Abstract
The mesencephalic trigeminal nucleus (MesV) contains the somata of primary afferent neurons that innervate muscle spindles in masticatory muscles and mechanoreceptors in the periodontal ligaments. There are conflicting reports about additional peripheral targets of MesV, such as the extraocular muscles, as well as about its central targets. In addition, only limited primate data are available. Consequently, we examined MesV projections in macaque monkeys. The retrograde tracer wheat germ agglutinin conjugated horseradish peroxidase (WGA-HRP) was injected into masticatory or extraocular muscles to define the peripheral targets of the primate MesV. Numerous labeled neurons were found in ipsilateral MesV following masticatory muscle injections. The scattered distribution of labeled cells, and their presence amongst clusters of unlabeled cells, suggests the muscle representations overlap. Just a few MesV neurons were labeled after extraocular muscle injections. This correlates with the small number of muscle spindles present in macaque extraocular muscles, suggesting MesV cells supplying extraocular muscle spindles may contribute a minor component to oculomotor proprioception. To examine the central connections of MesV, biotinylated dextran amine (BDA) was injected into the spinal trigeminal nucleus (Vs). The presence of retrogradely labeled MesV cells indicated a projection to Vs from MesV. These injections also anterogradely labeled terminals that lay in close association with MesV cells, suggesting an ascending projection from Vs to MesV. Finally, a small number of MesV neurons were labeled following WGA-HRP injections into the upper cervical spinal cord. This pattern of central connections indicates MesV and Vs information is combined to guide mastication.
Keywords: Mastication, Oculomotor, Proprioception, Somatosensory, Extraocular Muscle
INTRODUCTION
Despite its central location, the mesencephalic trigeminal nucleus (MesV) contains the somata of primary afferent neurons whose peripheral processes are associated with the muscle spindles of jaw-closing muscles and mechanoreceptors within the periodontal ligaments. The distribution of MesV spans the mesencephalon and the rostral portion of pons, but it is not organized as a discrete nucleus. Instead, it consists primarily of a set of large cells arrayed individually and in clusters along the edge of the midbrain periaqueductal gray (PAG). More caudally, additional MesV neurons are distributed adjacent to the mesencephalic tract. True to their identity as primary afferents, the somata of MesV neurons resemble those of cells in the dorsal root ganglion; i.e., they are pseudounipolar neurons and most appear to lack conventional dendritic trees (Johnston, 1909; Freeman, 1925; although see Nomura et al., 1985). Within the central nervous system, the process of each MesV neuron bifurcates into peripherally and centrally directed branches near the trigeminal motor nucleus (Corbin, 1942; Luo and Dessem, 1995). The peripheral process extends through the mesencephalic tract and exits the brainstem via the motor root of the trigeminal nerve. While it has been found to supply the masticatory muscles (Alvarado-Mallart et al., 1975; Capra et al., 1985; Shigenaga et al., 1988a), and periodontal ligaments (Jerge, 1963; Capra et al., 1984; Byers et al., 1986; Shigenaga et al., 1989) of a variety of species, only limited data are available on the peripheral targets of Mes V in primates (Yassin and Leong, 1979; Hassanali, 1997).
It has been proposed that MesV has another peripheral target: the extraocular muscles, but this remains a matter of dispute. There is general agreement that conventional sensory input is supplied to the extraocular muscles by cells located in the trigeminal ganglion. However, some reports find that a small number of extraocular muscle afferent neurons are also located in MesV (cat: Alvarado-Mallart et al., 1975; Buisseret-Delmas and Buisseret, 1990). Other studies argue that MesV does not contain any cells subserving proprioception in the extraocular muscles (pigeon: Eden et al., 1982; cat: Porter and Spencer, 1982; Ogasawara et al., 1987; Porter and Donaldson, 1991; monkey: Porter et al., 1983). It has been suggested that one reason for the differences between these findings is that MesV neurons may specifically supply muscle spindles in the extraocular muscles, and the number of these varies between species (Billig et al., 1995; Buisseret-Delmas and Buisseret, 1990).
The central processes of MesV neurons descend within the tract of Probst. These central processes carry information from masticatory muscles and periodontal ligaments directly to brainstem targets. MesV target neurons have previously been described in the rat and cat (Alvarado-Mallart et al., 1975; Appenteng et al., 1978; Capra and Wax, 1989, Luo and Dessem, 1995; Luo et al., 1995; Dessem and Luo, 1999; Lazarov, 2000). Within the pons, MesV axons give off branches to the supratrigeminal region, trigeminal motor nucleus and principal trigeminal nucleus to supply motoneurons and premotoneurons controlling mastication (Luo et al., 1995). Axons descending in the tract of Probst and the parvocellular reticular formation (PcRt) provide collaterals to other cranial nerve nuclei; particularly the spinal trigeminal nuclei and, in some cases, the facial and hypoglossal nuclei. These descending axons may reach as far as the C3 segment. Others have suggested that the central projections of MesV neurons access the vestibular nuclei and cerebellum (Buisseret-Delmas et al., 1997; Billig et al., 1995; Pinganaud et al., 1999). This widespread pattern of connections suggests that in addition to modifying jaw movements during eating, MesV neurons may play an important role in modulating associated movements of the face, tongue and even the neck (Shingenaga et al., 1988b; Luo et al., 1995; Dessem and Luo, 1999). However, there are, to our knowledge, no studies directly concerned with assessing the central projections of MesV in primates.
In order to better support the contention that the pattern of peripheral and central connections of Mes V found in non-primates represents a general mammalian plan applicable to the human condition (Usunoff et al., 1997), it seems reasonable to extend the examination of these features to the primate. Consequently, in the present investigation, neuronal tracers were used to define the location and projections of MesV neurons in macaque monkeys. The peripheral injection targets we tested were the masticatory muscles and the extraocular muscles. We examined other monkeys in which central injections of tracer were made into spinal trigeminal nucleus (Vs) or upper cervical spinal cord, in order to assess whether MesV afferent information, and information conveyed by neurons whose somata reside in the trigeminal ganglion, converge within the spinal trigeminal nucleus.
MATERIALS AND METHODS
Surgery
Material from 22 macaque monkeys was utilized in the present study. These animals were all used in other non-conflicting studies, and the results described here are from a retrospective investigation of these cases. In all cases, animals were sedated with ketamine hydrochloride (10 mg/kg, im) and anesthetized with isoflurane via an endotracheal airway. Atropine sulfate (0.05 mg/kg, im) was given to control airway secretions, and dexamethasone (2.5 mg/kg, iv) was given to preclude edema. Pulse, respiratory rate, temperature and respiratory gasses were monitored and maintained within normal limits during the surgery. The animal’s head was stabilized in a stereotaxic apparatus to allow precise application of the tracer. Either Buprenorphine (0.1 mg/kg, im) or Buprenex (0.01mg/kg, im) was administered following recovery to alleviate postsurgical discomfort. All of the surgical procedures were done under sterile conditions. They were performed in accordance with N.I.H. Guidelines for the Care and Use of Animals and with IACUC approval.
Monkeys were divided into four groups with respect to injection sites. The first group (n=5) received masticatory muscle injections. These injections consisted of 10–15μl of 1.0% wheat germ agglutinin conjugated horseradish peroxidase (WGA-HRP) combined with 10 % HRP (Sigma). This was injected into the masseter or medial pterygoid muscle on one or both sides. Injections were placed in up to 3 sites to ensure that the tracer was well distributed within the muscles. Intramuscular injections were made by inserting a 25 gauge needle attached to a 25 μl Hamilton syringe through the skin or oral mucosa. In the case of the masseter muscle, the anterior edge of the muscle was palpated over the teeth, and the injections were placed in muscle belly posterior to this edge.
In the second group (n=5), 5–16 μl of 1.0% WGA-HRP were injected into each targeted extraocular muscle. The targets included the superior rectus (n=3), medial rectus (n=4) and levator palpebrae (n=3) muscles, and in each case at least 2 muscles within the same orbit were injected. This was accomplished by incising the brow above the orbital edge of the frontal bone. The orbicularis oculi muscle was then cut from its origins to allow the levator palpabrae and underlying superior rectus muscles to be isolated. The medial rectus muscle was isolated after rotating the eyeball by pulling laterally on the superior rectus tendon, and catching the medial rectus with a muscle hook. The needle of a 10 μ1 Hamilton syringe was inserted into each muscle belly and advanced parallel to its long axis. Tracer was injected while retracting the needle. The orbicularis oculi muscle was then re-attached, and the incision was closed.
The third group of monkeys (n=4) received injections of 10% biotinylated dextran amine (BDA) into the spinal trigeminal nucleus (Vs). To approach Vs, the stereotaxic frame was inclined to flex the neck 30–45° nose down. After making an incision along the midline that extended from the external occipital protuberance to the C2 vertebra, the underlying neck muscles were freed from their superior attachment to the skull, incised along the ligamentum nuchae and retracted laterally. This revealed the atlanto-occipital membrane, which was incised and reflected to visualize the dorsal medulla. Surface landmarks were used to guide the needle of a 1.0 μl Hamilton syringe, angled perpendicular to the brainstem. Specifically, the needle was aimed at either pars caudalis or pars interpolaris of Vs by using the obex as a guide. It was inserted through the tuberculum cinereum, which overlies Vs. Approximately 0.1–0.2 μl of BDA was injected at each site. Several sites, varying only in their rostral-caudal position, were injected in an attempt to better fill this columnar target. The defect in the atlanto-occipital membrane was closed with Gelfilm. The muscles were re-attached in layers and the incision was closed. An additional 4 monkeys in which WGA-HRP was injected into the medullary reticular formation using an approach similar to that described above were analyzed as control cases.
In the last group of monkeys (n=4) an injection of 1–2 % WGA-HRP and 10 % HRP was made into the upper cervical spinal cord. The same surgical approach was used here as for the Vs injections. However, the posterior portion of the vertebral arch of C1 was removed to better reveal the rostral end of the cervical spinal cord. The syringe was placed more medially, and advanced deeper into the tissue (1.5–2.5 mm). The injection volume ranged from 0.01 to 0.05 μl. Two tracks were made at different rostrocaudal sites, producing a total volume 0.02 to 0.1 μl. The closing procedure was the same as for the Vs injection.
After a survival time of 24–48 h for the WGA-HRP injections or 3 weeks for the BDA injections, the animals were sedated with ketamine HCl (10 mg/kg, im) and deeply anesthetized with sodium pentobarbital (50–70 mg/kg, ip). They were then perfused through the heart with 1.0 l of buffered saline, followed by 2.0–3.0 l of fixative containing 1.0% paraformaldehyde and 1.25% glutaraldehyde in 0.1 M, pH 7.4 phosphate buffer (PB). Each brain was blocked in the frontal plane, post-fixed for 1 hour, and stored in cold PB. Brain blocks were cut on a vibratome into a series of 100 μm thick frontal sections.
Histochemistry and Analysis
To reveal the WGA-HRP labeling, a tetramethylbenzidene (TMB) procedure was utilized (Perkins et al., 2006, Olucha et al., 1985). For each case, at least one series of sections spaced 300 μm apart was pre-incubated for 20 minutes in 0.1 M, pH 6.0 PB containing 0.25% ammonium molybdate and 0.005 % TMB. Hydrogen peroxide was then added (final concentration - 0.0125%) to initiate the reaction; producing a blue reaction product. After incubating at 4°C overnight, sections were transferred to a stabilizing solution containing 5% ammonium molybdate in 0.1M, pH 6.0 PB. In some cases, the sections were then further incubated in a 0.5% diaminobenzidine (DAB) in 0.1 M, pH 7.2 PB for 10 minutes. Hydrogen peroxide was added (0.005%) to catalyze the reaction and produce a light brown reaction product.
To demonstrate the BDA labeling, a procedure similar to that described in Chen and May (2002) was followed. At least one series of sections spaced 300 μm apart was rinsed and transferred into a solution containing 0.1 % Triton X-100, in 0.1 M, pH 7.2 PB. Then they were incubated with agitation in a 1:500 avidin-HRP (Vector) solution in this same 0.1 % Triton X-100 PB for 24 hrs at 4 °C. Next, they were reacted in 0.5 % DAB solution containing 0.01% cobalt chloride and nickel ammonium sulfate. Hydrogen peroxide (0.005%) was added to catalyze the reaction and reveal the BDA as a black reaction product.
In all cases, sections were then mounted, counterstained with cresyl violet, dehydrated in ethanols, cleared in toluene and coverslipped. Sections containing the TMB and DAB stained cells were charted using an Olympus BH-2 microscope equipped with a drawing tube. Photomicrographs were taken using a color digital camera (Nikon DXM1220F) mounted on a Nikon photomicroscope (Eclipse D600). Images were obtained using Metamorph software. This allows up to 15 z-axis focal planes to be combined into a single picture using the “stack arithmetic, minimum summation” feature. The brightness, color and contrast were adjusted to match that observed through the microscope by use of Photoshop software. In the first group, only cases in which retrogradely labeled motoneurons were constrained to the dorsolateral subdivision of the motor trigeminal nucleus, and were absent from the facial nucleus were analyzed (Mizuno et al., 1981). In the second group, the pattern of labeling in the oculomotor nucleus was examined to make sure muscle specific injections occurred (Porter et al., 1983; 1989). In addition, the trochlear, abducens, facial and trigeminal motor nuclei were inspected, and were found to be free of labeled motoneurons in all cases used, in order to assure that spread of tracer inside or outside the orbit had not occurred.
RESULTS
Masticatory Muscle Injection
WGA-HRP injections in the medial pterygoid or masseter muscles, which resulted in the labeling of motoneurons constrained to the dorsolateral trigeminal motor nucleus, also labeled MesV neurons with somata that were large (long axis . 30 – 35 μm) and spherical or ovoid in shape (arrows, Fig. 1A–C). Most of these cells were found on the border of the periaqueductal gray (PAG). Some labeled neurons were found among clusters of unlabeled cells (arrowheads) whose morphology, as revealed by the counterstain, suggested they were also MesV neurons (Fig. 1A&C). Others lay as isolated neurons along the border of the PAG (Fig. 1B).
Figure 1.
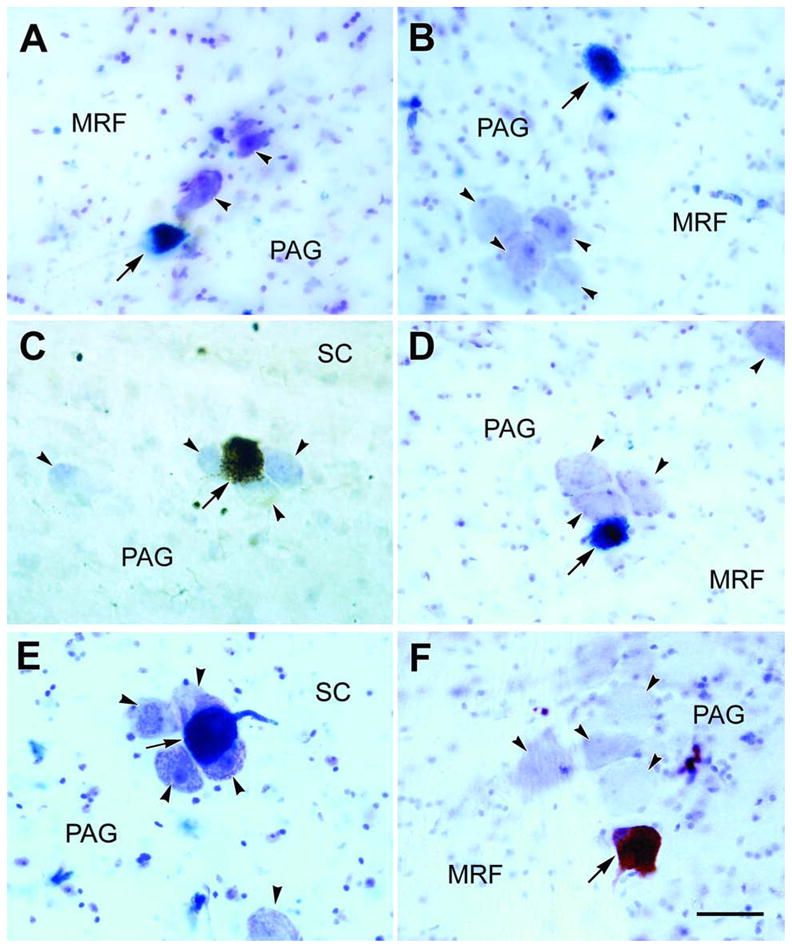
Photomicrographs of counterstained (arrowheads) and WGA-HRP labeled (arrows) macaque MesV neurons. Labeled MesV neuron and the adjacent unlabeled cells are shown following an injection into the medial pterygoid muscle (A) and the masseter muscle (B&C). In D&E labeled neurons within clusters of counterstained MesV cells are shown following WGA-HRP injections into the superior rectus and levator palpbrae muscles, and the medial rectus, superior rectus and levator palpbrae muscles, respectively. F shows a labeled MesV neuron and adjacent unlabeled cells following a WGA-HRP injection in the ipsilateral upper cervical cord. Scale bar = 50 μm.
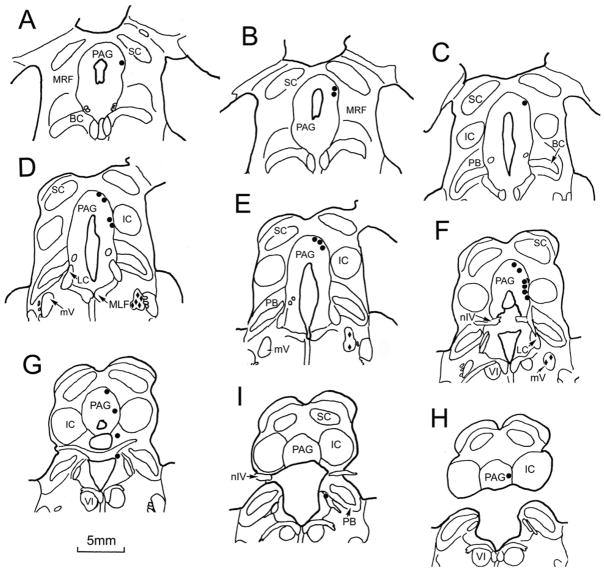
Figure 2 shows the distribution of retrogradely labeled cells after injections of WGA-HRP into the medial pterygoid muscle. Labeled motoneurons were present in the ipsilateral motor trigeminal nucleus (diamonds, Fig. 2D–F). The MesV cells labeled from this masticatory muscle injection (dots) had a widespread distribution. Numerous labeled cells (n.72 neurons) were scattered along the ipsilateral edge of the PAG from the level of the olivary pretectal nucleus (Fig. 2A) to the level of the abducens nucleus (Fig. 2H). In addition to the cells labeled in the PAG portion of MesV, a few cells were located along the mesencephalic tract near the parabrachial nuclei and locus coeruleus (Fig. 2G&I). Three examples of the pattern of MesV labeling following a masseter muscle injection are shown in figure 3. These sections (Fig. 3A–C) are from the caudal end of MesV. They show the outlines of counterstained, presumptive MesV cells located along the border of the PAG. The MesV population consists of both isolated cells and clusters. Most of the labeled cells (filled outlines) were arranged as isolated individuals along the PAG border, but others (Fig. 3C) were found amongst clusters of unlabeled somata. Looking across cases, no obvious topography was observed in the distribution of labeled MesV neurons with respect to muscle. It is noteworthy that following tracer injection in each masticatory muscle, only 1–2 cells were labeled within any cluster of cells in MesV.
Figure 2.
The distribution of labeled MesV neurons (dots) following a WGA-HRP injection into the right medial pterygoid muscle of a monkey. A rostral to caudal series of frontal sections spaced approximately 600 μm apart is shown. Retrogradely labeled neurons are located along the ipsilateral margins of the PAG. The labeled trigeminal motor neurons are indicated by solid diamonds in D–F.
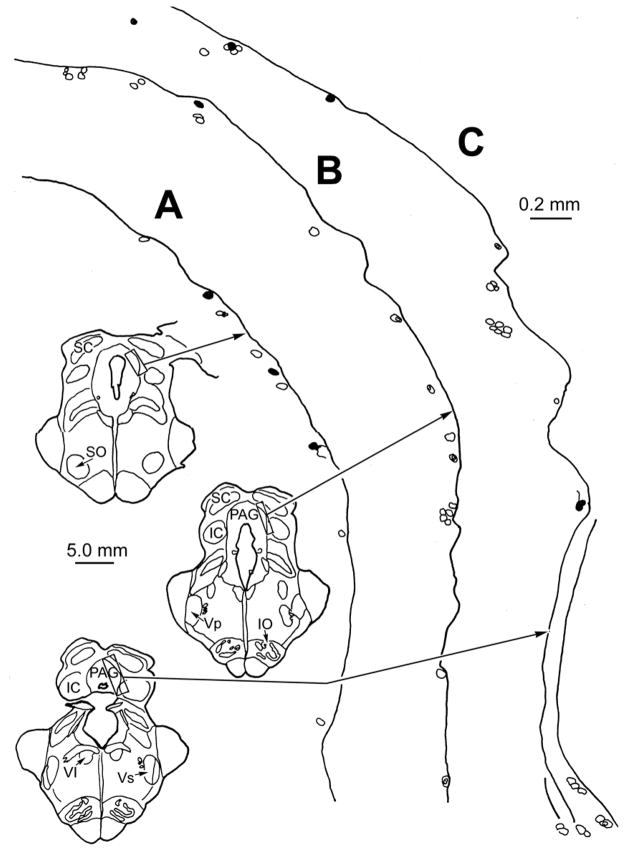
Figure 3.
Detailed chartings of MesV neurons following a WGA-HRP injection into the right masseter muscle in the monkey. Counterstained, unlabeled MesV cells (indicated by cell outlines) lie singly and in clusters formed by 2–7 neurons along the margins of the PAG. Labeled neurons (filled outlines) are found as isolated neurons (A–C) and among the clusters (C), but rarely cluster together (C). Illustrated sections through MesV are spaced approximately 900 μm apart.
Extraocular and Levator Muscle Injections
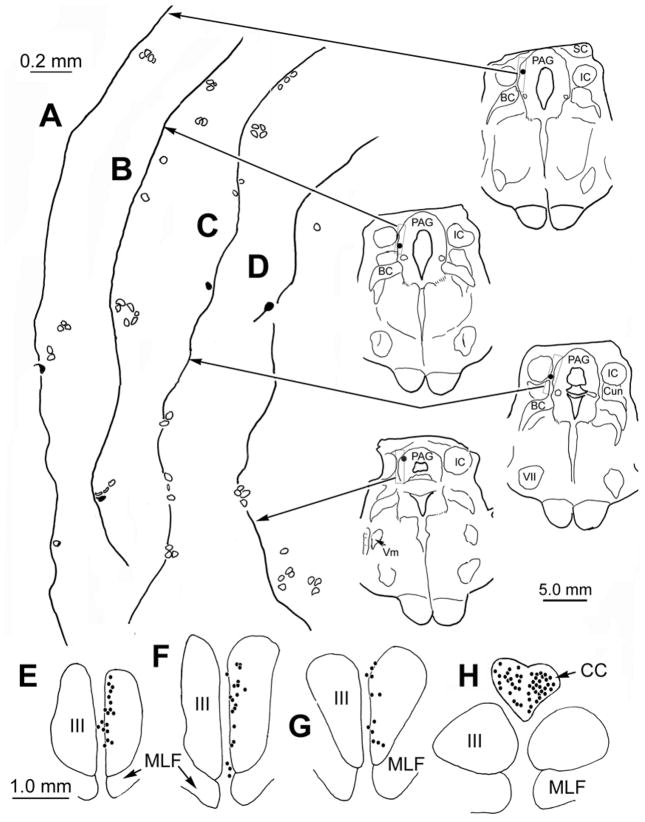
In each case in which WGA-HRP was injected into extraocular muscles, labeled neurons were observed in the corresponding subdivision of the oculomotor nucleus (Porter et al., 1983; 1989). This served to confirm that the desired muscles were injected, as spread of tracer from the injection site would have produced retrogradely labeled motoneurons in other subdivisions of the oculomotor nucleus. While no labeled neurons were found in the trigeminal motor nucleus, a small number of cells were retrogradely labeled in MesV. A case in which the superior rectus and levator palpbrae muscle were injected with WGA-HRP is shown in figure 4. Labeled levator palpebrae motoneurons were observed bilaterally in the caudal central subdivision (Fig. 4H), and labeled superior rectus motoneurons were present contralaterally in oculomotor nucleus (Fig. 4E–G). As shown in the chartings of the caudal midbrain (Fig. 4A–D), labeled Mes V neurons (dots) were present ipsilateral to the injected extraocular muscles. They were few in number (n.12), and were not concentrated in any specific area of the nucleus. Consequently, they were more widely scattered than those observed following masticatory muscle injections. Both isolated cells (Fig. 4A, C&D) and cells located in clusters of counterstained presumptive MesV neurons (Fig. 4B) were present. No obvious topographic pattern was observed when different cases were compared. The morphology of the MesV cells labeled from a case in which the superior rectus and levator palpebrae muscle were injected, and a case in which the medial rectus, superior rectus and levator palpbrae muscles were injected is shown photographically in figure 1 (D&E, respectively) . Like those observed following masticatory muscle injections, these somata varied from spherical to ovoid in shape, with long axes that lay within the same size range. As shown in these two examples, they were sometimes part of a MesV cluster.
Figure 4.
The distribution of labeled neurons observed following a WGA-HRP injection into the left superior rectus and levator palpebrae muscles. Labeled Mes V neurons were fewer in number than those seen following injections of muscles of mastication (filled outlines and dots in A–D). Most presented as isolated cells, but a few lay in MesV clusters (B). No labeled neurons are present in the ipsilateral trigeminal motor nucleus (D). However, the injection labeled motoneurons in the appropriate areas of the contralateral oculomotor nucleus and bilaterally in the caudal central subdivision (dots in E–H). Illustrated sections through MesV are spaced approximately 300 μm apart.
Central Projections
To examine whether MesV neurons send central projections to other parts of the trigeminal sensory complex, we inspected cases where BDA was injected into the spinal trigeminal nucleus (Vs) (Fig. 5A–C; T1-4). MesV labeling was noted in cases where the injections were located in either pars interpolaris or caudalis of Vs, with spread to into the adjacent medullary reticular formation. However, MesV neurons were not labeled following control injections of the medullary reticular formation (Fig. 5A–C; R1-4). Figure 6 shows the pattern of labeling in an example where the injection site spread from caudal pars interpolaris (Fig. 6A&B) into rostral pars caudalis (Fig. 6C–E). The tracer also extended ventrally into adjacent areas of the medullary reticular formation (MdRF). BDA-labeled neurons (n.21) were observed in ipsilateral MesV (Fig. 6F–H). In this example, most of the labeled neurons were observed along the dorsal edge of the caudal PAG, but the other cases showed different distributions. No obvious trend in their distribution was seen when the cases were compared.
Figure 5.
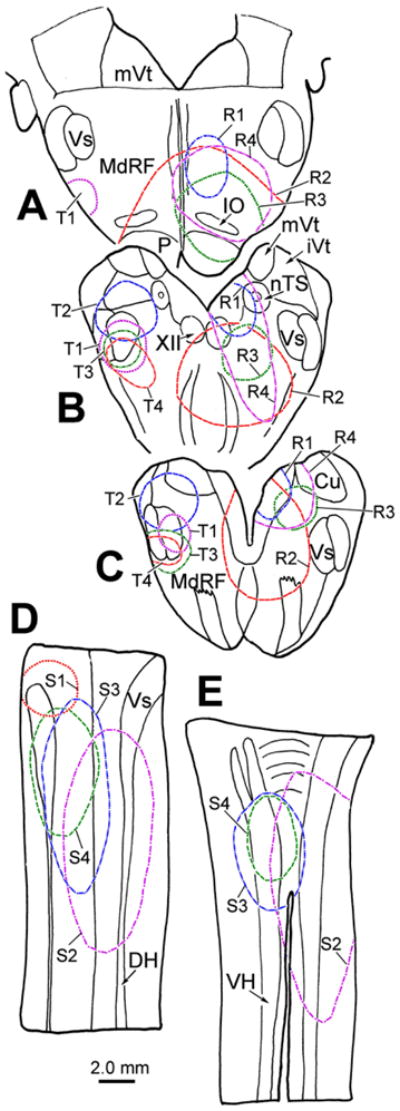
Central injection sites for the brainstem (A–C) and spinal cord (D&E) cases analyzed for this study. The 4 BDA injections of the spinal trigeminal nucleus (T1-4) are charted on the left in A–C. The 4 WGA-HRP injections centered in the medullary reticular formation (R1-4) are charted on the right in A–C. The 4 WGA-HRP injections into the rostral spinal cord (S1-4) are charted on dorsal (D) and ventral (E) longitudinal sections through the cervical region.
Figure 6.
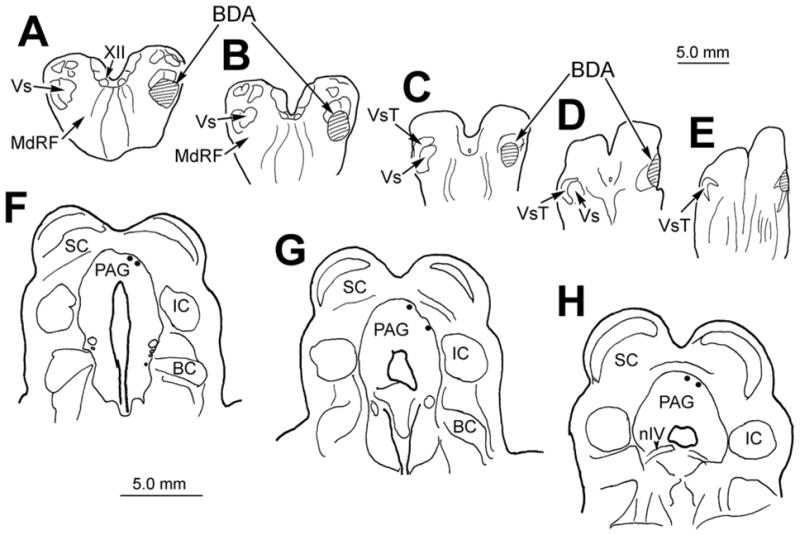
Chartings demonstrating the injection site (A–E) and the distribution of labeled MesV neurons (dots in F–H) following a BDA injection into the right spinal trigeminal nucleus pars interpolaris and caudalis. The injection site did not cover the whole extent of the nucleus, but it extended ventrally into the medullary reticular formation. Illustrated sections through MesV are spaced approximately 600 μm apart.
The morphology and location of the neurons labeled via their central projections resembled those labeled following masticatory muscle injections; i.e., they had large round or ovoid somata located along the border of PAG (Fig. 7A–D). Once again, some of these labeled cells were isolated cells and others were found among clusters of unlabeled, presumptive MesV neurons. In addition to retrogradely labeling cells, the BDA injections in Vs anterogradely labeled axons. Labeled fibers with small bouton-like enlargements were present bilaterally within the lateral portion of the PAG. Some of these labeled boutons were found in the immediate vicinity of MesV cells, and displayed close associations (arrowheads) with the somata of labeled (Fig. 7A&B) or unlabeled (Fig. 7C&D) MesV neurons.
Figure 7.
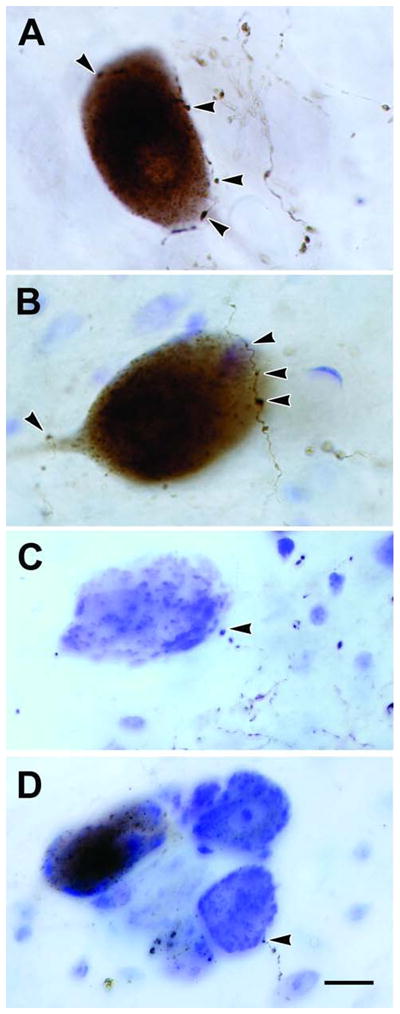
Retrogradely labeled cells and anterogradely labeled axons in MesV following BDA injections of the spinal trigeminal nucleus. Close associations (arrowheads) suggestive of synaptic contacts were observed between labeled boutons and retrogradely labeled MesV neurons (A&B), as well as unlabeled MesV neurons located singly (C) or in clusters (D). Scale bar = 10 μm (A–D). Number of Z-axis slices combined, A&B = 1, C=3, D=8.
Finally, we examined cases in which WGA-HRP injections in cervical spinal cord covered much of the anterior and posterior horns, as well as the white matter from the C1 to C3 level (Fig. 5D&E; S1-4). They involved the portion of Vs pars caudalis present at these levels as well. These injections resulted in very sparse labeling of neurons in MesV. Only 3–6 labeled neurons were found in each case. As shown in figure 1F, the somata of these labeled cells displayed the same morphology and location as those labeled following injections of the masticatory muscles. It should be noted that in one case where the injection area only covered a part of lateral funiculus and did not involve the gray matter (not illustrated), MesV was devoid of labeled neurons. This indicates the observed MesV labeling was not due to fiber-of-passage uptake by axons terminating below the levels injected.
DISCUSSION
The present study confirmed the innervation of masticatory muscles by the peripheral processes of MesV neurons in macaque monkeys. It also provides evidence that a small number of primate MesV neurons supply axons to the extraocular muscles. The central processes of MesV neurons were found to project to the caudal brainstem, and rostral spinal cord. One likely target of this projection was the spinal trigeminal nucleus (Vs). Evidence that Vs and/or the adjacent parvocellular reticular formation (PcRt) also sends axons bilaterally back to MesV was observed. The many similarities in the pattern of connection for this primate species and previously described non-primate species suggest the findings observed in non-primates are likely applicable to the human condition.
Masticatory Muscles
Mastication is a complex rhythmical behavior, which is produced by the premotor neurons of a brainstem central pattern generator subject to conscious control (Dellow and Lund, 1971; Nozaki et al., 1986; Lund and Kolta, 2006). Sensory feedback from muscle spindles and periodontal receptors that carry information about muscle contraction and bite force modulates the output of this central pattern generator (Lund and Kolta, 2006). It is likely that Mes V central projections to the reticular formation subnuclei immediately surrounding motor V (e.g. the supratrigeminal nucleus) and to the pontomedullary reticular formation provide this modulatory input (Lazarov, 2000; Shigenaga et al., 1988a&b). We have expanded the investigation of MesV organization to the primate because a number of differences in the regulatory circuits for mastication have been reported between species. These may be related to mastication complexity. For instance, most of the muscle spindles are clustered together in a restricted area within the rat masseter muscle, whereas in the cat and monkey masseter muscle, extensive fusion of the external capsules of adjacent spindles results in the formation of giant spindles (Rowlerson et al., 1988). The number of MesV neurons also varies. Although the rat masticatory muscles are much smaller than those of the cat and monkey, the rat has a greater number of neurons in MesV (Hinrichsen and Larramendi, 1969; Hassanali, 1997).
Previous studies in cats have indicated that MesV periodontal afferent neurons are mainly concentrated caudally (Cody et al., 1974; Linden, 1978; Nomura and Mizuno, 1985; Capra and Wax, 1989). In contrast, cat MesV jaw muscle afferents are more evenly distributed along the rostrocaudal extent of the nucleus (Jerge, 1963; Gottlieb et al., 1984; Capra et al., 1985; Nomura and Mizuno, 1985; Capra and Wax, 1989). The pattern in monkeys resembles that of cats. The MesV cells supplying the periodontal ligaments show a caudal bias in their distribution in the baboon and vervet monkey (Hassanali, 1997), and the present study in macaque monkeys did not reveal any rostrocaudal differences in the somatotopic distribution MesV neurons supplying masticatory muscles.
We found no somatotopic arrangement with respect to different masticatory muscles, which is consistent with previous findings (Nomura and Mizuno, 1985). However, for individual muscles, one remarkable cytoarchitectural feature of MesV was observed. Tracer from each individual masticatory muscle retrogradely labeled neurons in different cell clusters in MesV, but left most cells within the cluster unlabeled. It is possible that this could be due to incomplete labeling of the cells projecting to a single muscle, but in light of the widespread nature of this phenomenon in the monkey and other species (present results, Capra et al., 1985; Hassanali, 1997), it seems more likely that a single cluster may innervate different muscles, as has been directly demonstrated in the rat by Rokx and van Willigren (1988). This is a particularly striking finding, considering the fact each cell cluster may form a functional unit due to the presence of soma-somatic contacts, including gap junctions, between cells in a cluster (Hinrichsen and Larramendi, 1968; Hinrichsen, 1970; Baker and Llinás, 1971; Liem et al., 1991).
Extraocular and Palpebral Muscles
It appears that efference copy signals and visual sensory information, not extraocular muscle proprioception, direct eye movements while they are being produced and signal that they are accurate (Guthrie et al., 1983; Lewis et al., 2001). Furthermore, no conventional stretch reflex is present in these muscles (Keller and Robinson, 1971). Nevertheless, there is evidence that proprioceptive signals from the extraocular muscles are important for other aspects of oculomotor control. For example, removing or manipulating this afferent input affects static eye position and produces long term modifications in smooth pursuit, saccades and the vestibulocular reflex (van Donkelaar et al., 1997; Lewis et al., 1994; Kashii et al., 1989; Kimura et al., 1991). Proprioception in the extraocular muscles is also associated with development of proper visual function (Fiorentini et al., 1986; Knox et al., 2000; Weir et al., 2000; see Buisseret, 1995 for review). Thus, it is not surprising that proprioceptive signals from the extraocular muscles have been noted in a variety of central structures (see Donaldson, 2000 for review), most recently primary somatosensory cortex (Wang et al. 2007). However, it is not known whether these signals arise from muscle spindles, or other types of nerve specialization such as palisade endings (for review see Büttner-Ennever et al., 2006).
A long-standing debate exists on the location of the primary afferent neurons supplying the extraocular muscles. Retrograde labeling of a small number of MesV neurons following eye muscle injections has been reported in xenopus and cat (Alvarado-Mallart et al., 1975; Buisseret-Delmas and Buisseret, 1990; Buisseret-Delmas et al., 1997; Hiscock and Straznicky, 1982). However, others have obtained negative results in studies of the rat, cat, pig, monkey and pigeon extraocular muscles (Porter and Spencer, 1982; Porter et al., 1983; Bortolami et al., 1987; Daunicht et al., 1985; Eden et al., 1982) and in monkey levator palpebrae muscles (Van der Werf et al., 1997). Porter and Donaldson (1991) argued that it is the trigeminal ganglion, not MesV, that is the site of all the extraocular muscle afferent cell somata, and that their terminal fields lie in Vs at the spinomedullary junction. They suggested that the MesV labeling observed by others was caused by leakage of tracer into other targets of this central nucleus. In the present study, the trigeminal motor nucleus was examined following individual extraocular muscle injections and no evidence was found to indicate that axons of masticatory muscles had taken up tracer. This is not surprising, given that the bony orbit of the monkey is complete, separating its contents from the masticatory muscles. The possibility of spread to the periodontal ligament also seems unlikely for this reason. Branches of the ophthalmic division of the trigeminal nerve do traverse the orbit, but these supply the face around the orbit and the nasal cavity, and do not supply the palate and periodontal ligament. Furthermore, extraocular motoneuron labeling was muscle specific, as evidenced by the lack of labeling in the trochlear nucleus, despite the fact the superior oblique muscle lies between the two injected rectus muscles. This strongly argues against tracer spread within the orbit. Consequently, we believe the tracer we observed in labeled MesV neurons originated from the injected extraocular muscles, and was not due to fiber-of-passage uptake by intact orbital nerves.
Greene and Jampel (1966) reported that muscle spindles are only present in small numbers in the extraocular muscles of macaque monkeys. The small number of muscle spindles found in the macaque extraocular muscle correlates well with the small numbers of MesV neurons labeled following muscle injections in the present study. While such a correlation does not represent proof of this connection, it may explain the divergent findings across studies; i.e., a small and variable number of spindles may lead to variable labeling of a small number of MesV cells. While palisade endings are the only type of fiber specialization consistently found in vertebrate extraocular muscles, a number of other types of putative proprioceptors have been observed (Billig et al., 1997; Blumer et al., 2006; see Büttner-Ennever et al., 2006 for review). In fact, the quantity, distribution and subtypes of extraocular muscle endings vary between species, and perhaps even between individuals (Maier, 2000; Büttner-Ennever et al., 2003). It is very likely that other sensory fibers with somata in the trigeminal ganglion and terminals in Vs supply these non-spindle sensors (Billig et al., 1997; Porter and Spencer, 1982; Porter et al., 1983; Porter, 1986). Thus, the labeled MesV neurons observed in the present study following extraocular muscle injections do not exclude a proprioceptive projection to the spinal trigeminal nucleus by way of neurons residing in the trigeminal ganglion. In this regard, our results do not differ from those of Porter (1986) in that we have also observed terminals in the Vs following extraocular muscle injections. Consequently, our findings support the contention that primate extraocular muscle proprioceptive cells have a dual location: in the trigeminal ganglion and, to a lesser extent, in MesV. As MesV central projections include Vs, extraoculomotor proprioceptive information from both sources may converge on Vs neurons for dispersal to targets that participate in eye movement control (Porter and Spencer, 1982; Porter, 1986; Lazarov, 2000).
Central Projections
Anatomical and electrophysiological studies in non-primates (Shigenaga et al., 1988a&b; Capra and Wax, 1989, Billig et al., 1995; Luo and Dessem, 1995; Luo et al., 1995; Pombal et al., 1997) have reported that in addition to terminating in and around the motor and principal trigeminal nuclei, MesV central processes also send collaterals to numerous brainstem nuclei including Vs. These studies suggest that Vs neurons provide relays whereby MesV proprioceptive information reaches the cerebellum and cerebral cortex. For example, experiments in rats indicate that the projections of jaw-muscle spindle afferents to pars oralis and interpolaris relay orofacial proprioceptive signal to the cerebellum and to the thalamus (Luo and Dessem, 1995). The projection to pars caudalis may also be part of long-latency stretch reflex circuits accessing the trigeminal motor nucleus (Luo et al., 1995). In the present study, we provide initial evidence for these same projections in a primate. We found retrogradely labeled Mes V neurons following injections including pars interpolaris and caudalis, suggesting the pathways seen in the rat are also present in the monkey (Fig. 5A–C; T1-4). These injections extended beyond the boundary of Vs, to include subjacent portions of the reticular formation. This parvocellular reticular formation (PcRt) area, is also reported to be a target of rat central MesV projections (Luo et al., 1995). Thus, the retrogradely labeled cells seen in the present study could also provide proprioceptive information to PcRt (Ro and Capra, 1999). It should be noted that in other cases where medial medullary injections did not extend into Vs, no labeled MesV cells were observed, even when the injections included the hypoglossal nucleus (Fig. 5A–C; R1-4). Consequently, the current study provides evidence for caudal projections to Vs in monkeys, but does not provide evidence of the hypoglossal projections observed in rats (Zhang et al., 2001).
It has been suggested that Vs could send afferents to MesV based on projections seen in the rat (Buisseret-Delmas et al., 1997). Furthermore, the rat PcRt has been shown to send afferents to the contralateral Mes V (Minkels et al., 1991). We observed anterogradely labeled terminals in the vicinity of the macaque Mes V on both sides of the midbrain following unilateral injections of Vs that extended into the subjacent reticular formation. Moreover, we observed close associations between labeled boutons of these axons and the somata of labeled and unlabeled MesV neurons. Although ultrastructural evidence is necessary to prove the presence of a monosynaptic connection, these close associations suggest Vs or PcRt afferents synaptically contact MesV neurons. Certainly, synaptic contacts of unknown origin are present on MesV neuronal somata in other species (Hinrichsen and Larramendi, 1969; Liem et al., 1991). Furthermore, similar inputs from the principal trigeminal nucleus have been observed (Buisseret-Delmas et al., 1997). The presence of these ascending projections to MesV from its Vs and PcRt targets suggests a feedback circuit is present. However, it is unclear how these synapses on MesV cells might modulate the activity in MesV central projections. Presumably, action potentials traveling in the peripheral processes continue directly along the central processes and would be unaffected by synaptic activity in MesV somata. Thus, synaptic inputs to MesV cells may instead modify the capacity of individual somata to transfer activity into adjacent somata within cell clusters, or it may induce activity in the MesV central arbor that is not related to that produced in the periphery.
A cervical spinal cord projection of MesV was not consistently found in the previous studies. Lucchi and colleagues (1997) reported that fluorescent tracers injected into the C2-C3 did not label any MesV neurons in the duck, rat and rabbit. In contrast, Dessem and Luo (1999) found a small number of HRP-labeled neurons in MesV after cervical cord injection in the rat. In the present study, large injections of the upper cervical spinal cord labeled just a handful of neurons in MesV, indicating this small projection is present in monkeys, as well. The direct projection from the MesV to the cervical spinal cord has been suggested to coordinate jaw and neck movement during mastication and biting (Dessem and Luo, 1999). However, as all our effective injections included the cervical portion of pars caudalis, these descending axons may target parts of Vs, in agreement with the findings of Shigenaga and colleagues (1988b), and may not directly influence ventral horn activity controlling the neck.
Acknowledgments
We would like to express our appreciation to Drs. John Naftel and Susan Warren for many helpful suggestions made on earlier versions of this manuscript.
Supported by NIH Grants # EY09762 and EY014263
ABBREVIATIONS
- III
oculomotor nucleus
- IV
trochlear nucleus
- VI
abducens nucleus
- BC
brachium conjunctivum
- BP
brachium pontis
- CC
caudal central subdivision
- Cu
cuneate nucleus
- DH
dorsal horn
- IC
inferior colliculus
- IO
inferior olive
- iVt
inferior vestibular nucleus
- LC
locus coeruleus
- MRF
midbrain reticular formation
- MdRF
medullary reticular formation
- MesV
mesencephalic trigeminal nucleus
- MLF
medial longitudinal fasciculus
- mV
motor trigeminal nucleus
- mVt
medial vestibular nucleus
- nIV
trochlear nerve
- nTS
nucleus of the solitary tract
- P
pyramid
- PAG
periaqueductal gray
- PB
parabrachial nucleus
- PcRt
parvocellular reticular formation
- SC
superior colliculus
- VH
ventral horn
- Vp
principal trigeminal nucleus
- Vs
spinal trigeminal nucleus
- VsT
spinal trigeminal tract
- XII
hypoglossal nucleus
LITERATURE CITED
- Alvarado-Mallart MR, Batini C, Buisseret-Delmas C, Corvisier J. Trigeminal representations of the masticatory and extraocular proprioceptors as revealed by horseradish peroxidase retrograde transport. Exp Brain Res. 1975;23:167–179. doi: 10.1007/BF00235459. [DOI] [PubMed] [Google Scholar]
- Appenteng K, O’Donovan MJ, Somjen G, Stephens JA, Taylor A. The projection of jaw elevator muscle spindle afferents to fifth nerve motoneurones in the cat. J Physiol. 1978;79:409–423. doi: 10.1113/jphysiol.1978.sp012353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R, Llinás R. Electrotonic coupling between neurons in the rat mesencephalic nucleus. J Physiol. 1971;212:45–63. doi: 10.1113/jphysiol.1971.sp009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig I, Buisseret-Delmas C, Buisseret P. Identification of nerve endings in cat extraocular muscles. Anat Rec. 1997;248:566–575. doi: 10.1002/(SICI)1097-0185(199708)248:4<566::AID-AR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Billig I, Yatim N, Compoint C, Buisseret-Delmas C, Buisseret P. Cerebellar afferences from the mesencephalic trigeminal nucleus in the rat. Neuroreport. 1995;27:2293–6. doi: 10.1097/00001756-199511270-00006. [DOI] [PubMed] [Google Scholar]
- Blumer R, Konacki KZ, Streicher J, Hoetzenecker W, Blumer MJ, Lukas JR. Proprioception in the extraocular muscles of mammals and man. Strabismus. 2006;14:101–106. doi: 10.1080/09273970600701192. [DOI] [PubMed] [Google Scholar]
- Bortolami R, Lucchi ML, Pettorossi VE, Callegari E, Manni E. Localization and somatotopy of sensory cells innervating the extraocular muscles of lamb, pig and cat. Histochemical and electrophysiological investigation. Arch Ital Biol. 1987;125:1–15. [PubMed] [Google Scholar]
- Buisseret P. Influence of extraocular muscle proprioception on vision. Phys Rev. 1995;75:323–338. doi: 10.1152/physrev.1995.75.2.323. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Buisseret P. Central projections of extraocular muscle afferents in cat. Neurosci Lett. 1990;109:48–53. doi: 10.1016/0304-3940(90)90536-i. [DOI] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Pinganaud G, Compoint C, Buisseret P. Projection from trigeminal nuclei to neurons of the mesencephalic trigeminal nucleus in rat. Neurosci Lett. 1997;229:189–192. doi: 10.1016/s0304-3940(97)00452-7. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Eberhorn A, Horn AK. Motor and sensory innervation of extraocular eye muscles. Ann N Y Acad Sci. 2003;1004:40–49. doi: 10.1111/j.1749-6632.2003.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Konakci KZ, Blumer R. Sensory control of extraocular muscles. Prog Brain Res. 2006;151:81–93. doi: 10.1016/S0079-6123(05)51003-3. [DOI] [PubMed] [Google Scholar]
- Byers MR, O’Connor TA, Martin RF, Dong WK. Mesencephalic trigeminal sensory neurons of cat: axon pathways and structure of mechanoreceptive endings in periodontal ligament. J Comp Neurol. 1986;250:181–191. doi: 10.1002/cne.902500205. [DOI] [PubMed] [Google Scholar]
- Capra NF, Wax TD. Distribution and central projections of primary afferent neurons that innervate the masseter muscle and mandibular periodontium: a double-label study. J Comp Neurol. 1989;279:341–352. doi: 10.1002/cne.902790302. [DOI] [PubMed] [Google Scholar]
- Capra NF, Anderson KV, Pride JB, Jones TE. Simultaneous demonstration of neuronal somata that innervate the tooth pulp and adjacent periodontal tissues, using two retrogradely transported anatomic markers. Exp Neurol. 1984;86:165–170. doi: 10.1016/0014-4886(84)90077-3. [DOI] [PubMed] [Google Scholar]
- Capra NF, Anderson KV, Atkinson RC., III Localization and morphometric analysis of masticatory muscle afferent neurons in the nucleus of the mesencephalic root of the trigeminal nerve in the cat. Acta Anat (Basel) 1985;122:115–125. doi: 10.1159/000145992. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. Premotor circuits controlling eyelid movements in conjunction with vertical saccades in the cat: I. The rostral interstitial nucleus of the medial longitudinal fasciculus. J Comp Neurol. 2002;450:183–202. doi: 10.1002/cne.10313. [DOI] [PubMed] [Google Scholar]
- Cody FW, Harrison LM, Taylor A, Weghofer B. Distribution of tooth receptor afferents in the mesencephalic nucleus of the fifth cranial nerve. J Physiol. 1974;239:49P–51P. [PubMed] [Google Scholar]
- Corbin KB. Probst’s tract in the cat. J Comp Neurol. 1942;77:455–467. [Google Scholar]
- Daunicht WJ, Jaworski E, Eckmiller R. Afferent innervation of extraocular muscles in the rat studied by retrograde and anterograde horseradish peroxidase transport. Neurosci Lett. 1985;56:143–148. doi: 10.1016/0304-3940(85)90120-x. [DOI] [PubMed] [Google Scholar]
- Dellow PG, Lund JP. Evidence for central timing of rhythmical mastication. J Physiol. 1971;215:1–13. doi: 10.1113/jphysiol.1971.sp009454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessem D, Luo P. Jaw-muscle spindle afferent feedback to the cervical spinal cord in the rat. Exp Brain Res. 1999;128:451–459. doi: 10.1007/s002210050868. [DOI] [PubMed] [Google Scholar]
- Donaldson IM. The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci. 2000;355:1685–1754. doi: 10.1098/rstb.2000.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden AR, Correia MJ, Steinkuller PG. Medullary proprioceptive neurons from extraocular muscles in the pigeon identified with horseradish peroxidase. Brain Res. 1982;237:15–21. doi: 10.1016/0006-8993(82)90554-6. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Cenni MC, Maffei L. Impairment of stereoacuity in cats with oculomotor proprioceptive deafferentation. Exp Brain Res. 1986;63:364–368. doi: 10.1007/BF00236853. [DOI] [PubMed] [Google Scholar]
- Freeman W. The relationship of the radix mesencephalica trigemini to the extraocular muscles. Arch Neurol Psychiatry. 1925;14:111–113. [Google Scholar]
- Gottlieb S, Taylor A, Bosley MA. The distribution of afferent neurones in the mesencephalic nucleus of the fifth nerve in the cat. J Comp Neurol. 1984;228:273–283. doi: 10.1002/cne.902280212. [DOI] [PubMed] [Google Scholar]
- Greene T, Jampel R. Muscle spindles in the extraocular muscles of the macaque. J Comp Neurol. 1966;126:547–549. [PubMed] [Google Scholar]
- Guthrie BL, Porter JD, Sparks DL. Corollary discharge provides accurate eye position information to the oculomotor system. Science. 1983;221:1193–1195. doi: 10.1126/science.6612334. [DOI] [PubMed] [Google Scholar]
- Hassanali J. Quantitative and somatotopic mapping of neurones in the trigeminal mesencephalic nucleus and ganglion innervating teeth in monkey and baboon. Arch Oral Biol. 1997;42:673–682. doi: 10.1016/s0003-9969(97)00081-2. [DOI] [PubMed] [Google Scholar]
- Hiscock J, Straznicky C. Peripheral and central terminations of axons of the mesencephalic trigeminal neurons in Xenopus. Neurosci Lett. 1982;32:235–240. doi: 10.1016/0304-3940(82)90299-3. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF. Coupling between cells of the trigeminal mesencephalic nucleus. J Dent Res. 1970;49(Suppl):1369–1373. doi: 10.1177/00220345700490063701. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Larramendi LM. Synapses and cluster formation of the mouse mesencephalic fifth nucleus. Brain Res. 1968;7:296–299. doi: 10.1016/0006-8993(68)90105-4. [DOI] [PubMed] [Google Scholar]
- Hinrichsen CF, Larramendi LM. Features of trigeminal mesencephalic nucleus structure and organization. I. Light microscopy. Am J Anat. 1969;126:497–505. doi: 10.1002/aja.1001260408. [DOI] [PubMed] [Google Scholar]
- Jerge CR. Organization and function of the trigeminal mensencephalic nucleus. J Neurophysiol. 1963;26:379–392. doi: 10.1152/jn.1963.26.3.379. [DOI] [PubMed] [Google Scholar]
- Johnston JB. The radix mesencephalica trigemini. J Comp Neurol. 1909;19:593–664. [Google Scholar]
- Kashii S, Matsui Y, Honda Y, Ito J, Sasa M, Takaori S. The role of extraocular proprioception in vestibulo-ocular reflex of rabbits. Invest Ophthalmol Vis Sci. 1989;30:2258–2264. [PubMed] [Google Scholar]
- Keller EL, Robinson DA. Absence of a stretch reflex in extraocular muscles of the monkey. J Neurophysiol. 1971;34:908–919. doi: 10.1152/jn.1971.34.5.908. [DOI] [PubMed] [Google Scholar]
- Kimura M, Takeda T, Maekawa K. Contribution of eye muscle proprioception to velocity-response characteristics of eye movements: involvement of the cerebellar flocculus. Neurosci Res. 1991;12:160–168. doi: 10.1016/0168-0102(91)90108-b. [DOI] [PubMed] [Google Scholar]
- Knox PC, Weir CR, Murphy PJ. Modification of visually guided saccades by a nonvisual afferent feedback signal. Invest Ophthalmol Vis Sci. 2000;41:2561–2565. [PubMed] [Google Scholar]
- Lazarov NE. The mesencephalic trigeminal nucleus in the cat. Adv Anat Embryol Cell Biol. 2000;153:1–103. doi: 10.1007/978-3-642-57176-3. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Zee DS, Gaymard BM, Guthrie BL. Extraocular muscle proprioception functions in the control of ocular alignment and eye movement conjugacy. J Neurophysiol. 1994;72:1028–1031. doi: 10.1152/jn.1994.72.2.1028. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Zee DS, Hayman MR, Tamargo RJ. Oculomotor function in the rhesus monkey after deafferentation of the extraocular muscles. Exp Brain Res. 2001;141:349–358. doi: 10.1007/s002210100876. [DOI] [PubMed] [Google Scholar]
- Linden RW. Properties of intraoral mechanoreceptors represented in the mesencephalic nucleus of the fifth nerve in the cat. J Physiol. 1978;279:395–408. doi: 10.1113/jphysiol.1978.sp012352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem RS, Copray JC, van Willigen JD. Ultrastructure of the rat mesencephalic trigeminal nucleus. Acta Anat (Basel) 1991;140:112–119. doi: 10.1159/000147045. [DOI] [PubMed] [Google Scholar]
- Lucchi ML, Scapolo PA, Barazzoni AM, Clavenzani P, Lalatta CG, Berardinelli P, Bortolami R. Mesencephalic trigeminal nucleus neurons supplying the jaw closing muscles have no spinal projection: a fluorescent double-labeling study in birds and mammals. Anat Rec. 1997;249:255–258. doi: 10.1002/(SICI)1097-0185(199710)249:2<255::AID-AR13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia. 2006;21:167–174. doi: 10.1007/s00455-006-9027-6. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Goodwin GM. Patterns of mandibular movement and jaw muscle activity during mastication in the monkey. J Neurophysiol. 1974;37:954–966. doi: 10.1152/jn.1974.37.5.954. [DOI] [PubMed] [Google Scholar]
- Luo P, Dessem D. Inputs from identified jaw-muscle spindle afferents to trigeminothalamic neurons in the rat: a double-labeling study using retrograde HRP and intracellular biotinamide. J Comp Neurol. 1995;353:50–66. doi: 10.1002/cne.903530107. [DOI] [PubMed] [Google Scholar]
- Luo P, Wong R, Dessem D. Projection of jaw-muscle spindle afferents to the caudal brainstem in rats demonstrated using intracellular biotinamide. J Comp Neurol. 1995;358:63–78. doi: 10.1002/cne.903580104. [DOI] [PubMed] [Google Scholar]
- Maier A. Morphological variability and specializations in bovine extraocular muscle spindles. Ann Anat. 2000;182:259–267. doi: 10.1016/S0940-9602(00)80031-X. [DOI] [PubMed] [Google Scholar]
- Minkels RF, Juch PJ, Ter Horst GJ, van Willigen JD. Projections of the parvocellular reticular formation to the contralateral mesencephalic trigeminal nucleus in the rat. Brain Res. 1991;547:13–21. doi: 10.1016/0006-8993(91)90569-h. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Konishi A, Matsuda K, Nomura S, Itoh K, Nakamura Y, Sugimoto T. Myotopical arrangement and synaptic morphology of masticatory motoneurons, with special reference to commissural interneurons. In: Kawamura Y, Dubner R, editors. Oral-facial sensory and motor functions. Tokyo: Quintescence; 1981. pp. 113–120. [Google Scholar]
- Nomura S, Mizuno N. Differential distribution of cell bodies and central axons of mesencephalic trigeminal nucleus neurons supplying the jaw-closing muscles and periodontal tissue: a transganglionic tracer study in the cat. Brain Res. 1985;359:311–319. doi: 10.1016/0006-8993(85)91442-8. [DOI] [PubMed] [Google Scholar]
- Nomura S, Konishi A, Itoh K, Sugimoto T, Yasui Y, Mitani A, Mizuno N. Multipolar neurons and axodendritic synapses in the mesencephalic trigeminal nucleus of the cat. Neurosci Let. 1985;55:337–342. doi: 10.1016/0304-3940(85)90458-6. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Iriki A, Nakamura Y. Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol. 1986;55:806–825. doi: 10.1152/jn.1986.55.4.806. [DOI] [PubMed] [Google Scholar]
- Ogasavara K, Onodera S, Shiwa T, Ninomiya S, Tazawa Y. Projections of extraocular muscle primary afferent neurons to the trigeminal sensory complex in the cat as studied with the transganglionic transport of horseradish peroxidase. Neurosci Lett. 1987;73:242–246. doi: 10.1016/0304-3940(87)90252-7. [DOI] [PubMed] [Google Scholar]
- Olucha F, Martínez-García F, López-García C. A new stabilizing agent for the tetramethyl benzidine (TMB) reaction product in the histochemical detection of horseradish peroxidase (HRP) J Neurosci Methods. 1985;13:131–138. doi: 10.1016/0165-0270(85)90025-1. [DOI] [PubMed] [Google Scholar]
- Perkins E, Warren S, Lin RC, May PJ. Projections of somatosensory cortex and frontal eye fields onto incertotectal neurons in the cat. Anat Rec A. 2006;288:1310–1329. doi: 10.1002/ar.a.20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinganaud G, Bourcier F, Buisseret-Delmas C, Buisseret P. Primary trigeminal afferents to the vestibular nuclei in rat: existence of a collateral projection to the vestibulocerebellum. Neurosci Ltr. 1999;264:133–136. doi: 10.1016/s0304-3940(99)00179-2. [DOI] [PubMed] [Google Scholar]
- Pombal MA, Alvarez-Otero R, Rodicio MC, Anadón R. A tract-tracing study of the central projections of the mesencephalic nucleus of the trigeminus in the guppy (Lebistes reticulatus, teleostei), with some observations on the descending trigeminal tract. Brain Res Bull. 1997;42:111–118. doi: 10.1016/s0361-9230(96)00205-5. [DOI] [PubMed] [Google Scholar]
- Porter JD. Brainstem terminations of extraocular muscle primary afferent neurons in the monkey. J Comp Neurol. 1986;247:133–143. doi: 10.1002/cne.902470202. [DOI] [PubMed] [Google Scholar]
- Porter JD, Donaldson IM. The anatomical substrate for cat extraocular muscle proprioception. Neuroscience. 1991;43:473–481. doi: 10.1016/0306-4522(91)90309-c. [DOI] [PubMed] [Google Scholar]
- Porter JD, Spencer RF. Localization of morphology of cat extraocular muscle afferent neurones identified by retrograde transport of horseradish peroxidase. J Comp Neurol. 1982;204:56–64. doi: 10.1002/cne.902040107. [DOI] [PubMed] [Google Scholar]
- Porter JD, Guthrie BL, Sparks DL. Innervation of monkey extraocular muscles: localization of sensory and motor neurons by retrograde transport of horseradish peroxidase. J Comp Neurol. 1983;218:208–219. doi: 10.1002/cne.902180208. [DOI] [PubMed] [Google Scholar]
- Porter JD, Burns LA, May PJ. Morphological substrate for eyelid movements: innervations and structure of primate levator palpebrae superioris and orbicularis oculi muscles. J Comp Neurol. 1989;287:64–81. doi: 10.1002/cne.902870106. [DOI] [PubMed] [Google Scholar]
- Ro JY, Capra NF. Physiological evidence for caudal brainstem projections of jaw muscle spindle afferents. Exp Brain Res. 1999;128:425–434. doi: 10.1007/s002210050865. [DOI] [PubMed] [Google Scholar]
- Rokx JT, van Willigen JD. Organization of neuronal clusters in the mesencephalic trigeminal nucleus of the rat: fluorescent tracing of temporalis and masseteric primary afferents. Neurosci Lett. 1988;86:21–26. doi: 10.1016/0304-3940(88)90176-0. [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Mascarello F, Barker D, Saed H. Muscle-spindle distribution in relation to the fibre-type composition of masseter in mammals. J Anat. 1988;161:37–60. [PMC free article] [PubMed] [Google Scholar]
- Shigenaga Y, Mitsuhiro Y, Yoshida A, Cao CQ, Tsuru H. Morphology of single mesencephalic trigeminal neurons innervating masseter muscle of the cat. Brain Res. 1988a;445:392–399. doi: 10.1016/0006-8993(88)91206-1. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol. 1988b;268:489–507. doi: 10.1002/cne.902680403. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Doe K, Suemune S, Mitsuhiro Y, Tsuru K, Otani K, Shirana Y, Hosoi M, Yoshida A, Kagawa K. Physiological and morphological characteristics of periodontal mesencephalic trigeminal neurons in the cat intra-axonal staining with HRP. Brain Res. 1989;505:91–110. doi: 10.1016/0006-8993(89)90119-4. [DOI] [PubMed] [Google Scholar]
- Usunoff KG, Marani E, Schoen JH. The trigeminal system in man. Adv Anat Embryol Cell Biol. 1997;136:1–126. doi: 10.1007/978-3-642-60779-0. [DOI] [PubMed] [Google Scholar]
- Van der Werf, Aramideh M, Ongerboer de Visser BW, Baljet B, Speelman JD, Otto JA. A retrograde double fluorescent tracing study of the levator palpebrae superior muscle in cynomologous monkey. Exp Brain Res. 1997;113:174–179. doi: 10.1007/BF02454155. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Gauthier GM, Blouin J, Vercher JL. The role of ocular muscle proprioception during modifications in smooth pursuit output. Vis Res. 1997;37:769–774. doi: 10.1016/s0042-6989(96)00239-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci. 2007;10:640–6. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
- Weir CR, Knox PC, Dutton GN. Does extraocular muscle proprioception influence oculomotor control? Br J Ophthalmol. 2000;84:1071–1074. doi: 10.1136/bjo.84.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin IBHM, Leong SK. Localization of neurons supplying the temporalis muscle in the rat and cat. Neurosci Let. 1979;11:63–68. doi: 10.1016/0304-3940(79)90057-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Luo P, Pendlebury WW. Light and electron microscopic observations of a direct projection from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Brain Res. 2001;917:67–80. doi: 10.1016/s0006-8993(01)02911-0. [DOI] [PubMed] [Google Scholar]