Abstract
Fragile X-associated tremor/ataxia syndrome, a neurodegenerative disorder associated with premutation alleles (55–200 CGG repeats) of the FMR1 gene, affects many carriers in late-life. Patients with fragile X-associated tremor/ataxia syndrome typically have cerebellar ataxia, intranuclear inclusions in neurons and astrocytes, as well as cognitive impairment. Dementia can also be present with cognitive deficits that are as severe as in Alzheimer’s disease, however frontosubcortical type impairment is more pronounced in fragile X-associated tremor/ataxia syndrome. We sought to characterize the P600 and N400 word repetition effects in patients with fragile X-associated tremor/ataxia syndrome, using an event-related potential word repetition paradigm with demonstrated sensitivity to very early Alzheimer’s disease. We hypothesized that the fragile X-associated tremor/ataxia syndrome-affected participants with poor declarative verbal memory would have pronounced abnormalities in the P600 repetition effect. In the event-related potential experiment, subjects performed a category decision task whilst an electroencephalogram was recorded. Auditory category statements were each followed by an associated visual target word (50% ‘congruous’ category exemplars, 50% ‘incongruous’ nouns). Two-thirds of the stimuli (category statement–target word pairs) were repeated, either at short-lag (∼10–40 s) or long-lag (∼100–140 s). The N400 and P600 amplitude data were submitted to split-plot analyses of variance. These analyses of variance showed a highly significant reduction of the N400 repetition effect (F = 22.5, P < 0.001), but not of the P600 repetition effect, in mild fragile X-associated tremor/ataxia syndrome (n = 32, mean age = 68.7, mean Mini-Mental State Examination score = 26.8). Patients with fragile X-associated tremor/ataxia syndrome had significantly smaller late positive amplitude (550–800 ms post-stimulus onset) to congruous words (P = 0.04 for group effect). Reduced P600 repetition effect amplitude was associated with poorer recall within fragile X-associated tremor/ataxia syndrome patients (r = 0.66) and across all subjects (r = 0.52). Larger P600 amplitude to new congruous words also correlated significantly with higher free recall scores (r = 0.37, P < 0.01) across all subjects. We found a correlation between the amplitude of late positivity and CGG repeat length in those with fragile X-associated tremor/ataxia syndrome (r = 0.47, P = 0.006). Higher levels of FMR1 mRNA were associated with smaller N400s to incongruous words and larger positive amplitudes (between 300 and 500 ms) to congruous words. In conclusion, event-related potential word repetition effects appear sensitive to the cognitive dysfunction present in patients with mild fragile X-associated tremor/ataxia syndrome. Their more severe reduction in N400 repetition effect, than P600, is in contrast to the reverse pattern reported in amnestic mild cognitive impairment and incipient Alzheimer’s disease (Olichney et al., 2008).
Keywords: electroencephalogram/event-related potential, late positive component/P600, memory, language processing, aging
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS), a recently described neurodegenerative disorder (Hagerman et al., 2001; Jacquemont et al., 2003) associated with CGG expansions of the gene for fragile X mental retardation 1 protein (FMR1) affects many premutation carriers (55–200 CGG repeats) in late-life [full-mutation (alleles with >200 repeats) causes fragile X syndrome, the most common cause of inherited cognitive impairment]. Patients with FXTAS typically have cerebellar ataxia, peripheral neuropathy and may develop cognitive impairment, usually in the later stages. These impairments are mainly in frontal executive function, attention and short-term memory (Grigsby et al., 2008). FXTAS dementia involves significant cognitive deficits that may be as severe as those in Alzheimer’s disease. However, ‘frontosubcortical’ type impairment is more pronounced in FXTAS (Grigsby et al., 2008; Seritan et al., 2008). At autopsy, most patients with FXTAS have numerous intranuclear inclusions in the hippocampus and neocortex, both in neurons and astrocytes (Greco et al., 2002, 2006).
Cognitive event-related potentials (ERPs), composed mainly of summated excitatory and inhibitory post-synaptic potentials, provide an excellent tool for measuring the precise timing of neural events that mediate a variety of cognitive processes (Nunez, 2006). ERPs have an unsurpassed temporal resolution (millisecond level) and good signal to noise ratios. ERPs, like evoked-potentials, have proven very sensitive to a variety of neuropathological entities and to numerous task manipulations.
Cognitive ERP studies have found that a late positive component, sometimes called the P600, is related to both memory encoding and retrieval processes (e.g. Paller and Kutas, 1992; Düzel et al., 1997; Fernández et al., 1998, 1999; Olichney et al., 2000). Specifically, larger P600 word repetition effects (new > old) have correlated with superior verbal memory abilities (Olichney et al., 2000, 2002). Previous work in our lab has shown that decrements in ERP word repetition effects (e.g. of either the late positive component/P600, or of the ‘N400’) are associated with greater likelihood of subsequent Alzheimer’s dementia in patients with mild cognitive impairment (Olichney et al., 2008). The N400 component has been related to semantic processing load and may also be relevant to verbal learning (e.g. per invasive ERP studies in epilepsy; Helmstaedter et al., 1997). However, our prior studies have shown relatively normal N400s (with significant congruity and repetition effects) in patients with chronic well-circumscribed amnesia. Thus, we have suggested the N400 repetition effect primarily reflects semantic ‘priming’, or other implicit memory processes (Graf and Schacter, 1985; Shimamura et al., 1987; Keane et al., 1997; Olichney et al., 2000; Fernández et al., 2001).
This study examined these ERP components (N400, P600), relevant to different aspects of memory, in patients with mild or early-stage FXTAS [mean Mini-Mental State Examination (MMSE) score = 26.8]. We also examined the relationship of ERP word repetition effects to declarative memory, FMR1 gene expression (mRNA levels), and CGG repeat length.
Materials and methods
Participants
Participants were 32 FXTAS patients and 16 normal elderly controls who served as volunteers after providing informed consent according to the guidelines of the University of California, Davis Institutional Review Board. The majority of participants were male (24 FXTAS, 12 normal controls). All were right-handed except for three left-handed patients with FXTAS (12.5%) and one left-handed normal control (8.3%). The mean age and education ( ± SD) were 66.5 ± 7.7 years and 15.5 ± 3.5 years in the FXTAS group, which was not significantly different from age (62.9 ± 8.2) and education (16.7 ± 2.7) in the group of normal controls (age: t = 1.55, P = 0.13; education: t = 1.05, P = 0.30). Participants were referred from the MIND institute (Neurotherapeutics Research Institute), where they received extensive clinical research evaluations, including medical history, neurological examination, Unified Parkinson’s Disease Rating Scale, laboratory tests and neuropsychological testing. The neuropsychological test battery included tests of global abilities [MMSE, Wechsler Adult Intelligence Scale (WAIS) full scale, verbal and performance IQs], verbal and non-verbal memory [e.g. California Verbal Learning Test (CVLT), Wechsler Memory Scale (WMS-III) with logical memory, auditory—immediate and delayed memory and visual—immediate and delayed memory], language [WAIS-III vocabulary, category and letter fluency (Controlled Oral Word Association Test), Spanish and English Neuropsychological Assessment Scales object naming], visuospatial (WAIS-III block design, Spanish and English Neuropsychological Assessment Scales pattern recognition and spatial localization), executive/abstraction/problem solving function [e.g. Stroop colour and word test, Behavioural Dyscontrol Scale-2 (Grigsby and Kaye, 1996); WAIS-III similarities and arithmetic; and attentional (WAIS-III digit span) abilities (Salmon and Butters, 1992; Mungas et al., 2000; Olichney et al., 2000)]. Participants also received the Beck Anxiety Inventory (Beck et al., 1988) and the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders 4th edition-I (First et al.., 2005).
At the time of ERP testing, all patients with FXTAS met the criteria for probable or possible FXTAS (Jacquemont et al., 2003), with a FXTAS stage (Grigsby et al., 2006) ranging from 2 to 5 (mean = 3.1). While this indicates that, on average, our patients with FXTAS had moderate physical disabilities due to tremor and/or ataxia, most did not have dementia or marked cognitive impairment. The mean MMSE score was not significantly different between groups (FXTAS: 26.8 ± 1.7, normal controls: 27.1 ± 2.3, t = 0.48, P = 0.63). Four of the 32 FXTAS patients met clinical criteria for mild dementia (MMSE range = 22–28). The clinical diagnosis of dementia was established using the National Alzheimer’s Coordinating Center criteria (impairment in two or more cognitive domains, progression of deficits and functional decline; NACC, 2006). Affected cognitive domains included executive function, memory, attention, language, visuospatial function and personality. Functional impairment had to be clearly established as due to cognitive, and not motor dysfunction (Seritan et al., 2008). In the four FXTAS patients with dementia, only one was on pharmacological treatment for dementia (cholinesterase therapy: donepezil 10 mg/day). We did not exclude subjects taking primary CNS-active medications (e.g. antidepressants) due to their common use in FXTAS. Secondary analyses were performed to compare patients not taking any primary CNS-active medications with patients who were, and with the normal control group. Three of the normal controls were on CNS-active medications (two on lorazepam, one on carbamazepine). Exclusions for both groups were history of stroke, schizophrenia, traumatic brain injury, history of alcohol abuse or dependence and other neurodegenerative disorders.
Procedure
Participants were fit with an electrode cap and seated 100 cm from a video monitor. Category statements were read aloud, each followed (∼1 s later) by a visually presented target word (duration = 300 ms). The target word appeared within a 9.75 × 2.25 cm rectangle. Participants were instructed to sit quietly for 3 s following the target, then to say the perceived word followed by ‘yes’ or ‘no’, indicating whether or not it was an exemplar of the defined category. The ERPs were recorded in three blocks of 144 trials, each lasting slightly over 20 min.
Immediately after the ERP recordings were completed, three unanticipated paper and pencil memory tests (free recall, cued recall and multiple-choice recognition) were administered in that order. In the free recall task, subjects were instructed to recall as many target words as possible, regardless of whether they were congruous or incongruous. In the cued-recall task, patients were provided with a list of 40 categories (18 of which had preceded incongruous words, 22 had preceded congruous words) and subjects were asked to fill in the word that had been presented during the experiment, or to provide the ‘first word that comes to mind’ if they could not recall the associated target word. The multiple-choice recognition task consisted of category statements followed by six possible completions: four congruous category exemplars and two incongruous nouns. The five foils were words never presented during the ERP experiment.
Stimuli
The stimuli were 216 phrases describing a category (e.g. ‘a breakfast food’), each followed by a target word. Categories and targets were selected with the aid of published norms and locally administered normative questionnaires. Half of the target words (‘congruous’ words) were medium-typicality category exemplars (e.g. ‘pancake’ for ‘a breakfast food’). The other half of the targets were concrete nouns ‘incongruous’ with their associated category, but matched to the congruous target words for length and frequency of usage.
Each subject was randomly assigned to one of three counterbalanced stimulus lists, which included 36 congruous targets presented once, 36 presented twice, 36 presented three times and equal numbers of incongruous targets in the same repetition conditions, for a total of 432 trials. Half of the stimuli were congruous and half incongruous; half were new and half were repeats. Repeated targets always appeared with the same category as on first presentation. For singly repeated category–target pairings, the lag between first and second presentations was 0–3 intervening trials (spanning 10–40 s). For doubly repeated items, the lag for both second and third presentations was 10–13 intervening trials (∼120 s).
Electrophysiological recording
The electroencephalogram (EEG) was recorded from tin electrodes embedded in an elastic cap from midline (Fz, Cz, Pz, Poz), lateral frontal (F3, F4, F7, F8, FC1, FC2, Fp1, Fp2), temporal (T5, T6), parietal (P3, P4, Cp1, Cp2) and occipital sites (O1, O2, PO7, PO8) defined by the International 10–20 system. Additional lateral sites included electrode pairs which approximate Broca’s area (Bl, Br), Wernicke’s area (Wl, Wr) and their right hemisphere homologues, and an electrode pair 33% of the interaural distance lateral to Cz over the superior temporal lobe (41L and 41R). All scalp electrodes and the right mastoid electrode were referenced on-line to the left mastoid, and then re-referenced off-line to an average of the left and right mastoids. Vertical and horizontal eye movements were monitored by electro-oculogram recording from four electrodes, one below and one at the outer canthus of each eye. The EEG was recorded with a 0.016–100 Hz bandpass and digitized using a 250 Hz sampling rate. ERPs to the visual target words were averaged after off-line rejection of trials contaminated by eye movements or other artefacts. In normal controls, 28.8% of the trials were rejected versus 42.1% from patient with FXTAS (t = 2.63, P = 0.012), most commonly due to eye-blinks, leaving an average of 308 trials accepted in normal controls (range 145–411) and 250 trials in FXTAS (range 111–388). In order to be included in this report, we required an absolute minimum of 25 accepted trials for each of the main stimulus conditions (i.e. congruous new, congruous old, incongruous new, incongruous old).
ERP analyses
For the N400 word repetition effect (new versus old incongruous words) and congruity effect (incongruous versus congruous new words), the ERP data from 300 to 550 ms were submitted to split-plot ANOVAs with the between-subject factor of Group and the within-subject factors of Condition (either repetition or congruity) and Electrode. For the congruous word repetition effect (new versus old), the ERP data were analysed from 300 to 800 ms with the additional factor of latency. The data were submitted to split-plot ANOVAs with the between-subject factor of Group, and three within-subject factors: Repetition, Latency (300–550 ms and 550–800 ms epochs were used to quantify the ‘early’ late positive component and P600 time windows, respectively), and Electrode. Filtered (low-pass, 15 Hz cut-off) ERP data were collapsed across all stimulus (repetition/congruity) conditions in order to identify the N150 peak amplitude and latency (local peak negativity between 110 and 250 ms). These data were submitted to split-plot ANOVAs with the between-subject factor of group, and the within-subject factor of electrode. Two-tailed P-values of ≤0.05 were considered significant. The Greenhouse–Geiser correction (Geisser and Greenhouse, 1959) was applied where appropriate to correct for violations of sphericity. ANOVAs were also performed with the additional factor of time lag (short versus long lag) between new and repeated stimuli, testing for possible differential effects of time delay and have been omitted for brevity. Only statistically significant effects and interactions are reported here.
Additional ANOVAs were performed with the FXTAS group subdivided into high and low memory groups based on a median split of cued recall scores. In a separate analysis, the FXTAS group was subdivided into two groups based on whether the individual was currently taking primary CNS-active medications (as was the case for n = 16). These analyses revealed no significant effects of medication status and all such negative results have been omitted for brevity.
Correlational analyses
Pearson’s correlations examined the relationship of our main ERP measures to memory performance (i.e. free recall, cued recall and multiple choice recognition scores for the experimental stimuli; CVLT delayed recall and discriminability) and genetic factors (i.e. gene expression as measured by FMR1 mRNA levels, and number of CGG repeats of the FMR1 gene). In order to control for the multiple correlations performed and possible Type I error, P ≤ 0.01 were considered significant correlations, one-tailed for correlations with a directional hypothesis (e.g. larger P600 amplitude with higher memory scores) and two-tailed for all other correlations (with non-directional hypotheses).
Our main ERP measures were mean amplitudes of the ‘P600 repetition effect’ (mean voltage difference between 550 and 800 ms at Pz for new–old congruous words), and the N400 repetition effect (defined as the mean voltage between 300 and 550 ms at T6 for old–new incongruous words, in order to assign positive values to effects in the normal direction). These electrode sites were chosen because this is where normal subjects most consistently show large effects in their ERPs (Olichney et al., 2006). Mean amplitudes of the N400 and P600 were also measured to new incongruous and congruous words, respectively, using these same channels and time windows.
Results
Behavioural results
Performance on the semantic category decision task was near ceiling in both groups (99.7 ± 0.2% correct in normal controls, 99.0 ± 2.3% correct in patients with FXTAS) and did not differ significantly between groups (P = 0.23). Performance did not differ significantly across congruous words (99.8% ± 0.2% correct in normal controls, 99.6 ± 0.7% correct in patients with FXTAS, t = 1.57, P = 0.124) or incongruous words (99.8 ± 0.2% correct in normal controls, 99.3 ± 1.8% correct in patients with FXTAS, t = 1.077, P = 0.24).
Performance on subsequent memory tests (free recall, cued recall, multiple-choice recognition of stimuli) tended to be lower in FXTAS than in the normal control group, particularly for free recall; however, intergroup differences were non-significant (two-tailed P-values for t-tests and CVLT measures shown in Table 1).
Table 1.
Memory performance data for patients with FXTAS and normal controls
| Normal controls (n = 16) | FXTAS (n = 32) | t | P | |
|---|---|---|---|---|
| FXTAS stage | 0.0 ± 0.0 | 3.1 ± 0.96 | ||
| MMSE | 27.70 ± 2.29 | 26.84 ± 1.71 | 0.48 | 0.63 |
| Free recall | 17.27 ± 14.27 | 11.53 ± 10.01 | 1.57 | 0.12 |
| Cued recall (%) | 49.0 ± 8.0 | 45.0 ± 17.0 | 0.90 | 0.38 |
| Multiple-choice recognition (%) | 74.21 ± 16.9 | 68.72 ± 21.1 | 0.86 | 0.40 |
| CVLT list A, trials 1–5 | 47.36 ± 11.32 | 42.74 ± 12.58 | 1.15 | 0.26 |
| CVLT short delay free recall | 9.71 ± 2.64 | 8.67 ± 3.92 | 0.90 | 0.36 |
| CVLT short delay cued recall | 11.0 ± 2.22 | 10.04 ± 3.45 | 0.95 | 0.35 |
| CVLT long delay free recall | 9.71 ± 3.07 | 8.85 ± 3.65 | 0.76 | 0.45 |
| CVLT long delay cued recall | 10.64 ± 2.68 | 9.89 ± 3.22 | 0.75 | 0.46 |
| CVLT discrimination | 91.67 ± 5.99 | 89.1 ± 11.18 | 0.80 | 0.43 |
ERP results
N150 potential
The N150 potential was defined as the largest local negative peak occurring between 110 and 250 ms post-stimulus onset. Four posterior electrode sites (T5, T6, O1, O2), where the N150 was most prominent and reliably present across subjects, were used in the ANOVAs for peak latency and amplitude. A main effect of Group was found in the analysis of N150 latency [F(1,46) = 9.50, P = 0.003], with the FXTAS patients showing a delayed N150 component (mean = 160.8 ms averaged across channels) as compared to the normal controls (mean = 141.4 ms). ANOVA of the N150 peak amplitude showed a non-significant trend for a group effect [F(1,46) = 3.82, P = 0.057], in which the mean peak amplitude was −3.33 µV (SD = 4.11) in normal controls and −1.43 ± 3.62 µV in patients with FXTAS.
Semantic congruity
Figure 1A shows the ERPs elicited by the initial presentation of congruous (dark line) and incongruous (grey line) category words in normal controls (left) and patients with FXTAS (right). The N400 elicited by incongruous words is most prominent in the right temporal and posterior channels. The congruity effect, i.e. the difference between ERPs to incongruous and congruous words, began around 300 ms after stimulus onset and peaked at about 450 ms (Fig. 1B).
Figure 1.
The N400 congruity effect. (A) Grand average ERPs to initial presentation of congruous (dark line) and incongruous (grey line) words in normal controls (left) and participants with FXTAS (right). The N400 congruity effect is shaded where present in channels T6 and Wr. The N150 is labelled ‘N1’ in the left occipital (O1) channel. (B) Topographical maps of the congruity effect (incongruous–congruous words). FXTAS high and low memory subgroups shown on right.
There was a main effect for Congruity [F(1,46) = 30.00, P < 0.0001] across all electrode sites, with initial incongruous words producing larger negativities (N400s) than congruous words. There was a Congruity × Electrode interaction [F(25,1150) = 5.62, P = 0.02], indicating that different scalp distributions were elicited by congruous compared to incongruous words. There was no significant Group × Congruity interaction [F(1,46) = 2.67, P = 0.11], indicating that the congruity effect is relatively intact in the FXTAS group. There was a significant congruity effect within both groups (normal controls: F = 29.05, P < 0.0008; FXTAS: F = 12.42, P < 0.001). Similar amplitudes and distributions were found for FXTAS with high versus low memory performance (right side of Fig. 1B). In an ANOVA that compared FXTAS subgroups with high versus low memory scores, there was a main effect for congruity [F(1,30) = 12.07, P = 0.002] and Congruity × Electrode interaction [F(25,750) = 4.34, P < 0.37], but no group effect [F(1,30) = 0.39, P = 0.54] or Group × Congruity interaction [F(1,30) = 0.14, P = 0.71].
Repetition of incongruous words
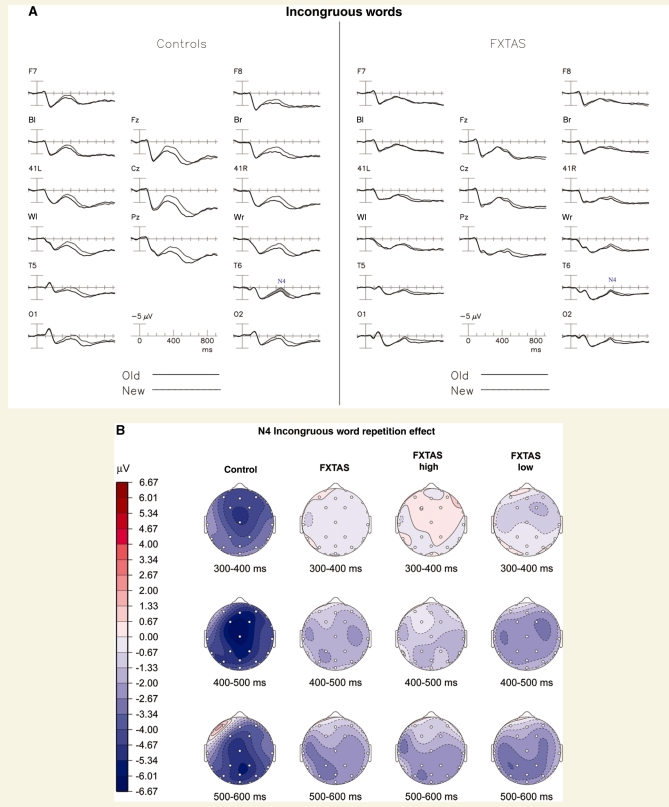
Figure 2A shows the ERPs elicited by the initial (grey line) and repeated (dark line) presentations of incongruous category words, collapsed across repetition lag in normal controls (left) and participants with FXTAS (right). The new incongruous words elicited a large negativity starting around 250 ms, which peaked at 450 ms in right temporal channels (as is typical of the N400) and was reduced with repetition. The ‘incongruous word repetition effect’ (i.e. the reduction of the N400 component amplitude) in the normal control group was largest at the vertex and right posterior channels. In contrast, the FXTAS group did not have an appreciable incongruous repetition effect (compare left and right sides of Fig. 2A). In normal controls, the repetition effect began around 300 ms after stimulus onset and peaked at about 450 ms (Fig. 2B).
Figure 2.
The incongruous word repetition effect. (A) Grand average ERPs to initial (grey line) and repeated (dark line) presentations of incongruous category words, collapsed across repetition lag in normal controls (left) and participants with FXTAS (right). The N400 repetition effect is shaded where present in channel T6. (B) Topographical maps of the repetition effect (new–old words).
Mean amplitudes within the latency window of 300–550 ms post-stimulus (relative to a 100 ms pre-stimulus baseline) defined the N400 amplitudes. These data were subjected to ANOVA as described above. This ANOVA showed a main effect for Repetition [F(1,46) = 98.41, P < 0.0001] across all electrodes sites, with initial words producing larger negativities (N400s) than repeated words, and a significant Group × Repetition interaction [F(1,46) = 22.45, P < 0.001], with the FXTAS group showing less of an attenuation to repeated words than the normal control group. The scalp distribution of each group’s repetition effect for incongruous words is shown in Fig. 2B. In a separate ANOVA that excluded all FXTAS patients on primary CNS-active medications, and compared the remaining patients (n = 16) to the normal control group, we found the same pattern of results, with a highly significant Group × Repetition interaction (P = 0.0047). An ANOVA comparing FXTAS subgroups with high versus low memory scores (see right side of Fig. 2B), revealed a main effect of Repetition [F(1,30) = 6.11, P = 0.019] and Group × Repetition × Electrode interaction [F(25,750) = 11.37, P = 0.003], but no Group × Repetition interaction [F(1,30) = 0.86, P = 0.36] or main effect of Group [F(1,30) = 0.14, P = 0.71]. While nearly all the normal controls (14 of 16) had N400 repetition effects greater than 0.5 µV at electrode T6 (mean ± SD = 1.18 ± 0.80), only 53% (17/32) of the patients with FXTAS had N400 repetition effects larger than this cut-off (χ2 = 5.51, P = 0.019) with a mean amplitude of 0.49 µV (SD = 0.99 µV).
Repetition of congruous words
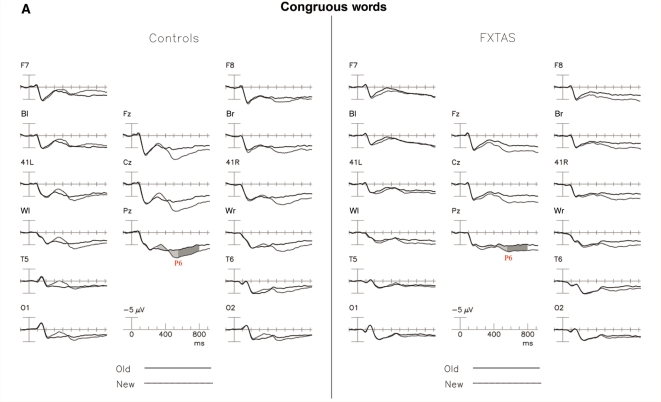
Figure 3A shows the ERPs elicited by the initial (grey line) and repeated (dark line) presentations of congruous category words, collapsed across repetition lag in normal controls (left) and participants with FXTAS (right). In normal controls, the repetition effect, i.e. the difference between the ERPs to new and old words, began around 250 ms after stimulus onset and changed polarity at about 450 ms (Fig. 3). The new congruous words elicited a late positivity of extended duration that was reduced with repetition. This ‘congruous word repetition effect’ (i.e. the reduction of a late positivity) in the normal control group was largest at midline electrode sites and was present from 450 to beyond 900 ms. In contrast, in the FXTAS group, the early congruous repetition effect was absent, and effects in the late ‘P600’ window were slightly reduced compared to normal controls (Fig. 3B).
Figure 3.
The congruous word repetition effect. (A) Grand average ERPs to initial (grey line) and repeated (dark line) presentations of congruous category words, collapsed across repetition lag in normal controls (left) and participants with FXTAS (right). The congruous repetition effect is shaded at Pz where present (P600 epoch shaded dark). (B) Topographical maps of the repetition effect (new–old words, difference plotted at bottom). FXTAS high and low memory subgroups shown on right.
The main ANOVA (300–800 ms) revealed main effects for Group [F(1,46) = 4.20, P = 0.046], and for Repetition [F(1,46) = 68.84, P < 0.0001]. This indicated that the late positive component was smaller in the FXTAS group across repetition conditions. Separate ANOVAs done within the early (300–550 ms) and late (550–800 ms) portion of the late positive component showed the main effect of Group narrowly missed significance [early: F(1,46) = 3.40, P = 0.07; late: F(1,46) = 3.19, P = 0.08]. In both time windows, there was a main effect for Repetition [early: F(1,46) = 42.9, P < 0.0001; late: F(1,46) = 79.5, P < 0.0001] across all electrode sites, with new words producing larger positivities (P600s) than repeated words.
An ANOVA (of the 300–800 ms epoch) that compared FXTAS subgroups with high versus low memory scores revealed a main effect for Latency [F(1,30) = 8.86, P = 0.006], Repetition [F(1,30) = 0.69, P = 0.004] and Electrode [F(25,750) = 6.22, P = 0.15] as well as a significant Epoch × Repetition [F = 17.9, P < 0.001] and Repetition × Electrode [F(25,750) = 9.59, P = 0.036] interaction. From 300 to 550 ms, there was a main effect of repetition [F(1,30) = 11.38, P = 0.002] and Group × Repetition interaction [F(1,30) = 5.13, P = 0.031]. Figure 3B illustrates this interaction with the high-memory performance patients showing decreased positivity to repeated words over frontocentral channels (left slightly greater than right) between 300 and 500 ms, but little to no repetition effect in the low-memory performance patients until 500–800 ms (with a right frontal maximum). The ANOVA of the 550–800 ms epoch showed significant main effects of Repetition [F(1,30) = 18.92, P < 0.0001], Electrode [F(25,750) = 8.80, P = 0.006] and a Repetition × Electrode interaction [F(25,750) = 4.98, P = 0.27], but no significant Group effect or Group × Repetition interaction.
Correlation results
As expected, FMR1 mRNA levels and number of CGG repeats were significantly correlated in FXTAS (r = 0.60, P < 0.001) and across all subjects (r = 0.76, P < 0.0005). Premutation carriers with longer CGG repeats have higher mRNA levels, which are CGG repeat number dependent, but lower fragile X mental retardation protein (FMRP) level, due to less efficient translation of mRNA to protein (Primerano et al., 2002; Hagerman and Hagerman, 2004). Neither mRNA level nor CGG repeat number correlated with subsequent recall or recognition (all r’s < 0.21, P’s > 0.28).
The mean amplitude of the P600 repetition effect (new–old congruous words) was strongly correlated with free recall (r = 0.66, one-tailed P < 0.0005) within the FXTAS group and across all subjects (n = 48; r = 0.52, one-tailed P < 0.0005). Moderate correlations of borderline significance were present between P600 repetition effect amplitude and cued recall (r = 0.40, one-tailed P = 0.012) and between free recall scores and P600 amplitude to congruous new words (r = 0.40, one-tailed P = 0.015) in patients with FXTAS. The P600 amplitude to new congruous words also correlated significantly with free recall (r = 0.37, one-tailed P = 0.0065) across all subjects.
Amplitude of the N400 repetition effect tended to correlate significantly with cued recall across all participants (recall for all items: r = 0.30, two-tailed P = 0.04; recall for incongruous items: r = 0.33, P = 0.03), but these did not reach our threshold for significance and were weaker within the FXTAS group (r = 0.20, P = 0.27). Similarly, across all subjects, we found non-significant correlations between N400 repetition effect amplitude and multiple measures of the CVLT (with list A trials 1–5: r = –0.35, P = 0.026; with short delay cued recall: r = –0.33, P = 0.038; with long delay free recall: r = –0.38, P = 0.017; with long delay cued recall, r = –0.32, P = 0.046). Within the FXTAS group, these correlations were weak to moderate and did not reach significance (i.e. the strongest correlation was with acquisition on List A trials 1–5: r = –0.37, P = 0.059).
Smaller N400 amplitude (i.e. greater positivity between 300 and 550 ms) to new incongruous words was significantly correlated with higher cued recall scores for incongruous words (r = 0.48, P = 0.007) in the FXTAS group, but was non-significantly correlated with higher free recall scores (r = 0.41 with free recall for incongruous words, P = 0.02; r = 0.36 with free recall for congruous words, P = 0.049). There were no significant correlations found within the normal controls group (e.g. r = –0.16 with free recall, P = 0.58).
Other correlational analyses between the main ERP measures and genetic factors revealed significant correlations between the P600 amplitude (550–800 ms at channel Pz, elicited by new congruous words) and CGG repeat length in those with FXTAS (r = 0.47, two-tailed P = 0.006). Higher CGG repeat number was also associated with larger positive amplitudes to new congruous words in the earlier (300–550 ms) epoch (r = 0.46, P = 0.008).
Within the FXTAS patients, higher mRNA levels were associated with smaller N400s to new incongruous words (r = 0.48, P = 0.008) (Fig. 4) and a non-significant trend for larger positive amplitudes in this same epoch (300–550 ms) to new congruous words (r = 0.39, P = 0.037). Within the normal control group, there was no significant correlation of N400 amplitude with either FMR1 mRNA level (r = –0.34, P = 0.28) nor number of CGG repeats (r = –0.06, P = 0.83). There was no significant correlation between P600 amplitude to new words and mRNA levels within either group. Neither the N400 repetition effect nor the P600 repetition effect correlated with mRNA levels (r’s < 0.12 within FXTAS).
Figure 4.
Inverse correlation of FMR1 mRNA level and N400 amplitude to incongruous new words within FXTAS.
Discussion
This is the first report on cognitive ERPs in patients with FXTAS, a recently identified neurodegenerative disease. While a few previous ERP studies (St Clair et al., 1987; Castrén et al., 2003) have found abnormalities in children with mental impairment due to fragile X syndrome in whom there is no production of the FMR1 encoding gene product FMRP, none used language paradigm similar to the present study. The main ERP finding in our FXTAS patient group was a robust reduction in their N400 repetition effect (in response to semantically incongruous words). While repeating semantically incongruous words in a fixed context normally results in a decrement in the N400 amplitude, this effect was markedly attenuated in patients with mild FXTAS. Robust intergroup differences were present despite our patients with FXTAS generally having only very mild cognitive impairments (e.g. they had MMSE and verbal memory scores comparable to the normal control group and only 4 of 32 patients met criteria for mild dementia). This decrement of N400 repetition effect in FXTAS is in contrast to patients with chronic well-circumscribed amnesia due to medial temporal lobe or diencephalic lesions, who showed sparing of this ERP effect (Olichney et al., 2000). Thus, we have interpreted the N400 repetition effect as most likely representing processes related to conceptual/semantic priming, or other types of implicit memory (Olichney et al., 2000; Fernández et al., 2001).
However, other investigators have suggested that the N400 may be related to verbal learning (rather than retention) abilities, such as was found from subdural recordings over lateral temporal cortex of epileptic patients (Helmstaedter et al., 1997). The main N400 generators are believed to reside in the bilateral anterior fusiform cortex (Nobre et al., 1994) and the superior temporal sulcus (Halgren et al., 2002). The anterior fusiform contains higher visual association cortex (e.g. Brodmann area 36) thought to be particularly important for visual object recognition (Gaffan, 1995; Suzuki, 1996). The superior temporal sulcus has both higher visual and auditory association cortex (Saleem et al., 2000), and is important in language comprehension.
A moderate correlation of borderline significance (0.01 < P < 0.05) was present between amplitude of the N400 repetition effect and cued recall across all participants, but this did not approach significance within the FXTAS group. Thus, these borderline correlations appear to be driven by intergroup differences. While we have previously failed to find significant correlations between the N400 repetition effect and subsequent memory (generally assessed 20–60 min after stimulus presentation), we have suggested that the N400 repetition effect may reflect a form of shorter term memory useful for immediate comprehension, perhaps facilitating language comprehension at the sentence or paragraph level (Olichney et al., 2000). However, before discounting the possibility that N400 abnormalities in FXTAS are unrelated to their learning and memory, it should be kept in mind that N400 repetition effect amplitude tended to correlate with short and long delayed recall measures on the CVLT across all subjects. While these correlations may be driven by intergroup difference, it is also plausible that some patients with FXTAS have a deficit in a shorter term memory system, which facilitates learning/acquisition, but does not guarantee its longer term retention (which is likely to be dependent on the hippocampus and neighbouring medial temporal lobe structures). We also found that greater positive amplitude (i.e. smaller N400) to new incongruous words correlated with higher cued recall for the incongruous target words within the FXTAS group.
Another possible interpretation is that the decreased N400 repetition effect in FXTAS may indicate glutamatergic-signalling deficits. Prior invasive electrophysiological studies in the anteromedial temporal lobe have shown diminution of the anteromedial temporal lobe-N4 and elimination of N400 repetition effects with the NMDA-antagonist ketamine (Grunwald et al., 1999). Glutamate signalling dysregulation including enhanced downstream mGluR5 activity is of primary importance in fragile X syndrome due to the FMR1 full mutation (Bear et al., 2004; Hagerman et al., 2009). FMRP inhibits protein synthesis-dependent long-term depression and lessens AMPA receptor internalization. Thus, it may be an important determinant of synaptic strength and cause a fundamental modulation of ERP amplitude. Although FMRP levels are usually normal in those with the premutation, they can be lowered in those with a high-end premutation. Perhaps, lower FMRP level leads to greater long-term depression, less spreading cortical activation and attenuated N400 amplitude. Elevated FMR1-mRNA, present in all premutation carriers, may also lead to glutamate-mediated excitotoxicity in those with FXTAS (Jacquemont et al., 2007). We are currently conducting ERP studies as part of a clinical drug trial of memantine for FXTAS, which we are hopeful may improve glutamatergic signalling and reverse some of their clinical deficits. It will be of great interest to see if N400 amplitude and N400 repetition effect are affected by this treatment.
In contrast to their reduced N400 repetition effects, our patients with mild FXTAS showed generally intact N400 congruity effects (contrasting new semantically incongruous versus congruous words). This suggests relatively normal semantic expectancy and organization of semantic network structure in these patients with FXTAS. This result contrasts with prior ERP studies of mild Alzheimer’s disease (Iragui et al., 1996; Ford et al., 2001; Olichney et al., 2006) and patients with mild cognitive impairment who later convert to Alzheimer’s disease (Olichney et al., 2008) that have shown abnormalities in the N400 amplitude or latency elicited by new words.
We also found reduced P600/late positive component amplitudes in FXTAS, but did not find significant decreases in their P600 word repetition effects to congruous words, as we had hypothesized. This group of patients with mild FXTAS, overall, performed comparably to controls on verbal memory tests, and this sparing of declarative memory ability and lack of rapid forgetting may explain this negative finding. However, as hypothesized, the low-memory performance FXTAS subgroup showed significantly smaller early repetition effects than the high-memory FXTAS group. Across the FXTAS sample, P600 repetition effect amplitude was a strong predictor of subsequent free recall for the experimental stimuli, and also correlated moderately with cued recall scores. This is consistent with our past ERP studies of this word repetition paradigm, with strong correlations found both within normal subjects (Olichney et al., 2000) and in patients with memory disorders (Olichney et al., 2002). In the present study, P600 amplitude also showed significant correlations with free recall ability across our entire sample. Potential P600 generators include the hippocampus, parahippocampal gyrus, cingulate cortex and several regions of higher association cortex (Halgren et al., 1994; Guillem et al., 1999). Thus, decreased P600 amplitude and the smaller P600 repetition effect observed in some patients with FXTAS may reflect an early failure of the medial temporal lobe and its neocortical interconnections (Taylor and Olichney, 2007).
Our analysis of the N150 peak amplitude showed a nearly significant trend for smaller N150 amplitude in FXTAS (P = 0.057). Analysis of the N150 peak latency, however, revealed that N150 peak latencies in FXTAS were significantly later than those in normal controls (P = 0.003). This result was not attributable to CNS-active medications, as the FXTAS subgroup on these medications had slightly faster mean N150 latencies than the other patient subgroup (156.6 ± 13.5 ms versus 163.6 ± 15.8 ms). This finding implicates abnormal speed of conduction along visual white matter tracts in FXTAS and is consistent with prior neuropathological and neuroimaging studies that have demonstrated abnormalities in the white matter of FXTAS brains. Many enlarged astrocytes have intranuclear mRNA-containing inclusions in FXTAS (Greco et al., 2006). White matter hyperintensities are increased in both male and female FXTAS patients, relative to healthy controls (Adams et al., 2007).
Our correlational analyses found significant correlations between the amplitude of late positivity and CGG repeat length in those with FXTAS. This correlation, in which larger P600s to new words was associated with larger CGG repeat expansions, was not hypothesized, and is in need of independent replication. It is interesting to note that the decrease in late positive component amplitude observed in our patients with FXTAS is less pronounced in those with longer CGG repeats and higher FMR1 mRNA levels. We also found that higher FMR1 mRNA levels were correlated with smaller N400 amplitude. A decrease in N400 amplitude might reflect facilitation of semantic integration for the incongruous target words, which usually require greater processing load (Kutas and Van Petten, 1994; Chwilla et al., 1995). This greater ease of semantic integration (i.e. smaller N400 amplitude) also appears to be associated with better learning of these incongruous items. Many past ERP studies have associated larger late positive amplitudes with successful encoding and improved subsequent recall or recognition (Paller et al., 1987, 1988; Duzel et al., 1997; Fernandez et al., 1998, 1999) often referred to as the ‘Dm’ effect. This suggests a possible compensatory mechanism in which greater FMR1–mRNA levels may be helpful in both decreasing N400 amplitude and increasing the early portion of the late positive component. Increased mRNA levels are also associated with decreased FMRP levels and this lowering of FMRP may buffer the neurotoxicity effect of higher mRNA in FXTAS (Brouwer et al., 2008). Another possibility is that FXTAS patients with increased CGG repeat length and mRNA levels are compensating by voluntarily allocating more attentional resources to the stimuli and using more effortful encoding strategies. The abnormalities in the N150 suggest impaired afferent inputs, which may interact with later stages of processing (N400, late positive component) and alter processes such as rehearsal and elaborating encoding. The late positive component, like the P300 (Johnson, 1986), can be modulated by attention and effort, e.g. its amplitude is decreased when subjects are provided cues to not remember specific stimuli (Paller, 1990).
Limitations of the present study include the decreased signal to noise ratio in our FXTAS group data, secondary to a greater number of rejected trials with EEG artefacts. Most commonly, these trials had blinks or other involuntary eye movements that may reflect decreased mental control in the patients with mild FXTAS, who are known to have predominantly ‘frontal’-type cognitive dysfunction (Grigsby et al. 2008). However, we did have adequate signal to noise ratio in our individual subject ERP tracings to identify clear N150 and N400 potentials in all the included FXTAS cases. We doubt that our main ERP findings (e.g. decreased N400 repetition effects, delayed N150 peak latencies) are due to poorer signal to noise ratio in the FXTAS data. First, the intersubject variability was not significantly higher than in the normal control group ERP data. Second, the very small N400 repetition effects present in approximately half of our patients with FXTAS shows that the N400 component was in a sense ‘replicated’ in new versus old incongruous words. This, along with most of the patients with FXTAS demonstrating the normal N400 congruity effect (larger N400s for new incongruous than congruous words), implies that the signal to noise ratio was indeed adequate in the ERP data of the FXTAS group.
Another study limitation is that multiple correlations (79 total) were performed that could result in Type I error, particularly for the non-hypothesized correlations. To control Type I error, we chose an alpha level of 0.01 for our correlations, which we think is appropriate for the first ERP study of FXTAS. More stringent correction procedures (e.g. Bonferroni) would decrease our ability to detect moderate correlations of potential clinical significance. Therefore, our reported correlations of ERP measures with mRNA level and CGG repeat number are in need of independent replication, for which larger sample sizes will be most helpful.
In summary, a distinct pattern of late ERP component abnormalities was found in patients with mild FXTAS using a word repetition paradigm that reliably elicits and modulates the N400 and late positive component. The finding of abnormal N400 repetition effects in early stage FXTAS, but spared P600 repetition effects, is in contrast to mild cognitive impairment converters [who usually show early loss of P600 repetition effect and later N400 abnormalities (Olichney et al., 2008)] and patients with chronic amnesia [who usually show absent P600 repetition effect and spared N400 effects (Olichney et al., 2000)]. In mild Alzheimer’s disease, severe decrements in both the N400 and P600 repetition effects are present (Olichney et al., 2006). A different stage of cognitive processing appears to be primarily affected in FXTAS than in these amnestic disorders. While the intranuclear inclusions of FXTAS are most numerous in hippocampal neurons, they are also common among neurons and astrocytes in temporal and other neocortical regions (Greco et al., 2002, 2006). As discussed above, abnormal glutamatergic signalling may underlie the observed large decrement in the N400 repetition effect. We also suggest a possible compensatory role of increased FMR1 mRNA levels, associated with larger positive late ERP amplitudes, but not with improved P600 repetition effects or better memory retention. ERPs may provide a valuable biomarker sensitive to increases in mRNA level, perhaps even reflecting brain levels or activity of FMRP within cortical neurons.
Funding
National Institutes of Health Roadmap Interdisciplinary Research Consortium Grant [Grant Numbers UL1DE019583 (NIDCR), RL1AG032115 (NIA), RL1AG032119 (NIA)]; National Institute on Aging (NIA) (Grant AG18442).
Conflict of interest: Randi Hagerman receives research support from Neuropharm, Seaside therapeutics, Forest, Johnson and Johnson and Roche and consultation with Novartis for fragile X research studies.
Acknowledgements
Special thanks to the co-authors, Rawi Nanakul, Lannah Lua, the MIND Institute and the Center for Mind & Brain. We would like to thank Jeremy Phillips and Chris Brick for technical assistance.
Glossary
Abbreviations
- CVLT
California Verbal Learning Test
- ERP
event-related potential
- FMR1
gene for fragile X mental retardation 1 protein
- FMRP
fragile X mental retardation protein
- FXTAS
fragile X-associated tremor/ataxia syndrome
- MMSE
Mini-Mental State Examination
- WAIS
Wechsler Adult Intelligence Scale
References
- Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–9. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile x mental retardation. Trends Neurosci. 2004;27:370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer TA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Brouwer JR, Huizer K, Severijnen LA, Hukema RK, Berman RF, Oostra BA, et al. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008;107:1671–82. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén M, Pääkkönen A, Tarkka IM, Ryynänen M, Partanen J. Augmentation of auditory N1 in children with fragile X syndrome. Brain Topogr. 2003;15:165–71. doi: 10.1023/a:1022606200636. [DOI] [PubMed] [Google Scholar]
- Chwilla DJ, Brown CM, Hagoort P. The N400 as a function of the level of processing. Psychophysiology. 1995;32:274–85. doi: 10.1111/j.1469-8986.1995.tb02956.x. [DOI] [PubMed] [Google Scholar]
- Düzel E, Yonelinas AP, Mangun GR, Heinze HJ, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proc Natl Acad Sci USA. 1997;94:5973–8. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dümpelmann M, et al. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285:1582–5. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- Fernández G, Heitkemper P, Grunwald T, Van Roost D, Urbach H, Pezer N, et al. Inferior temporal stream for word processing with integrated mnemonic function. Hum Brain Mapp. 2001;14:251–60. doi: 10.1002/hbm.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández G, Weyerts H, Tendolkar I, Smid HG, Scholz M, Heinze HJ. Event-related potentials of verbal encoding into episodic memory: dissociation between the effects of subsequent memory performance and distinctiveness. Psychophysiology. 1998;35:709–20. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. New York: Biometrics Research, New York State Psychiatric Institute; Structured Clinical Interview for DSM-IV-TR Axis I Disorders – Non-patient Edition (SCID-I/NP, 4/2005 revision) 10032. [Google Scholar]
- Ford JM, Askari N, Mathalon DH, Menon V, Gabrieli JD, Tinklenberg JR, et al. Event-related brain potential evidence of spared knowledge in Alzheimer’s disease. Psychol Aging. 2001;16:161–76. doi: 10.1037/0882-7974.16.1.161. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transaction and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1995;99:411–22. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Geisser S, Greenhouse S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Graf P, Schacter DL. Implicit and explicit memory for new associations in normal and amnesic subjects. J Exp Psychol Learn Mem Cogn. 1985;11:501–18. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–55. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–71. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248:227–33. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Kaye K. 2nd. BDS: Denver, CO; 1996. The Behavioral Dyscontrol Scale: Manual. [Google Scholar]
- Grunwald T, Beck H, Lehnertz K, Blümcke I, Pezer N, Kurthen M, et al. Evidence relating human memory to hippocampal N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 1999;96:12085–9. doi: 10.1073/pnas.96.21.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem F, Rougier A, Claverie B. Short- and long-delay intracranial ERP repetition effects dissociate memory systems in the human brain. J Cogn Neurosci. 1999;11:437–58. doi: 10.1162/089892999563526. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74:805–16. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–90. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–30. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K. Spatio-temporal stages in face and word processing 1 Depth-recorded potentials in the human occipital, temporal and parietal lobes. J Physiol Paris. 1994;88:1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, VanPetten C, Marinkovic K, Lewine JD, et al. N400-like MEG responses modulated by semantic context, word frequency, and lexical class in sentences. NeuroImage. 2002;17:1101–16. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Linke DB, Elger CE. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain Cogn. 1997;33:135–50. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- Iragui V, Kutas M, Salmon DP. Event-related brain potentials during semantic categorization in normal aging and senile dementia of the Alzheimer's type. Electroencephalogr Clin Neurophysiol. 1996;100:392–406. [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–78. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6:45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–84. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Kaye K, Grigsby J, Robbins LJ, Korzun B. Prediction of independent functioning and behavior problems in geriatric patients. J Am Geriatr Soc. 1990;38:1304–10. doi: 10.1111/j.1532-5415.1990.tb03452.x. [DOI] [PubMed] [Google Scholar]
- Keane MM, Gabrieli JD, Monti LA, Fleischman DA, Cantor JM, Noland JS. Intact and impaired conceptual memory processes in amnesia. Neuropsychology. 1997;11:59–69. doi: 10.1037//0894-4105.11.1.59. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten CK. Psycholinguistics electrified. In: Gernsbacher MA, editor. Handbook of psycholinguistics. San Diego: Academic Press; 1994. pp. 83–143. [Google Scholar]
- Mungas D, Reed BR, Marshall SC, González HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14:209–23. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- National Alzheimer's Coordinating Center User Data Set. Uniform Data Set Coding Guidebook. Seattle: NACC; 2006 [Google Scholar]
- Nobre AC, Allison TA, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–3. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. 2nd. New York: Oxford University Press; 2006. Electric fields of the brain: the neurophysics of EEG; pp. 163–6. [Google Scholar]
- Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M. Absent event-related potential (ERP) word repetition effects in mild Alzheimer’s disease. Clin Neurophysiol. 2006;117:1319–30. doi: 10.1016/j.clinph.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, et al. Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73:377–84. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, et al. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763–70. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia: Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–63. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Paller KA. Recall and stem-completion priming have different electrophysiological correlates and are modified differentially by directed forgetting. J Expt Psychol Learn Mem Cogn. 1990;16:1021–32. doi: 10.1037//0278-7393.16.6.1021. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. J Cogn Neurosci. 1992;4:375–91. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol. 1987;67:360–71. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Paller KA, McCarthy G, Wood CC. ERPs predictive of subsequent recall and recognition performance. Biol Psychol. 1988;26:269–76. doi: 10.1016/0301-0511(88)90023-3. [DOI] [PubMed] [Google Scholar]
- Primerano B, Tassone F, Hagerman RJ, Hagerman P, Amaldi F, Bagni C. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA. 2002;8:1482–8. [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Suzuki W, Tanaka K, Hashikawa T. Connectiosn between anterior inferotemporal cortex and superior temporal sulcus regions in the macaque monkey. J Neurosci. 2000;20:5083–101. doi: 10.1523/JNEUROSCI.20-13-05083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Butters NM. Neuropsychological assessment of dementia in the elderly. In: Katzman R, Rowe J, editors. Principles of geriatric neurology. Philadelphia: FA Davis; 1992. pp. 144–63. [Google Scholar]
- Seritan AL, Nguyen DV, Farias ST, Hinton L, Grigsby J, Bourgeois JA, et al. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): Comparison with Alzheimer’s disease. Am J Med Genet B: Neuropsychiatr Genet. 2008;147B:1138–44. doi: 10.1002/ajmg.b.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Salmon DP, Squire LR, Butters N. Memory dysfunction and word priming in dementia and amnesia. Behav Neurosci. 1987;101:347–51. doi: 10.1037//0735-7044.101.3.347. [DOI] [PubMed] [Google Scholar]
- St Clair DM, Blackwood DH, Oliver CJ, Dickens P. P3 abnormality in fragile X syndrome. Biol Psychiatry. 1987;22:303–12. doi: 10.1016/0006-3223(87)90148-x. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. The anatomy, physiology and functions of the perirhinal cortex. Current Opinion in Neurobiology. 1996;6:179–86. doi: 10.1016/s0959-4388(96)80071-7. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olichney JM. From amnesia to dementia: ERP studies of memory and language. [Review] Clin EEG Neurosci. 2007;38:8–17. doi: 10.1177/155005940703800106. [DOI] [PubMed] [Google Scholar]