Abstract
Prostate cancer vaccines attempt to induce clinically relevant, cancer-specific systemic immune responses in patients with prostate cancer and represent a new class of targeted, nontoxic therapies. With a growing array of vaccine technologies in preclinical or clinical development, autologous antigen-presenting cell vaccines loaded with the antigen, prostate acid phosphatase, and poxvirus vaccines targeting prostate-specific antigen have recently demonstrated a significant survival benefit in randomized trials of patients with metastatic castration-resistant prostate cancer, whereas others have failed to demonstrate any benefit. The combination of vaccines with chemotherapy, radiotherapy, and other biologic agents is also being evaluated. Efforts to optimize vaccine approaches and select ideal patient populations need to continue to build on these early successes.
Key words: Immunotherapy, Vaccine, Prostate cancer
The conventional initial systemic therapy for locally advanced or metastatic prostate cancer (PCa) is androgen deprivation therapy (ADT). However, ADT is associated with deleterious effects on quality of life and cardiovascular and bone health. The disease then progresses inexorably to a phase when ADT alone fails to control the malignancy despite castrate testosterone levels, and is termed castration-resistant prostate cancer (CRPC). Currently, docetaxel chemotherapy is accepted as the conventional frontline chemotherapy for metastatic CRPC based on randomized phase III trials that demonstrated a modest extension of median survival of approximately 2 to 3 months over controltreated patients.1,2 Ongoing frontline randomized trials are evaluating the value of combining docetaxel with biologic agents (eg, bevacizumab, aflibercept, atrasentan, ZD4054, dasatinib). Effective salvage therapy following prior docetaxel is lacking, as only modest efficacy has been demonstrated with mitoxantrone or ixabepilone. Novel agents targeting the androgen pathway, including abiraterone and MDV3100, have shown promise in this setting and are both currently in phase III trials in both chemotherapy-treated and chemotherapy-naive patients. Given the elderly male population with CRPC with a multitude of attendant comorbidities, nontoxic and targeted agents are desirable and are under investigation. This review discusses emerging vaccines for the therapy of PCa.
Rationale for Vaccine Therapy in Androgen-Independent Prostate Cancer
Immunoregulatory Pathways
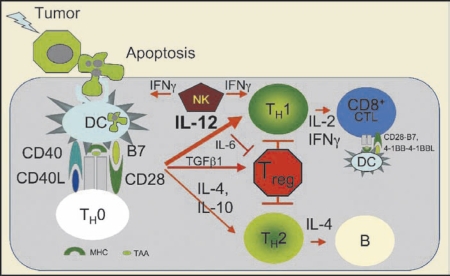
Improved knowledge of immunoregulatory pathways has enabled novel immunotherapeutic agents including vaccines.3,4 Endogenous protein-derived peptides, including tumor antigens, are “cross-presented” on the surface of antigen-presenting cells (APCs) (including dendritic cells [DCs], the most effective APC) in the context of major histocompatibility complex (MHC) class I molecules to T-cell receptors (TCR) on cytotoxic CD8-expressing T lymphocytes (CTLs) (Figure 1). A second set of endogenous, largely nonoverlapping peptides is presented in the context of MHC class II molecules to TCRs on helper CD4-expressing T lymphocytes, which are required for a maximum CTL response and optimum establishment of long-lived antigenic “memory.” Another set of stimulatory antigen-independent interactions occurs between B7 (B7.1/CD80 and B7.2/CD86) on APCs and CD28 on Tcells. Additional B7-related (eg, ICOSL to ICOS/CD278) and B7-unrelated interactions (eg, 4-1BBL/CD137L to 4-1BB/CD137) can also contribute to fine-tuning immunity. Conversely, interaction between B7 and CTLA-4/CD152 (cytotoxic T-lymphocyte-associated antigen) engenders immune-inhibitory signals leading to tolerance. The balance between stimulatory and inhibitory signals modulates T-cell activation and the corresponding immune response.
Figure 1.
Immunoregulatory pathways. Proapoptotic stimuli (radiation, chemotherapy) trigger apoptosis of tumor cells and phagocytosis by antigen-presenting dendritic cells (DCs). Tumor-associated antigens (TAA) are processed and presented by major histocompatibility complex (MHC) class II molecules on the DC surface to naïve CD4}+ thelper T cells (TH0). A subset of peptides is also delivered into the cytoplasm of DCs and “cross-presented” by MHC class I molecules to CD8+ T cells. If the DCs are sufficiently activated, they upregulate costimulatory B7 (CD80, CD86) and the CD40 receptor. If a cognate T cell exists, it is activated by the combination of MHC-peptide and costimulation, leading to upregulation of CD40L, which provides a retrograde signal for optimum activation of DCs. Fully activated or “licensed” DCs make TH1 polarizing cytokines, like interleukin (IL)-12, which drive differentiation to the TH1 cytokine-producing pathway. Subsequently, activated DCs can activate TAA-specific CD8+ T cells, which proliferate and differentiate into cytotoxic T lymphocytes. Insufficient DC activation can lead to polarization of helper TH2 cells (which favors humoral immunity, ie, B cells) and expansion of inhibitory regulatory Treg cells. IFN, interferon; TGF, transforming growth factor.
Tumor-Associated Antigens
Tumor-associated antigens (TAAs) are usually self-antigens that are poorly or nonimmunogenic owing to the induction of self-tolerance via several mechanisms. Tumors can downregulate MHCs and are enriched for “Treg,” or regulatory CD4(+)CD25(hi) T-cells that can downregulate the immune response.5 Tumor escape is accomplished through the activation of molecular mechanisms that inhibit immune cell functions or induce apoptosis of immune effector cells. For example, myeloid-derived suppressor cells (MDSCs) can accumulate within tumors and release reactive oxygen and nitrogen species and arginase that are toxic to T cells.6 Development of effective vaccines that can induce a powerful tumor antigenspecific immune response must overcome these barriers. Tumor antigens chosen for targeting should be ideally expressed exclusively on tumor cells, so that an antigen-specific immune response may target the tumor and avoid exposing patients to toxicities typically observed with DNA-replication-targeting or other less targeted chemotherapeutic approaches. Usually, tumor antigens are tissue/organ specific (ideally a nonessential organ like the prostate) rather than tumor specific (eg, prostate-specific antigen [PSA] and prostate-specific membrane antigen [PSMA], expressed in both PCa and normal prostate tissue) or overexpressed antigens also found in other normal tissues not bearing the tumor (eg, MUC-1, EGFR, CEA). Administration of adjuvants (eg, granulocyte-macrophage colonystimulating factor [GM-CSF] or Tolllike receptor [TLR] ligands) may further bolster the immune response.
Dendritic Cell Vaccines
Vaccines consisting of autologous antigen-presenting cells, including DCs manipulated to enhance the presentation of tumor antigens to CTLs, have advanced to mature stages of clinical development. DCs are efficient APCs that express several costimulatory molecules that participate in the activation of T cells.7 Mature DCs can be generated in the laboratory by exposing multipotent CD34+ hematopoietic progenitor cells first to stem cell factor (SCF) and FLT3 ligand and second to GM-CSF, interleukin (IL)-4 and tumor necrosis factor (TNF)-α or by exposing myeloid progenitor CD14+ cells to GM-CSF and IL-4, which can then be pulsed with the TAA. The desired results are APCs/DCs presenting both MHC-I- and MHC-II-derived TAA on the cell surface. The most common ex vivo technique is to pulse DCs with TAA proteins or peptides, which are then phagocytosed, processed, and presented by the DCs, or with messenger RNA (mRNA) of the TAA or derived from tumor cells, enabling the cell’s own genetic machinery to produce the TAA proteins, enhancing presentation by the MHC-I pathway. The optimal method of production, and the route and schedule of administration of DC vaccines, are unknown and may vary depending on the target cancer type.
Sipuleucel-T
Sipuleucel-T (Provenge®; APC8015, Dendreon Corp, Seattle, WA) is a cellular product consisting of autologous peripheral blood mononuclear cells obtained by leukapheresis and enriched for a CD54+ DC fraction pulsed with PA2024, a prostatic acid phosphatase (PAP)-GM-CSF construct.8 GM-CSF functions to enable efficient GM-CSF receptor-mediated uptake of the PAP antigen moiety. Following promising results in early trials, 127 previously untreated men with asymptomatic, metastatic CRPC were randomized 2:1 in a phase III clinical trial (D9901) to receive sipuleucel-T or placebo as intravenous (IV) infusions every 2 weeks × 3.9 Crossover to the vaccine was allowed for progressing placebo patients. Eligible patients were not on steroids, had no visceral metastasis, and > 25% of cancer cells were required to be positive for the expression of PAP. The primary endpoint of time to progression (TTP) displayed a trend to statistical significance for the superiority of sipuleucel-T (P = .052). The median overall survival was 25.9 months for those on sipuleucel-T compared with 21.4 months for those on placebo (P = .01). At the preplanned 3-year survival analysis, 34% of sipuleucel-T-treated patients were alive compared with 11% of placebo-treated patients (P = .0046). PCa-specific survival was also improved with a hazard ratio (HR) of 2.04 (P = .002). Concerns were raised regarding the relatively small size of the study and a potential imbalance in prognostic factors. However, after adjusting for 8 prognostic factors, therapy with sipuleucel-T remained a significant predictor of survival benefit (P = .002). In addition, a similar proportion of patients in both arms received docetaxel or other chemotherapy following sipuleucel-T. This trial reinforced questions about the utility of TTP as an appropriate endpoint in vaccine trials (ie, progression may occur before the biologic effect of vaccines) and the appropriateness of a vaccine approach for rapidly progressing patients. Treatment was generally well tolerated and low-grade fever and rigor were the most common adverse events. Sipuleucel-T patients also induced an average 8-fold increase in the T-cell stimulation index ratio (counts per minute with antigen/counts per minute without antigen, T-cell proliferation to sipuleucel-T was evaluated by 3H-thymidine uptake). The D9902A trial, which was originally designed to be the companion randomized study to D9901, was discontinued in 2002 after 98 patients were enrolled.10 Analysis showed a trend toward improved survival in patients treated with sipuleucel-T compared with placebo (19.0 vs 15.7 months; HR 1.27; P = .331). The 36-month survival in the sipuleucel-T group was 50% higher than in the placebo group (31.6% vs 21.2%). The D9902A protocol was amended to become the D9902B or IMmunotherapy for Prostate AdenoCarcinoma Treatment (IMPACT) pivotal double-blind, randomized, phase III study (Table 1). The IMPACT trial randomized 512 men with asymptomatic chemonaive metastatic CRPC in a 2:1 ratio to sipuleucel-T or placebo IV infusions every 2 weeks × 3 in a 2:1 ratio (Table 1). A presentation at the 2009 American Urological Association annual meeting reported that the median survival was 25.8 months with sipuleucel-T compared with 21.7 months with placebo, and the 3-year survival also improved significantly (31.7% vs 23.0%; P = .032). The treatment effect remained consistent after adjustment for docetaxel use following investigational therapy. PCa-specific survival also favored the sipuleucel-T arm. However, once again, there was no significant delay in the time to objective disease progression. Toxicities were manageable, with chills reported in 54.1% of patients (vs 12.5% with placebo), fever in 29.3% (vs 13.7% with placebo), headache in 16.1% (vs 5% with placebo), and flu-like symptoms in 9.8% (vs 4.3% with placebo).11 Formal approval by regulatory agencies is anticipated based on these data. In addition, sipuleucel-T alone or in combination with bevacizumab appears feasible and active in patients with castration-sensitive nonmetastatic PCa with PSA progression.12,13
Table 1.
Recent Frontline Randomized Trials of Vaccines for CRPC
| Institution (Trial) | Population | Standard Arm | Experimental Arm | Results |
| Multicenter (IMPACT or D9902B) | Asymptomatic metastatic chemonaïve CRPC | Placebo | Sipuleucel-T | Improved overall survival with vaccine |
| Multicenter (VITAL-1) | Asymptomatic metastatic chemonaïve CRPC | Docetaxel +prednisone | GVAX | Closed prior to completion for futility |
| Multicenter (VITAL-2) | Symptomatic metastatic chemonaïve CRPC | Docetaxel +prednisone | GVAX + docetaxel | Closed after interim analysis showed inferiority for vaccine arm |
| Multicenter phase II randomized trial | Asymptomatic metastatic chemonaïve CRPC | Placebo | Prostvac + GM-CSF | Improved survival with vaccine |
| ECOG (PARADIGM) | Nonmetastatic CRPC | GM-CSF | Prostvac + GM-CSF | Planned |
CRPC, castration-resistant prostate cancer; ECOG, European Cooperative Oncology Group; GM-CSF, granulocyte-macrophage colony-stimulating factor. GVAX is manufactured by BioSante Pharmaceuticals, Lincolnshire, IL..
Genetically Modified DC Vaccines Designed to Enhance Tumor Immunogenicity
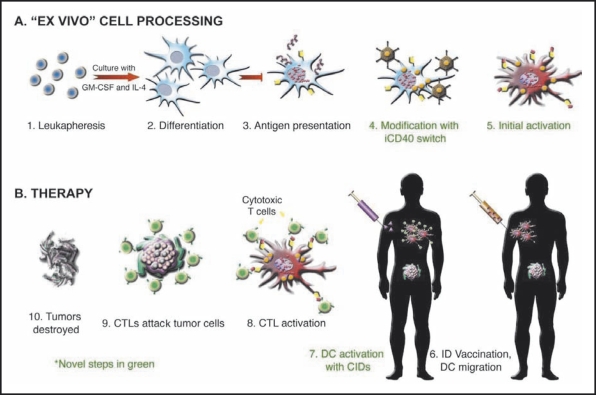
DC vaccines employing autologous DCs pulsed with PSMA, PSA, or telomerase reverse transcription (hTERT) have demonstrated activity and immunogenicity in early trials.14–16 CD40, a receptor of the TNF family, plays a critical role in the priming and activation of DCs, and is an attractive target for manipulation to augment antigen presentation. Some investigators have shown that CD40 agonistic monotherapy is sufficient for the induction of an effective immune response.17 Unlike other DC-expressed receptors that interact with the proinflammatory cytokines or pathogen-associated molecules that DCs encounter throughout the periphery, the DC-expressed CD40 receptor is engaged by CD4+ T-helper cells within the lymph node paracortex through its cognate ligand, CD40L.18,19 This signal enhances the expression of antigen-presenting and costimulatory molecules, soluble cytokines, and several antiapoptotic molecules, ultimately enabling DCs to activate CTLs. Recent studies have also shown that CD40 stimulation enables DCs to cross-present antigen and overcome peripheral T-cell tolerance. In one ongoing effort to enhance immunogenicity of an autologous DC vaccine, a potent, druginducible CD40 (iCD40) receptor was engineered that permits temporally controlled, lymphoid-localized, DCspecific activation.20 iCD40 is composed of a membrane-localized cytoplasmic domain of CD40 fused to drug-binding domains, allowing it to respond to a lipid-permeable, high-affinity dimerizer drug (AP1903) while circumventing ectodomain-dependent negative-feedback mechanisms. These modifications permit prolonged activation of iCD40-expressing DCs in vivo, resulting in more potent CD8(+) T-cell effector responses, including the preclinical eradication of previously established solid tumors, relative to the standard clinical practice of ex vivo activation (P < .01). In addition, iCD40-mediated DC activation exceeded that achieved by stimulating the full-length, endogenous CD40 receptor both in vitro and in vivo. Because iCD40 is insulated from the extracellular environment and can be activated within the context of an immunologic synapse, iCD40-expressing DCs have a prolonged lifespan and should lead to more potent vaccines, perhaps even in immune-compromised patients. Phase I safety and dose range-finding studies with AP1903 have shown that this dimerizing agent reached effective serum concentrations without generating adverse side effects.20 An open phase I/IIa clinical trial at the University of Texas Health Science Center-Houston is evaluating the intradermal administration of an autologous DC vaccine pulsed with a form of PSMA and transduced with inducible human (ih)-CD40, followed 24 hours later by IV infusion of AP1903, in men with up to one prior systemic regimen for metastatic CRPC (Figure 2). In a related and potentially synergistic approach designed to enhance DC survival, introduction of activated Akt into DCs holds potential for enhancing the efficacy of DC vaccines.21
Figure 2.
Design of phase I clinical trial to evaluate a novel intradermal (ID) autologous dendritic cell (DC) vaccine pulsed with prostate-specific membrane antigen and transduced with inducible CD40 (iCD40) followed 24 hours later by dimerizing/activating agent AP1903. CID, chemical inducer of dimerization; CTL, cytotoxic T lymphocytes; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin.
GM-CSF-Modified Tumor Cell Vaccines
GM-CSF stimulates myeloid progenitor cells and induces antitumor immunity and has been demonstrated to have biologic activity in metastatic CRPC as well as biochemical hormonenaive disease.22 An increase in the number of circulating monocytes and DCs was observed after 14 days of GM-CSF treatment. Patients on long-term GM-CSF tended to have lower initial T stage, Gleason score, and pretreatment PSA, suggesting that lower stage and more indolent disease may optimally benefit from immunotherapy. Although irradiated autologous tumor cell vaccines transfected with the GM-CSF gene have exhibited immunogenicity and antitumor activity in small trials, the need for harvesting an adequate number of autologous tumor cells followed by ex vivo manipulation is onerous. The GM-CSF-secreting vaccine GVAX (Cell GeneSys, Inc., South San Francisco, CA; now part of BioSante Pharmaceuticals) was a mixture of the PCa cell lines PC-3 and LNCaP transduced with a replication-defective retrovirus containing cDNA for GM-CSF and then irradiated. In an earlier trial, GVAX platform-based immunotherapy was administered to 34 patients with metastatic chemonaive CRPC.23 This trial demonstrated a complete PSA response (PSA level dropped to 0.1 ng/mL) in 1 patient, a reduced PSA velocity in 73% of patients, stabilized or decreased levels of a biomarker of osteolytic activity in 69% of patients, and produced median survival times of 34.9 and 24 months with the high and low doses of immunotherapy, respectively. The agent was subsequently modified to increase GM-CSF production. A phase I–II, multicenter, open-label study was designed to characterize the safety and activity of this modified product in patients with metastatic CRPC.24 Eighty men with progressive asymptomatic, chemotherapy-naive PCa with castration-resistant disease were treated with different dose levels of the vaccine product. The most common adverse effect was injection-site erythema and a maximal tolerated dose was not established. The median survival time was 35 months in the high-dose group, 20 months in the mid-dose, group, and 23.1 months in the low-dose group. However, data on administration of postvaccine docetaxel were unavailable and may have affected outcomes and there was no control arm that did not receive GVAX. PSA stabilization occurred in 15 patients (19%), and a > 50% decline in PSA was seen in 1 patient. The proportion of patients who generated an antibody response to 1 or both cell lines increased with dose and included 10 of 23 (43%) in the low-dose group, 13 of 18 (72%) in the mid-dose group, and 16 of 18 (89%) in the high-dose group. Serum GMCSF levels peaked 1 to 3 days post-treatment and transient increases in white blood cells, neutrophils, and eosinophils were observed, thereby confirming bioactivity of GM-CSF, and all levels returned to baseline before subsequent treatment.
GVAX was then re-engineered to secrete 5- to 10-fold higher levels of GM-CSF in an attempt to improve responses. A phase III trial (VITAL-1) was scheduled to randomize 600 metastatic CRPC patients without pain to GVAX or docetaxel/prednisone, and another phase III trial (VITAL-2) was designed to evaluate GVAX plus docetaxel compared with docetaxel/prednisone in metastatic CRPC patients with pain (Table 1). Disappointingly, preliminary analysis of the VITAL-2 trial demonstrated a survival advantage for docetaxel/prednisone over GVAX/docetaxel.25 The study was prematurely terminated after accrual of 408 patients due to an imbalance in deaths, with 67 deaths in the GVAX/docetaxel and 47 deaths in the standard arm. Overall survival was shorter in the GVAX-containing arm with median survival of 12.2 months versus 14.1 months (P = .0076). An unplanned futility analysis of the VITAL-1 trial was conducted by the Independent Data Monitoring Committee (IDMC) following the termination of VITAL-2, which indicated that the trial had less than a 30% chance of meeting its predefined primary endpoint of an improvement in survival.26 Therefore, this trial, which was fully enrolled in 2007 with 626 patients, was also terminated. In another recent trial, GVAX demonstrated activity in hormone-naive patients with PSA relapse.27 Novel combination approaches with other immunotherapeutic agents, for example, GVAX plus ipilimumab, a monoclonal antibody (mAb) that targets CTLA-4, demonstrated activity, although endocrinopathy with hypophysitis was observed at larger doses.28 However, further directions for the clinical development of GVAX PCa remain unclear owing to the negative results from the large phase III trials.
Poxvirus Vaccines
The use of viral vaccines offers several potential advantages, including the inherent immunogenicity of the virus and high levels of gene expression. The poxviruses represent a family of related double-stranded DNA viruses distinguished by their host specificity and have been extensively studied as vaccines in preclinical models.29 Similar to other poxviruses, the vaccinia virus replicates within the cytoplasm of infected cells and induces cell lysis, releasing new virion capable of infecting surrounding cells. The host immune response to vaccinia virus, including foreign transgenes expressed by recombinant vectors, includes strong neutralizing antibody titers and a significant cell-mediated T-cell response. The ability to express large eukaryotic genes, induce potent immunity, and lack of nuclear integration suggested that recombinant poxviruses could be useful for vaccines targeting highly specific antigens. The rapid appearance of strong neutralizing antibodies against the vaccinia vector itself appeared to inhibit the ability to boost immunity against weak foreign transgenes expressed by recombinant vectors. The avipox viruses are a family of poxviruses that infect birds and are unable to replicate in mammalian cells. Because infection with avipox viruses does not produce new virions, the degree of neutralizing antibodies generated following mammalian infection is quite low. This allows viral particles to persist for a longer period of time and express foreign transgenes resulting in significantly enhanced T-cell immunity. Further studies in animal models suggested that heterologous prime-boost vaccination schedules using 2 different poxvirus vectors expressing tumorantigen and costimulatory factors induced stronger immune responses against foreign antigens compared with single-agent immunization protocols. A TRIad of COstimulatory Molecules (TRICOM) consists of co-stimulatory molecules including intercellular adhesion molecule (ICAM)-1, B7.1, and leukocyte function-associated antigen-3 (LFA-3). Preclinical studies using TRICOM were previously demonstrated to be superior to those containing only 1 or 2 of the costimulatory molecules.
Following a phase I trial, a phase II study randomized 32 chemonaive patients with progressive metastatic CRPC into 1 of 4 cohorts.29 All cohorts received initial vaccine consisting of priming rV-PSA-TRICOM followed by monthly boosting with rF-PSATRICOM (Prostvac®-VF; Therion Biologics, Cambridge, MA). Patients randomized to cohort 1 received vaccine alone, cohort 2 received vaccine with recombinant GM-CSF protein, and cohorts 3 and 4 received vaccine with 2 different doses of fowlpox-GM-CSF. PSA-survival for the majority of patients exceeded predicted survival. The median survival was 26.3 months, whereas the nomogram-predicted median survival was 17.4 months. Eleven of 32 patients were alive with a median follow-up of 44.6 months. Twelve patients (37.5%) displayed some decrease of PSA, and 14 of 30 (46.7%) evaluable patients displayed decreases in PSA velocity. Immune responses to PSA were demonstrated by ELISpot (IFN-γ secretion in vitro by T cells in response to PSA peptide). The ability of patients to mount a ≥ 6-fold increase in T-cell responses was associated with an increase in survival. In a recently reported double-blind, randomized, phase II trial of patients enrolled between November 2003 and July 2005, 122 patients with chemonaive minimally symptomatic metastatic CRPC, Gleason score ≤ 7, and no visceral metastasis were treated with Prostvac-VF or placebo in a 2:1 ratio.30 The primary endpoint was progression-free survival (PFS) defined as 2 new lesions on bone scan or Response Evaluation Criteria In Solid Tumors (RECIST)-defined progression. PFS was similar in the 2 groups (P = .56) and originally, the trial was reported as negative. However, with greater follow-up, Prostvac-treated patients experienced a significantly greater median survival (25.1 vs 16.6 months, P = .0061) (Table 1). Additionally, Prostvac-VF patients had a better 3-year survival (30% vs 17%). The authors suggested an emerging theme in phase III vaccine studies in advanced PCa cancer was one of prolonged survival, without a demonstrable signal of tumor shrinkage or delay in short-term disease progression. Confirmation of these data in a phase III trial is planned in this setting. The vaccine will also be evaluated in earlier stages of nonmetastatic CRPC by ECOG (E1805, Paradigm) in a phase III trial of PROSTVAC/GM-CSF versus GMCSF (Table 1).
Measurement of Immune Response With Vaccines
The optimal measure of immune response is unclear. Correlation of immune response with clinical activity is vital to validate vaccine therapy. T-cell- and antibody-based immunoassays are used to determine if a given vaccine can elicit an immune response. The ELISpot assay reproducibly measures cytokine (eg, IFN-γ) release from T cells and can detect a peptide-specific T-cell response.31 Recently, MHC-peptide tetramer (or pentamer) assays have been widely used to quantify the number of antigen-specific T cells in animal models.32 The intracellular cytokine FastImmune assay uses flow cytometry to detect intracellular cytokines and allows the examination of multiple cytokines within T cells.33 Pre- and posttreatment T-cell proliferation assays in response to specific antigens have also been used to measure cell-mediated immunity.34 Delayed-type hypersensitivity skin tests have been used to crudely evaluate cell-mediated immunity to specific antigens. A humoral response is usually considered positive if a 4-fold increase in enzyme-linked immunosorbent assay (ELISA)-measured antigen-specific titer occurs compared with pretreatment levels with no cross reactivity against an unrelated patient antigen.
Other Emerging Immunotherapeutic Agents for PCa Therapy
Anti-CTLA-4 mAbs are emerging as active agents and single nucleotide polymorphisms (SNPs) within the CTLA-4 gene may predict responses.35 There are several antibodies (eg, ipilimumab, tremelimumab) in various stages of preclinical/clinical development that have the potential for clinical efficacy. These include antibodies to CD137, a costimulatory molecule that is induced on T cells after activation and enhances T-cell activation/proliferation on crosslinking; programmed death (PD)-1 receptor, a receptor that binds to the negative T-cell costimulatory molecule; programmed death ligand-1, promoting T-cell apoptosis and dampening the immune response; and OX-40 (CD134), expressed on Tregs and a negative regulator of their activity. The combination of such antibodies with vaccine as well as other modalities may merit further study to potentiate the immune response.
Endpoints and Patient Selection in Trials Evaluating Immunotherapy for CRPC
The choice of a primary endpoint for a phase II trial of CRPC is difficult, as advanced PCa is characterized by a poor ability to measure response due either to immeasurable bone-only metastases or PSA-only disease. Although a ≥ 30% or ≥ 50% PSA decline within 3 months may be a useful surrogate for long-term outcomes with chemotherapeutic agents, its validity with biologic agents is unknown.36 Vaccines may potentially induce a transient rise in PSA by provoking an immune reaction in the normal and malignant prostate tissue. Kinetic PSA endpoints are invalidated as intermediate surrogates for improved clinical outcomes, but may be a consideration. Other useful intermediate surrogates for outcomes with traditional cytotoxic chemotherapy, such as circulating tumor cells (CTCs) require further validation, especially in the context of biologic agents. Alternatively, time-to-event endpoints may be clinically useful surrogates and are currently recommended by the Prostate Cancer Clinical Trials Working Group-2 guidelines.36 In particular, PFS defined as a composite endpoint constituted by symptomatic or radiologic progression may be a clinically relevant primary endpoint and preliminarily appeared to be a useful intermediate surrogate for survival in the setting of frontline chemotherapy. However, progression may continue to remain an endpoint fraught with problems for vaccine therapy if none can reliably induce an effect on measurable disease in the short term, leaving overall survival the only currently reliable endpoint for trial of vaccine therapy in metastatic CRPC.9,11,30 Optimal patient selection is critical for trials evaluating vaccines and other immunotherapeutic agents for PCa. Although a heterogeneous group of patients with advanced PCa may be suitable for early phase I trials, further development should probably rely on signals of activity in subsets that appear to optimally benefit. These subsets may be patients with biologically indolent or early disease and those with expression of certain tumor or host tissue genomic and proteomic biomarkers. Biomarkers for immune modulation correlating with outcomes need to be studied, because no consistent correlations have been found between a specific immune response to used antigens and enhanced clinical outcomes. Preclinical data from animal models should also inform the decision to select patients for clinical trials.
Conclusions
Vaccines are emerging as a legitimate, safe, and active modality for the therapy of CRPC, with sipuleucel-T potentially becoming the first cancer vaccine therapy US Food and Drug Administration-approved for the treatment of cancer later this year. The failure of GVAX in phase III trials coupled with the promising data in more recently reported randomized phase II trials for Prostvac-VF highlight both the pitfalls and promise inherent to this new class of therapy. Efforts to optimize vaccine approaches, select ideal patient populations, and discover optimal doses and routes of administration need to continue building on these early successes. The combination of vaccines with other modalities should be developed cautiously, given the inferior outcomes seen with the combination of GVAX and docetaxel. The development of vaccine approaches, either alone or in combination with other modalities, that may lead to objective measurable disease responses or delay in short-term disease progression would be a significant advance in the field and may lead to a more rapid and feasible pathway for their clinical development.
Main Points.
Sipuleucel-T appears promising as a vaccine that specifically targets prostate cancer (PCa) with minimal toxicities. The IMmunotherapy for Prostate AdenoCarcinoma Treatment (IMPACT) double-blind randomized phase III study of 512 men with asymptomatic chemonaïve metastatic castration-resistant prostate cancer (CRPC) reported that the median survival was 25.8 months with sipuleucel-T compared with 21.7 months with placebo, and the 3-year survival also improved significantly (31.7% vs 23.0%; P = .032). The treatment effect remained consistent after adjustment for docetaxel use following investigational therapy. Formal approval by mid-2010 is expected, which would make this the first vaccine therapy US Food and Drug Administration (FDA)- approved for the treatment of cancer, after a plethora of previous phase III failures of cancer vaccines in other tumor types.
The granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting vaccine GVAX (Cell Genesys, South San Francisco, CA) was a mixture of the PCa cell lines, PC-3 and LNCaP, transduced with a replication-defective retrovirus containing cDNA for GM-CSF and then irradiated. Disappointingly, both the VITAL-1 trial that evaluated GVAX or docetaxel/prednisone for asymptomatic metastatic CRPC patients, and the VITAL-2 trial that evaluated GVAX plus docetaxel or docetaxel/prednisone in symptomatic metastatic CRPC patients, did not demonstrate improved outcomes with GVAX, leading to early termination of both trials.
Poxviruses represent a family of related double-stranded DNA viruses distinguished by their host specificity. A poxvirus (Prostvac)-expressing prostate-specific antigen and a triad of costimulatory molecules (TRICOM) have been studied in a double-blind placebo-controlled randomized phase II trial of 122 patients with chemonaïve minimally symptomatic metastatic CRPC. This trial demonstrated that Prostvac extended median survival (25.1 vs 16.6 mo; P = .0061) as well as 3-year survival (30% vs 17%).
An emerging theme in phase III studies of vaccines (eg, sipuleucel-T and Prostvac) in advanced PCa is one of prolonged survival, without a demonstrable signal of tumor shrinkage or delay in short-term disease progression. The development of vaccine approaches, either alone or in combination with other modalities, that may lead to objective measurable disease responses or delay in short-term disease progression would be a significant advance in the field and may lead to a more rapid and feasible pathway for their clinical development.
Optimal patient selection is critical for trials evaluating vaccines and other immunotherapeutic agents for PCa. Preclinical data from animal models should inform the decision to select patients for clinical trials.
Footnotes
Relevant conflicts of interest: Dr. Guru Sonpavde has received research funding from Bellicum Pharmaceuticals, Houston, TX; Dr. Kevin M. Slawin is co-founder and chief scientist at Bellicum Pharmaceuticals; Dr. David M. Spencer is co-founder and chief scientific officer at Bellicum Pharmaceuticals.
References
- 1.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival of the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354:1166–1176. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 4.Webster WS, Small EJ, Rini BI, Kwon ED. Prostate cancer immunology: biology, therapeutics, and challenges. J Clin Oncol. 2005;23:8262–8269. doi: 10.1200/JCO.2005.03.4595. [DOI] [PubMed] [Google Scholar]
- 5.Miller AM, Pisa P. Tumor escape mechanisms in prostate cancer. Cancer Immunol Immunother. 2007;56:81–87. doi: 10.1007/s00262-005-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 7.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 8.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 9.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 10.Dendreon reports preliminary D9902A trial data for Provenge in patients with advanced prostate cancer [press release] San Francisco, CA: Dendreon Corporation; 2005. [Google Scholar]
- 11.Schellhammer PF, Higano C, Berger ER, et al. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC) Abstract presented at: American Urological Association (AUA) Annual Meeting. 2009 [Google Scholar]
- 12.Rini BI, Weinberg V, Fong L, et al. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;107:67–74. doi: 10.1002/cncr.21956. [DOI] [PubMed] [Google Scholar]
- 13.Murphy GP, Tjoa BA, Simmons SJ, et al. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate. 1999;38:73–78. doi: 10.1002/(sici)1097-0045(19990101)38:1<73::aid-pros9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Perambakam S, Hallmeyer S, Reddy S, et al. Induction of specific T cell immunity in patients with prostate cancer by vaccination with PSA146-154 peptide. Cancer Immunol Immunother. 2006;55:1033–1042. doi: 10.1007/s00262-005-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su Z, Dannull J, Yang BK, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 16.Diehl L, den Boer AT, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 17.Schoenberger SP, Toes REM, Voort EIH, et al. Tcell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 18.Hanks BA, Jiang J, Singh RA, et al. Reengineered CD40 receptor enables potent pharmacological activation of dendritic-cell cancer vaccines in vivo. Nat Med. 2005;11:130–137. doi: 10.1038/nm1183. [DOI] [PubMed] [Google Scholar]
- 19.Lapteva N, Seethammagari MR, Hanks BA, et al. Enhanced activation of human dendritic cells by inducible CD40 and Toll-like receptor-4 ligation. Cancer Res. 2007;67:10528–10537. doi: 10.1158/0008-5472.CAN-07-0833. [DOI] [PubMed] [Google Scholar]
- 20.Iuliucci JD, Oliver SD, Morley S, et al. Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers. J Clin Pharmacol. 2001;41:870–879. doi: 10.1177/00912700122010771. [DOI] [PubMed] [Google Scholar]
- 21.Park D, Lapteva N, Seethammagari M, et al. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat Biotechnol. 2006;24:1581–1590. doi: 10.1038/nbt1262. [DOI] [PubMed] [Google Scholar]
- 22.Small EJ, Reese DM, Um B, et al. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]
- 23.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor-secreting, allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 24.Higano CS, Corman JM, Smith DC, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 25.Small E, Demkow T, Gerritsen WR, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) Proceedings of the 2009 Genitourinary Cancer Symposium. 2009 [Google Scholar]
- 26.Cell Genesys announces termination of VITAL-1 phase 3 trial of GVAX immunotherapy for prostate cancer [press release] South San Francisco, CA: Cell Genesys, Inc.; 2008. [Google Scholar]
- 27.Simons JW, Carducci MA, Mikhak B, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clin Cancer Res. 2006;12:3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 28.Gerritsen W, van den Eertwegh AJ, de Gruijl T, et al. Expanded phase I combination trial of GVAX immunotherapy for prostate cancer and ipilimumab in patients with metastatic hormone-refractory prostate cancer (mHPRC) J Clin Oncol. 2008;26(suppl) [Google Scholar]
- 29.Madan RA, Gulley JL, Dahut WL, et al. Overall survival (OS) analysis of a phase II study using a pox viral-based vaccine, PSA-TRICOM, in the treatment of metastatic, castrate-resistant prostate cancer (mCRPC): implications for clinical trial design. J Clin Oncol. 2008;26:(suppl) [Google Scholar]
- 30.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castrationresistant prostate cancer. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arlen P, Tsang KY, Marshall JL. The use of a rapid ELISPOT assay to analyze peptide-specific immune responses in carcinoma patients to peptide vs. recombinant poxvirus vaccines. Cancer Immunol Immunother. 2000;49:517–529. doi: 10.1007/s002620000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molldrem JJ, Lee PP, Wang C, et al. A PR1-human leukocyte antigen-A2 tetramer can be used to isolate low-frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chronic myelogenous leukemia. Cancer Res. 1999;59:2675–2681. [PubMed] [Google Scholar]
- 33.Storkus WJ, Howell DN, Salter RD, et al. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987;138:1657–1659. [PubMed] [Google Scholar]
- 34.Karanikas V, Hwang LA, Pearson J, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 36.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]