Abstract
Difficulties in social cognition are well recognized in individuals with autism spectrum conditions (henceforth ‘autism’). Here we focus on one crucial aspect of social cognition: the ability to empathize with the feelings of another. In contrast to theory of mind, a capacity that has often been observed to be impaired in individuals with autism, much less is known about the capacity of individuals with autism for affect sharing. Based on previous data suggesting that empathy deficits in autism are a function of interoceptive deficits related to alexithymia, we aimed to investigate empathic brain responses in autistic and control participants with high and low degrees of alexithymia. Using functional magnetic resonance imaging, we measured empathic brain responses with an ‘empathy for pain’ paradigm assessing empathic brain responses in a real-life social setting that does not rely on attention to, or recognition of, facial affect cues. Confirming previous findings, empathic brain responses to the suffering of others were associated with increased activation in left anterior insula and the strength of this signal was predictive of the degree of alexithymia in both autistic and control groups but did not vary as a function of group. Importantly, there was no difference in the degree of empathy between autistic and control groups after accounting for alexithymia. These findings suggest that empathy deficits observed in autism may be due to the large comorbidity between alexithymic traits and autism, rather than representing a necessary feature of the social impairments in autism.
Keywords: empathy, autism, alexithymia, interoception, anterior insula, mentalizing, theory of mind
Introduction
In recent years, the field of social neuroscience has made rapid progress in elucidating the neuronal basis of our capacity to understand mental states such as the thoughts and feelings of others. According to recent neuroscientific models (Decety and Jackson, 2004; Blair, 2005, 2008; Decety and Grèzes, 2006; de Vignemont and Singer, 2006; Singer, 2006; Singer and Lamm, 2009), at least two different routes to the understanding of other minds can be distinguished: our ability to understand the abstract beliefs and intentions of others, which is referred to as Theory of Mind, cognitive perspective taking or mentalizing (Premack and Woodruff, 1978; Frith and Frith, 2003) and our ability to share the feelings of others, which is referred to as empathy (Wispé, 1986; Eisenberg and Strayer, 1987; Eisenberg and Fabes, 1990; Eisenberg, 2000; Hoffman, 2000; Preston and de Waal, 2002; Singer et al., 2004, 2006; Blair, 2005; Decety and Lamm, 2006; de Vignemont and Singer, 2006; Keysers and Gazzola, 2007). Empathy in turn involves at least two major components: an affective component, which allows us to share the feelings of others, and a cognitive component, which is related to our capacity for self-other distinction. When we empathize, we vicariously experience the emotional state of another person, realizing that what we are feeling is not our own emotional state but that of the other person (e.g. Eisenberg, 2000; Decety and Lamm, 2006; de Vignemont and Singer, 2006).
Even though empathizing and Theory of Mind are usually simultaneously engaged in social cognition, recent imaging studies have suggested that the two postulated routes to understanding others rely on distinct neural networks. Theory of Mind has been mainly linked to activity of the medial prefrontal cortex, the superior temporal sulcus and the adjacent temporoparietal junction (for a review see Frith and Frith, 2006; Saxe, 2006; Saxe and Baron-Cohen, 2006; also Mitchell et al., 2002; Mitchell, 2008). In contrast, our ability to empathize with other people’s emotional states (such as disgust or pain) activates parts of those neuronal networks that are involved when the emotional states are experienced by the self. Thus, activation of brain areas relevant for emotion processing such as somatosensory, insular and anterior cingulate cortices have been observed during empathy (Carr et al., 2003; Wicker et al., 2003; Keysers et al., 2004; Morrison et al., 2004; Singer et al., 2004, 2006, 2008; Jackson et al., 2005, 2006; Jabbi et al., 2007). The most robust evidence for such shared networks in empathy stems from a multitude of studies on empathy for pain. These suggest the necessary involvement of anterior insula and, less consistently, the anterior cingulate cortices when people empathize with the suffering of others (Morrison et al., 2004, 2007; Singer et al., 2004, 2006, 2008; Jackson et al., 2005, 2006; Cheng et al., 2007; Gu and Han, 2007; Lamm et al., 2007a, b; Saarela et al., 2007). More generally, insular cortex, also called ‘interoceptive cortex’, has been shown to be involved in mapping internal bodily and subjective feeling states (Damasio, 1994; Craig, 2002, 2003, 2009; Critchley et al., 2004, 2005; Singer et al., 2009). These findings have led to the suggestion that cortical representations underlying the representation of feeling states in the self also underlie our ability to share the emotional state of the other (so-called ‘shared-network’ models).
Further evidence speaking to the existence of multiple and dissociable neural networks which underlie different socio-cognitive abilities comes from studies of patients with specific social disorders such as psychopathy or autism spectrum conditions. Psychopaths, for example, seem to have an impaired ability to empathize, but not an impaired ability to understand other people’s intentions and goals, a pattern reflected in the oft-reported Machiavellian nature of psychopaths (Blair, 2003, 2005). Conversely, individuals with autism spectrum conditions have a general deficit in the social domain, with evidence for reduced Theory of Mind (see Frith and Happé, 2005 for a review) and reduced activity of the brain network associated with this mentalizing capacity (see Frith and Frith, 2006 for a review). In addition, individuals with autism spectrum conditions have frequently been characterized as lacking in empathy (Gillberg, 1992; Shamay-Tsoory et al., 2002; Baron-Cohen and Wheelwright, 2004; McIntosh et al., 2006; Lombardo et al., 2007; Minio-Paluello et al., 2009). Baron-Cohen (2009) argues that individuals with autism spectrum conditions are best described as being low on empathizing (a construct which includes both cognitive perspective taking and empathy) and high on systemizing (a construct described as the drive to analyse or construct systems). In support of this characterization, individuals with autism spectrum conditions score lower on the Empathy Quotient (Baron-Cohen and Wheelwright, 2004; Johnson et al., 2009), which assesses the self-reported capacity to take another person’s mental perspective as well as the capacity to share their feelings. Further evidence is provided by reduced inhibition of corticospinal excitability in individuals with autism spectrum conditions when they observe a painful stimulus being applied to another (Minio-Paluello et al., 2009) and lower self-reported empathy in autism spectrum condition populations on empathy questionnaires such as the Interpersonal Reactivity Index (IRI: Davis, 1980; Lombardo et al., 2007; however, see Rogers et al., 2007 and Dziobek et al., 2008 for conflicting findings). Furthermore, when children with autism were shown vignettes depicting other children experiencing various emotions, they reported less emotional empathy (matching emotional states) with the characters depicted in the vignettes (Yirmiya et al., 1992).
The claim of a global empathy deficit in autism spectrum conditions does not always reflect, however, the more detailed distinction made between our capacities to mentalize and to empathize. For example, a test widely used in autism research as a marker for empathy is the ‘reading the mind in the eyes test’ (Baron-Cohen et al., 1996, 1997, 2001). However, this test does not directly assess emotional responses as it requires one to infer the expressed mental state from the eye region of emotional facial expressions, but does not directly measure the vicarious emotional response elicited by the expression.
A further, important complication with the ‘empathy-deficit’ characterization of autism spectrum conditions is the high comorbidity between autism spectrum conditions and alexithymia. Alexithymia has been described as a subclinical phenomenon marked by difficulties in identifying and describing feelings and difficulties in distinguishing feelings from the bodily sensations of emotional arousal (Nemiah et al., 1976). Alexithymia is thought to characterize 10% of the general population (Linden et al., 1995; Salminen et al., 1999). However, although neither a necessary nor sufficient feature of autism spectrum conditions, recent studies have found severe degrees of alexithymia in ∼50% of individuals with autism spectrum conditions, with the majority showing slight or severe impairments (Hill et al., 2004; Berthoz and Hill, 2005; see also Lombardo et al., 2007 and Silani et al., 2008). Thus, it is unclear whether the empathy deficit reported in individuals with autism spectrum conditions is a result of the autism spectrum condition, or whether it is a result of comorbid alexithymia. Indeed, a previous study suggests that the lack of empathy in autism spectrum conditions is a function of interoceptive deficits associated with alexithymia rather than a function of autism spectrum conditions per se (Silani et al., 2008). Silani et al. showed that the degree to which participants were able to understand their own emotions (i.e. their degree of alexithymia) was correlated with activity in the anterior insula during an interoceptive task (Silani et al. 2008). Importantly, the relationship between participants’ self-reported degree of alexithymia, and activity in the anterior insula when introspecting on their emotions, was the same for both the autism spectrum conditions and control groups. Participants with autism spectrum conditions but without alexithymia showed normal activity in the anterior insula during interoception, suggesting that they were unimpaired in understanding their own emotions. Furthermore, participants’ self-reported degree of alexithymia, and activity in the anterior insula when introspecting on emotion, were correlated with scores on a classical self-report measure of trait empathy (Davis, 1980).
The association between alexithymia and empathy is predicted by the previously described ‘shared network’ models of empathy: these models suggest that the networks responsible for processing emotions in the self are the same networks used to represent the emotions of others. Thus, a difficulty representing one’s own emotions would result in a deficit in representing others’ emotions (e.g. Singer et al., 2004, 2009). The findings of Silani et al. (2008) provide initial support for the hypothesized role of the anterior insula in alexithymia and empathy and suggest that degree of empathy within individuals with autism spectrum conditions is associated with their degree of alexithymia. However, Silani et al.’s (2008) study leaves at least two crucial questions unanswered. As the study included only a self-reported measure of empathy without testing empathy directly, it could only show that alexithymia was associated with the degree to which individuals with autism spectrum conditions were consciously aware of their empathic response. As alexithymia is a deficit in identifying and describing one’s own emotion, it is possible that the alexithymic individuals with autism spectrum conditions did have an empathic reaction, but were unable to identify and therefore report this reaction.
The second question left unanswered by Silani et al.’s (2008) study is whether there is a general empathic deficit associated with autism spectrum conditions that is not explained by alexithymia; that is, whether even non-alexithymic individuals with autism spectrum conditions show reduced empathy.
Therefore, in the present study, we aimed to test empathy in individuals with autism spectrum conditions directly and to determine whether any deficits are due to their autism spectrum condition and/or a result of the increased level of alexithymia in this group. We therefore tested empathy in a group of individuals with autism spectrum conditions selected to ensure a wide distribution of alexithymia scores and a matched control group (of individuals without autism spectrum conditions) with the same wide distribution of alexithymia scores. Significantly, we tested empathy in the domain of pain using a well-established empathy-for-pain functional magnetic resonance imaging (fMRI) paradigm that has been shown to involve activation of the interoceptive cortices but not the cognitive perspective taking network (Singer et al., 2004, 2006, 2008). Therefore, we contend that this task provides a purer measure of empathy than more commonly used tests and is minimally confounded by mentalizing. Furthermore, this paradigm has the advantage of assessing empathy in vivo by measuring the empathic brain responses of participants while their partners or friends receive pain. Thus, the social emotions are tested in a real social context. Importantly, the use of symbolic cues instead of pictorial material helps to overcome the significant methodological problems associated with testing empathy using pictures of emotional facial expressions in autism spectrum condition populations. Several studies have found that individuals with autism spectrum conditions show decreased attention to the face, and particularly to eye regions of the face, in comparison to non-autism spectrum conditions control groups (Boucher and Lewis, 1992; Klin et al., 1999, 2002; Blair et al., 2002) and may also have problems recognizing emotional facial expressions (Howard et al., 2000; Humphreys et al., 2007; see Adolphs et al., 2001 for conflicting findings). Using the present paradigm, any empathy deficit seen in the autism spectrum conditions group cannot be due to reduced attention to the eye regions, which may be crucial in signalling the pain of the other when pictures of facial emotion are presented (Adolphs, 2007, 2008), or a failure in interpreting the emotional state of the other. Finally, the present paradigm allows for the assessment of empathic responses without requiring verbal reports from participants, a feature which may facilitate finding empathic responses in alexithymic individuals and those with autism spectrum conditions.
Based on the findings of Silani et al. (2008), we hypothesized that autism spectrum conditions do not result in an empathy deficit per se. Rather, we hypothesized that empathy-related activity will vary as a function of the degree of alexithymia in both groups and be associated with activation in insular cortices.
Materials and methods
Participants
This study required an equal distribution of high and low alexithymic participants in control and autism spectrum condition groups. As the prevalence rate of alexithymia differs in autistic and normal control populations (Hill et al., 2004; Tani et al., 2004), we pre-screened a larger sample of participants with the 20-item Toronto Alexithymia Scale (TAS-20) (Bagby et al., 1994) as a measure of alexithymia to reach our final sample of 18 male participants with autism spectrum conditions and 18 male controls who were matched on alexithymia scores, age and IQ. Two-sample t-tests confirmed that the groups were not significantly different in terms of alexithymia (autism spectrum condition mean ± SD = 57.2 ± 11.8, range 37–80; control mean ± SD = 50.3 ± 14.5, range 27–72), age (autism spectrum condition mean ± SD = 34.6 ± 13.3, range 19–60; control mean ± SD = 35.0 ± 12.8, range 22–63), or IQ (autism spectrum condition mean ± SD = 115.8 ± 14.6, range 91–140; control mean ± SD = 118.8 ± 11.7, range 103–149), whereby IQ was assessed with the Wechsler Adult Intelligence Scale (WAIS®-III UK; Wechsler, 1999) (Tables 1 and 2). A Kolmogorov–Smirnov goodness of fit test confirmed that both samples are normally distributed on the TAS (autism spectrum condition D = 0.117, exact P = 0.942; controls D = 0.169, exact P = 0.624).
Table 1.
Demographic characteristics and pain thresholds
| Autism spectrum condition mean (SD) | Control mean (SD) | Autism spectrum condition versus controls | |
|---|---|---|---|
| Age (years) | 34.6 (13.3) | 35.0 (12.8) | P = 0.92 |
| Verbal IQ | 117.3 (13.4) | 118.9 (7.9) | P = 0.70 |
| Performance IQ | 110.2 (16.6) | 111.9 (11.8) | P = 0.75 |
| Full IQ | 115.8 (14.6) | 118.8 (11.7) | P = 0.52 |
Groups’ means, SD and P-values associated with an independent samples t-test on the differences between groups *P < 0.05, **P < 0.01.
Table 2.
Questionnaire data
| Autism spectrum condition mean (SD) | Control mean (SD) | Autism spectrum conditions versus controls | |
|---|---|---|---|
| TAS | 57.2 (11.8) | 50.3 (14.5) | P = 0.13 |
| BVAQ | 54.2 (8.4) | 51.4 (9.7) | P = 0.40 |
| IRI | 52.1 (15.4) | 59.7 (10.9) | P = 0.12 |
Mean and SD values for both groups, and P-values associated with an independent samples t-test on the differences between groups.
All participants in the autism spectrum condition group were high functioning and had previously received a diagnosis of autism or Asperger’s Syndrome from an independent clinician according to the standard Diagnostic and Statistical Manual of Mental Disorders-IV criteria (American Psychiatric Association, 1994). Fifteen participants had received a diagnosis of Asperger’s Syndrome and three of autism. In addition to the clinical diagnosis, we used the Autism Diagnostic Observational Schedule (ADOS-G; Lord et al., 2000) to characterize the current level of functioning for the autism spectrum conditions group further (Table 3). On this measurement, eight participants met ADOS criteria for autism and five participants met criteria for autistic spectrum disorders. Four participants scored above the cut-off point only in one of the two subscales and one participant was below the cut-off point in both subscales (see ‘Discussion’ section).
Table 3.
Diagnosis, ADOS-G and alexithymia scores
| Participant | Diagnosis | ADOS social interaction Cut-off = 4 | ADOS communication Cut-off = 2 | ADOS Total score Cut-off = 7 | TAS |
|---|---|---|---|---|---|
| 1 | AS | 3 | 4 | 7 | 37 |
| 2 | Autism | 5 | 10 | 15 | 41 |
| 3 | AS | 1 | 5 | 6a | 43 |
| 4 | AS | 1 | 1 | 2b | 44 |
| 5 | AS | 2 | 5 | 7 | 51 |
| 6 | AS | 4 | 7 | 11 | 48 |
| 7 | Autism | 4 | 6 | 10 | 52 |
| 8 | AS | 4 | 6 | 10 | 55 |
| 9 | AS | 2 | 8 | 9 | 59 |
| 10 | AS | 4 | 8 | 12 | 59 |
| 11 | AS | 3 | 4 | 7 | 61 |
| 12 | Autism | 3 | 8 | 11 | 62 |
| 13 | AS | 3 | 4 | 7 | 67 |
| 14 | Autism | 3 | 7 | 10 | 60 |
| 15 | AS | 2 | 4 | 6a | 80 |
| 16 | AS | 1 | 2 | 3a | 71 |
| 17 | AS | 0 | 5 | 5a | 66 |
| 18 | AS | 6 | 11 | 17 | 73 |
The diagnosis refers to the original clinical assessment provided by a qualified psychologist or psychiatrist (AS = Asperger’s syndrome). Scores on the ADOS-G are derived from the diagnostic algorithm and represent the behaviour of the participant at the time of the study. TAS represents scores on the TAS-20 Alexithymia questionnaire.
a: Below cut-off on one ADOS-G subscale.
b: Below cut-off on both ADOS-G subscale.
Control participants did not exhibit autistic features and were screened for any pre-existing neurological or psychiatric disorders using a questionnaire/interview. All participants gave their informed consent to participate in the study, which was approved by the Local Ethics Committee and conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
The empathy-for-pain paradigm used in this study (e.g. Singer et al., 2004) required participants to bring another individual (henceforth ‘partner’). In contrast to the original paradigm used by Singer et al. (2004), the participants’ partner was not necessarily their romantic partner. As the majority of the participants with autism spectrum conditions were not in a romantic relationship, participants were asked to bring a person with whom they had a significant relationship (family member, friend or carer). A total of 36 participant pairs took part in the experiment. Five participants in the autism spectrum condition group came with their romantic partner, nine with a family member and one with a close friend. Three participants in the autism spectrum condition group were not able to bring a partner so they completed the experiment with a researcher from the Institute of Cognitive Neuroscience, UCL, with whom they had spent considerable time during previous testing sessions and thus had developed a friendly relationship. Ten of the control group came with their romantic partner, two with family members and five with friends. One participant from the control group was not able to bring a partner and was therefore also matched with a researcher with whom he had spent time during pre-testing. Comparison of the results of previous studies using this paradigm (Singer et al., 2004, 2006) suggests that empathy towards romantic partners may be higher than towards relative strangers. It is possible that the autism spectrum condition group would therefore exhibit less empathy as a result of their partner profiles, even in the absence of any true empathy deficit. Accordingly, the Relationship Closeness Inventory (Berscheid et al., 1989) was used to assess the quality and duration of the relationship between the participants and their respective partners. Analysis of the Relationship Questionnaire did not reveal any significant difference between the autism spectrum condition and control groups [t(24) = −0.8; P > 0.05] or a significant correlation with degree of alexithymia (autism spectrum condition: r = 0.239, P > 0.05; control: r = 0.119, P > 0.05), suggesting that any observed differences in empathic brain response were not due to a selection bias in the quality and duration of the relationship between the participant and their partner. As a final check, scores from the Relationship Closeness Inventory were entered into the analysis (reported below) as a covariate. Inclusion of the covariate did not change the reported results, and it was also not predictive of empathy-related brain activity in either the autism spectrum condition or control groups. It should be acknowledged that the validity of this analysis assumes that participants in both the autism spectrum conditions and control groups were equally able to complete the Relationship Closeness Inventory. At present this assumption has not been empirically tested. We contend, however, that the available data suggest that the different partner profiles between the autism spectrum condition and control groups do not explain the observed results.
Questionnaire measures
In addition to the brain measures, we also assessed individual differences in empathy with the IRI (Davis, 1980) and validated the TAS-20 alexithymia measure using an alternative alexithymia scale, the Bermond-Vorst Alexithymia Questionnaire (BVAQ; Vorst and Bermond, 2001).
Experimental paradigm and procedure
In this study, we adopted the same procedure as described by Singer et al. (2008). In brief, before entering the scanner room, participants were familiarized with the experimental task and individual pain thresholds were determined for each participant pair (see Singer et al., 2004 for a full description of the procedure). Pain stimulation was obtained by passing electrical current through a bipolar concentric surface electrode placed on the dorsum of the left hand of the participants and on the dorsum of the right hand of their partners (square pulse waveform, 100 Hz, 4 ms pulse length, 1 s duration).
After determination of individual pain thresholds, participants were placed into the scanner and the partner was seated next to the scanner. The participant’s left hand and the partner’s right hand were placed on a tilted board which enabled the participant to see both hands with the help of a mirror system. Coloured arrows indicating the person who was to receive the next painful stimulation were projected onto a large screen placed in front of the participant. Stimulation intensity was indicated by the brightness of the arrow, light arrows indicating non-painful low stimulation and dark arrows indicating painful high stimulation. After each trial, participants rated the subjective level of unpleasantness on an analogue scale ranging from −10 (very unpleasant) to +10 (very pleasant) by moving a cursor along the scale with their right index and middle fingers. Each trial consisted of the presentation of an anticipatory cue (the arrow) which was followed after 3.5 s by a small circle of the same colour centred on the screen indicating the beginning of the electrical stimulation. After 2 s, the rating scale appeared on the screen for a total duration of 4 s. In order to reduce socially desirable responding when the participant rated how unpleasant they found their partner’s pain, the rating scale was presented in a position on the screen which was not visible to the partner. The invisibility of the participant’s response to their partner was emphasized to each participant. The scanning phase consisted of two 9 min sessions and a 10 min structural scan. Each session consisted of 20 trials for each condition (painful high stimulation and non-painful low stimulation) and 50% null events where only a fixation cross was presented. The two sessions were blocked with respect to the recipient of the stimulation. During the first session only the partner was stimulated (‘other’ condition) and during the second session only the participant was stimulated (‘self’ condition). Throughout both sessions the only part of the partner’s body viewable to the participant was the partner’s hand.
Imaging data acquisition
MRI brain images were acquired with a 1.5 Tesla system (Siemens Sonata). Functional whole brain data were obtained using a T2* echoplanar sequence sensitive to blood oxygen level dependent contrast (44 slices, 3 mm thickness, gap 0.75 mm, echo time 90 ms, repetition time 3960 ms per volume). To reduce inhomogeneities in amygdala and orbitofrontal cortex, a sequence with axial slices tilted by 30° and a flip angle of 90° was used (Deichmann et al., 2002). The functional data were acquired in 2 sessions; the first six volumes of each session were discarded to allow for T1 equilibration effects. Stimulus presentation began after the sixth volume. A total of 308 full-brain volumes for each participant were acquired. Structural images were obtained with a T1 sequence using a phased-array head coil at the end of the two functional sessions.
Imaging data analysis
fMRI data were analysed using Statistical Parametric Mapping (SPM)-5 (Wellcome Department of Imaging Neuroscience, London; www.fil.ion.ucl.ac.uk/spm). During preprocessing, functional images were realigned to the first volume, spatially normalized to a standard template with a resampled voxel size of 3 × 3 × 3 mm, and smoothed using a Gaussian kernel width of 10 mm full width at half maximum (6 mm at the first level, 8 mm at the second level) (Friston et al., 1995a). After preprocessing, functional images were analysed in an event-related fashion (Worsley and Friston, 1995), using the general linear model (Friston et al., 1995b).
The paradigm is based on a 2 × 2 × 2 factorial design with within-subject factors of ‘Pain’ (pain versus no-pain) and ‘Target’ (self versus other) and a between-subjects factor of ‘Group’ (autism spectrum conditions versus control). To create regressors of interest, each condition was modelled by convolving a delta function at each trial onset (presentation of the anticipatory cue) and at each rating onset (presentation of the rating scale) with a canonical haemodynamic response function over the duration of the event (5.5 and 4 s, respectively). Residual effects of head motion were corrected for by including the six estimated motion parameters for each participant as regressors of no interest. Contrast images were then calculated by applying appropriate linear contrasts to the parameter estimates for the regressors of interest.
Region of interest analyses
For our main analysis, we chose a region of interest approach (ROI) based on two independent empathy-for-pain studies performed previously with a similar paradigm in male populations only (Singer et al., 2006, 2008). The ROIs were formally defined by reanalysing functional data from these two previous studies to identify areas that were more active in response to high pain than low pain in the ‘other’ condition in conjunction with high versus low pain in the self condition. Thus, contrast images for the contrast Other High Pain–Other Low Pain and Self High Pain–Self Low Pain were entered into a second-level random effects model using SPM5. An ANOVA, thresholded at P < 0.05, familywise error corrected for the whole brain, identified a cluster in left anterior insula (centre of mass −36, 33, 3; volume 108 mm3; max/min x − 39/−33, max/min y 30/33, max/min z 3/3) that defined the ROI. A statistical threshold of P < 0.05 was used for all ROI analyses.
In order to investigate brain responses outside the a priori ROIs, additional whole brain analyses were performed. Results of these additional analyses are reported in Supplementary Table 1 at a threshold of P < 0.001, uncorrected.
Results
Questionnaires
As expected, the two questionnaire measures of alexithymia (TAS and BVAQ) were highly correlated (autism spectrum condition: r = 0.772, P < 0.01; control: r = 0.703, P < 0.01), suggesting that individual differences in alexithymia could be reliably measured in both groups. As in our previous study (Silani et al., 2008), a significant negative correlation was found between scores on the alexithymia questionnaire (TAS-20) and scores on the IRI (r = −0.422, P < 0.05), suggesting a relationship between degree of alexithymia and self-reported empathy. An independent samples t-test revealed that the autism spectrum condition and control groups did not differ significantly on self-reported trait empathy as measured by the IRI [t(30) = −1.6; P > 0.05].
Stimulus sensitivity and behavioural ratings of unpleasantness
In order to test for differences in stimulus sensitivity between groups, the amplitude of the stimulation measured in mA (i.e. the participants’ high and low pain thresholds) was entered in a repeated measures ANOVA with a within-subjects factor of Pain (high versus low pain) and a between-subjects factor of Group (autism spectrum conditions versus control). The analysis revealed a main effect of Pain [F(1,29) = 37.4, P < 0.001], but the main effect of Group was not significant [F(1,29) = 1.24, P > 0.05] (Table 4).
Table 4.
Pain stimulation thresholds and pain ratings
| Autism spectrum condition mean (SD) | Control mean (SD) | Autism spectrum condition versus controls | |
|---|---|---|---|
| Pain threshold (mA) | |||
| Low | 0.29 (0.13) | 0.33 (0.17) | P = 0.55 |
| High | 1.50 (1.40) | 2.08 (1.52) | P = 0.24 |
| Pain rating | |||
| Self low | −0.8 (2.7) | 2.0 (3.6) | P = 0.02* |
| Self high | −5.6 (2.3) | −5.9 (2.7) | P = 0.68 |
| Other low | −1.2 (3.1) | 2.1 (3.4) | P = 0.01** |
| Other high | −4.6 (3.4) | −5.5 (2.5) | P = 0.43 |
Statistics applied: independent samples t-test. *P < 0.05, **P < 0.01.
In order to corroborate the subjective nature of the pain thresholding procedure, we performed an ANOVA on the unpleasantness ratings for low and high pain stimulation during the self and the other conditions with two within-subjects factors (Pain: pain versus no-pain; Target: self versus other) and one between-subjects factor (Group: autism spectrum condition versus control). The ANOVA revealed a main effect of Pain [F(1,29) = 80.2, P < 0.001] and a significant interaction between Pain and Group F(1,29) = 7.49, P < 0.011]. Follow-up t-tests revealed that the groups gave significantly different unpleasantness ratings for the low pain condition {both for the self [t(29) = −2.7; P < 0.01, unpaired t-test] and the other [t(29) = −2.4; P < 0.05, unpaired t-test]}, but not for the high pain condition. Inspection of the mean ratings shows that the autism spectrum condition group judged the unpleasantness of the low pain stimulation to be close to zero, while the controls rated the low pain as slightly pleasant (Table 4).
Interestingly, a significant correlation was not found between participants’ self-reported level of alexithymia and ratings of unpleasantness in either the self or the other condition [self condition: autism spectrum condition: r = −0.127, P > 0.05; control: r = −0.164, P > 0.05; other condition: autism spectrum condition: r = −0.055, P > 0.05; control: r = 0.153, P > 0.05]. These results are in line with the findings of our previous study (Silani et al., 2008), in which behavioural ratings did not differ as a function of alexithymia in spite of clear differential brain responses in anterior insula for high and low alexithymic participants during interoception on emotions. Although speculative, this finding may suggest that alexithymic individuals are able to use a cognitive rule, perhaps based on social desirability, to make their response when tasks are as simple as those used in these studies.
Functional imaging results
To determine whether the often-reported empathy deficit in autism spectrum conditions is due to the alexithymia comorbidity within this group or to the presence of an autism spectrum condition, we sought to investigate: (i) whether empathic brain responses were correlated with degree of alexithymia in autism spectrum condition and control groups; (ii) whether the relationship between degree of alexithymia and empathic brain response varied as a function of autism spectrum condition diagnosis; and (iii) whether the autism spectrum condition and control groups exhibited differential levels of empathic brain activity after accounting for levels of alexithymia.
To perform these analyses, mean contrast values in the ROI were extracted using the MaRsBaR toolbox (Brett et al., 2002) for the contrast High Pain in the Other–Low Pain in the Other group. These values served as an index of empathic brain response and were entered as the dependant variable into regression models including TAS-20 scores for each group separately, and in combined models.
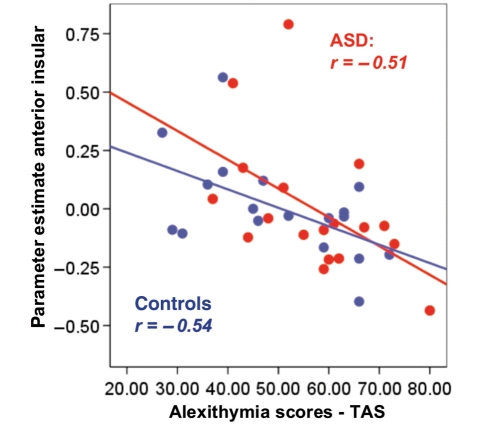
Our first analysis revealed that mean activity in the left anterior insula was significantly negatively correlated with TAS scores in both groups (Fig. 1). The higher the self-reported degree of alexithymia, the lower the empathy-related activity in this region when the partner received pain (autism spectrum condition: r = −0.506, P < 0.05; control, r = −0.536, P < 0.05).
Figure 1.
Mean activation levels (parameter estimates) of the voxels lying in the left anterior insula [–36 33 3] defined by the independent mask (see ‘Materials and methods’ section for details about ROI analyses) during empathy-related conditions (Pain–No pain in other) are significantly correlated with individual differences in alexithymia as measured by the TAS in both autism spectrum condition (ASD) (red dots) and control (blue dots) participants. The line represents the linear best fit. All correlations are significant at the P < 0.05 level.
Secondly, in order to investigate whether the relationship between empathic brain responses and alexithymia varies as a function of group, we performed a Potthoff (1966) analysis to test the null hypothesis of no difference between groups in the correlation coefficients, slopes, and intercepts of the two regressions. This analysis revealed that neither the degree of association (r) between activity in the anterior insula and alexithymia, nor the slope of the regression line, nor the intercept differed significantly between groups [F(2.32) < 1, P = 0.548, t(32) = −0.749, P = 0.459, t(32) = 0.919, P = 0.365, respectively].
Thirdly, in order to test for any group differences in empathic brain activity in the anterior insula independent of the degree of alexithymia, activity in the anterior insula was entered into an ANCOVA with Group (autism spectrum condition versus control) as a between-subjects factor and the TAS scores as a covariate. This analysis showed that the groups were not significantly different [F(1,35) < 1], indicating that there were no differences in empathic brain activity due to the presence or absence of an autism spectrum condition diagnosis after controlling for the degree of alexithymia.
In subsequent analyses, we explored the validity of our ROI analyses using whole brain analyses. First, in order to replicate the typical pattern of empathic brain activation observed in previous empathy-for-pain studies and confirm the relationship between empathy and alexithymia, participants were divided into high and low alexithymic groups (median split low alexithymia, mean ± SD = 42.3 ± 8.2, n = 18 and high alexithymia, mean ± SD = 65.2 ± 5.8, n = 18) and activity in interoceptive cortices were compared (Supplementary Table 1). As in previous studies (Singer et al., 2004, 2006, 2008), pain-related empathic brain responses were identified by masking the contrast of painful versus non-painful trials in the other condition with the contrast of painful versus non-painful trials in the self condition. This procedure allows us to identify brain regions that are activated by both the processing of pain in the self and empathy for pain in the other (i.e. a shared pain network for the self and other). Consistent with our ROI analysis, the results of this analysis revealed peak activation in left anterior insula in the low alexithymic group (−33, 30, 0; z = 3.79, P < 0.001, uncorrected) and for the difference between low and high alexithymic groups (−33, 33, 0; z = 3.33, P < 0.001, uncorrected).
We further tested for group differences between patients with autism spectrum conditions and control participants to guard against the possibility that empathic differences between the autism spectrum condition and control groups occur in areas outside the pre-defined ROI. This is especially pertinent as the ROIs were defined on the basis of a sample of individuals without an autism spectrum condition, and although one would expect that this would result in a relative ‘advantage’ for the control group in demonstrating empathic brain responses, it is important to test for empathic differences between groups across the whole brain. The contrast High Pain–Low Pain in the other, masked on High Pain–Low Pain in the Self, revealed that the only area in which the control group showed increased activity in response to pain in the other was in visual cortex (24 − 81 −9; z = 3.72, P < 0.001, uncorrected), which is not part of the typical pain matrix, nor a part of the empathy or Theory of Mind networks.
A final analysis was conducted to investigate a possible concern with respect to the current study: that alexithymia scores are a proxy for symptom severity in autism spectrum conditions. If true, the present findings could be explained by hypothesizing that controlling for degree of alexithymia before testing for group differences in empathy causes all variance due to autism spectrum condition symptom severity to be removed. This would result in a spurious null result and a false conclusion of there being no empathy deficit in autism spectrum conditions after controlling for alexithymia. Such a possibility is made plausible by the inclusion of participants who, despite having received a clinical diagnosis of autism or Asperger’s Syndrome, do not meet ADOS-G cut-off in the sample of individuals with autism spectrum conditions. These individuals may raise the mean empathic brain response in the autism spectrum condition group and mask any differences in empathy due to diagnosis of an autism spectrum condition (if alexithymia scores are a proxy for autism spectrum condition symptom severity the corollary of this would also be true; highly alexithymic participants in the control group may also have high levels of autism spectrum condition symptoms). To guard against the possibility that any null effects observed in the data could be caused by overly inclusive diagnostic classification, or statistical covariance between ADOS scores and alexithymia scores, the ADOS scores were regressed against empathy-related brain data and alexithymia scores as measured by the TAS. ADOS scores were unrelated to these measures (all correlations P > 0.4). Inspection of scatterplots (Supplementary Figs 1–3) showing the relationship between the ADOS and empathy-related brain data, TAS and BVAQ scores, reveals that it is not the case that participants with low ADOS scores are clustered at the extremes of the distributions of any measure. In addition, the relationship between alexithymia (TAS scores) and empathic brain responses was found in both the autism spectrum condition and control groups, who were matched for degree of alexithymia. Thus, it is unlikely that any of the observed effects are an artefact of inappropriate diagnosis, or a statistical artefact due to high covariance between autism spectrum conditions symptom severity and degree of alexithymia.
Discussion
The main goal of this study was to test the widespread assumption that autistic spectrum conditions are associated with a general lack of empathy. In order to test for specific empathy deficits in autism spectrum conditions, we first provided a fine-graded distinction between two different capacities underlying social cognition: mentalizing ability (Theory of Mind) and empathic ability. Whereas autism has been shown to be associated with a deficit in Theory of Mind (see Frith and Happé, 2005 for a review), it is much less clear whether individuals with autism spectrum conditions also suffer from interoceptive and empathy deficits. An earlier fMRI study focussing on interoceptive awareness in individuals with an autism spectrum condition with and without alexithymic symptoms (Silani et al., 2008) suggested that it is the degree of alexithymia, which is frequently elevated in individuals with an autism spectrum condition, rather than the autism spectrum conditions per se, that is predictive of activation in interoceptive cortex (specifically, anterior insula), and therefore reduced levels of empathy. To test this hypothesis, we chose an empathy-for-pain paradigm that counteracts possible problems associated with the measurement of empathy in autistic populations (i.e. a deficit in facial emotion recognition, or reduced attention to the eye region of faces).
The results of the present study confirm the main hypotheses that the degree of alexithymic traits assessed with the TAS-20 would be associated with the level of empathic brain activation in anterior insula when participants witnessed a close partner suffering pain. More importantly, this association was not significantly different for control and autism spectrum condition groups, and after accounting for degree of alexithymia, individuals with an autism spectrum condition were not different from control participants in terms of empathic brain responses in the ROI, or in the rest of the brain. These results suggest that it is not autism per se, but high levels of alexithymia (in both individuals with and without an autism spectrum condition diagnosis) that are predictive of reduced empathic brain responses. Note, however, that the present samples of control participants and patients with an autism spectrum condition are not representative with respect to their distribution of alexithymic traits within each group, as we aimed to achieve an equal distribution of alexithymic scores in both samples. Far higher rates of alexithymia are reported across those with autism spectrum conditions than in the typical population. Thus, if we were to replicate these findings in a representative sample of control and individuals with an autism spectrum condition, we would expect to observe weaker empathy-related brain activation in anterior insula in the autism spectrum condition group, reflecting the higher prevalence of alexithymia in the autism spectrum condition population.
These results replicate previous findings of a crucial role for insular cortex in pain-related empathy (Morrison et al., 2004, 2007; Singer et al., 2004, 2006, 2008; Jackson et al., 2005, 2006; Cheng et al., 2007; Gu and Han, 2007; Lamm et al., 2007a, b; Saarela et al., 2007). Thus, the activation observed in left anterior insula when participants empathized with their partners when they were suffering overlapped with coordinates of empathic brain responses in anterior insula described in previous empathy studies (Singer et al., 2004, 2006, 2008). More importantly, we extended previous findings that showed modulation of empathic brain responses in insular cortices as a function of contextual appraisal and affective link (Singer et al., 2006; Lamm et al., 2007a, b; Hein and Singer, 2008) to the domain of individual trait characteristics. Here we show that individual characteristics such as the ability to interocept upon one’s own emotions are also a modulatory factor for empathic brain responses.
Taken together, the previous (Silani et al., 2008) and the present findings suggest that people with interoceptive deficits reflected in high levels of alexithymia show reduced activation in insular cortices while interocepting on their own emotions as well as when empathizing with others who are feeling pain. In both studies, high correlations between an alexithymia scale (TAS-20) and a classical trait empathy scale (Davis IRI), were observed. This pattern of results is consistent with the notion that our ability to empathize relies on the same neural circuitries underlying our capacity to understand our own feeling states and that these capacities are intimately linked with functions of the anterior insula cortices (Singer et al., 2004, 2009).
Studying individual differences in alexithymia within a population of individuals with an autism spectrum condition enabled us to obtain a more detailed picture of the social deficits observed in autism spectrum conditions. This picture illustrates the heterogeneity of individuals with an autism spectrum condition with regard to empathy deficits. Thus, not all individuals with an autism spectrum condition, but only a subgroup with interoceptive deficits, seem to be impaired on the empathic route to social cognition. This finding is in agreement with earlier research pointing to a large heterogeneity in cognitive profiles within the autistic populations (Pellicano et al., 2006; White et al., 2009a, b) and cautions against overgeneralization of deficits commonly attributed to autism spectrum conditions to every individual on the autistic spectrum. Despite this heterogeneity within individuals with autism spectrum conditions, it is clear that an outstanding research question in this area relates to the increased prevalence of high levels of alexithymia in this group compared to neurotypical individuals.
Finally, these findings speak to the differentiation between Theory of Mind and empathy and point to a dissociation between these two streams of social cognition. Interestingly, analysis of the subscale scores from the Davis IRI (Supplementary Table 2) support the previously-reported deficit in cognitive perspective taking in autism spectrum conditions (Frith and Happé, 2005). The autism spectrum condition group reported significantly less perspective taking (see Rogers et al., 2007 who reported a similar pattern of subscale scores). Demonstrations of a Theory of Mind deficit in autism spectrum conditions, together with intact empathy shown by the present study, support the suggestion that empathy and Theory of Mind are dissociable. It should be noted however, that Theory of Mind was not directly tested in the present study. Therefore, future research should focus on testing four groups of individuals, individuals with and without autism spectrum conditions with low and high degrees of alexithymia, using pure empathy and Theory of Mind tasks in order to test whether participants with an autism spectrum condition show Theory of Mind but not empathy deficits, and those with alexithymia empathy but not theory of mind deficits. Evidence for such a double dissociation would not only inform the development of clinical interventions tailored to the specific difficulties of these groups, but could also help us to obtain a more sophisticated picture of the different neural networks underlying social cognition in adults.
Funding
The National Centre of Competence in Research (NCCR) for the Affective Sciences, Geneva, the University of Zurich (Research Priority Program on the Foundations of Human Social Behaviour), and the European Research Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement n°205557 EMPATHICBRAIN (to T.S.). In addition, it was funded by a Medical Research Council UK (Grant No. G9617036) (to U.F.), and by the Wellcome Trust.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We thank Stephanie Spengler for her help running the subjects, Claus Lamm for providing the mask used for the ROI analyses and the engineering, physics and radiology teams at the Wellcome Department of Functional Imaging for their invaluable help. We also thank Dr Elizabeth Hill and Prof. Chris Frith for helpful comments on an earlier draft of this manuscript.
Glossary
Abbreviations
- ADOS-G
Autism Diagnostic Observational Schedule
- BVAQ
Bermond-Vorst Alexithymia Questionnaire
- fMRI
functional magnetic resonance imaging
- IRI
Interpersonal Reactivity Index
- ROI
region of interest
- TAS-20
20-item Toronto Alexithymia Scale
References
- Adolphs R. Looking at other people: mechanisms for social perception revealed in subjects with focal amygdala damage. Novartis Found Symp. 2007;278:146–59. [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cognitive Neurosci. 2001;13:232–40. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Spezio ML, Parlier M, Piven J. Distinct face processing strategies in parents of autistic children. Curr Biol. 2008;18:1090–3. doi: 10.1016/j.cub.2008.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: Author; 1994. [Google Scholar]
- Bagby RM, Parker JD, Taylor GJ. The Twenty-item Toronto Alexithymia Scale–II. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994;38:33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Autism: the empathizing-systemizing (E-S) theory. Ann NY Acad Sci. 2009;1156:68–80. doi: 10.1111/j.1749-6632.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Jolliffe T. Is there a "language of the eyes"? Evidence from normal adults, and adults with autism or Asperger syndrome. Visual Cognition. 1997;4:311–31. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "reading the mind in the eyes" test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–51. [PubMed] [Google Scholar]
- Baron-Cohen S, Riviere A, Fukushima M, French D, Hadwin J, Cross P, et al. Reading the mind in the face: a cross-cultural and developmental study. Visual Cognition. 1996;3:39–59. [Google Scholar]
- Berscheid E, Snyder M, Ornoto AM. The Relationship Closeness Inventory: assessing the closeness of interpersonal relationships. J Pers Soc Psychol. 1989;57:792–807. [Google Scholar]
- Berthoz S, Hill EL. Reliability of the Bermond-Vorst Alexithymia Questionnaire: data from adults with autism spectrum disorder, their relatives and normal controls. Eur Psychiat. 2005;20:291–8. doi: 10.1016/j.eurpsy.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Neurobiological basis of psychopathy. Br J Psychiatry. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Frith U, Smith N, Abell F, Cipolotti L. Fractionation of visual memory: agency detection and its impairment in autism. Neuropsychologia. 2002;40:108–18. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. Q J Exp Psychol. 2008;61:157–70. doi: 10.1080/17470210701508855. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic children. J Child Psychol Psychiat. 1992;33:843–59. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16:S497. [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences of the USA. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, et al. Expertise modulates the perception of pain in others. Curr Bioly. 2007;17:1708–13. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurol. 2003;13:500–05. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel – now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural system supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' error and the future of human life. Sci Am. 1994;271:144. doi: 10.1038/scientificamerican1094-144. [DOI] [PubMed] [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalogue of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:435–41. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J. The power of simulation: Imagining one's own and other's behavior. Brain Res. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. Sci World J. 2006;6:1146–63. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Josephs O, Hutton C, Corfield DR, Turner R. Compensation of susceptibility-induced BOLD sensitivity losses in echoplanar fMRI imaging. Neuroimage. 2002;15:120–35. doi: 10.1006/nimg.2001.0985. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET) J Autism Dev Disord. 2008;38:464–73. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Ann Rev Psychol. 2000;51:665–97. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA. Empathy: conceptualization, measurement, and relation to prosocial behavior. Motiv Emotion. 1990;14:131–49. [Google Scholar]
- Eisenberg N, Strayer JA. Empathy and its development. Cambridge, MA: Cambridge University Press; 1987. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiack RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;3:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiack RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–34. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Happé F. Autism spectrum disorder. Curr Biol. 2005;15:R786–90. doi: 10.1016/j.cub.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Gillberg CL. The Emanuel Miller memorial lecture 1991: autism and autistic-like conditions: Subclasses among disorders of empathy. J Child Psychol Psychiat. 1992;33:813–42. doi: 10.1111/j.1469-7610.1992.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36:256–67. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–8. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U. Brief report: cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. J Autism Dev Disord. 2004;34:229–35. doi: 10.1023/b:jadd.0000022613.41399.14. [DOI] [PubMed] [Google Scholar]
- Hoffman ML. Empathy and moral development: implications for caring and justice. Cambridge, MA: Cambridge University Press; 2000. [Google Scholar]
- Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, et al. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:1931–5. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Minshew N, Leonard GL, Behrmann M. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia. 2007;45:685–95. doi: 10.1016/j.neuropsychologia.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–53. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Filliter JH, Murphy RR. Discrepancies between self- and parent-perceptions of autistic traits and empathy in high functioning children and adolescents on the autism spectrum. J Autism Dev Disord. 2009;39:1706–14. doi: 10.1007/s10803-009-0809-1. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn Sci. 2007;11:194–6. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–46. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiat. 2002;59:809–16. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007a;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007b;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden W, Wen F, Paulhus DL. Measuring alexithymia: Reliability, validity, and prevalence. In: Butcher J, Spielberger C, editors. Advances in personality assessment. Hillsdale, NJ: Erlbaum; 1995. pp. 51–95. [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS ONE. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, EHJr Cook, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- McIntosh DN, Reichmann-Decker A, Winkielman P, Wilbarger JL. When the social mirror breaks: deficits in automatic, but not voluntary, mimicry of emotional facial expressions in autism. Dev Sci. 2006;9:295–302. doi: 10.1111/j.1467-7687.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- Minio-Paluello I, Baron-Cohen S, Avenanti A, Walsh V, Aglioti SM. Absence of embodied empathy during pain observation in Asperger syndrome. Biol Psychiatry. 2009;65:55–62. doi: 10.1016/j.biopsych.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex. 2008;18:262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA. 2002;99:15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Peelen MV, Downing PE. The sight of others' pain modulates motor processing in human cingulate cortex. Cereb Cortex. 2007;17:2214–22. doi: 10.1093/cercor/bhl129. [DOI] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cogn Affect Behav Neurosci. 2004;4:270–8. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Nemiah JC, Freyberger H, Sifneos PE. Alexithymia: a view of the psychosomatic process. In: Hill OW, editor. Modern trends in psychosomatic medicine. London: Butterworths; 1976. pp. 430–9. [Google Scholar]
- Pellicano E, Maybery M, Durkin K, Maley A. Multiple cognitive capabilities/deficits in children with an autism spectrum disorder: ”Weak“ central coherence and its relationship to theory of mind and executive control. Dev Psychopathol. 2006;18:77–98. doi: 10.1017/S0954579406060056. [DOI] [PubMed] [Google Scholar]
- Potthoff RF. Statistical aspects of the problem of biases in psychological tests (Institute of Statistics Mimeo Series No. 479) Chapel Hill, NC: University of North Carolina, Department of Statistics; 1966. [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:515–26. [Google Scholar]
- Preston SD, de Waal FBM. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–72. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Rogers K, Dziobek I, Hassenstab J, Wolf OT, Convit A. Who cares? Revisiting empathy in Asperger syndrome. J Autism Dev Disord. 2007;37:709–15. doi: 10.1007/s10803-006-0197-8. [DOI] [PubMed] [Google Scholar]
- Saarela MV, Hluschuk Y, Williams ACdC, Schürmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another's face. Cereb Cortex. 2007;17:230–7. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Salminen JK, Saarijarvi S, Aarela E, Toikka T, Kauhanen J. Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. J Psychosom Res. 1999;46:75–82. doi: 10.1016/s0022-3999(98)00053-1. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Baron-Cohen S. The neuroscience of theory of mind. Soc Neurosci. 2006;1:i–ix. doi: 10.1080/17470910601117463. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Yaniv S, Aharon-Peretz J. Empathy deficits in Asperger syndrome: a cognitive profile. Neurocase. 2002;8:252. doi: 10.1093/neucas/8.3.245. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: An fMRI study. Soc Neurosci. 2008;3:97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30:855–63. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Year Cogn Neurosci 2009: Ann NY Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Snozzi R, Bird G, Petrovic P, Silani G, Heinrichs M, et al. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8:781–91. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani P, Lindberg N, Joukamaa M, Nieminen-von Wendt T, von Wendt L, Appelberg B, et al. Asperger syndrome, alexithymia and perception of sleep. Neuropsychobiology. 2004;49:64–70. doi: 10.1159/000076412. [DOI] [PubMed] [Google Scholar]
- Vorst HCM, Bermond B. Validity and reliability of the Bermond-Vorst Alexithymia Questionnaire. Pers Indiv Diff. 2001;30:413–34. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – 3rd UK Edition (WAIS®-III UK) London: The Psychological Corporation; 1999. [Google Scholar]
- White S, O'Reilly H, Frith U. Big heads, small details and autism. Neuropsychologia. 2009b;47:1274–81. doi: 10.1016/j.neuropsychologia.2009.01.012. [DOI] [PubMed] [Google Scholar]
- White S, Hill E, Happé F, Frith U. Revisiting the strange stories: revealing mentalizing impairments in autism. Child Development. 2009a;80:1097–117. doi: 10.1111/j.1467-8624.2009.01319.x. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wispé L. The distinction between sympathy and empathy: to call forth a concept, a word is needed. J Pers Soc Psychol. 1986;50:314–21. [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited–again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Sigman MD, Kasari C, Mundy P. Empathy and cognition in high-functioning children with autism. Child Development. 1992;63:150–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.