Abstract
Conversion disorder is characterized by neurological signs and symptoms related to an underlying psychological issue. Amygdala activity to affective stimuli is well characterized in healthy volunteers with greater amygdala activity to both negative and positive stimuli relative to neutral stimuli, and greater activity to negative relative to positive stimuli. We investigated the relationship between conversion disorder and affect by assessing amygdala activity to affective stimuli. We conducted a functional magnetic resonance imaging study using a block design incidental affective task with fearful, happy and neutral face stimuli and compared valence contrasts between 16 patients with conversion disorder and 16 age- and gender-matched healthy volunteers. The patients with conversion disorder had positive movements such as tremor, dystonia or gait abnormalities. We also assessed functional connectivity between the amygdala and regions associated with motor preparation. A group by affect valence interaction was observed. Post hoc analyses revealed that whereas healthy volunteers had greater right amygdala activity to fearful versus neutral compared with happy versus neutral as expected, there were no valence differences in patients with conversion disorder. There were no group differences observed. The time course analysis also revealed greater right amygdala activity in patients with conversion disorder for happy stimuli (t = 2.96, P = 0.006) (with a trend for fearful stimuli, t = 1.81, P = 0.08) compared with healthy volunteers, with a pattern suggestive of impaired amygdala habituation even when controlling for depressive and anxiety symptoms. Using psychophysiological interaction analysis, patients with conversion disorder had greater functional connectivity between the right amygdala and the right supplementary motor area during both fearful versus neutral, and happy versus neutral ‘stimuli’ compared with healthy volunteers. These results were confirmed with Granger Causality Modelling analysis indicating a directional influence from the right amygdala to the right supplementary motor area to happy stimuli (P < 0.05) with a similar trend observed to fearful stimuli (P = 0.07). Our data provide a potential neural mechanism that may explain why psychological or physiological stressors can trigger or exacerbate conversion disorder symptoms in some patients. Greater functional connectivity of limbic regions influencing motor preparatory regions during states of arousal may underlie the pathophysiology of motor conversion symptoms.
Keywords: conversion disorder, arousal, amydala, affect, psychogenic movement disorder
Introduction
Conversion disorder is characterized in the Diagnostic and Statistical Manual of Mental Disorders, Version IV, 1994 by neurological signs and symptoms such as movements, seizures or sensory symptoms unrelated to an underlying neurological or medical disorder. Unexplained neurological symptoms are common and reported in 30% of general neurology clinics (Carson et al., 2000) and are the cause of prominent disability (Carson et al., 2003), yet the mechanisms are very poorly understood.
Functional imaging studies have focused on conversion paralysis or the absence of movement. The main hypotheses to explain conversion paralysis include either impairments in the generation of motor intention (Spence et al., 2000; Roelofs et al., 2002; Burgmer et al., 2006) or that motor intention is intact but execution is disrupted (Marshall et al., 1997; Cojan et al., 2009). Furthermore, impairments in self-monitoring (de Lange et al., 2007, 2008; Cojan et al., 2009), limbic processing or higher order regulation (Tiihonen et al., 1995; Marshall et al., 1997) have been proposed to inhibit motor execution (reviewed in Nowak and Fink, 2009). In this study, we focused on conversion disorder with positive motor symptoms such as tremor, dystonia, chorea, tics and gait disorders rather than conversion paralysis. We hypothesize that there may be mechanistic differences between conversion disorders resulting in the absence or presence of movement. For instance, the generation of positive conversion motor symptoms may be characterized by abnormalities in the conversion motor representation possibly through implicit learning processes, or implicate abnormal action selection processes such as excessive facilitation or impaired inhibition. Whether the abnormalities of conversion motor symptoms versus conversion paralysis may implicate a different process (e.g. abnormal action selection at the level of initiation rather than inhibition of motor execution), and hence a different neural network, is not known. Although motor network abnormalities may possibly differ, we further hypothesize that similar to that of conversion paralysis, upstream inputs such as emotion, arousal or hyperactive self-monitoring may also play a role in interfering with these processes. In this study, we focus on these upstream inputs of emotion or arousal and its role in the pathophysiology of conversion disorder with positive motor symptoms.
Several lines of evidence suggest a relationship between conversion disorder and psychological issues. For instance, patients with conversion disorder have a high frequency of comorbid depressive and anxiety symptoms (Bowman and Markand, 1996; Sar et al., 2004) and conversion disorder symptom severity is associated with more frequent early and later adverse life events (Bowman, 1993; Roelofs et al., 2005). In an early paper, Lader and Sartorius (1968) demonstrated that patients with mixed active conversion disorder symptoms failed to habituate skin conductance to repeated auditory stimuli compared with control patients with anxiety and to healthy volunteers (Lader and Sartorius, 1968). The patients with conversion disorder also had higher baseline arousal levels as measured by the rate of spontaneous fluctuation in skin resistance as compared with the control patients with ‘anxiety’ and healthy volunteers. As the failure to habituate has been inversely correlated with high arousal level, other authors (Horvath et al., 1980) have suggested that the Lader and Sartorius (1968) findings may be related to the demonstrated high arousal levels. Horvath et al. (1980) extended these findings in patients with remitted mixed conversion disorder symptoms compared with control subjects with ‘free floating anxiety’ emphasizing a failure to habituate in skin conductance response to repeated acoustic stimuli with normal baseline responses (Horvath et al., 1980). The authors suggest the findings may reflect either greater arousal or a failure to inhibit the orienting response to a familiar stimulus, which may be a risk factor for the development of conversion disorders. More recently, patients with non-epileptic seizures have been shown to have higher basal cortisol levels compared with healthy volunteers, which is a marker of stress levels, unrelated to seizure frequency, physical activity or acute psychological stress (Bakvis et al., in press). Patients with non-epileptic seizures also have greater vigilant attentional bias towards threat stimuli (angry faces) in a masked emotional Stroop task, a bias that positively correlates with baseline cortisol levels (Bakvis et al., 2009a, b). Both the baseline cortisol levels and increased threat vigilance were more likely in patients with a history of sexual abuse (Bakvis et al., 2009a, b, in press). Patients with non-epileptic seizure also have reduced heart rate variability and increased threat vigilance compared with healthy volunteers (Bakvis et al., 2009a, b). Finally, patients with psychogenic movement disorder, another form of conversion disorder characterized by abnormal movements, were demonstrated to have greater startle response to both positive and negative affective stimuli compared with healthy volunteers linking arousal to a reflexive motor response (Seignourel et al., 2007). In summary, patients with conversion disorder mixed symptoms appear to be associated with greater arousal during the illness state (e.g. galvanic skin response, baseline cortisol, reduced heart rate variability, greater threat vigilance, greater startle response to arousing stimuli). The extent of previous exposure to childhood sexual abuse may also modulate measures including the baseline cortisol levels and threat vigilance (Roelofs and Spinhoven, 2007).
Preliminary data from neuroimaging studies provide information on possible networks engaging limbic and motor regions that may be involved in conversion paralysis. Studies demonstrate the engagement of regions in the limbic-motor interface to attempted or imagined movement (ventromedial prefrontal cortex) and non-noxious brush stimuli (caudate/putamen) in conversion paralysis. These regions have been suggested as potential nodal points for emotional stimuli to influence motor mechanisms (Marshall et al., 1997; Vuilleumier et al., 2001; de Lange et al., 2007, 2008). Furthermore, a patient with conversion paralysis was demonstrated to have greater amygdala activity and lower motor cortex activity to recall of a personal emotionally distressing event (Kanaan et al., 2007). Finally, a comparison of one patient with conversion paralysis and 30 healthy volunteers showed greater functional connectivity between the right motor cortex and posterior cingulate during a go/no-go task, leading the authors to suggest that internal monitoring of memories or emotional states may play a role in interfering with motor execution (Cojan et al., 2009).
The literature suggests a potential role between arousal and conversion disorder that may play a role in modulating motor networks, resulting in the abnormal conversion motor symptom. We sought to investigate the relationship between affect or arousal and conversion disorder with positive motor symptoms (herein referred to as motor conversion disorder) by investigating amygdala activity in association with viewing affective stimuli in a large patient sample size. We use an affective task that has been extensively investigated in healthy volunteers and patients with psychiatric disorders. Healthy volunteers have been well-documented to have greater amygdala activity to both negative and positive emotional stimuli relative to neutral stimuli along with greater activity to negative relative to positive stimuli (Breiter et al., 1996; Morris et al., 1996; Costafreda et al., 2008). We hypothesized that motor conversion disorder would be associated with greater amygdala activity to both positive and negative affective stimuli.
Methods
Subjects
Patients with motor conversion disorder were recruited from the Human Motor Control Section clinic at the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH). The Human Motor Control Section specializes in movement disorders with a specific interest in psychogenic movement disorders with presentations of unexplained tremor, dystonia, gait abnormalities, chorea or tics (Williams et al., 1995). Patients with either documented or clinically established levels of certainty for the diagnosis of psychogenic movement disorders (Williams et al., 1995) were included in this study. Psychogenic movement disorders do not require an association with a psychological issue whereas this criterion is necessary for a conversion disorder diagnosis, namely Criteria B—‘psychological factors are judged to be associated with the symptom or deficit because the initiation or exacerbation of the symptom or deficit is preceded by conflicts or other stressors’ (Diagnostic and Statistical Manual of Mental Disorders, Version IV, 1994). In our psychiatric assessment, we included diagnoses such as major depression or generalized anxiety disorder when considering ‘psychological factors’ and considered factors influencing either symptom ‘initiation or exacerbation’ as per the diagnostic criteria. The diagnosis of psychogenic movement disorders does not differentiate the voluntary production of the symptom whereas conversion disorder does include this criterion. However, in practice, and in this study along with any other published studies in conversion disorder, the diagnosis of factitious disorder and malingering is not feasible without either the patient admitting the voluntary nature of the symptom or surreptitious observation of the patient.
Inclusion criteria for patients with motor conversion disorder included diagnostic confirmation by at least two neurologists and one psychiatrist, no movement symptoms at rest for the imaging study, movement symptoms not affecting the head or neck, no history of traumatic brain injury and not on antidepressants. All patients had a clinical structural MRI as part of their diagnostic workup and additional tests such as EEG, nerve conduction studies, lumbar punctures or other blood work as clinically indicated. Other inclusion criteria for patients with motor conversion disorder and healthy volunteers included being 19 years of age or older, with exclusion criteria including having a serious medical or neurological illness, current major depression, panic disorder, post-traumatic stress disorder, substance abuse or other major affective or psychotic disorders (Diagnostic and Statistical Manual of Mental Disorders, Version IV, 1994), being on antidepressants and contraindications for MRI. Psychiatric diagnoses were screened using a semi-structured clinician-administered interview, the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Version IV Disorders (V.V. and R.A.). As we were interested in commonalities in the exacerbation of motor symptoms, we did not select for specific motor presentations. Age- (±5 years) and gender-matched healthy volunteers were recruited from the National Institutes of Health healthy volunteer database. All subjects were assessed by a movement disorders neurologist (M.H.) to confirm the diagnosis of psychogenic movement disorder using Williams’ criteria (Williams et al., 1995) and by a psychiatrist (V.V.) to confirm the diagnosis of motor conversion disorder and screen for psychiatric disorders using the Diagnostic and Statistical Manual of Mental Disorders, Version IV diagnostic criteria. The study was approved by the National Institutes of Health Institutional Review Board and all subjects signed informed consent.
Incidental affective task
Subjects viewed affective face stimuli in three different block conditions of fearful, happy and neutral stimuli. Seventeen 24 s blocks (16 stimuli per block) of four runs were shown interspersed with 11 s fixation rest. The conditions were pseudorandomized over four runs. Stimuli were presented centrally for 1 s with a fixation cross present for 0.5 s between trials. Stimuli from the Karolinska Directed Emotional Faces (12 different faces; 6 male and 6 female) (1998) were used and were similar for ratings arousal [as measured in a validation study using the 9 point Self-Assessment Manikin rating scale, which shows graphic figure representations of arousal: F 3.90 (SD 0.38) and H 4.02 (SD 0.38)] (Goeleven et al., 2008) and intensity [as measured on a nine point Likert rating scale of intensity: F 6.12 (SD 0.78) and H 6.61 (SD 0.53)] (Goeleven et al., 2008) between fearful and happy (arousal: df = 22, t = 0.77, P = 0.44) (intensity: df = 22, t = 1.8, P = 0.09). The images were pseudorandomized within blocks. The neutral face consisted of 25% happy morphed with 75% neutral as 100% neutral faces have been demonstrated to be experienced as potentially negative stimuli (Phillips et al., 1998). Subjects were asked to perform a gender identification task during stimuli presentation by pressing, as rapidly as possible, the right button for male and left button for female on a Lumina response box using two fingers on the dominant hand. We assessed reaction time and excluded reaction times in which subjects either failed to respond (<1% of the overall stimuli) or responded after more than 1000 ms. The task was coded in e-PRIME. Subjects also completed the Beck Depression Inventory (BDI) and the Beck Anxiety Inventory (BAI).
Image acquisition and preprocessing
To control for head movement, the subject’s head was secured with an elastic bandage and dense foam packing. MRI scanning was performed on a 1.5 T General Electric scanner with an eight channel head coil. Forty-two axial slices (slice thickness = 2 mm, gap 1 mm) were acquired using T2*-weighted echo planar images at a temporal resolution of 3 s, echo time 33 ms, flip angle 90°, matrix 64 × 64 with interleaved acquisition. The first four echo planar image volumes were discarded from analysis as dummy scans to allow for magnetization to reach steady state. The imaging data were preprocessed and analysed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). The data were adjusted for slice timing, realigned to the first image of the first run, normalized to the Montreal Neurological Institute atlas and smoothed using an 8 mm Gaussian kernel. Head motion parameters were used as regressors of no interest in the first level analysis.
Statistical analyses
Age, BDI and BAI scores were compared between groups using an unpaired t-test. The reaction time to the affective conditions were compared as a mixed model ANOVA with Valence as a within subject factor and Group as a between subject factor. P < 0.05 was considered significant. Statistical Package for the Social Sciences 16.0 was used for statistical analyses.
Imaging analyses
The blocks were modelled as a box car function convolved with the haemodynamic response function based on the time of onset of the block and the block duration. First, we compared the contrasts of fearful versus neutral (F–N) and happy versus neutral (H–N) from all 4 runs between patients with motor conversion disorder and healthy volunteers using a mixed measures ANOVA with Valence as a within subject factor and Group as a between subject factor. To ensure that the task was associated with amygdala activity, we also looked at the overall effect of F–N and H–N in both subject groups. To study the Valence and Group effects independently from the depression and anxiety effects, we repeated the analysis using the BDI and BAI scores as covariates of no interest. We also compared F–R (rest) and H–R using the same analysis to ensure that the response was independent of amygdala activity to neutral stimuli. Given our specific hypotheses focusing on the amygdala, P < 0.001 whole brain uncorrected confirmed with P < 0.05 region of interest corrected was considered significant. The region of interest analysis was defined using the anatomical definition of the amygdala from MarsBaR (MARSeille Boîte À Région d’Intérêt) (http://marsbar.sourceforge.net). Next, we extracted the time course of right amygdala activity using the Finite Impulse Response function (peri-stimulus time histogram) using MarsBaR. For the amygdala time course analysis for the fearful and happy conditions, the central coordinates were defined based on the peak voxel of the right amygdala identified in the overall effect of F–N and H–N in patients with motor conversion disorder and healthy volunteers with a radius of 8 mm. The area under the time course curve was compared between patients with motor conversion disorder and healthy volunteers using unpaired t-tests with P < 0.05 considered significant. Then, to assess the relationship between depressive and anxiety symptoms and amygdala activity in patients with motor conversion disorder, we conducted regression analyses between amygdala blood oxygen level dependent (BOLD) activity and BDI and BAI scores in patients with motor conversion disorder. For the regression analysis, P < 0.001 was considered significant.
To assess the relationship between the right amygdala and regions associated with motor preparation during exposure to emotional stimuli, we conducted a psychophysiological interaction analysis to assess functional connectivity (Friston et al., 1997) assessing the contrasts of F–N and H–N. The seed volume of interest for the psychophysiological interaction was the right amygdala defined based on the peak voxel identified in the overall effect of F–N and H–N in patients with motor conversion disorder and healthy volunteers along with a radius of 8 mm. For the psychophysiological interaction, P < 0.001 whole brain uncorrected was considered significant. The group analysis was conducted using mixed measures ANOVA with Valence as a within-subject factor and Group as a between-subject factor. All the second level analyses including the psychophysiological interaction were repeated with reaction time as a covariate to control for reaction time as a confounder.
Since the psychophysiological interaction analysis identified an interaction between the right amygdala and right supplementary motor area, we used Granger Causality Modelling (GCM) (Roebroeck et al., 2005) to assess the directionality of the functional connectivity using R, an open source platform-independent modelling program (Chen et al., 2009). GCM assumes that a region causally connected to a second region would have similar signal patterns following a time-lag delay and that this latency suggests a causal relationship between the two regions. The central coordinates for the seed volumes of interest for the right amygdala were defined as above with the psychophysiological interaction analysis, and the right supplementary motor area was defined based on the peak voxel of the Group effect of the psychophysiological interaction analysis of F–N and H–N in patients with motor conversion disorder versus healthy volunteers with an 8 mm radius. The time courses were separately extracted for the right amygdala and right supplementary motor area for fearful and happy conditions for each motor conversion disorder subject. Using a vector auto-regressive model with a time lag equivalent to one repetition time (3 s), the two volumes of interest were separately compared for both conditions for each subject resulting in path coefficients and t-values corresponding to either the amygdale, to supplementary motor area direction or the opposite. A second-stage group analysis based on the signed path coefficients to determine network connectivity was conducted for fearful and happy conditions (Chen et al., 2009).
Results
Subject characteristics
We scanned 16 patients with motor conversion disorder [10 female, mean age 40.56 (SD 6.32) (range 21–66)] and 16 age- and gender-matched right handed healthy volunteers [10 female, mean age 38.29 (SD 7.85)] (t = 0.62, df = 30, P = 0.54). One patient with motor conversion disorder was left handed. In the motor conversion disorder group, predominant symptoms included tremor (n = 10, all bilateral; seven upper extremities and three both upper and lower extremities), dystonia (not fixed) (n = 2), gait disorder (n = 2), mixed tremor/dystonia/gait disorder (n = 2) with mean symptom duration prior to study 6.4 (3.9) years (range 1–25 years). Psychological issues at motor conversion disorder symptom onset or involved in symptom exacerbation included major depression (n = 5), dysthymia (n = 3), adjustment disorder (n = 2), panic attacks (n = 2), generalized anxiety disorder (n = 4) on Axis I of the Diagnostic and Statistical Manual of Mental Disorders, Version IV and psychosocial stressors (n = 9) on Axis IV of the Diagnostic and Statistical Manual of Mental Disorders, Version IV (e.g. difficulties with relationships with a spouse or a child, coping with a death, an accident occurring at work, relationship with a superior at work). Two patients with motor conversion disorder had a history of childhood sexual abuse (obtained by clinician interview). There were no diagnoses of post-traumatic stress disorder at motor conversion disorder symptom onset. Past diagnoses were in remission at the time of scanning; only one patient had a diagnosis of generalized anxiety disorder at the time of the study. Three patients were on nocturnal benzodiazepines (prescribed for sleep or for the movement symptoms) and one patient on an antiepileptic medication (prescribed for the movement symptoms), which were withheld the night before the study. Mean depression and anxiety scores were higher in patients with motor conversion disorder [BDI: 10.42 (SD 8.32), BAI: 13.38 (SD 6.38)] compared with healthy volunteers [BDI: 2.75 (SD 2.03), BAI: 3.63 (SD 2.92)] (BDI: t = 3.58, df = 30, P = 0.001; BAI: t = 5.56, df = 30, P < 0.001) despite the lack of categorical diagnoses of depressive or anxiety disorders at the time of scanning.
Behavioural effects
We first analysed the reaction time data. Overall, there were no differences in reaction time between patients with motor conversion disorder [mean reaction time in milliseconds, N: 593.71 (SD 56.33); F: 600.19 (SD 59.74); H: 588.40 (SD 53.48)] and healthy volunteers [mean reaction time in milliseconds, N: 603.32 (SD 59.24); F: 611.48 (SD 61.19); H: 605.76 (SD 58.38)] [F(1,30) = 1.11, P = 0.20]. There were no main or interaction effects of Valence (all P > 0.05). Patients with motor conversion disorder responded to 98.6% (SD 3.09) of stimuli and healthy volunteers responded to 98.9% (SD 1.09) (t = 1.12, P = 0.25) of stimuli. Patients with motor conversion disorder correctly identified 98.92% (SD 1.78) and healthy volunteers correctly identified 99.5% (SD 1.11) (t = 1.14, P = 0.26).
Imaging effects
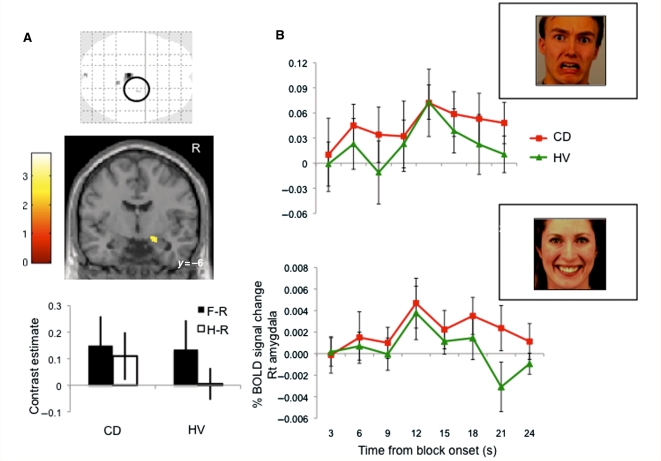
We then analysed the Valence and Group effects on the BOLD signal amplitude focusing on the amygdala. There was a positive effect overall of bilateral amygdala activity for the contrasts of H–N and F–N in both subject groups thus confirming that the affective stimuli were associated with an increase of amygdala activity (P < 0.05 region of interest corrected). There was no main effect of Group or Valence. We observed a Group by Valence interaction effect in the right amygdala (local peak Montreal Neurological Institute coordinates x, y, z = 24, −8, −16 mm, Z = 3.29, P < 0.001 uncorrected, P < 0.05 region of interest corrected) (Fig. 1A). Post hoc t-tests demonstrated that healthy volunteers had greater amygdala activity to F–N compared with H–N (P < 0.05 region of interest corrected), as expected. In contrast, there was no significant difference between F–N and H–N in patients with motor conversion disorder. The Finite Impulse Response time course of the right amygdala for the fearful and happy conditions in patients with motor conversion disorder and healthy volunteers are illustrated in Fig. 1B. In the happy condition, there was greater right amygdala activity of the area under the time course curve in patients with motor conversion disorder [0.05 (SD 0.03)] compared with healthy volunteers [0.0.1 (SD 0.04)] (t = 2.96, df = 30, P = 0.006). In the fearful condition, there was a trend towards greater right amygdala activity of the area under the time course curve in patients with motor conversion disorder [0.97 (SD 0.71)] compared with healthy volunteers [0.52 (SD 0.69)] (t = 1.81, df = 30, P = 0.08). To ensure that these results of the right amygdala were unrelated to depressive and anxiety scores, we repeated the analysis including BDI and BAI as covariates of no interest. Again, there were no main effects but there was a Group by Valence interaction effect in the right amygdala but at a lower local peak maximum (local peak Montreal Neurological Institute coordinates x, y, z = 22, −8, −16 mm, Z = 2.75, P = 0.003 uncorrected, P < 0.05 region of interest corrected). We also repeated the analysis without the left handed patient. Similarly, there were no main effects but there was a Group by Valence effect in the right amygdala (local peak coordinates x, y, z = 24, −8, −16 mm, Z = 3.23, P < 0.001 uncorrected). We repeated the analysis comparing the contrast of Runs 1 + 2 versus Runs 3 + 4 for fearful and happy using a mixed measures ANOVA with Valence as a within-subject factor and Group as a between-subject factor to assess for differences between runs that may be related to habituation effects. We did not observe any main or interaction effects even with a liberal threshold of P < 0.05 uncorrected.
Figure 1.
Amygdala activity to emotional stimuli in conversion disorder (CD). (A) Patients with motor conversion disorder (CD) were compared with healthy volunteers (HV) using an incidental affective task with a mixed measures ANOVA with patient Group as a between-subjects factor and Valence as a within-subjects factor. The glass brain, statistical parametric map image and contrast estimates show the significant patient Group by Valence interaction of fearful versus rest (F–R) and happy versus rest (H–R) contrasts between patients with conversion disorder and healthy volunteers localized to the right amygdala (mixed measures ANOVA) (right amygdala local peak Montreal Neurological Institute coordinates x, y, z = 24, −4, −24 mm, Z = 3.83, P < 0.001 uncorrected, P < 0.05 region of interest corrected). The glass brain is shown at P < 0.001 uncorrected cluster threshold >4. The statistical parametric map image is shown at P < 0.005 uncorrected cluster threshold > 4. (B) Right amygdala time course activity. The area under the curve for the right amygdala time course (Finite Impulse Response function time-locked to block onset) was compared between patients with conversion disorder and healthy volunteers for the fearful (top; t = 1.81, P = 0.08) and happy (bottom; t = 2.96, P = 0.006) conditions. Error bars represent standard deviation.
To confirm that the interaction effect was not related to the effect on neutral stimuli, the same analysis was conducted comparing F–R and H–R. We observed a main effect of Group: patients with motor conversion disorder had greater right amygdala activity compared with healthy volunteers (local peak x, y, z = 24, −4, −24 mm, Z = 3.83, cluster size = 4, P < 0.001 uncorrected, P < 0.05 region of interest corrected). There was a similar Group by Valence interaction effect of the right amygdala (local peak x, y, z = 24, −8, −15 mm, Z = 3.31, P < 0.001 uncorrected, P < 0.05 region of interest corrected). There were no differences in amygdala activity when the neutral condition was compared between patients with motor conversion disorder and healthy volunteers (two sample t-test) even with conservatively lowering the threshold to P < 0.01 uncorrected.
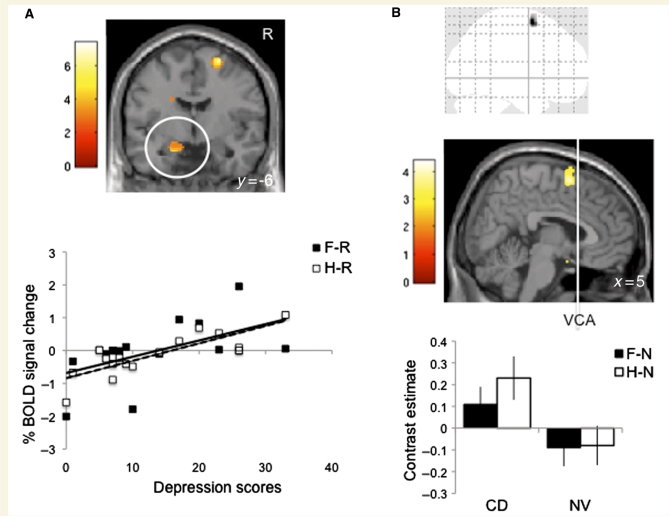
We also assessed the relationship between depressive and anxiety symptoms and amygdala activity in patients with motor conversion disorder by conducting regression analyses focusing on bilateral amygdala activity. The BDI score in patients with motor conversion disorder was positively correlated with greater left amygdala activity to both F–R (local peak x, y, z = −14, −4, −20 mm, Z = 3.11, P < 0.001 uncorrected) and H-R (local peak x, y, z = −18, −6, −22 mm, Z = 4.24, cluster size = 20, P < 0.001 uncorrected) contrasts (Fig. 2A). The BDI score did not correlate with right amygdala activity and the BAI score did not correlate with amygdala activity including with lowering the threshold to P < 0.01 uncorrected.
Figure 2.
Depression scores and functional connectivity. (A) The statistical parametric map image shows the regression analysis of the Beck Depression Inventory (BDI) scores and the happy versus rest (H–R) contrast of the left amygdala in patients with motor conversion disorder (CD). The scatter plot show the regression analyses of the depression scores and the % BOLD signal change of the left amygdala in patients with motor conversion disorder during fearful versus rest (F–R) (solid line: R2 = 0.31, P < 0.001) and happy versus rest (dashed line: R2 = 0.66, P < 0.001). The statistical parametric map image is shown at P < 0.005 uncorrected cluster threshold >4. (B) The glass image, scanning probe microscopy image and plot show the significant Group effect of the functional connectivity map of the right amygdala (seed voxel not shown) [mixed measures ANOVA with within-subjects Valence factor (F–N and H–N) and between-subjects Group (motor conversion disorder and healthy volunteers) of the psychophysiological interaction analysis]. The glass image is shown at P < 0.001 uncorrected cluster threshold >4. The statistical parametric map image is shown at P < 0.005 uncorrected cluster threshold > 4. Error bars are in standard deviation. VCA = vertical anterior commisure line.
In the psychophysiological interaction analysis, there was a Group effect with patients with motor conversion disorder demonstrating greater functional connectivity between the right amygdala and the right supplementary motor area during both F–N and H–N relative to healthy volunteers (global peak x, y, z = 10, 6, 64 mm, Z = 4.22, cluster size = 129, P < 0.001 uncorrected) (Fig. 2B). There was no Valence effect or a Group by Valence interaction effect. The seed voxel for the psychophysiological interaction and time course analyses was x, y, z = 24, −8, −16 mm with an 8 mm radius. The path matrix and P-matrix scores of the Granger Causality Mapping group analysis are reported in Table 1. During the group analysis of the happy condition, there was a significant positive correlation in the path from the right amygdala to the right supplementary motor area (P < 0.05). During the fearful condition, there was a trend towards a positive correlation in the path from the amygdala to supplementary motor area (P < 0.07). The central coordinates used for the right amygdala were x, y, z = 24, −8, −16 mm and for the right supplementary motor area, x, y, z = 10, 6, 64 mm.
Table 1.
Path matrix and P-matrix values
| Fearful |
Happy |
|||
|---|---|---|---|---|
| Amygdala | SMA | Amygdala | SMA | |
| Path matrix | ||||
| Amygdala | −0.09 | 0.20 | −0.20 | 0.20 |
| SMA | −0.12 | 0.17 | −0.01 | −0.071 |
| P-matrix | ||||
| Amygdala | 0.42 | 0.06 | 0.0003 | 0.02 |
| SMA | 0.15 | 0.13 | 0.11 | 0.33 |
The Path matrix and P-matrix values of the Group section of the GCM analysis. The sign of the Path matrix indicates a positive or negative correlation. The table is read from column (seed volume of interest) to row. SMA = supplementary motor area.
Discussion
Using an incidental affective functional MRI task, we demonstrated that whereas healthy volunteers have the expected pattern of greater right amygdala activity to negative as compared with positive emotional stimuli, patients with motor conversion disorder did not have such a differential response to valence. Moreover, the time course activity suggested the right amygdala in patients with motor conversion disorder may be less likely to habituate with repeated exposure to positive stimuli, and possibly also to negative stimuli. Using psychophysiological interaction, we show a greater interaction between the right amygdala and right supplementary motor area during fearful and happy in patients with motor conversion disorder relative to healthy volunteers. Using Granger Causality Mapping analysis, we further show that with positive stimuli, activity in the right amygdala may influence a region associated with motor initiation, the right supplementary motor area. A similar trend was also seen with negative stimuli.
Implications of amygdala activity
The amygdala is a crucial structure in the modulation of motivated attention and preparation for action (reviewed in Lang and Davis, 2006; Whalen et al., 2009). The basolateral amygdala receives input from the thalamus, hippocampus and cerebral cortex and has outputs to the dorsal and ventral striatum (believed to modulate secondary reinforcement learning and avoidance behaviour), hippocampus and orbitofrontal cortex. The basolateral amygdala projects to the central nucleus of the amygdala and lateral basal nucleus of the stria terminalis (‘extended’ amygdala) which then project to the periaqueductal grey (freezing), lateral hypothalamus (autonomic responses) and midbrain cell bodies such as the ventral tegmental area and locus coeruleus (attention, vigilance and arousal) (Lang and Davis, 2006). The amygdala is implicated in the orienting response to facilitate attention to a stimulus. The basolateral amygdala plays a role in associative learning and thus is believed to assign motivational significance to stimuli; and the central nucleus is believed to play a role in maintaining the stimulus as the source of attention (Lang and Davis, 2006). Multiple studies demonstrate that both valence and arousal play a critical role in modulating amygdala activity (reviewed in Whalen et al., 2009). In healthy volunteers, the amygdala is robustly activated to negative emotional stimuli relative to positive stimuli and is activated particularly to fearful stimuli. This valence specific activity suggests a crucial role in determining biologically salient or threatening stimuli in the environment. The amygdala also responds to level of intensity or arousal, with a recent meta-analysis demonstrating not only the association with negative stimuli but also arousing positive stimuli (Breiter et al., 1996; Morris et al., 1996; Costafreda et al., 2008). The fact that we see, in patients with motor conversion disorder, a loss of this asymmetric activity between fearful and happy observed in healthy volunteers would suggest either a valence specific effect to positive stimuli or a general effect to arousing stimuli. Several lines of evidence point towards a general effect of arousal. In our study, we selected positive and negative face images with a similar level of arousal and intensity. There was trend for the happy stimuli to be of greater emotional intensity than fearful stimuli and this suggests that our results may reflect the effects of arousal on amygdala activity in motor conversion disorder. These results also dovetail with a study in patients with psychogenic movement disorder demonstrating a greater startle response to both positive and negative emotional stimuli; in healthy volunteers, positive stimuli are associated with a startle inhibition and negative stimuli are associated with a startle facilitation (Seignourel et al., 2007). The authors in this study proposed that a greater startle response to arousing stimuli may underlie these observations in psychogenic movement disorders. A general arousal effect is also consistent with the literature on conversion disorder demonstrating greater baseline arousal as measured using galvanic skin response, baseline cortisol levels and heart rate variability relative to healthy volunteers (Lader and Sartorius, 1968; Bakvis et al., 2009a,b, in press).
The amygdala plays a critical role in evaluating the initial relevance of sensory stimuli and rapidly habituates with repeated presentations, thus allowing for a shift of attentional resources to more salient stimuli (Breiter et al., 1996). The right amygdala in particular has been implicated in responding to dynamic situations with more rapid habituation to emotionally valenced stimuli (Fischer et al., 2000; Wright et al., 2001). Our data suggest that in patients with motor conversion disorder, the right amygdala may fail to habituate to emotionally salient stimuli compared with healthy volunteers. This pattern was significant for positive stimuli with a trend towards significance for negative stimuli. This observation dovetails with reports of a failure of habituation of galvanic skin response to acoustic stimuli in both active and remitted conversion disorder patients relative to patients with anxiety symptoms and healthy volunteers (Lader and Sartorius, 1968; Horvath et al., 1980). Impairments in habituation in motor conversion disorder may suggest either a role for generalized increased arousal or alternatively, a failure of adaptation of the attentional process in evaluating salience relevance with repeated presentations. Further studies incorporating an event-related design would be useful to clarify the role of habituation in these patients.
The generalized findings for happy and fearful face processing may at first sight contrast with previous findings demonstrating greater attentional bias to threatening stimuli (angry faces relative to happy and neutral faces) using a masked emotional Stroop in non-epileptic seizure patients compared with healthy volunteers (Bakvis et al., 2009a,b). However, there are several reasons for the observed differences. First, the tasks are different with the prior one assessing reaction time to the interference effect of masked non-conscious angry faces whereas our task assessed amygdala BOLD activity to fearful faces in a block design with a gender-identification task. Second, the differences may be related to differences in patient group between motor conversion disorder and non-epileptic seizure, not just to symptom presentation but also to the high comorbidity of affective disorders in the non-epileptic seizure group (15/19 patients). However, more intriguingly, the threat bias in the non-epileptic seizure group was positively correlated with previous sexual trauma reported in 14/19 non-epileptic seizure patients. In contrast, in our current study, only 2/16 patients with motor conversion disorder conversion disorder had a history of sexual abuse, as determined by clinical interview. This potentially lower incidence of sexual abuse in motor conversion disorder compared with non-epileptic seizure is consistent with a previous report of lower rates of incest in patients with conversion motor symptoms compared with patients with non-epileptic seizures (Stone et al., 2004). Thus, differences in exposure to sexual abuse during childhood development may also contribute to the observed differences between the studies (Roelofs and Spinhoven, 2007).
These findings were independent of depressive or anxiety symptoms as the patients with motor conversion disorder did not have current diagnoses of depressive disorders, and depressive and anxiety scores were used as covariates in the imaging analysis. Neither depressive nor anxiety scores correlated with right amygdala activity whereas depressive scores correlated positively with left amygdala activity. However, the patients with motor conversion disorder did have elevated depressive and anxiety scores relative to the healthy volunteers, which may have several implications. Although subjects were screened with a semi-structured clinician interview for psychiatric diagnostic categories, neither minor depression nor depression in partial remission, both of which are active states (Thase, 2009), were diagnosed. We have previously suggested that these states may influence antidepressant efficacy in the management of conversion motor symptoms (Voon and Lang, 2005). Furthermore, patients with conversion disorder may be less likely to endorse or report emotional symptoms. Thus, patients with unrecognized or under-reported forms of depression may have been included in the sample and depression or anxiety may indeed have some influence on the right amygdala findings. However, we argue that this is not likely to be a major issue because (i) depressive symptoms should be associated with greater amygdala activity to negative stimuli rather than positive stimuli whereas we observed the opposite; (ii) we controlled for BDI and BAI scores and even with lowering the thresholds, these scores were not correlated with right amygdala activity; and (iii) the BDI scores were positively correlated with left but not the right amygdala activity; however, this was observed to both positive and negative stimuli and therefore argues against a simple role for depression, but rather, an effect of general arousal. The elevated depressive and anxiety scores may be markers for elevated physiological arousal with concomitant somatic symptoms rather than mood states per se. Conversion disorder commonly also presents with higher levels of somatic symptoms and depression and anxiety scales not only assess mood, but also cognitive and somatic items. In neurological disorders such as Parkinson’s disease, which are characterized by higher somatic symptoms, this is a prominent issue and studies demonstrate that higher cut-off scores are associated with depression in Parkinson’s disease as compared with the cut-off scores used in the general population (Schrag et al., 2007). Thus, it may also be that higher scores on depression and anxiety scales in motor conversion disorder are not necessarily indicative of depression or anxiety. Alternatively, while comorbid psychiatric disorders are distinct conditions, it has been hypothesized that mood and anxiety symptoms may be an integral part of the presentation of conversion disorder (LaFrance and Barry, 2005). As this current study excluded patients with diagnoses of major depression or severe anxiety disorders to assess a more homogeneous patient population, we are unable to comment on whether these comorbid disorders may play a role in the mechanisms described here. Investigating whether comorbid psychiatric disorders (which also have their own neurobiological underpinnings) have a similar or different physiological influence on conversion symptoms would be of great interest as a follow up study.
We also note that the psychiatric disorders of major depression and post-traumatic stress disorder are associated with exaggerated amygdala activity to negative compared with positive affective stimuli relative to healthy volunteers (Rauch et al., 2000; Sheline et al., 2001). Our data suggest that amygdala activity may distinguish motor conversion disorder from these disorders. Our study provides neural evidence suggesting a potential role for psychological or physiological arousal in the pathophysiology of motor conversion disorder. We emphasize that further studies are required to investigate whether this explains the initial triggers and onset of motor conversion disorder symptoms, a response to the motor conversion disorder symptoms or a perpetuating feature, or an underlying predisposing state. The results may also be consistent with a reaction to the behavioural consequences of motor conversion disorder.
Limbic motor interaction
There are several mechanisms by which amygdala activity may modulate motor behaviours. In response to a threatening stimulus, an animal becomes highly alert and attentive; with increasing proximity of the threat, the animal then will either dart away or attack (Lang and Davis, 2006). Electrical stimulation of the amygdala at low levels initially is association with freezing behaviours or cessation of behaviour with accompanying bradycardia reflecting the orienting attentional reflex (Applegate et al., 1983). With increasing stimulation, the animal becomes more active and subsequently attempts to escape the stimulation reflecting defensive behaviours. Amygdala projections to the periaqueductal grey area are believed to mediate these responses with the ventral region modulating freezing and dorsal regions modulating action. Lesions of the central nucleus block freezing but not escape whereas lesions of the basolateral nucleus block avoidance behaviours (Killcross et al., 1997). The projections of the basolateral nucleus to the dorsal or ventral striatum have been suggested to play a role in avoidance learning. The amygdala has also been implicated in conditioned approach behaviours. In rodent studies of autoshaping, bilateral lesions of the central nucleus blocks approach behaviours towards learned conditioned stimuli paired with reward and are hypothesized to be mediated by projections to the mesolimbic dopaminergic cell bodies (Parkinson et al., 2000).
In our study, we demonstrated aberrant limbic-motor interactions in patients with motor conversion disorder that may underlie the influence of affect or arousal on motor function. Patients with motor conversion disorder had greater functional connectivity from the right amygdala to the right supplementary motor area to happy with a trend observed for fearful. Although there are no direct neuroanatomical projections between the amygdala and supplementary motor area, the amygdala projects to the nucleus accumbens core and dorsal striatum, which have projections via the pallidum and thalamus to the supplementary motor area (Groenewegen et al., 1997). Alternatively, amygdala projections to the periacqueductal grey and midbrain cell bodies (Lang and Davis, 2006) may also have downstream effects on supplementary motor area activity.
The supplementary motor area is a major source of input to the corticospinal tract and is reciprocally connected to the primary motor cortex and basal ganglia. The supplementary motor complex is implicated in self-initiated action, although its role is not without controversy (reviewed in Nachev et al., 2008; Passingham et al., 2009). The supplementary motor area is believed to be one source of the Bereitschaftspotential or ‘readiness potential’, a slowly increasing negative potential that precedes movement onset (Shibasaki and Hallett, 2006). The supplementary motor area has also been implicated in non-conscious motor inhibition. A study involving three patients with well-characterized focal supplementary motor area and superior eye field lesions demonstrated a failure of motor inhibition (Sumner et al., 2007). In healthy individuals, masked stimuli that are briefly presented and not consciously observed can act as a prime to initially facilitate a response but the response is then inhibited. In the lesion patients, the responses were normally facilitated but not subsequently inhibited suggesting a potential role of the supplementary motor area in non-conscious motor response inhibition. Thus, we speculate that effect of arousal on amygdala activity may influence motor symptoms either through a general effect on initiation of the motor conversion symptom or possibly through a failure of inhibition of the motor conversion symptom.
The study of motor conversion disorder can be subdivided into: (i) the study of motor function (e.g. initiation or inhibition); (ii) potential upstream influences such as limbic influences as in this study; or (iii) the question of why a movement symptom using the same voluntary motor pathways should be experienced as involuntary. We have recently demonstrated that the comparison of non-intentional conversion tremor versus intentional mimicked tremor in patients with motor conversion disorder is associated with lower right temporoparietal junction activity (Voon et al., 2010). While there is a range of potential explanations, the temporoparietal junction has been implicated as a comparator of predicted versus actual outcomes. We thus speculated that the hypoactivity may be due to the lack of an appropriate prediction outcome signal of the conversion tremor. Without the predicted outcome signal, there would be no comparison between the predicted versus the actual sensory outcome of the conversion movement and hence the temporoparietal junction hypoactivity and the sensation that the movement is not under one’s control. Whether the signal is indeed impaired and its source is in motor conversion disorder is not known; the involvement of the supplementary motor area in this this study may implicate this region as a potential source in motor conversion disorder.
Strengths and limitations
Our study strength lies in the use of a paradigm that has been extensively investigated in healthy volunteers and patients with psychiatric and neurologic disorders. The study also has a large sample size whereas previous imaging studies in conversion disorder have reported sample sizes from one to eight (Vuilleumier et al., 2001; Ghaffar et al., 2006; Roelofs et al., 2006; Kanaan et al., 2007; de Lange et al., 2008; Cojan et al., 2009). Another strength is the inclusion of only patients with conversion motor presentations (i.e. we excluded other forms of conversion disorder such as conversion blindness, paralysis and anaesthesia) but which also limits generalization to other types of conversion disorder. We would expect a similar effect of arousal on amygdala activity in patients with conversion disorder presenting with other neurological symptoms but may differ depending on the presence of depression or a history of sexual abuse. However, we would expect that connectivity between the amygdala and motor preparatory regions may differ. For instance, greater limbic connectivity with sensory processing regions may explain conversion anaesthesia or with another area involved in motor preparation or execution may explain conversion paralysis. We used a block design in order to optimize the psychophysiological interaction analysis. However, an event related design would provide further insights into the early orienting response of the amygdala and further information on habituation. The use of auto-regressive analysis in Granger Causality Modeling is not without criticism (Friston, 2009); however, our results from two separate analyses of functional connectivity concur. We also note that like all other studies in conversion disorders, the assessment of a partial or complete voluntary component to the symptom (e.g. factitious or malingering) cannot be reliably conducted and may be a confounder. Finally, as the patients did not have movements while at rest in the scanner, the results may also be related to underlying ongoing background brain activity rather than necessarily related to the generative process of conversion disorder.
Conclusion
Motor conversion disorder is characterized by greater amygdala activity to arousal and potential impairments in habituation to arousing stimuli. Amygdala activity may have a downstream influence on the supplementary motor area, a region involved in motor initiation and non-conscious response inhibition. Our findings add to the current conceptualization of potential contributors to conversion disorder. A model of contributors may include the presence of both positive and negative emotional events, major personally relevant crises or minor repeated daily stressors, constant exposure to physiologically arousing events such as lack of sleep or a neurobiological recurrence of depression symptoms leading to greater arousal and the onset or exacerbation of conversion motor symptoms. Finally, our study suggests that biological or psychological treatments targeting arousal may be worthy of controlled study in motor conversion symptoms.
Funding
This study was funded by and conducted at the intramural National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Glossary
Abbreviations
- BAI
Beck Anxiety Inventory
- BDI
Beck Depression Inventory
- BOLD
blood oxygen level dependent
- GCM
Granger Causality Modelling
References
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav. 1983;31:353–60. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- Bakvis P, Roelofs K, Kuyk J, Edelbroek PM, Swinkels WA, Spinhoven P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia. 2009a;50:1001–11. doi: 10.1111/j.1528-1167.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- Bakvis P, Spinhoven P, Roelofs K. Basal cortisol is positively correlated to threat vigilance in patients with psychogenic nonepileptic seizures. Epilepsy Behav. 2009b;16:558–60. doi: 10.1016/j.yebeh.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Bakvis P, Spinhoven PH, Giltav EJ, Kuyk J, Edelbroek PM, Zitman FG, et al. Basal hypercortisolism and trauma in patients with psychogenic non-epileptic seizures. Epilepsia; (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Bowman ES. Etiology and clinical course of pseudoseizures. Relationship to trauma, depression, and dissociation. Psychosomatics. 1993;34:333–42. doi: 10.1016/S0033-3182(93)71867-8. [DOI] [PubMed] [Google Scholar]
- Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153:57–63. doi: 10.1176/ajp.153.1.57. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Burgmer M, Konrad C, Jansen A, Kugel H, Sommer J, Heindel W, et al. Abnormal brain activation during movement observation in patients with conversion paralysis. Neuroimage. 2006;29:1336–43. doi: 10.1016/j.neuroimage.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Carson AJ, Best S, Postma K, Stone J, Warlow C, Sharpe M. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897–900. doi: 10.1136/jnnp.74.7.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson AJ, Ringbauer B, Stone J, McKenzie L, Warlow C, Sharpe M. Do medically unexplained symptoms matter? A prospective cohort study of 300 new referrals to neurology outpatient clinics. J Neurol Neurosurg Psychiatry. 2000;68:207–10. doi: 10.1136/jnnp.68.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Hamilton P, Thomason ME, Gotlib IH, Saad ZS. ISMRM 17th Scientific Meeting. Hawaii: Granger causality via vector auto-regression tuned for FMRI data analysis. 2009. [Google Scholar]
- Cojan Y, Waber L, Carruzzo A, Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage. 2009;47:1026–37. doi: 10.1016/j.neuroimage.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Washington: DC: American Psychiatric Association; 1994. [Google Scholar]
- de Lange FP, Roelofs K, Toni I. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia. 2007;45:2051–8. doi: 10.1016/j.neuropsychologia.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Roelofs K, Toni I. Motor imagery: a window into the mechanisms and alterations of the motor system. Cortex. 2008;44:494–506. doi: 10.1016/j.cortex.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fischer H, Furmark T, Wik G, Fredrikson M. Brain representation of habituation to repeated complex visual stimulation studied with PET. Neuroreport. 2000;11:123–6. doi: 10.1097/00001756-200001170-00024. [DOI] [PubMed] [Google Scholar]
- Friston K. Dynamic causal modeling and Granger causality comments on: the identification of interacting networks in the brain using fMRI: model selection, causality and deconvolution. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.09.031. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Ghaffar O, Staines WR, Feinstein A. Unexplained neurologic symptoms: an fMRI study of sensory conversion disorder. Neurology. 2006;67:2036–8. doi: 10.1212/01.wnl.0000247275.68402.fc. [DOI] [PubMed] [Google Scholar]
- Goeleven E, de Raedt R, Leyman L, Berschuere B. The Karolinska Directed Emotional Faces: a validation study. Cognition and Emotion. 2008;22:1094–118. [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Horvath T, Friedman J, Meares R. Attention in hysteria: a study of Janet's hypothesis by means of habituation and arousal measures. Am J Psychiatry. 1980;137:217–20. doi: 10.1176/ajp.137.2.217. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Craig TK, Wessely SC, David AS. Imaging repressed memories in motor conversion disorder. Psychosom Med. 2007;69:202–5. doi: 10.1097/PSY.0b013e31802e4297. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–80. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Lader M, Sartorius N. Anxiety in patients with hysterical conversion symptoms. J Neurol Neurosurg Psychiatry. 1968;31:490–5. doi: 10.1136/jnnp.31.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFrance WC, Jr, Barry JJ. Update on treatments of psychological nonepileptic seizures. Epilepsy Behav. 2005;7:364–74. doi: 10.1016/j.yebeh.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. CD ROM from Department of Clinical Neuroscience, Psychology section. Karolinska Instituet; 1998. The Karolinska Directed Emotional Faces - KDEF. [Google Scholar]
- Marshall JC, Halligan PW, Fink GR, Wade DT, Frackowiak RS. The functional anatomy of a hysterical paralysis. Cognition. 1997;64:B1–B8. doi: 10.1016/s0010-0277(97)00020-6. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–15. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Fink GR. Psychogenic movement disorders: aetiology, phenomenology, neuroanatomical correlates and therapeutic approaches. Neuroimage. 2009;47:1015–25. doi: 10.1016/j.neuroimage.2009.04.082. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–13. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Bengtsson SL, Lau HC. Medial frontal cortex: from self-generated action to reflection on one's own performance. Trends Cogn Sci. 2010;14:16–21. doi: 10.1016/j.tics.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, et al. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res. 1998;83:127–38. doi: 10.1016/s0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–42. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Roelofs K, Spinhoven P. Trauma and medically unexplained symptoms towards an integration of cognitive and neuro-biological accounts. Clin Psychol Rev. 2007;27:798–820. doi: 10.1016/j.cpr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Roelofs K, de Bruijn ER, van Galen GP. Hyperactive action monitoring during motor-initiation in conversion paralysis: an event-related potential study. Biol Psychol. 2006;71:316–25. doi: 10.1016/j.biopsycho.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Roelofs K, van Galen GP, Keijsers GP, Hoogduin CA. Motor initiation and execution in patients with conversion paralysis. Acta Psychol (Amst) 2002;110:21–34. doi: 10.1016/s0001-6918(01)00068-3. [DOI] [PubMed] [Google Scholar]
- Roelofs K, Spinhoven P, Sandijck P, Moene FC, Hoogduin KA. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508–14. doi: 10.1097/01.nmd.0000172472.60197.4d. [DOI] [PubMed] [Google Scholar]
- Sar V, Akyuz G, Kundakci T, Kiziltan E, Dogan O. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161:2271–6. doi: 10.1176/appi.ajp.161.12.2271. [DOI] [PubMed] [Google Scholar]
- Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, et al. Depression rating scales in Parkinson's disease: critique and recommendations. Mov Disord. 2007;22:1077–92. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seignourel PJ, Miller K, Kellison I, Rodriguez R, Fernandez HH, Bauer RM, et al. Abnormal affective startle modulation in individuals with psychogenic [corrected] movement disorder. Mov Disord. 2007;22:1265–71. doi: 10.1002/mds.21451. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–56. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Spence SA, Crimlisk HL, Cope H, Ron MA, Grasby PM. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet. 2000;355:1243–4. doi: 10.1016/S0140-6736(00)02096-1. [DOI] [PubMed] [Google Scholar]
- Stone J, Sharpe M, Binzer M. Motor conversion symptoms and pseudoseizures: a comparison of clinical characteristics. Psychosomatics. 2004;45:492–9. doi: 10.1176/appi.psy.45.6.492. [DOI] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, et al. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron. 2007;54:697–711. doi: 10.1016/j.neuron.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME. Update on partial response in depression. J Clin Psychiatry. 2009;70(Suppl 6):4–9. doi: 10.4088/JCP.8133su1c.01. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Viinamaki H, Lehtonen J, Partanen J. Altered cerebral blood flow during hysterical paresthesia. Biol Psychiatry. 1995;37:134–5. doi: 10.1016/0006-3223(94)00230-Z. [DOI] [PubMed] [Google Scholar]
- Voon V, Lang AE. Antidepressant treatment outcomes of psychogenic movement disorder. J Clin Psychiatry. 2005;66:1529–34. doi: 10.4088/jcp.v66n1206. [DOI] [PubMed] [Google Scholar]
- Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–8. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124:1077–90. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Davis C, Oler JA, Kim H, Kim J, Neta M. Human amygdala responses to facial expressions of emotion. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York: The Guildford Press; 2009. pp. 265–88. [Google Scholar]
- Williams DT, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol. 1995;65:231–57. [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]