Abstract
Aims
Sepsis is a major cause of morbidity and mortality in the elderly population. In prior studies, we have shown that in vivo, the inflammatory response in aged animals is exaggerated as compared to young animals and that this response likely accounts for the increased morbidity and mortality. Part of this uncontrolled inflammatory response in sepsis is due to the innate immune response. However, recent studies have shown that the pathogenesis of sepsis is much more complex. The adrenergic autonomic nervous system is now thought to play a key role in modulating the inflammatory response in sepsis. In this study, we hypothesize that not only is the innate immune response enhanced in response to lipopolysaccharide (LPS) in aged animals, but that the adrenergic nervous system also plays a role in the release of excess inflammatory cytokines.
Main Methods
Male Fisher 344 rats (young: 3 months; aged: 24 months) were used. Endotoxemia was induced by intravenous injection of lipopolysaccharide (LPS, 15 mg/kg BW). Splenic tissues were harvested and mRNA and protein were extracted. The protein expression of CD14 and TLR4, key mediators of LPS in the innate response, as well as alpha-2A adrenergic receptor (α2A-AR) and phosphodiesterase 4D (PDE4D), as the means by which the autonomic nervous system exerts its effects were analyzed.
Key Findings
Splenic tissue concentrations of α2A-AR, PDE4D, CD14, TLR4 were significantly increased in septic aged rats as compared to aged sham rats and septic young rats. The increased expression of α2A-AR in septic aged rats was further confirmed by immunohistochemical staining of splenic tissues.
Significance
These data support the hypothesis that not only is the innate immune response increased in aged animals during sepsis, but that there is also an upregulated response of the adrenergic autonomic nervous system that contributes to excess proinflammatory cytokine release.
Keywords: aging, inflammation, endotoxemia, sepsis, α2A-adrenoceptor, CD14, TLR4
Introduction
It is well known that the morbidity and mortality associated with sepsis increases exponentially with age. Approximately 750,000 people in the United States develop sepsis every year, with the majority of patients being 65 years and older (Angus et al. 2001). With the rapidly expanding elderly population, this has become an increasingly major health issue, impacting not just patient care, but health care costs as well. The mechanism by which sepsis exerts its detrimental effects on the elderly remains unclear. Generally, it has been accepted that there is a decline in immune function in the elderly, leading to an inadequate inflammatory response, and therefore resulting in increased morbidity and mortality (Renshaw et al. 2002; Renshaw et al. 2002). This statement is supported by many in vitro studies that show there is a marked decrease in proinflammatory cytokine release in aged animals exposed to LPS (Renshaw et al. 2002). What appears to be the more likely mechanism, however, is that the impairment in immune function is not due to a decreased inflammatory response, but an uncontrolled inflammatory response leading to excess release of proinflammatory cytokines. This is supported by many other prior studies that have shown a marked increase in the release of proinflammatory cytokines, specifically TNF-α and IL-6, in association with more severe tissue injury (Tateda et al. 1996; Wu et al. 2009).
The interaction between the immune system and the autonomic nervous system (ANS) has also become an active area of interest as more and more studies demonstrate the importance of this relationship in the inflammatory response. Studies have shown that during sepsis, there is an increase in the peripheral sympathetic nervous system activity resulting in increased plasma levels of norepinephrine (NE) (Hahn et al. 1995; Yang et al. 2000; Zhou et al. 2005). It is through the α2A-AR that NE is able to exert its effects on the inflammatory response by upregulating TNF-α production in Kupffer cells (KC) (Miksa et al. 2009; Spengler et al. 1990; Yang et al. 2001; Zhou et al. 2001). We have also found that there is a hyperresponsiveness to α2A-AR stimulation by NE due to an increased expression of this receptor (Miksa et al. 2009). Similarly, it has been shown that the administration of α2A-AR antagonists such as rauwolscine or yohimbine protects hepatocellular function and attenuates TNF-α upregulation during early sepsis or after NE administration, underscoring the importance of α2A-AR activation in inflammation (Yang et al. 2001; Yang et al. 2000).
Phosphodiesterase (PDE) inhibitors have also been shown to have important anti-inflammatory effects (Ariga et al. 2004; Torphy 1998). In particular, PDE4 inhibitors have been shown to attenuate TNF-alpha production in mononuclear cells by increasing the cAMP expressed in inflammatory cells (Ariga et al. 2004; Jin and Conti 2002; Sinha et al. 1995; Spengler et al. 1990). In human circulating monocytes, inhibition of PDE4 by rolipram markedly suppresses TNF-α synthesis and release in response to LPS (Torphy 1998). Inhibition of the PDE4 enzyme has been shown to correlate with decreased TNF-α release from LPS-stimulated whole blood, a cellular marker of nonselective PDE4 inhibition (Muise et al. 2002; Robichaud et al. 2002).
We have previously shown that an increase in α2A-AR expression in Kupffer cells is in part responsible for the increased proinflammatory response during sepsis. PDE4A, 4B and 4D have been found to be expressed in most inflammatory cells (Ariga et al. 2004; Giembycz 2000; Houslay et al. 1998; Torphy 1998). It is also well documented that the LPS induced signaling is mediated by the CD14 and the toll-like receptor 4 (TLR4) receptors (Lu et al. 2008). To explore the role of the ANS and the innate immune response in the increased susceptibility to inflammation in aging, we examined the expression of the α2A-AR, PDE4D as well as CD14 and TLR4 in splenic tissues during sepsis.
Material and methods
Experimental animals
Male Fischer-344 rats (young: 3 month-old; aged: 24 month-old) were obtained from the National Institute on Aging (NIA). They were housed in a temperature controlled room, placed on a 12 hour light/dark cycle and fed a standard Purina rat chow diet. Prior to the induction of endotoxemia, the rats were fasted overnight, but were allowed water ad libitum. All experiments were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Animal model of endotoxemia
The male Fischer-344 rats were anesthesized with isoflurane inhalation. The inguinal region was shaved and then washed with 10% povidone-iodine. A short subinguinal incision was made through which the femoral vein was then carefully separated from the artery. The femoral vein was cannulated with a catheter through which a bolus injection of lipopolysaccharide (LPS, 15 mg/kg BW; E.coli 055:B5 in 200-μl normal saline; Sigma, St. Louis, MO) was given. Tissue samples were collected 4 hours after LPS injection.
Determination of protein levels of IL-10 in splenic tissues
Splenic levels of IL-10 were quantified using an enzyme-linked immunosorbent assay (ELISA) kit specifically for rat IL-10 (BD Biosciences, San Diego, CA). Splenic tissues were homogenized in a lysis buffer and the supernatant was collected and the protein concentration was determined using DC protein assay kit (BioRad, Hercules, CA). A 96-well plate was coated with a specific capture primary antibody for rat IL-10. 100 μg protein/well were loaded into the pre-coated plate and the assay was carried out according to the manufacturer’s instructions.
Determination of protein expression of α2A-AR, PDE4D, CD14 and TLR4 in splenic tissues
Protein expression was determined by using Western blot analysis. Tissue samples were lysed and homogenized with lysis buffer and centrifuged at 12,000 g for 15 minutes at 4°C. The samples were then diluted to 1:100 in Tris-buffered saline (TBS; 10 mM Tris-HCl, pH 7.6, 150 mM NaCl) and measured by a DC protein assay kit. 50-60 μg protein was fractionated on 4-12% Bis-Tris gels and transferred to 0.2 μm-nitrocellulose membranes. The nitrocellulose blots were blocked in 5% milk in TBS-0.1% Tween 20 (TBST) for 1 hour. The blots were incubated at 4°C overnight with either 1:300 dilution of anti-α2A-AR, 1:500 dilution of anti-PDE4D, 1:1000 dilution of each of anti-CD14 or anti-TLR4 polyclonal antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The blots were then washed in TBST followed by incubation in 1:10,000 dilution of HRP labeled secondary antibody in 2.5% milk in TBST for 1 hour at room temperature. The blots were then washed and reacted with chemiluminescent peroxidase substrate (ECL Plus, Amersham Biosciences) and exposed to X-ray film. The band densities were measured using the Bio-Rad image system.
α2A-AR immunohistochemistry
The paraffin sections of splenic tissues were dewaxed and rehydrated followed by microwave antigen retrieval and immunostaining procedure as described previously (Wu et al. 2009). The slides were blocked in 3% albumin in TBS, rinsed twice and then incubated with 1:50 dilution of goat anti-α2A polyclonal antibody in TBS-0.2% Triton-X-100-1.5% albumin at 4°C overnight. The slides were then washed, incubated with 1:200 dilution of biotinylated anti-goat IgG (H+L) (Vector Labs, Burlingame, CA) in TBS-1% FBS for 1 hour at room temperature. Vectastain ABC reagent and DAB kit (Vector Labs) were used to reveal the immunohistochemical reaction.
Statistical analysis
All data are expressed as mean ± standard error (SE). They are compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test for multiple group analysis. Differences in values were considered significant if p<0.05.
Results
Splenic TNF-α and IL-6 mRNA expression is increased in septic aged rats
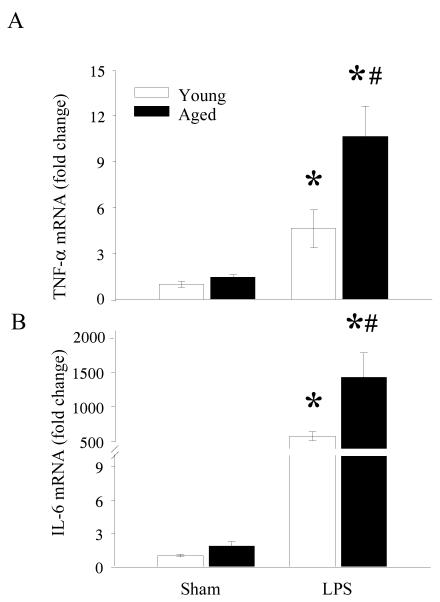
Previously we have shown that plasma levels of TNF-α and IL-6 were significantly elevated in septic aged rats as compared to aged sham or young LPS exposed rats (Wu et al. 2009). These results clearly showed that a more severe hyperinflammatory response occur in aging even in the presence of the same concentrations of LPS. This elevation in plasma levels of cytokine could be caused by the increased production of cytokines from distant organs presumably due to increased susceptibility to LPS. To further determine if spleen is such a distant organ, we examined the mRNA expressions of TNF-α and IL-6 from splenic tissues of young and aged sham and LPS exposed rats. As shown in Figure 1A, TNF-α expression increased from similar sham levels in both young and aged rats to 4 fold and 11 fold in LPS exposed young and aged rats, respectively. Likewise, IL-6 expression was markedly elevated in LPS exposed young and aged rats (Figure 1B). Interestingly, these levels in LPS exposed aged rats were elevated by 2.47 fold as compared to LPS exposed young rats. These data suggest that the observed increase in plasma levels of TNF-α and IL-6 in LPS exposed aged rats could be due to increased production of these cytokines from the spleen in response to LPS.
Figure 1. Increased mRNA expressions of TNF-α and IL-6 in splenic tissues of septic aged rats.
RNA was extracted from splenic tissues of sham and LPS exposed young and aged rats and examined for TNF-α (A) and IL-6 (B) mRNA expressions by real time Q-PCR and presented as fold change over GAPDH. Data are presented as mean ± SE (n=6) and compared by two-way ANOVA and Student-Newman-Keuls method: * p<0.05 versus respective sham; #p <0.05 versus young sham or LPS.
Splenic IL-10 protein expression is increased in septic aged rats
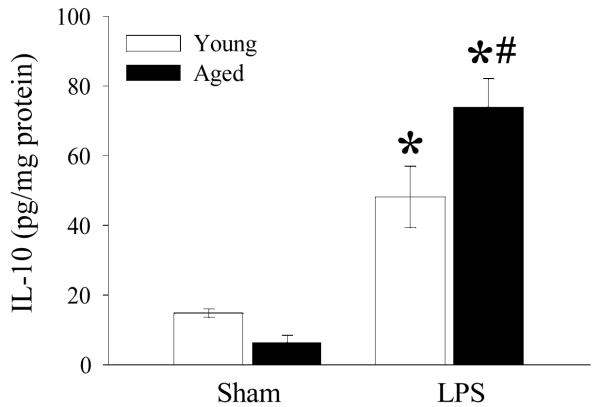
Previously we have shown that splenic TNF-α and IL-6 protein levels were markedly elevated in septic aged rats as compared to septic young rats (Zhou 2010, in press). Given the relationship between cAMP and IL-10 and IL-10’s regulatory effect on TNF-α and IL-6, we examined whether IL-10 protein level is affected by aging and endotoxemia. As shown in Figure 2, IL-10 protein levels, though not significant, were slightly decreased in aged sham rats as compared to young sham rats. In contrast, endotoxemia caused a significant increase of IL-10 in both young and aged rats. Interestingly, IL-10 protein in LPS exposed aged rats was significantly elevated from that of young rats.
Figure 2. Increased protein levels of IL-10 in splenic tissues of septic aged rats.
Protein were extracted from splenic tissues of sham and LPS exposed young and aged rats and analyzed for IL-10 using enzyme linked immunosorbent assay (ELISA) kit. Data are presented as mean ± SE (n=6) and compared by two-way ANOVA and Student-Newman-Keuls method: * p<0.05 versus respective sham; #p <0.05 versus young sham or LPS.
Splenic α2A-AR protein expression is increased in septic aged rats
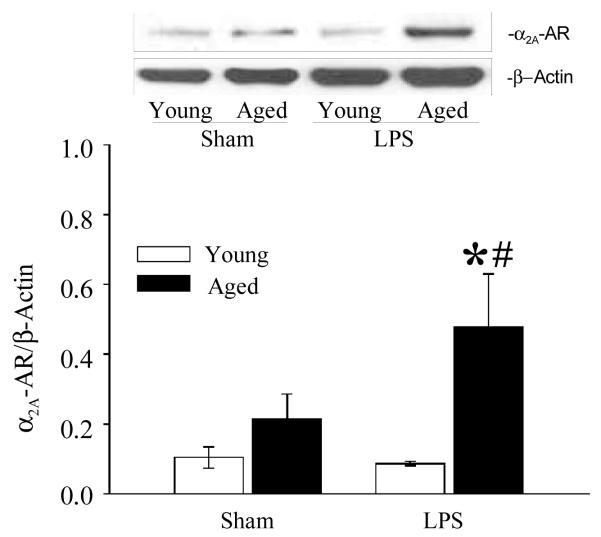
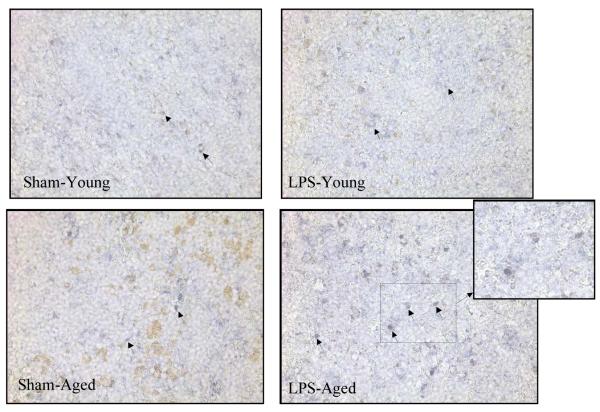
We have previously demonstrated that NE induced hepatic dysfunction in polymicrobial sepsis is mediated by the α2A-AR as evidenced by the increased expression of these receptors in Kupffer cells isolated from septic rats (Miksa et al. 2009). However, it is unknown that α2A-AR expression is altered in the splenic tissue of septic animals and more importantly, if there is any significant change in these receptor expressions between LPS exposed aged and young rats. As shown in Figure 3, protein expression of α2A-AR is significantly increased in LPS exposed aged animals as compared to aged sham or LPS exposed young rats. There is no difference in α2A-AR in LPS exposed young rats as compared to sham young rats. In addition, immunohistochemistry staining of splenic tissue with α2A-AR specific antibodies showed increased expression of α2A-AR in LPS exposed aged rats as compared to aged sham rats (Figure 4). No significant difference was observed in α2A-AR expression between sham and LPS exposed young rats. This supports the role of the α2A-AR pathway by which sepsis leads to increased proinflammatory cytokine release in aged animals as compared to young animals.
Figure 3. Increased expression of α2A-AR in splenic tissues of septic aged rats.
Splenic tissues from sham and LPS exposed young and aged rats were extracted for protein and subjected to Western blotting using anti-α2A-AR antibody. β-actin antibody was used to correct for changes in protein loading. Data are shown as α2A-AR/β-actin ratio. Data are presented as mean ± SE (n=6) and compared by two-way ANOVA and Student-Newman-Keuls method: * p<0.05 versus respective sham; #p <0.05 versus young sham or LPS.
Figure 4. Increased immunohistochemical staining of α2A-AR in splenic tissues of septic aged rats.
Splenic tissues from sham and LPS exposed aged rats were paraffin embedded, sectioned and collected on slides. The slides were prepared for immunohistochemistry and reacted with anti-α2A-AR antibody followed by biotinylated secondary antibody and detected using Vector Kits. Representative photographs of X200 magnification are shown.
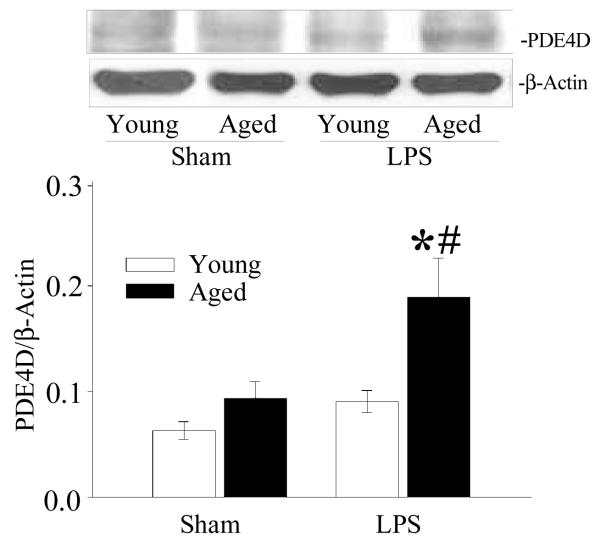
Splenic PDE4D protein expression is increased in septic aged rats
PDE4A, 4B and 4D have been found to be expressed in most inflammatory cells (Ariga et al. 2004; Giembycz 2000; Houslay et al. 1998; Torphy 1998). To examine if PDEs have any specific role in increased susceptibility to inflammation in aged rats, splenic tissues from young and aged sham and LPS exposed rats were examined for PDE4D expression. PDE4D protein expression is increased in LPS exposed aged rats as compared to aged sham rats, and LPS exposed young rats. Again, there is no significant difference in PDE4D protein expression in LPS exposed young rats as compared to young sham rats (Figure 5).
Figure 5. Increased expression of PDE4D in splenic tissues of septic aged rats.
Proteins were extracted from splenic tissues from sham and LPS exposed young and aged rats and examined for PDE4D protein by Western blotting. β-actin antibody was used to correct for changes in protein loading. Data are shown as PDE4D/β-actin ratio. Data are presented as mean ± SE (n=6) and compared by two-way ANOVA and Student-Newman-Keuls method: * p<0.05 versus respective sham; #p <0.05 versus young sham or LPS.
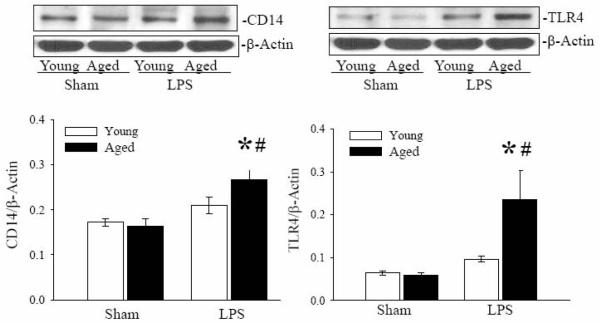
Splenic CD14 and TLR4 protein expressions are increased in septic aged rats
It is well recognized that LPS induced signaling is mediated by the CD14 and TLR4 receptors (Lu et al. 2008). To further delineate the signaling pathway(s) responsible for the susceptibility of inflammation in aged animals, protein expressions of CD14 and TLR4 in splenic tissues of young and aged sham and LPS exposed rats were examined by Western blotting. As shown in Figure 6, CD14 protein expression in splenic tissue was significantly increased in LPS-exposed aged animals as compared to sham and to LPS exposed young rats. CD14 protein expression in LPS exposed young rats as compared to sham rats was slightly increased, but not significant. Protein expression of TLR4 was also significantly increased in LPS exposed aged rats as compared to age-matched sham rats and to LPS exposed young rats. TLR4 expression in young LPS exposed rats was slightly increased as compared to young sham rats, but not significant (Figure 6).
Figure 6. Increased expressions of CD14 and TLR4 in splenic tissues of septic aged rats.
Proteins were extracted from splenic tissues of sham and LPS exposed young and aged rats and examined for CD14 (A) and TLR4 (B) protein by Western blotting. β-actin antibody was used as internal control for protein loading. Data are shown as CD14/β-actin ratio. Data are presented as mean ± SE (n=6) and compared by two-way ANOVA and Student-Newman-Keuls method: * p<0.05 versus respective sham; #p <0.05 versus young sham or LPS.
Discussion
We have previously shown that endotoxemia results in a hyperinflammatory state in aged animals (Wu et al. 2009). This is evidenced by increased plasma levels of liver enzymes (AST and ALT), lactate, TNF-α and IL-6, and more severe tissue injury than septic young rats (Wu et al. 2009). We have also shown that splenic TNF-α and IL-6 protein levels were significantly elevated in endotoxemia aged rats (Zhou et al. in press). In the present study, we show significant increases in the α2A-AR and PDE4D protein expressions and marked elevations in the CD14 and TLR4 protein expressions in the splenic tissues of LPS exposed aged rats as compared to aged sham or LPS exposed young rats. In addition, we show that splenic tissue IL-10 protein was also markedly elevated in LPS exposed aged rats. These results suggest that the increased susceptibility to inflammation in aging could be due to the concerted efforts of the ANS and the innate immune responses.
A study by Takeda et al. 1996 showed that aged mice are approximately 6.5 fold more sensitive to LPS toxicity than young mice and that serum TNF-α and IL-6 levels were significantly higher in aged mice as compared to young mice. Our results are in agreement with the previous study. Our studies showed that TNF-α and IL-6 plasma levels (Wu et al. 2009) were significantly elevated at 4 h after LPS challenge whereas, Takeda et al showed that these levels peaked at 1.5 h and 3 h following LPS administration, respectively. However, the discrepancy in the absolute value of TNF-α is possibly due to the delayed sampling time in our studies (i.e., 4 h vs 1.5 h). The TNF-α levels in the aged, therefore, were already significantly lowered from the peak value but still remained higher than the young rats. In terms of IL-6, the serum levels were comparable to that was observed by Takeda et al. Since splenic mRNA levels were not measured by the other study, it is difficult to compare the two observations.
The data presented herein is exclusively on the spleen of young and aged rats. It is well recognized that spleen is not the sole contributor for the release of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-10 in response to inflammation. In fact, Kupffer cells in the liver play a significant role in host immune responses through the upregulation and release of proinflammatory cytokines (Koo et al. 1999; Wang et al. 1997). Nonetheless, the spleen has a major role in the anti-inflammatory effects produced by the cholinergic system (Tracey 2009). In this regard, exogenous ligands such as LPS bind to receptors on the cells of the innate immune system and cause the release of proinflammatory cytokines. These cytokines activate the sensory arm of the inflammatory response. In parallel, the vagus nerve activates the cholinergic pathway and inhibits the innate immune responses in the spleen through the activation of the nicotinic acetylcholine receptor subunit α7, the receptor for acetylcholine expressed by cytokine producing immune cells. In addition, we have recently shown that milk fat globule EGF factor VIII (MFG-E8) predominately expressed by the spleen, attenuates the systemic inflammatory response in sepsis by enhancing apoptotic cell clearance by phagocytosis (Miksa et al. 2009). Thus, the spleen does play an integral part in systemic inflammatory response mediated by endotoxemia or sepsis.
The role of the ANS in the inflammatory response has been reported (Hahn et al. 1995; Miksa et al. 2009; Yang et al. 2000; Zhou et al. 2005). Studies have shown that during sepsis, there is an increase in the activation of the sympathetic nervous system leading to the release of NE from the gut. The gut released NE is able to activate the α2A-AR at least on Kupffer cells leading to the upregulation of pro-inflammatory cytokines. In addition, polymicrobial sepsis leads to an increased expression of the α2A-AR in Kupffer cells. This increased expression in α2A-AR appears to be correlated with increased release of TNF-α in the circulation of septic rats. In the present study, even though there is no significant increase in α2A-AR in LPS exposed young rats, there is a marked increase in these receptors in the LPS exposed aged rats suggesting a prominent role of these receptors in the inflammatory responses in the aged rats. This upregulation of the α2A-AR may lead to inhibition of cAMP and eventually decreased inhibition of NF-κB and increased production of TNF-α.
Consistent with the increase in α2A-AR expression, we found that PDE4D also is upregulated in LPS exposed aged rats. The increased expression of PDE4D could also lead to inactivation of cAMP and thereby contributes to the hyperinflammatory response as well. These results confirm the hypothesis that the adrenergic system does contribute to the hyperinflammatory response in aged animals by increasing the release of proinflammatory cytokines via the adrenergic pathway. Interestingly enough, while PDE4 has been shown to be critical in the inflammatory response, the isoform PDE4B specifically has been shown to be critical for LPS-activation of TNF-α. In the same study, PDE4D knockout mice were shown not affect the TNF-α levels (Robichaud et al. 2002). However, the specific role of PDE4D and its effects on outcomes in the septic response has yet to be fully elucidated.
PDE inhibitors have shown to possess anti-inflammatory effects (Ariga et al. 2004; Torphy 1998). These inhibitors are currently in clinical use for patients with depression (Hebenstreit et al. 1989), chronic obstructive pulmonary disease (Calverley et al. 2007; Rabe et al. 2005) and allergic asthma (Timmer et al. 2002). In animal models of septic shock, treatment with PDE inhibitor rolipram or the use of PDE4B knockout (Jin et al. 2005) ameliorated the inflammatory responses of peripheral blood cells and improved survival. Due to the fact that increased expression of PDE4D was observed in endotoxemic aged rats as compared to that of young rats, it is plausible that PDE4D inhibitor can be, in part, beneficial for reducing the hyperinflammatory response generally observed during endotoxemia in aging. Further studies are warranted for such a concept.
TLR4 and CD14 are crucial in mediating the effects of LPS. Our data show significant increase in expression of these receptors in aged animals exposed to LPS as compared to their sham controls and to young animals exposed to LPS. The finding of TLR4 and CD14 being upregulated during sepsis in aged animals is significant because prior studies have found that TLR4 expression is decreased in vitro and was postulated to be the reason for poor adaptive immune responses in aging (Renshaw et al. 2002). In contrast, our data suggest that instead of a decreased inflammatory response to LPS, there is an overactive response with likely uncontrolled recruitment of anti-inflammatory cells and release of inflammatory cytokines.
Whether the increase in TLR4 expression in LPS exposed aged rats translates to the direct action of α2A receptor signaling remains to be determined. We have previously shown that NE potentiates LPS-induced TNF-α release via the α2A receptor both in vivo and in vitro (Miksa et al. 2009). In this regard, endotoxemia itself or in concert with α2A signaling may upregulate these receptors.
Clearly, sepsis exerts its deleterious effects partly through the release of inflammatory cytokines, such as TNF-α, IL-6 and IL-10, regardless of age. It is generally accepted that sepsis upregulates TNF-α and IL-6; but IL-10 has been regarded as an anti-inflammatory cytokine. Studies have shown that cAMP elevating agents, PDE inhibitors such as rolipram and cicaprost enhance IL-10 synthesis by peripheral blood mononuclear cells while suppressing TNF production (Eigler et al. 1998). In contrast, another study showed that cAMP elevating agent, rolipram, inhibited IL-10 production by normal peripheral lymphocytes stimulated by T cell receptor mediated signaling (Liopeta et al. 2009). This discrepancy in IL-10 production could be cell type and stimulus specific. Nevertheless, our data showed that IL-10 protein levels were significantly elevated in the spleen of endotoxemic aged rats and this elevation was concurrent with splenic TNF-α and IL-6 protein increases. Our results are in agreement with others that showed while IL-10 dependent anti-inflammatory control and downregulation of TNF-α appears to be maintained in normal animals, endotoxemia in aging tend to loss this direct correlation (Donoso et al. 2008; Tateda et al. 1996).
It has been well regarded that the LPS model of sepsis does not adequately reflect the changes found in human sepsis. In fact, a number of studies by Ayala et al. (Ayala et al. 1994; Ayala et al. 1995; Ayala et al. 2000) showed that the immune dysfunction induced in sepsis by cecal ligation and puncture (CLP) in mice, a model that closely resembles peritonitis in humans, is not caused by endotoxin alone but is also due to the necrotic tissue and other microbial components. Nonetheless, LPS or endotoxin is an integral component of the systemic inflammatory response. Exposure to high doses of LPS produces the inflammatory response comparable to what is observed in CLP sepsis. However, it is questionable that LPS level that are seen in CLP sepsis is sufficient to induce the inflammatory response generally observed in CLP sepsis.
In agreement with our studies, others have shown that pro-inflammatory cytokines, TNF-α and IL-6 are hyperproduced after inflammatory challenges in aged humans (Bruunsgaard et al. 2001), rats (Donoso et al. 2008) and mice (Tateda et al. 1996). These studies indicated that in aging, there is an elevation of the maximum expression of these cytokines and appears to be a time delay in the induction of these cytokines in response to LPS challenge. However, the complexity of the results obtained in these studies made it difficult to conclude age-related effects in the regulatory mechanisms of cytokine production. In the present study, we suggest that the upregulation of α2A adrenergic and the CD14/TLR4 pathways lead to a synergistic release of inflammatory cytokines, likely leading to a prolonged inflammatory response, associated with increased tissue injury, and hence, morbidity and mortality. The synergistic upregulation of the two pathways may be responsible for the hyperinflammatory response that occurs in endotoxemia and aging.
Conclusion
Our study shows two distinct pathways by which endotoxemia induced immune response is enhanced in aging, one by the innate immune response mediated by the CD14/TLR4 signaling, and the other by the activation of the sympathetic nervous system. The finding of having multiple pathways activated during sepsis in aged animals suggests that targeting these pathways may overcome the hyperinflammatory response observed in aging. Further studies are warranted for such conclusions.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) grants R01 AG028352 and R01 GM053008 (PW). The authors wish to thank Weifeng Dong for his expert technical assistances.
Footnotes
Conflict of Interests None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, Jin SL, Conti M. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. Journal of Immunology. 2004;173(12):7531–7538. doi: 10.4049/jimmunol.173.12.7531. [DOI] [PubMed] [Google Scholar]
- Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-gamma release while increasing IL-4/IL-10 production. Journal of Surgical Research. 1994;56(6):579–585. doi: 10.1006/jsre.1994.1092. [DOI] [PubMed] [Google Scholar]
- Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Does endotoxin play a major role in inducing the depression of macrophage function during polymicrobial sepsis? Archives of Surgery. 1995;130(11):1178–1184. doi: 10.1001/archsurg.1995.01430110036007. discussion 1184-1175. [DOI] [PubMed] [Google Scholar]
- Ayala A, Song GY, Chung CS, Redmond KM, Chaudry IH. Immune depression in polymicrobial sepsis: the role of necrotic (injured) tissue and endotoxin. Critical Care Medicine. 2000;28(8):2949–2955. doi: 10.1097/00003246-200008000-00044. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Current Opinions in Hematology. 2001;8(3):131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2007;176(2):154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- Donoso V, Gomez CR, Orriantia MA, Perez V, Torres C, Coddou C, Nelson P, Maisey K, Morales B, Fernandez R, Imarai M, Huidobro-Toro JP, Sierra F, Acuna-Castillo C. The release of sympathetic neurotransmitters is impaired in aged rats after an inflammatory stimulus: a possible link between cytokine production and sympathetic transmission. Mechanism of Ageing and Development. 2008;129(12):728–734. doi: 10.1016/j.mad.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigler A, Siegmund B, Emmerich U, Baumann KH, Hartmann G, Endres S. Anti-inflammatory activities of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. Journal of Leukocyte Biology. 1998;63(1):101–107. doi: 10.1002/jlb.63.1.101. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. Phosphodiesterase 4 inhibitors and the treatment of asthma: where are we now and where do we go from here? Drugs. 2000;59(2):193–212. doi: 10.2165/00003495-200059020-00004. [DOI] [PubMed] [Google Scholar]
- Hahn PY, Wang P, Tait SM, Ba ZF, Reich SS, Chaudry IH. Sustained elevation in circulating catecholamine levels during polymicrobial sepsis. Shock. 1995;4(4):269–273. doi: 10.1097/00024382-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Hebenstreit GF, Fellerer K, Fichte K, Fischer G, Geyer N, Meya U, Sastre-y-Hernandez M, Schony W, Schratzer M, Soukop W, et al. Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry. 1989;22(4):156–160. doi: 10.1055/s-2007-1014599. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Sullivan M, Bolger GB. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. Advances in Pharmacology. 1998:44225–342. doi: 10.1016/s1054-3589(08)60128-3. [DOI] [PubMed] [Google Scholar]
- Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. Journal of Immunology. 2005;175(3):1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proceedings of National Academy of Sciences U S A. 2002;99(11):7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo DJ, Chaudry IH, Wang P. Kupffer cells are responsible for producing inflammatory cytokines and hepatocellular dysfunction during early sepsis. Journal of Surgical Research. 1999;83(2):151–157. doi: 10.1006/jsre.1999.5584. [DOI] [PubMed] [Google Scholar]
- Liopeta K, Boubali S, Virgilio L, Thyphronitis G, Mavrothalassitis G, Dimitracopoulos G, Paliogianni F. cAMP regulates IL-10 production by normal human T lymphocytes at multiple levels: a potential role for MEF2. Molecular Immunology. 2009;46(3):345–354. doi: 10.1016/j.molimm.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Miksa M, Das P, Zhou M, Wu R, Dong W, Ji Y, Goyert SM, Ravikumar TS, Wang P. Pivotal role of the alpha(2A)-adrenoceptor in producing inflammation and organ injury in a rat model of sepsis. PLoS One. 2009;4(5):e5504. doi: 10.1371/journal.pone.0005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y, Wang Z, Wang H, Ravikumar TS, Tracey KJ, Wang P. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor VIII. Journal of Immunology. 2009;183(9):5983–5990. doi: 10.4049/jimmunol.0802994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muise ES, Chute IC, Claveau D, Masson P, Boulet L, Tkalec L, Pon DJ, Girard Y, Frenette R, Mancini JA. Comparison of inhibition of ovalbumin-induced bronchoconstriction in guinea pigs and in vitro inhibition of tumor necrosis factor-alpha formation with phosphodiesterase 4 (PDE4) selective inhibitors. Biochemical Pharmacology. 2002;63(8):1527–1535. doi: 10.1016/s0006-2952(02)00903-6. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast--an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366(9485):563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. Journal of Immunology. 2002;169(9):4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, Liu S, Huang Z, Conti M, Chan CC. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. Journal of Clinical Investigation. 2002;110(7):1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Semmler J, Eisenhut T, Eigler A, Endres S. Enhanced tumor necrosis factor suppression and cyclic adenosine monophosphate accumulation by combination of phosphodiesterase inhibitors and prostanoids. European Journal of Immunology. 1995;25(1):147–153. doi: 10.1002/eji.1830250125. [DOI] [PubMed] [Google Scholar]
- Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. Journal of Immunology. 1990;145(5):1430–1434. [PubMed] [Google Scholar]
- Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infection and Immunity. 1996;64(3):769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer W, Leclerc V, Birraux G, Neuhauser M, Hatzelmann A, Bethke T, Wurst W. The new phosphodiesterase 4 inhibitor roflumilast is efficacious in exercise-induced asthma and leads to suppression of LPS-stimulated TNF-alpha ex vivo. Journal of Clinical Pharmacology. 2002;42(3):297–303. doi: 10.1177/00912700222011328. [DOI] [PubMed] [Google Scholar]
- Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. American Journal of Respiratory and Critical Care Medicine. 1998;157(2):351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nature Reviews, Immunology. 2009;9(6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Ba ZF, Chaudry IH. Mechanism of hepatocellular dysfunction during early sepsis. Key role of increased gene expression and release of proinflammatory cytokines tumor necrosis factor and interleukin-6. Archives of Surgery. 1997;132(4):364–369. doi: 10.1001/archsurg.1997.01430280038005. discussion 369-370. [DOI] [PubMed] [Google Scholar]
- Wu R, Zhou M, Dong W, Ji Y, Miksa M, Marini CP, Ravikumar TS, Wang P. Ghrelin hyporesponsiveness contributes to age-related hyperinflammation in septic shock. Annals of Surgery. 2009;250(1):126–133. doi: 10.1097/SLA.0b013e3181ad85d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Koo DJ, Zhou M, Chaudry IH, Wang P. Gut-derived norepinephrine plays a critical role in producing hepatocellular dysfunction during early sepsis. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2000;279(6):G1274–1281. doi: 10.1152/ajpgi.2000.279.6.G1274. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhou M, Chaudry IH, Wang P. Norepinephrine-induced hepatocellular dysfunction in early sepsis is mediated by activation of alpha2-adrenoceptors. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2001;281(4):G1014–1021. doi: 10.1152/ajpgi.2001.281.4.G1014. [DOI] [PubMed] [Google Scholar]
- Zhou M, Das P, Simms HH, Wang P. Gut-derived norepinephrine plays an important role in up-regulating IL-1beta and IL-10. Biochimica et Biophysica Acta. 2005;1740(3):446–452. doi: 10.1016/j.bbadis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Zhou M, Yang S, Koo DJ, Ornan DA, Chaudry IH, Wang P. The role of Kupffer cell alpha(2)-adrenoceptors in norepinephrine-induced TNF-alpha production. Biochimica et Biophysica Acta. 2001;1537(1):49–57. doi: 10.1016/s0925-4439(01)00055-2. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wu R, Dong W, Leong J, Wang P. Acclerated apoptosis contributes to aging-related hyperinflammation in endotoxemia. International Journal of Molecular Medicine. 2010 doi: 10.3892/ijmm_00000424. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]