Abstract
In healthy adult individuals, late life is a dynamic time of change with respect to the microstructural integrity of white matter tracts. Yet, elderly individuals are generally excluded from diffusion tensor imaging studies in schizophrenia. Therefore, we examined microstructural integrity of frontotemporal and interhemispheric white matter tracts in schizophrenia across the adult lifespan. Diffusion tensor imaging data from 25 younger schizophrenic patients (≤55 years), 25 younger controls, 25 older schizophrenic patients (≥56 years) and 25 older controls were analysed. Patients with schizophrenia in each group were individually matched to controls. Whole-brain tractography and clustering segmentation were employed to isolate white matter tracts. Groups were compared using repeated measures analysis of variance with 12 within-group measures of fractional anisotropy: (left and right) uncinate fasciculus, arcuate fasciculus, inferior longitudinal fasciculus, inferior occipito-frontal fasciculus, cingulum bundle, and genu and splenium of the corpus callosum. For each white matter tract, fractional anisotropy was then regressed against age in patients and controls, and correlation coefficients compared. The main effect of group (F3,92 = 12.2, P < 0.001), and group by tract interactions (F26,832 = 1.68, P = 0.018) were evident for fractional anisotropy values. Younger patients had significantly lower fractional anisotropy than younger controls (Bonferonni-corrected alpha = 0.0042) in the left uncinate fasciculus (t48 = 3.7, P = 0.001) and right cingulum bundle (t48 = 3.6, P = 0.001), with considerable effect size, but the older groups did not differ. Schizophrenic patients did not demonstrate accelerated age-related decline compared with healthy controls in any white matter tract. To our knowledge, this is the first study to examine the microstructural integrity of frontotemporal white matter tracts across the adult lifespan in schizophrenia. The left uncinate fasciculus and right cingulum bundle are disrupted in younger chronic patients with schizophrenia compared with matched controls, suggesting that these white matter tracts are related to frontotemporal disconnectivity. The absence of accelerated age-related decline, or differences between older community-dwelling patients and controls, suggests that these patients may possess resilience to white matter disruption.
Keywords: schizophrenia, diffusion tensor, ageing, white matter fibre pathways
Introduction
The theory of disrupted oligodendrocyte structure/function (i.e. white matter) as a core underlying pathophysiological mechanism in schizophrenia is compelling: converging evidence from electron microscopy, post-mortem gene expression, animal models, gene association, neurophysiology and neuroimaging has repeatedly pointed to myelin and oligodendrocyte abnormalities as a core feature of, and possible common pathway for, impaired connectivity in schizophrenia (Davis et al., 2003; Dwork et al., 2007; Kubicki et al., 2007; Voineskos, 2009).
Myelinated bundles of axons that form white matter tracts can be visualized and characterized using diffusion tensor imaging (DTI), a clinical neuroimaging technique sensitive to alterations in the brain white matter microstructure. Such alterations in the microstructure can be indexed by fractional anisotropy (FA) (Basser and Pierpaoli, 1996), the degree to which diffusion of water molecules is restricted by microstructural elements such as cell bodies, axons, myelin and other constituents of cytoskeleton (Beaulieu, 2002). The use of methods not optimally suited to DTI (e.g. voxel-based morphometry) (Jones et al., 2005b), underpowered samples and limited reporting of effect size have limited interpretability and created challenges for replicating findings from DTI studies of schizophrenia (Konrad and Winterer, 2008). More recent DTI tractography approaches allow for the measurement of tissue integrity along white matter tracts, provide an anatomically based sample of the target fibre (Catani et al., 2002) and permit reconstruction of white matter tracts consistent with known neuroanatomy (Mori et al., 1999). However, most common methods for isolating fibre bundles based on streamline tractography still require some manual placement of multiple regions of interest, which can introduce bias. We have developed a white matter tract ‘clustering’ segmentation method that eliminates the need to place regions of interest manually in order to identify fibre bundles (O'Donnell et al., 2006) and is also successful in measuring frontotemporal and interhemispheric white matter tracts (Voineskos et al., 2009).
Disruption in frontotemporal and interhemispheric white matter tracts may underlie frontotemporal and interhemispheric disconnectivity, respectively, in schizophrenia. However, much less is known about the relationship of such deficits with age. A recent DTI study (Friedman et al., 2008) demonstrated accelerated age-related decline in the inferior longitudinal fasciculus, an occipitotemporal tract, and in certain subdivisions of the corpus callosum, but in contrast, such deficits were not present at the first episode of psychosis. To our knowledge, the possible progression across the life span of frontotemporal white matter tract deficits has not yet been studied. Rather, age-related studies of frontotemporal white matter tracts have been limited to mid-life patients with chronic schizophrenia (i.e. 20–55 years) with conflicting results (Jones et al., 2006; Rosenberger et al., 2008).
The absence of studies comparing DTI white matter tract measures in older patients with schizophrenia and older healthy individuals is striking. Late-life is a particularly dynamic time in terms of white matter change, where healthy individuals are susceptible to decline in white matter integrity (Salat et al., 2005; Sullivan and Pfefferbaum, 2006), and such decline may explain cognitive changes in normal ageing (Hedden and Gabrieli, 2004; Sullivan and Pfefferbaum, 2007; Ziegler et al., 2008). Similarly, in patients with schizophrenia, late-life may also be a time when significant changes in white matter may occur, at least in a subset of individuals. Clinical studies suggest that some, but not all, patients undergo progressive decline in cognition and function in late-life (Rajji and Mulsant, 2008). It is possible that white matter changes may represent the neurobiological correlates of such divergent outcomes in schizophrenia. The increasing numbers of older individuals with schizophrenia and the finding that schizophrenia in late-life is one of the most expensive of medical disorders (Jeste and Nasrallah, 2003) underscore the need for understanding the synergies between schizophrenia and ageing with respect to white matter changes.
Using whole-brain tractography and our clustering segmentation approach, we examined intrahemispheric frontotemporal association fibre tracts (left and right): the uncinate fasciculus, cingulum bundle, arcuate fasciculus, inferior occipitofrontal fasciculus; the main occipitotemporal association fibre tract (left and right), the inferior longitudinal fasciculus; and interhemispheric fibre tracts comprising the genu and splenium of the corpus callosum. We focused on patients with chronic schizophrenia, since the bulk of evidence suggests that white matter abnormalities in schizophrenia may not be present at illness onset (Friedman et al., 2008; Konrad and Winterer, 2008). Our study had three main hypotheses: (i) that differences in frontotemporal white matter tracts would be present in younger patients with chronic schizophrenia compared with healthy controls; (ii) that similar or even greater differences would be present in older patients with schizophrenia and age-matched controls; and (iii) that patients with schizophrenia would demonstrate similar or greater age-effects on white matter tract integrity across adult life compared with healthy controls.
Methods
Study participants
Participants were recruited at the Centre for Addiction and Mental Health (CAMH) in Toronto, Canada, via referral, study registries and advertisements. All clinical assessments occurred at CAMH while DTI scans were performed at a nearby general hospital in Toronto. All participants were administered the Mini Mental Status Exam to screen for dementia (Folstein et al., 1975), the Structured Clinical Interview from Diagnostic and Statistical Manual of Mental Disorders-IV (First et al., 1995) and were interviewed by a psychiatrist to ensure diagnostic accuracy. The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) was administered to characterize clinical symptoms further. Comorbid illness burden was measured by administrating the Clinical Information Rating Scale for Geriatrics (Miller et al., 1992). Medication histories were recorded via self-report and verified when necessary from the patient’s treating psychiatrist and chart review. Patients or controls with current substance abuse or any history of substance dependence were excluded, and urine toxicology screens were performed on all subjects. Individuals with previous head trauma and loss of consciousness or neurological disorders were also excluded. A history of a primary psychotic disorder in first-degree relatives was also an exclusion criterion for controls. Criteria used to match controls with patients were: age within 5 years, gender, handedness (Edinburgh handedness inventory) (Oldfield, 1971) and IQ (Wechsler Test for Adult Reading) (Wechsler, 2001). Initially, we attempted to match based on parental socioeconomic status (Hollingshead four-factor index of social position) (Hollingshead, 1975); however, several older subjects were unable to report parental education and occupation confidently. After complete description of the study to the subjects, written and informed consent was obtained. The study was approved by the Centre for Addiction and Mental Health Ethics Review Board.
Although 143 subjects met initial screening criteria, 19 were unable to complete all protocols (e.g. due to failure to return for DTI procedure, claustrophobia in MRI scanner, request to withdraw from study). Thus, 124 subjects completed all DTI and clinical procedures. Three DTI scans were deemed unusable due to excessive artefact and DTI data from 21 subjects were not used in the present study since no suitable match was found. Thus, 100 subjects are included in this report. They were distributed in the following four groups, each including 25 subjects: patients with chronic schizophrenia 55 years and younger; younger matched healthy control participants; patients with schizophrenia 56 years and older; and older healthy controls. The choice of 55 years as the dividing point between age groups was intended to reflect a commonly chosen upper age limit in neuroimaging studies of patients with chronic schizophrenia that, typically, have excluded older patients (e.g. Kubicki et al., 2002; Jones et al., 2006; Rosenberger et al., 2008).
Image acquisition
DTI images were acquired using an eight-channel head coil on a 1.5 Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, WI), which permits maximum gradient amplitudes of 40 mT/m. A single shot spin echo planar sequence was used with diffusion gradients applied in 23 non-collinear directions and b = 1000 s/mm2. Two b = 0 images were obtained. Whole brain coverage was obtained (no gap), oblique to the axial plane. Slice thickness was 2.6 mm and voxels were isotropic. The field of view was 330 mm and the size of the acquisition matrix was 128 × 128 mm2, with echo time = 85.5 ms and repetition time = 15 000 ms. To improve the signal to noise ratio, the entire sequence was repeated three times. Inversion recovery prepped spoiled gradient recall and fast-spin echo T2-weighted images were also acquired in the event of need for registration and to ensure anatomical accuracy.
Image analysis and tractography
Diffusion weighted images were transferred to a workstation for analysis. The three repetitions were co-registered to the first b = 0 image in the first repetition using FMRIB Software Library (v. 4.0) http://www.fmrib.ox.ac.uk to produce a new averaged image, with gradients re-oriented according to the registration transformation. A final diffusion tensor was then estimated based on all 75 aligned volumes using a weighted least-squares approach. Registration corrects eddy current distortions and subject motion, important artefacts that can affect the data, and averaging improves the signal to noise ratio. A brain ‘mask’ was then generated. Points were seeded throughout each voxel of the brain. Whole-brain tractography was performed with a deterministic (streamline) approach (Runge–Kutta order 2 tractography with a fixed step size of 0.5 mm). More detailed descriptions of our tractography approach and our clustering segmentation algorithm have been recently published (O'Donnell et al., 2006; Voineskos et al., 2009) and are summarized here. Threshold parameters for tractography were based on the linear anisotropy measure CL, where CL = (λ1 − λ2)/λ1 and λ1 and λ2 are the two largest eigenvalues of the diffusion tensor sorted in the descending order. Thresholds were based on the CL rather than on FA, because FA can be relatively high in regions of planar anisotropy, which may indicate tract crossings or branching (Ennis and Kindlmann, 2006). The three threshold parameters for tractography were Tseed, Tstop and Tlength. The Tseed and Tstop are anisotropy thresholds based on CL. The Tlength threshold was 20 mm to prevent very short fibres from being generated (O'Donnell and Westin, 2007), in order to permit reliable selection of clusters that comprise the major neuroanatomic tracts of interest (Voineskos et al., 2009). Tractography and creation of white matter fibre tracts were performed using 3D Slicer (http://www.slicer.org) and MATLAB 7.0 (http://www.mathworks.com).
A pairwise fibre trajectory similarity was quantified by first computing a pairwise fibre distance, and then employing a mean closest point distance. The directed distances between fibres ‘A’ and ‘B’ were converted to a symmetric pairwise fibre distance. Each distance was then converted to an affinity measure suitable for spectral clustering via a Gaussian kernel (Wij) =  (Shi and Malik, 2000). The role of σ (σ = 60 mm) is to define the size scale of the problem by setting the distance over which fibres can be considered similar. A spectral embedding of fibres was then created based on the eigenvectors of the fibre affinity matrix. We used the top 15 eigenvectors of the fibre similarity matrix to calculate the most important shape similarity information for each fibre, using a k-way normalized cuts clustering algorithm (O'Donnell et al., 2006).
(Shi and Malik, 2000). The role of σ (σ = 60 mm) is to define the size scale of the problem by setting the distance over which fibres can be considered similar. A spectral embedding of fibres was then created based on the eigenvectors of the fibre affinity matrix. We used the top 15 eigenvectors of the fibre similarity matrix to calculate the most important shape similarity information for each fibre, using a k-way normalized cuts clustering algorithm (O'Donnell et al., 2006).
Once the whole brain cluster model was produced, a trained operator (A.N.V.) combined the clusters that correspond to a given fibre tract. Left and right uncinate fasciculus, inferior occipitofrontal fasciculus, cingulum bundle, inferior longitudinal fasciculus, arcuate fasciculus, and genu and splenium (parietal, temporal, occipital fibres) of the corpus callosum were selected (Voineskos et al., 2009) (Fig. 1). As reported elsewhere (Voineskos et al., 2009), two individuals, blind to participant information, performed the entire clustering procedure separately on 10 individuals with schizophrenia and 10 healthy controls, and achieved excellent spatial and quantitative reliability using this clustering method (i.e. both voxel overlap and scalar measures of the tensor showed high agreement). MATLAB (v. 7.0) was then used to calculate FA (Basser and Pierpaoli, 1996). Presented data represent the mean values along the selected tracts. With the exception of participants included in the reliability study, this is a new cohort of subjects, with data reported for the first time.
Figure 1.
White matter tracts of interest superimposed on fractional anisotropy grey scale images. (A) Left to right: left uncinate fasciculus, left inferior occipitofrontal fasciculus, left arcuate fasciculus. (B) Left to right: left inferior longitudinal fasciculus, right cingulum bundle, genu and splenium of corpus callosum (in same panel, both coloured red).
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences v.15.0. For groupwise comparisons, a repeated measures ANOVA model was used. The four ‘diagnosis-age’ groups were compared: younger controls, younger schizophrenic patients, older controls and older schizophrenic patients. Some reports have indicated that gender may affect microstructural integrity of white matter (Schmithorst et al., 2008; Huster et al., 2009). Therefore, gender was also included as a between-group factor. There were 12 within-group factors; the mean FA for each of the 12 white matter tracts was studied. Post hoc t-tests comparing the younger control to the younger schizophrenia groups and the older control to the older schizophrenia groups were used to discover where significant differences in tract FA were present between age-specific diagnostic groups. For individual tract analyses, Bonferroni correction for 12 comparisons (P < 0.05/12) (i.e. P < 0.0042) was applied. When a significant difference was found, tract volume and chlorpromazine equivalents of medication dosage were regressed against FA for that fibre tract, to correct for any influence of these variables.
Rather than conducting post hoc within diagnostic group comparisons of the younger schizophrenia to older schizophrenia groups and the younger control to older control groups, we sought to examine the age-related change in each white matter tract within the diagnostic group by using correlational analysis across adult life separately for all schizophrenic patients and then for all controls. We then compared correlations of age-related FA decline between the schizophrenia group and the control group for each white matter tract across adult life. Significance of the differences between correlations (to compare age-related FA changes for each white matter tract in schizophrenic patients versus healthy controls) was calculated by dividing the difference between Fischer’s Z-score transformation of Pearson’s r by the standard error of difference between the two correlations (Blalock, 1972).
Since previous studies have found evidence of potential differences in asymmetry (Kubicki et al., 2002) in schizophrenia, a laterality index was calculated for each bilateral hemispheric tract (i.e. uncinate fasciculus, arcuate fasciculus, inferior longitudinal fasciculus, inferior occipitofrontal fasciculus, cingulum bundle), as in Catani et al. (2007): laterality index = (left tract FA – right tract FA)/[(left tract FA + right tract FA)]/2. A repeated measures ANOVA was performed, with the five-tract laterality index within group measures and the diagnosis-age group as the between-group measure. To examine for age-related effects, the laterality index for each tract pair was regressed against age.
Exploratory correlational analyses for tract FA values were performed with positive and negative symptom subscale scores from the PANSS in each schizophrenia group (younger schizophrenia group, older schizophrenia group). For each laterality index, correlations with PANSS scores were performed for all schizophrenic patients, since age was not related to laterality.
The relationship of Mini Mental Status Exam scores to tract FA was also explored using separate Pearson correlational analyses for patients with schizophrenia and controls.
Results
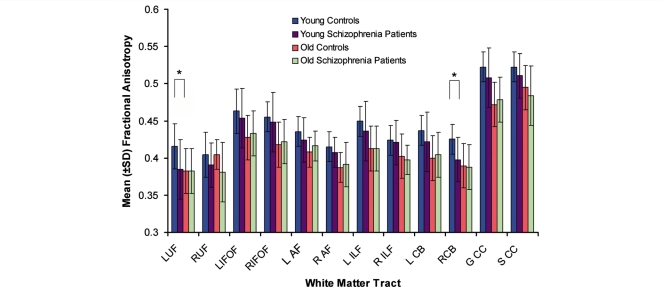
Demographic and clinical characteristics of the 100 subjects are presented in Table 1. Figure 2 illustrates the mean FA values for each ‘diagnosis-age’ group at each white matter tract. There was a significant main effect of group (F3,92 = 12.2, P < 0.001) and a group by tract interaction (F33,1012 = 1.68, P = 0.010) (Greenhouse–Geiser correction: F26,832 = 1.68, P = 0.018) (observed power = 0.989). There was no significant main effect of gender (F1,92 = 0.53, P = 0.47), nor was there a significant group by gender (F3,92 = 1.68, P = 0.18) or group by gender by tract interaction (F33,1012 = 1.17, P = 0.26).
Table 1.
Demographic and clinical characteristics of subjects
| Demographic | Young controls (n = 25) | Young SZ (n = 25) | Old controls (n = 25) | Old SZ (n = 25) |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 38 (10) | 40 (12) | 66 (7) | 65 (6) |
| Educationa | 15 (2) | 13 (3) | 15 (2) | 13 (3) |
| WTAR (IQ) | 114 (8) | 110 (12) | 115 (10) | 112 (13) |
| MMSE | 29 (1) | 29 (1) | 29 (1) | 29 (1) |
| CIRS-Gb | 1 (2) | 5 (3) | 4 (3) | 8 (4) |
| Age of onsetc | NA | 23 (5) | NA | 30 (13) |
| Duration of illness | NA | 17 (12) | NA | 33 (15) |
| Chlorpromazine equivalent (mg) | NA | 305 (220) | NA | 236 (188) |
| PANSS | ||||
| Positive | NA | 14 (6) | NA | 16 (6) |
| Negative | NA | 17 (6) | NA | 15 (7) |
| General | NA | 27 (9) | NA | 27 (6) |
| Diagnosis, n | NA | 22 SZ, 3 SA | NA | 22 SZ, 3 SA |
| Gender, n | 16 M, 9 F | 16 M, 9 F | 13 M, 12 F | 13 M, 12 F |
| Handedness, n | 24 R, 1 L | 24 R, 1 L | 24 R, 1 L | 24 R, 1 L |
| Ethnicity, n | 18 C, 3 As, 4 Af | 21 C, 4 As | 22 C, 1 As, 2 Af | 23 C, 2 As |
| Currently smoking, n | 8 | 12 | 10 | 12 |
| Antipsychotic treatment,dn | NA | 21°, 22 2° | NA | 11°, 23 2° |
SZ = schizophrenic; SA = schizoaffective; NA = not applicable; 1° = first-generation antipsychotic; 2° = second-generation antipsychotic; M = male; F = female; WTAR = Wechsler Test for Adult Reading; MMSE = Mini Mental State Examination; CIRS-G = Clinical Information Rating Scale, Geriatrics; C = Caucasian; As = Asian; Af = African (based on self-report).
a,b Young controls were compared with young schizophrenic patients; and old controls to old schizophrenic patients using t-tests on age, education, WTAR, MMSE and CIRS-G. Significant differences (i.e. P < 0.05) were present on education (t48 = 2.8, P = 0.008) (young schizophrenic patients < young controls and old schizophrenic patients < old controls) and CIRS-G (t48 = 5.5, P < 0.001) (young controls < young schizophrenic patients) and (t48 = 4.0, P < 0.001) (old controls < old schizophrenic patients).
c Age of onset is higher in the older schizophrenia group; however, when the three late-onset (i.e. > 45 years of age) individuals were removed from the older schizophrenic group, the schizophrenia groups were no longer different in the age of onset, and all analyses of group FA differences were unchanged.
d In each schizophrenia group, one individual was not on antipsychotic medication.
Figure 2.
Fractional anisotropy by diagnostic-age group for 12 white matter tracts. *Significant differences (that survived Bonferroni correction) between young controls and young schizophrenic patients are noted on the figure: left uncinate fasciculus t48 = 3.7, P = 0.001; and right cingulum bundle t48 = 3.6, P = 0.001. L UF = left uncinate fasciculus; R UF = right uncinate fasciculus; L IFOF = left inferior occipitofrontal fasciculus; R IFOF = right inferior occipitofrontal fasciculus; L AF = left arcuate fasciculus; R AF = right arcuate fasciculus; L ILF = left inferior longitudinal fasciculus; R ILF = right inferior longitudinal fasciculus; L CB = left cingulum bundle; R CB = right cingulum bundle; G CC = genu of corpus callosum; S CC = splenium of corpus callosum.
Three individuals in the schizophrenia group experienced ‘late-onset schizophrenia’ (i.e. onset of illness at age 45 and older) (Jeste et al., 1995), which may represent a milder version of illness (Almeida et al., 1995), resulting in a significant difference in the age-of-onset in the two schizophrenia groups (t48 = 2.5, P = 0.02); thus, the analysis was repeated without these three individuals (the age-of-onset was no longer different between the schizophrenia groups: t45 = 1.4, P = 0.16). Significant results were unchanged following this new analysis: a significant main effect of group (F3,89 = 14.1, P < 0.001) and a group by tract interaction (F33,979 = 1.68, P = 0.010) (Greenhouse–Geiser correction: F26,784 = 1.68, P = 0.018) were still present, while no significant main effects or interaction effects of gender were present.
The post hoc comparisons of the younger control and the younger schizophrenia groups demonstrated statistically significant differences (threshold set at P < 0.0042) with relatively large effect size for the left uncinate fasciculus (t48 = 3.7, P = 0.001) (Cohen’s d = 1.05) and the right cingulum bundle (t48 = 3.6, P = 0.001) (Cohen’s d = 1.02). There was no relationship between FA and the fibre tract volume for the left uncinate fasciculus and right cingulum bundle, nor with FA and the mean medication dose. No differences in FA were present between the older groups for any white matter tract (P > 0.10 for all tracts).
Significant inverse correlations between age and FA were found in healthy controls (P ≤ 0.001 for all white matter tracts), and in schizophrenic patients, except for the left uncinate fasciculus (r = −0.26, P = 0.07), right uncinate fasciculus (r = −0.32, P = 0.02), left arcuate fasciculus (r = −0.24, P = 0.10), right arcuate fasciculus (r = −0.35, P = 0.01) and right cingulum bundle (r = −0.36, P = 0.01), which did not reach significance after applying the P value corrected for the 12 comparisons (Fig. 3). There was no statistically significant difference in correlation coefficients measuring the age-related decline of FA between diagnostic groups for any white matter tract (all P > 0.05).
Figure 3.
Relationship between age and fractional anisotropy for each white matter tract in both schizophrenic patients and healthy controls. No significant differences (when comparing correlation coefficients) in age-related decline of fractional anisotropy were present in any white matter tract between schizophrenic patients and controls.
For the laterality index, there were no main effects of group (F3,96 = 1.05, P = 0.37) nor group by tract interaction (F12,384 = 1.10, P = 0.36). No relationship was present between age and laterality index for any bilateral tract in either the controls or schizophrenia samples.
No significant relationships were found for PANSS scores with FA in either schizophrenia group. A significant relationship between the cingulum bundle laterality index and negative symptoms (r = 0.47, P = 0.001) across all schizophrenia subjects was found.
No relationship between the Mini Mental Status Exam score and the tract FA was found for schizophrenic patients, and no such relationship was found for healthy controls.
Discussion
We conducted a comprehensive DTI study of the microstructural integrity of frontotemporal and interhemispheric white matter tracts in 50 patients with schizophrenia stratified into a younger and an older group and 50 individually matched controls. This study has three main findings: (i) the left uncinate fasciculus and right cingulum bundle showed a significant decrease in microstructural integrity (FA) in younger patients compared with younger controls; (ii) no differences in FA were observed/detected between older patients and older controls; and (iii) age-related FA decline occurs in both patients and controls; however, no exaggerated ageing effects in schizophrenia were found.
The decreased FA we observed in the left uncinate faciculus in younger patients with schizophrenia is consistent with the literature suggesting frontotemporal disconnectivity in schizophrenia (McIntosh et al., 2008), although there have been negative studies (Jones et al., 2006). Reduced oligodendrocyte number and gene expression in frontal and temporal cortices in schizophrenia have been reported (Hakak et al., 2001; Uranova et al., 2001; Katsel et al., 2005), which align with our finding of reduced FA in the left uncinate fasciculus. Reduced oligodendrocyte number in schizophrenia may lead to disrupted myelin, or insufficient myelin mediated inhibition of neuritic sprouting (Budel et al., 2008), which in turn may contribute to axonal disorganization, and hence reduced FA in the left uncinate fasciculus (Voineskos, 2009). The uncinate fasciculus may also have disrupted asymmetry in schizophrenia (Kubicki et al., 2002); however, we found no difference in our laterality index. A role for the uncinate fasciculus in self-regulation, self-awareness and goal-directed behaviour is suggested by the specific frontotemporal regions it connects (Kubicki et al., 2002) and by results from lesion studies. Therefore, disrupted uncinate fasciculus integrity in schizophrenia may be related to impaired social cognition, characterized mainly by theory of mind and empathy deficits (Benedetti et al., 2009). However, these dimensions of the disease are not measured by the PANSS scale or conventional neuropsychological testing.
We also observed decreased FA of the right cingulum bundle in younger schizophrenic patients compared with controls, consistent with frontotemporal disconnectivity. Increased white matter volume in the right cingulate but not the left (Mitelman et al., 2005) has been shown, and right cingulate metabolism has shown preferential decrease during the Stroop task in schizophrenia (Nordahl et al., 2001). Glial cell loss has been demonstrated in cingulate cortex in schizophrenia, and oligodendrocyte-related genes are downregulated in three regions connected only by the cingulum bundle, the cingulate, frontal and temporal cortices (Davis et al., 2003). Recent work has also demonstrated downregulation of oligodendrocyte genes in cingulate white matter, namely the quaking gene, myelin-associated glycoprotein gene, 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene and the transferrin gene (McCullumsmith et al., 2007). Dowregulation of such genes in cingulate white matter in schizophrenia may be related to decreased FA in the cingulum bundle measured with DTI. However, previous DTI studies have shown decreases in FA in both left and right cingulum bundles, while others have not (Konrad and Winterer, 2008). Disrupted cingulum integrity in schizophrenia may be related to negative symptoms and impaired executive function (Kubicki et al., 2007). In our analysis, we found no relationship with negative symptoms in left or right cingulum. However, a significant correlation between the cingulum laterality index and negative symptom burden was present across both schizophrenia age groups (though this should be viewed as preliminary given the large number of comparisons made with tract FA and the PANSS). Nevertheless, it is possible that greater asymmetry of the cingulum may represent a pathophysiological mechanism underlying negative symptoms in schizophrenia. Others have also shown that increasing asymmetry may be disadvantageous: while asymmetry of the arcuate fasciculus is normal, healthy individuals with the greatest asymmetry performed most poorly in the California Verbal Learning Task (Catani et al., 2007).
We found no differences in FA in the arcuate fasciculus, inferior longitudinal fasciculus, inferior occipitofrontal fasciculus or corpus callosum. For the left inferior longitudinal fasciculus, recent tractography findings in early onset schizophrenia adolescents have shown reduced FA, where individuals with visual hallucinations showed further reduced FA (Ashtari et al., 2007). On the other hand, higher FA in both the arcuate fasciculus (Shergill et al., 2007) and cingulum bundle (Hubl et al., 2004) has been associated with auditory hallucinations in schizophrenia. Despite our negative findings, continued investigation of the arcuate fasciculus, inferior longitudinal fasciculus, inferior occipitofrontal fasciculus and corpus callosum in schizophrenia is important, particularly given their crucial roles in language, visuo-emotional processing, visuo-spatial function and interhemispheric communication, respectively (Gazzaniga, 2000; Catani et al., 2003; Kier et al., 2004; Kubicki et al., 2007; Catani and Mesulam, 2008).
To our knowledge, this is the first report examining frontotemporal white matter tracts in schizophrenia across the adult lifespan. Our data showed no excess in the expected age-related decline in older patients compared with healthy controls. While it is possible that we were insufficiently powered to detect differences between the older groups, the detectable differences and large effect sizes observed between the younger groups make such a possibility less likely. Other studies using DTI tractography have also found differences between groups with similar or smaller sample sizes compared with ours (Ashtari et al., 2007; McIntosh et al., 2008). Another DTI tractography study (Jones et al., 2005a) examined elderly patients with ‘very-late-onset schizophrenia-like’ psychosis compared with healthy elderly controls and found no differences in FA measured along frontotemporal white matter tracts. Though the patients in that study did not have schizophrenia and had a much shorter duration of illness (since the onset of illness was 60 years or greater), one important similarity with our elderly patients was that all individuals had Mini Mental Status Exam scores of 25 or greater. Our findings are unlike the recent finding of exaggerated age-related decline in schizophrenia in the forceps minor of the corpus callosum and the inferior longitudinal fasciculus (Friedman et al., 2008). One explanation for this difference might be due to different methodological approaches, whereby Friedman et al. (2008) used a region-of-interest-based approach, and thus sampled specific anatomic portions of the corpus callosum and inferior longitudinal fasciculus, whereas via our tractography approach, our FA results represent microstructural integrity measured along each fibre tract. In addition, Friedman et al. (2008) included long-term institutionalized patients, who have been shown to experience dramatic cognitive decline, with cognitive scores consistent with severe dementia (Rajji and Mulsant, 2008). Our patients were community-dwelling with cognitive scores in the non-demented range. Unlike institutionalized patients, older community-dwelling patients may be more resilient, given the otherwise dramatically decreased life-expectancy in individuals with schizophrenia (Marder et al., 2004; Tiihonen et al., 2009). Our lack of finding of a relationship between Mini Mental Status Exam score and tract FA was not surprising given the narrow range of Mini Mental Status Exam scores in our sample, and the simplified nature of the Mini Mental Status Exam as a neurocognitive assessment. Efforts are underway by our group to comprehensively investigate the relationship between white matter integrity and cognition in schizophrenia across the adult lifespan. Further characterization of such a relationship, particularly in late-life, may provide insight into neurobiological underpinnings of cognitive and functional outcomes, and neural correlates of successful ageing in schizophrenia.
The absence of differences between our older groups provides some insight regarding the potential confounding effects of medication on FA. Like ours, most studies demonstrate no relationship between medication and FA. These cross-sectional findings do not prove that reduced FA in chronic schizophrenia samples is not medication-related. However, they help mitigate such concerns. If medication had a significant effect on FA, the group with the longer medication exposure (i.e. the elderly group) might have had a greater reduction in FA than the control group. Furthermore, antipsychotic medications may lead to white matter repair, possibly reflected by increased FA (Garver et al., 2008), and, in mice, the atypical antipsychotic, quetiapine, has been shown to facilitate oligodendrocyte development (Xiao et al., 2008).
Our study has several limitations. First, even though they may provide a protective effect for oligodendrocytes, antipsychotic medication effects on FA are still not well understood. Second, ageing is associated with a larger cumulative burden of exposure to environmental factors that may change white matter integrity, such as smoking, alcohol or comorbid physical disorders. In patients with schizophrenia, these factors are present at a higher rate than in the general population (Marder et al., 2004). Third, post-mortem studies of schizophrenic patients that demonstrate reduced myelin gene expression have been shown with both younger chronic (Torrey et al., 2000; Tkachev et al., 2003) and older chronic subjects (Hakak et al., 2001), though the older subjects had ‘chronic intractable schizophrenia, each with at least 35 years of hospitalization’ (Hakak et al., 2001). Since these post-mortem studies do not include elderly community-dwelling subjects, conclusions regarding the relationship of post-mortem myelin gene downregulation reported in the literature to the lack of FA differences between our elderly groups cannot be drawn. Regarding study design, a longitudinal DTI study is needed to provide information about white matter decline in each individual, and it would protect against a survivor selection bias, which can be present in a cross-sectional design, such as ours. Fourth, we did not study institutionalized patients, who may have a more severe form, or different variant of illness. They may experience progressive deterioration, and accelerated decrease in white matter integrity. Recently, prominent FA changes in schizophrenia have emerged from datasets that include individuals who have severe disease phenotypes, such as patients with early-onset schizophrenia (Ashtari et al., 2007), institutionalized inpatients (Friedman et al., 2008) and deficit syndrome patients (Rowland et al., 2009). Finally, while we are confident in our measurement of FA given that previous work has demonstrated at least 20 unique sampling orientations as necessary for a robust measurement of anisotropy (Jones, 2004) (we obtained 23 unique sampling orientations), for robust estimations of tensor orientation and mean diffusivity at least 30 unique sampling orientations are required (Jones, 2004).
In summary, we investigated 12 susceptible corticocortical white matter tracts in schizophrenia across the adult lifespan. We studied a relatively large, carefully matched group, assessing both age- and group-effects for each white matter tract. Statistically significant reductions in FA with large effect sizes were observed in the left uncinate and right cingulum of younger patients with chronic schizophrenia. However, these differences were not present in older patients. Future DTI investigations of these elderly patients in conjunction with more detailed cognitive measures may ultimately reveal specific mechanisms or biomarkers of resilience in schizophrenia. At the same time, a focus on white matter in individuals with schizophrenia who have a severe, deteriorating course of illness may reveal which patients experience age-related decline that exceeds that of healthy ageing, thus providing increased capability to discover biological substrates of prognosis in schizophrenia across adult life.
Funding
Canadian Institutes of Health Research Clinician Scientist Award (to A.N.V.); APA/APIRE Astra-Zeneca Young Minds in Psychiatry Award (to A.N.V.); Canadian Institutes of Health Research Fellowship (to T.K.R.); National Institutes of Health (NIH) (R01, grant number MH074794) (to M.E.S.), (R01, grant number MH 50740) (to M.E.S.), NIH (1P50, grant number MH08272) (to M.E.S.), NIG/HS NIH (U54 grant number GM072977-01) (to M.E.S.), VA MERIT (to M.E.S.), VA Schizophrenia Center Grant (to M.E.S.), NIH (R01, grant number MH082918) (to S.B.), the Sandra A. Rotman Research Institute (to B.G.P.) and the Centre for Addiction and Mental Health.
Glossary
Abbreviations
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- PANSS
Positive and Negative Syndrome Scale
References
- Almeida OP, Howard RJ, Levy R, David AS. Psychotic states arising in late life (late paraphrenia) psychopathology and nosology. Br J Psychiatry. 1995;166:205–14. doi: 10.1192/bjp.166.2.205. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64:1270–80. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Bosia M, Cavallaro R, Dallaspezia S, Falini A, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114:154–60. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Blalock H. Social Statistics. New York: McGraw-Hill; 1972. [Google Scholar]
- Budel S, Padukkavidana T, Liu BP, Feng Z, Hu F, Johnson S, et al. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci. 2008;28:13161–72. doi: 10.1523/JNEUROSCI.3828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, et al. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. 2007;104:17163–8. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–61. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–56. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. Int J Neuropsychopharmacol. 2007;10:513–36. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med. 2006;55:136–46. doi: 10.1002/mrm.20741. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Strucutured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York: Biometrics Research; 1995. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–32. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Garver DL, Holcomb JA, Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int J Neuropsychopharmacol. 2008;11:49–61. doi: 10.1017/S1461145707007730. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–68. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, Schweiger E, Wittling W. Hemispheric and gender related differences in the midcingulum bundle: a DTI study. Hum Brain Mapp. 2009;30:383–91. doi: 10.1002/hbm.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Harris MJ, Krull A, Kuck J, McAdams LA, Heaton R. Clinical and neuropsychological characteristics of patients with late-onset schizophrenia. Am J Psychiatry. 1995;152:722–30. doi: 10.1176/ajp.152.5.722. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Nasrallah HA. Schizophrenia and aging: no more dearth of data? Am J Geriatr Psychiatry. 2003;11:584–7. doi: 10.1176/appi.ajgp.11.6.584. [DOI] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51:807–15. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O'Sullivan M, et al. A diffusion tensor magnetic resonance imaging study of frontal cortex connections in very-late-onset schizophrenia-like psychosis. Am J Geriatr Psychiatry. 2005a;13:1092–9. doi: 10.1176/appi.ajgp.13.12.1092. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O'Sullivan M, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27:230–8. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005b;26:546–54. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005;77:241–52. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–91. [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull. 2008;34:72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–20. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334–49. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, et al. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Maniega SM, Lymer GK, McKirdy J, Hall J, Sussmann JE, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–92. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Carter CS, Salo RE, Kraft L, Baldo J, Salamat S, et al. Anterior cingulate metabolism correlates with stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology. 2001;25:139–48. doi: 10.1016/S0893-133X(00)00239-6. [DOI] [PubMed] [Google Scholar]
- O'Donnell LJ, Kubicki M, Shenton ME, Dreusicke MH, Grimson WE, Westin CF. A method for clustering white matter fiber tracts. AJNR Am J Neuroradiol. 2006;27:1032–6. [PMC free article] [PubMed] [Google Scholar]
- O'Donnell LJ, Westin CF. Automatic tractography segmentation using a high-dimensional white matter atlas. IEEE Trans Med Imaging. 2007;26:1562–75. doi: 10.1109/TMI.2007.906785. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102:122–40. doi: 10.1016/j.schres.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Rosenberger G, Kubicki M, Nestor PG, Connor E, Bushell GB, Markant D, et al. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2008;102:181–8. doi: 10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW. White matter alterations in deficit schizophrenia. Neuropsychopharmacology. 2009;34:1514–22. doi: 10.1038/npp.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Kanaan RA, Chitnis XA, O'Daly O, Jones DK, Frangou S, et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164:467–73. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Shi J, Malik J. Normalized cuts and image segmentation. IEEE Trans Pattern Anal Mach Intell. 2000;22:888–905. [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–61. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroradiological characterization of normal adult ageing. Br J Radiol. 2007;80 (Spec No 2):S99–108. doi: 10.1259/bjr/22893432. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2009;374:620–7. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The stanley foundation brain collection and neuropathology consortium. Schizophr Res. 2000;44:151–5. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Voineskos AN. Converging evidence for the Nogo-66 receptor gene in schizophrenia. J Neurosci. 2009;29:5045–7. doi: 10.1523/JNEUROSCI.0477-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, O'Donnell LJ, Lobaugh NJ, Markant D, Ameis SH, Niethammer M, et al. Quantitative examination of a novel clustering method using magnetic resonance diffusion tensor tractography. Neuroimage. 2009;45:370–6. doi: 10.1016/j.neuroimage.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. New York: Harcourt Assessment; 2001. [Google Scholar]
- Xiao L, Xu H, Zhang Y, Wei Z, He J, Jiang W, et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry. 2008;13:697–708. doi: 10.1038/sj.mp.4002064. [DOI] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging; 2008. Dec 15 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]