.7 g (Figure 1). Normalizing optimal tension to the cross sectional area of the aortic rings produced a pressure measurement of 70 mmHg in both strains.
Figure 1.
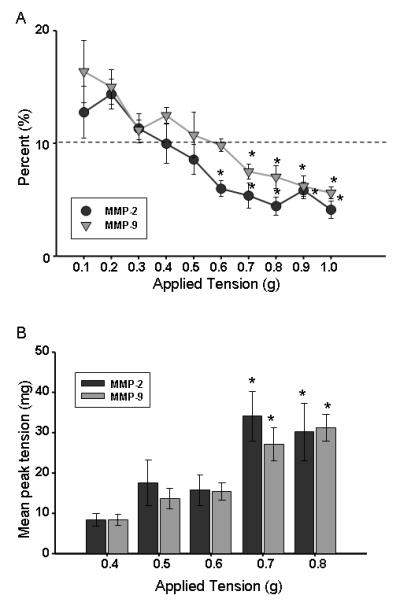
Determination of optimal tension for the each reporter strain. A. Percent decrease in tension observed during equilibration at each level of applied tension (n=8 for each). *p<0.05 from 10%; B. Mean peak contractile tension generated following addition of 100mM KCl is shown here (n=7 for MMP-2, n=8 for MMP-9). *p<0.05 from 0.4 g. Together these data define the optimal tension for the MMP-2 and MMP-9 reporter strains (0.7 g in each strain).
The fold expression of lacZ following 3 hours of applied pressure at 0, 70, 85, or 100 mmHg, was calculated with regard to the baseline setting of 70 mmHg (Figure 2). Additionally, MMP-2 reporter mouse aortic rings maintained at 0 mmHg, displayed a decrease in lacZ expression, while the application of 100 mmHg produced an increase. MMP-9 promoter activation was neither induced nor inhibited by manipulating wall tension (Figure 2).
Figure 2.
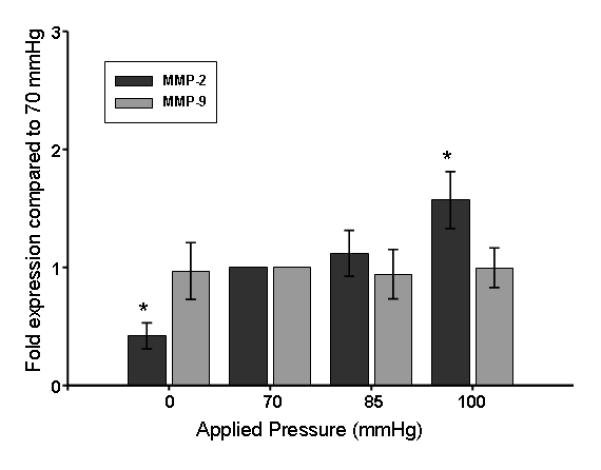
Utilizing optimal tension (70 mmHg) as the baseline, MMP promoter activity was determined by fold expression of lacZ calculated at each pressure gradient. (n=6 for each). *p<0.05 from 1.0.
Endogenous transcripts for MMP-2 were measured from a pool of three CD-1 wild-type aortic rings. Elevated wall tension induced MMP-2 mRNA levels by 1.97-fold at 85 mmHg and 1.55-fold at 100 mmHg (data not shown).
Discussion
Vascular remodeling involves morphologic alterations in the blood vessel wall in response to a stressor. The matrix metalloproteinase (MMP) family of endopeptidases mediate vessel wall ECM restructuring, particularly through the action of MMP-2 and MMP-9.(2) Neurohormonal mediators(14-16) and reactive oxygen species(17, 18) can regulate vascular wall MMP activity, and hemodynamic forces such as shear stress and wall tension likely contribute to the events initiating MMP induction. In fact, there is a documented association between hypertension and MMP driven forms of vascular remodeling such as intimal hyperplasia(19) and aortic aneurysm development.(20) However, no study has demonstrated that a mechanical stimulus alone can induce type-specific MMP promoter activity. Therefore, utilizing a vascular ring apparatus and unique transgenic mice carrying MMP promoter/reporter gene constructs, the presence of wall tension-induced MMP promoter activation was investigated. The unique findings of this study were twofold. First, we demonstrated that a physiologically relevant mechanical stimulus was sufficient to differentially induce MMP promoter activation such that wall tension stimulated MMP-2, while MMP-9 promoter-dependent transcription was insensitive to this mechanical stimulus. Second, these data suggest that selective MMP induction occurs at wall tension values concordant with mild hypertension, indicating that a mechanical-molecular set point may exist which triggers adverse vascular wall remodeling, either alone or in combination with other factors.
Advantages of the vascular ring apparatus and reporter gene system
It is likely that numerous factors coincide and collaborate to induce vascular remodeling, therefore isolating and evaluating any one parameter can be challenging. The biggest advantage of the vascular ring apparatus utilized in this study, therefore, was that it afforded the ability to examine the effects of wall tension alone. To effectively utilize this system and measure the effect of tension independent of other factors, knowledge of the native material properties of the murine thoracic aorta was required, specifically the optimal tension. Optimal tension in each reporter strain utilized in this study was determined to be 0.7 g, and using the aortic dimensions and equations defined by He et al., this optimal tension was determined to be equivalent to 70 mmHg.(12) Accordingly, equilibrating the aortic rings at optimal tension corresponds to the in vivo resting state of the vessel. Furthermore, reporter gene transcription at this point can be considered baseline promoter activity.
Quantifying and comparing low copy-number genes such as MMP-2 and MMP-9 can be challenging; particularly in small tissue samples. To circumvent this limitation, MMP-2 and MMP-9 transcriptional activation was assessed using specific reporter mouse strains that carried multiple copies of a transgene containing the MMP-2 or MMP-9 promoter linked to the bacterial β-galactosidase gene (lacZ). Specific MMP promoter activity could then be measured through quantification of lacZ transcripts. The required length of treatment to evoke a detectable response was also considered. A previous study applying mechanical stress to cultured cells reported elevated MMP-2 in just 3 hours, suggesting that this short span of time may be sufficient to induce and detect transcriptional activity in vascular tissue, potentially modeling an early response to hypertension.(21) Ultimately, these transgenic reporter mice allowed for highly sensitive, specific measurements of promoter activation at early time points following the onset of increased wall tension. Together, the vascular ring apparatus and reporter mouse strains allow for a direct measurement of early MMP promoter activation in response to wall tension.
Relation of Wall Tension to Blood Pressure
The constant rather than cyclic application of tension by the vascular ring system corresponds to the effect of mean arterial pressure on the vessel wall. Following identification of optimal tension, elevated pressure levels were chosen based upon tension applications commonly utilized in relevant literature. More specifically, murine thoracic aortic rings maintained in a similar vascular ring apparatus have been set at 1.0g of wall tension to evaluate potency and efficacy of numerous vasoactive compounds.(22-24) Appropriate physiologic function was maintained at this tension level and no evidence of matrix destruction or medial cellular necrosis had been reported, therefore this value was chosen as a maximum tension application and in these mouse lines was equivalent to 100 mmHg. Normal murine blood pressure values closely approximate those of humans(25, 26) and mean arterial pressures of 85 and 100 mmHg, although only mildly hypertensive, would be well within the range of pressures experienced by a thoracic aorta in vivo. Maintaining vessel rings at 0 mmHg, on the other hand, would help define the pressure-sensitive state of the MMP promoter and furthermore would approximate general vessel dynamics downstream from an occlusion, another active site of vascular remodeling.
Wall Tension can induce MMP Promoter Activity
When subjected to elevated pressure, MMP-2 promoter activity was increased approximately 1.5-fold, but at the low pressure state, another potential condition requiring matrix remodeling, a decrease in MMP-2 promoter activity was recorded. Previous studies have demonstrated enhanced MMP-2 production in increased as well as decreased pressure states, however those investigations maintained vessels in organ culture for 72 hours and may have represented an advanced stage of vascular remodeling.(27) The differential response of the MMP-2 promoter to alterations in wall tension described in this investigation not only highlights diversity within the process of vascular remodeling, but suggests that this protease may selectively play an early role in hypertensive vascular remodeling.
Additionally, to confirm that elevated wall tension results in enhanced MMP-2 transcriptional activation, mRNA isolated from a pool of 3 CD-1 wild-type aortic rings was converted to cDNA and subjected to QPCR analysis using primers for the endogenous murine MMP-2 gene. Although steady-state MMP-2 mRNA levels are likely influenced by various post-transcriptional events in addition to promoter activity, the increased quantity of transcripts detected at both 85 and 100 mmHg (1.97-fold and 1.55-fold, respectively) confirmed that MMP-2 promoter activity is increased in response to elevated wall tension.
Conversely, MMP-9 promoter activity was not affected by an alteration in wall tension. Previous studies demonstrating an MMP-9 response to elevated intraluminal pressure utilized an organ bath model in which either shear stress or elevated wall tension had the potential to initiate MMP-9 transcription over the course of 72 hours.(27) Whether a longer duration of tension application could activate the MMP-9 promoter in this system has not yet been explored. Additionally, these two MMPs have markedly different promoter regions. The MMP-9 promoter has binding sites for transcription factors common to nearly all MMPs including AP-1 and PEA-3, as well as those associated with an inflammatory response such as NF-κB.(28) The MMP-2 promoter, on the other hand, is interesting in that it lacks the typical MMP transcription element binding sites.(28) The intracellular signaling cascades translating mechanical stimuli into gene expression remain unclear, but the obvious differences between MMP-2 and MMP-9 promoter regions suggest that diverse transcriptional regulatory mechanisms may exist.
Wall Tension, MMP Promoter Activity, and Vascular Remodeling
The present study has implications in vascular remodeling with regard to numerous vascular environments including the aorta and vascular bypass grafts. For example, increased protein levels of MMP-2 and MMP-9 have been associated with aortic aneurysms.(29-31) While little is understood about how the cycle of MMP secretion, matrix degradation, and aneurysm dilation begins, thoracic aortic aneurysms have been commonly associated with high blood pressure; an obvious source of elevated wall tension. Elevated MMP-2 expression in a mildly hypertensive state presents the possibility that poorly controlled hypertension may initiate the collagenolytic and elastolytic breakdown of the aortic media. Additionally, elevated wall tension has the potential to stimulate vascular remodeling in both coronary and peripheral saphenous vein grafts. When arterialized, and therefore subjected to markedly increased pressure, veins undergo intimal hyperplasia and medial hypertrophy that may consequently result in stenosis or occlusion.(32) Under normal physiologic conditions, MMP-2 levels are elevated in saphenous vein segments compared to other common coronary bypass conduits such as the internal mammary artery, potentially providing a predisposition to early graft remodeling and failure.(19) In an internal jugular-carotid artery interposition graft model, MMP-2 and MMP-9 displayed rapid and sustained elevation in activities,(32) suggesting that these proteases were instrumental to vascular remodeling in this environment and may have been stimulated by the rapid increase in wall tension. Understanding the transduction of mechanical stimuli to MMP transcriptional activity will allow for targeted inhibition of these proteases to ultimately slow or prevent vascular remodeling and preserve vessel function.
Limitations
While this project has provided unique insight into the forces influencing vascular remodeling, some limitations must be addressed. The study of tissue ex vivo raises the question of tissue viability over the duration of the three hour tension application. In an effort minimize tissue damage, several steps were taken. First, the experimental protocol was specifically designed to take advantage of reporter strains that could effect a large induction of gene transcription upon activation. Secondly, the time between ring excision and tension application was minimized and standardized to avoid potential deleterious effects on ring viability. Lastly, to be sure that the ex vivo tissue had maintained viability throughout the duration of the experiment, a contractile response was confirmed with the addition of KCl treatment at the end of the experimental period prior to RNA harvesting. Accordingly, all tested vessels maintained the ability to contract, suggesting that ring viability was sufficiently maintained.
Summary and Clinical Perspective
This project was designed to identify alterations in MMP promoter activation within reporter constructs at early time-points to target potential inciting events in MMP transcription. Therefore, this study’s demonstration of increased lacZ transcription within 3 hours of physiologically relevant applied tension in the MMP-2 reporter mouse strain not only supports the argument that elevated wall tension alone can modify MMP promoter activation, but may suggest that poorly controlled blood pressure itself can initiate pathologic vascular remodeling. Moreover, the decreased MMP-2 promoter activity documented at 0 mmHg may imply that wall tension is actually necessary for MMP-2 transcription and that a relationship exists between the amplitude of wall tension and the degree of MMP-2 promoter activation.
Pathologic remodeling is a common theme in cardiovascular disease. Clinical studies characterizing biomarkers indicative of heart failure or arterial stiffness in hypertensive patients have demonstrated that elevated serum levels of particular MMPs may identify those at increased risk for worsening cardiovascular disease.(33-36) This study has specifically shown that the MMP-2 promoter sequence responds rapidly to even mildly hypertensive pressure states, thereby identifying this protease as a target for further investigation. Incorporating knowledge of predictive biomarkers with the unique results of the present study to define the mechanical-molecular set point for induction of MMPs may have a significant impact on the prognostic and therapeutic management of patients at risk for progressive vascular remodeling.
Acknowledgments
This study was supported by NIH/NHLBI grant R01 HL075488-04.
Appendix A
A compilation of equations from Lalli, et al., and He, et al., was utilized to convert tension to pressure. (12, 13) Force (F) was calculated from Equation 1 with the applied tension serving as the mass and gravity as the acceleration. To account for the distribution of this force across the aortic ring, the force was normalized (FN, Equation 3) to the cross-sectional area (CSA) derived from Equation 2. The internal circumference (L) of the aortic ring was then determined by Equation 4, and the applied pressure (P) was computed utilizing a derivation of LaPlace’s Law, Equation 5.
Summary of calculated values for MMP-2 reporter mouse strain.
| Tension (g) | Force (mN) | Normalized Force (kPa) |
Pressure (mmHg) |
|---|---|---|---|
| 0.70 | 6.9 | 22.25 | 69.42 ∼ 70 |
| 0.85 | 8.3 | 26.87 | 83.84 ∼ 85 |
| 1.00 | 9.8 | 31.6 | 98.59 ∼ 100 |
References
- 1.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 3.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 5.Lehoux S, Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension. 1998;32:338–345. doi: 10.1161/01.hyp.32.2.338. [DOI] [PubMed] [Google Scholar]
- 6.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 7.Hall AJ, Busse EF, McCarville DJ, Burgess JJ. Aortic wall tension as a predictive factor for abdominal aortic aneurysm rupture: improving the selection of patients for abdominal aortic aneurysm repair. Ann Vasc Surg. 2000;14:152–157. doi: 10.1007/s100169910027. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee R, Mingoia JT, Bruce JA, Austin JS, Stroud RE, Escobar GP, McClister DM, Jr, Allen CM, Alfonso-Jaume MA, Fini ME, Lovett DH, Spinale FG. Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H2216–2228. doi: 10.1152/ajpheart.01343.2005. [DOI] [PubMed] [Google Scholar]
- 9.Mohan R, Rinehart WB, Bargagna-Mohan P, Fini ME. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during developmental and injury-related tissue remodeling. J Biol Chem. 1998;273:25903–25914. doi: 10.1074/jbc.273.40.25903. [DOI] [PubMed] [Google Scholar]
- 10.Bond BR, Zellner JL, Dorman BH, Multani MM, Kratz JM, Crumbley AJ, 3rd, Crawford FA, Jr., Spinale FG. Differential effects of calcium channel antagonists in the amelioration of radial artery vasospasm. Ann Thorac Surg. 2000;69:1035–1040. doi: 10.1016/s0003-4975(00)01132-2. discussion 1040-1031. [DOI] [PubMed] [Google Scholar]
- 11.Swafford AN, Jr., Harrison-Bernard LM, Dick GM. Knockout mice reveal that the angiotensin II type 1B receptor links to smooth muscle contraction. Am J Hypertens. 2007;20:335–337. doi: 10.1016/j.amjhyper.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 12.He GW, Angus JA, Rosenfeldt FL. Reactivity of the canine isolated internal mammary artery, saphenous vein, and coronary artery to constrictor and dilator substances: relevance to coronary bypass graft surgery. J Cardiovasc Pharmacol. 1988;12:12–22. doi: 10.1097/00005344-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Lalli J, Harrer JM, Luo W, Kranias EG, Paul RJ. Targeted ablation of the phospholamban gene is associated with a marked decrease in sensitivity in aortic smooth muscle. Circ Res. 1997;80:506–513. doi: 10.1161/01.res.80.4.506. [DOI] [PubMed] [Google Scholar]
- 14.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis VA, Persidskaia RN, Baca-Regen LM, Fiotti N, Halloran BG, Baxter BT. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J Surg Res. 2001;101:152–156. doi: 10.1006/jsre.2001.6281. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–308. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 18.Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20:E120–126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 19.Anstadt MP, Franga DL, Portik-Dobos V, Pennathur A, Bannan M, Mawulawde K, Ergul A. Native matrix metalloproteinase characteristics may influence early stenosis of venous versus arterial coronary artery bypass grafting conduits. Chest. 2004;125:1853–1858. doi: 10.1378/chest.125.5.1853. [DOI] [PubMed] [Google Scholar]
- 20.Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin. 1999;17:615–635. vii. doi: 10.1016/s0733-8651(05)70105-3. [DOI] [PubMed] [Google Scholar]
- 21.von Offenberg Sweeney N., Cummins PM, Birney YA, Cullen JP, Redmond EM, Cahill PA. Cyclic strain-mediated regulation of endothelial matrix metalloproteinase-2 expression and activity. Cardiovasc Res. 2004;63:625–634. doi: 10.1016/j.cardiores.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Brandes RP, Kim D, Schmitz-Winnenthal FH, Amidi M, Godecke A, Mulsch A, Busse R. Increased nitrovasodilator sensitivity in endothelial nitric oxide synthase knockout mice: role of soluble guanylyl cyclase. Hypertension. 2000;35:231–236. doi: 10.1161/01.hyp.35.1.231. [DOI] [PubMed] [Google Scholar]
- 23.Sutliff RL, Hoying JB, Kadambi VJ, Kranias EG, Paul RJ. Phospholamban is present in endothelial cells and modulates endothelium-dependent relaxation. Evidence from phospholamban gene-ablated mice. Circ Res. 1999;84:360–364. doi: 10.1161/01.res.84.3.360. [DOI] [PubMed] [Google Scholar]
- 24.Sutliff RL, Weber CS, Qian J, Miller ML, Clemens TL, Paul RJ. Vasorelaxant properties of parathyroid hormone-related protein in the mouse: evidence for endothelium involvement independent of nitric oxide formation. Endocrinology. 1999;140:2077–2083. doi: 10.1210/endo.140.5.6700. [DOI] [PubMed] [Google Scholar]
- 25.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 26.Hoit BD, Kiatchoosakun S, Restivo J, Kirkpatrick D, Olszens K, Shao H, Pao YH, Nadeau JH. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics. 2002;79:679–685. doi: 10.1006/geno.2002.6754. [DOI] [PubMed] [Google Scholar]
- 27.Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109:1041–1047. doi: 10.1161/01.CIR.0000115521.95662.7A. [DOI] [PubMed] [Google Scholar]
- 28.Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovasc Res. 2006;69:666–676. doi: 10.1016/j.cardiores.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, 3rd, Spinale FG, Gorman RC. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133:1028–1036. doi: 10.1016/j.jtcvs.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 30.Schmoker JD, McPartland KJ, Fellinger EK, Boyum J, Trombley L, Ittleman FP, Terrien C, 3rd, Stanley A, Howard A. Matrix metalloproteinase and tissue inhibitor expression in atherosclerotic and nonatherosclerotic thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;133:155–161. doi: 10.1016/j.jtcvs.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Tamarina NA, McMillan WD, Shively VP, Pearce WH. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery. 1997;122:264–271. doi: 10.1016/s0039-6060(97)90017-9. discussion 271-262. [DOI] [PubMed] [Google Scholar]
- 32.Sharony R, Pintucci G, Saunders PC, Grossi EA, Baumann FG, Galloway AC, Mignatti P. Matrix metalloproteinase expression in vein grafts: role of inflammatory mediators and extracellular signal-regulated kinases-1 and -2. Am J Physiol Heart Circ Physiol. 2006;290:H1651–1659. doi: 10.1152/ajpheart.00530.2005. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 34.Alla F, Kearney-Schwartz A, Radauceanu A, Dores S. Das, Dousset B, Zannad F. Early changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetes. Eur J Heart Fail. 2006;8:147–153. doi: 10.1016/j.ejheart.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Tan J, Hua Q, Xing X, Wen J, Liu R, Yang Z. Impact of the metalloproteinase-9/tissue inhibitor of metalloproteinase-1 system on large arterial stiffness in patients with essential hypertension. Hypertens Res. 2007;30:959–963. doi: 10.1291/hypres.30.959. [DOI] [PubMed] [Google Scholar]
- 36.Yasmin, McEniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR, Wilkinson IB. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]


