Abstract
The primate Edinger-Westphal nucleus (EW) contains perioculomotor preganglionic (pIIIPG) motoneurons that control the lens and pupil. Separate subdivisions have been described in EW and termed visceral columns, with the lateral visceral column (lvc) reportedly receiving pretectal inputs for the pupillary light reflex. However, choline acetyl transferase staining reveals a single paired column of cells dorsal to the oculomotor nucleus, suggesting the EW is not subdivided. We investigated this issue by transneuronal retrograde labeling of pIIIPG neurons in three monkey species. In all three, pIIIPG neurons were contained in a single column. We have also examined which part of the macaque pIIIPG population receives pretectal input. Injections of biocytin into the pretectum anterogradely labeled terminals that lay in close association with pIIIPG motoneurons retrogradely labeled by ciliary ganglion injections of WGA-HRP. These close associations were concentrated in the ventromedial portion of the middle third of EW, suggesting this pIIIPG region mediates pupillary control. In other cases, pretectal WGA-HRP injections, in addition to labeling terminals in the EW, produced a circular field of labeled neurons and terminals in the periaqueductal gray, dorsolateral to EW. This region may represent the previously designated lvc, but it does not contain pIIIPG motoneurons.
INTRODUCTION
We rarely move one joint in isolation. Instead, most actions involve coordination of multiple muscles to produce complex multi-joint movement combinations. Similarly, the extraocular muscles rarely move the eyes in isolation. Since the targets of gaze movements are located within three dimensional space, extraocular muscle action is coordinated with control of the intraocular muscles: the ciliary and sphincter pupillae muscles. Eye movements are thus accompanied by changes in lens accommodation and pupillary diameter to allow the eyes to focus on newly acquired targets (e.g., the near response). The pupils are also regulated with respect to the luminance of each new field of view, to optimize perception of the target in its surroundings. Signals for both extraocular and intraocular muscle contraction flow down the third cranial nerve (IIIn), but in the case of the intraocular muscles, the preganglionic parasympathetic motoneurons that supply the ciliary ganglion lie apart from the somatic motoneurons found in the oculomotor nucleus (III). They are traditionally described as forming a column dorsal to the oculomotor nucleus called the Edinger-Westphal nucleus (EW), which extends into the anteromedian nucleus (AM), rostrally.
While these preganglionic motoneurons were originally described as forming a single paired column in macaque monkeys (Akert et al., 1980), later investigators suggested that they lie in a series of discrete subgroups (Burde and Williams, 1989): AM, the nucleus of Perlia, and the dorsal, medial, and lateral visceral columns of EW. These subdivisions took on added importance when trans-synaptic anterograde tracers were utilized in an attempt to define the pupillary light reflex pathway in the monkey (Kourouyan and Horton, 1997). This study indicated that the lateral visceral column (lvc) represents the portion of EW that receives input from the retina, by way of the olivary pretectal nucleus (OPt). This finding paralleled evidence from conventional anterograde studies that also suggested the pretectum projects to the contralateral lvc (Baleydier et al., 1990; Büttner-Ennever et al., 1996). Our understanding of the structure of EW has been further challenged by studies that indicate that this nucleus also contains neurons projecting to central targets, instead of the ciliary ganglion (cat: Loewy et al., 1978; monkey: Burde, 1988), and that these centrally projecting neurons use neuropeptides, not acetylcholine as their transmitter (Maciewicz et al., 1983). This has recently been reinforced by discovery of a population of neurons that use urocortin, a neuropeptide tied to stress responses and ingestive behavior, within the rodent EW (Bachtell et al., 2002; Kozicz et al., 2001; Vaughan et al., 1995).
In order to better determine the true identity of EW, we recently investigated the populations of neurons found in the vicinity of the oculomotor nucleus, the perioculomotor (pIII) populations, in the cat and monkey (May et al., 2008). Specifically, we compared the locations of the cholinergic and urocortin peptidergic populations by using antibodies to choline acetyl transferase (ChAT) and urocortin I. The results of this study indicated that there are several pIII populations. In macaque monkeys, ChAT+ pIII preganglionic motoneurons (pIIIPG) lie in the EW and AM, while the perioculomotor population of urocortin+ cells (pIIIU) lies between EW and III, where it partially overlaps with the ChAT+ population of the S and C group motoneurons (pIIIS&C) that supply multiply innervated fibers (MIFs) in the extraocular muscles. In contrast, the cat EW primarily contains the pIIIU population, not the pIIIPG population. To clarify the problem posed by EW designating different cell populations in different species, we proposed that these species specific nuclei be designated EWPG (monkey) and EWU (cat and rodents). However, another striking feature of this work was the observation that the ChAT+ pIIIPG neurons in the macaque EWPG appeared to form a single paired column, and were not subdivided into several subgroups, as previously described. These findings concurred with those of Horn and colleagues (2008), who recently described the pIII populations in monkeys and man.
Consequently, in the present investigation, we utilized conventional and trans-synaptic retrograde tracers to re-examine the distribution of pIIIPG motoneurons in several primate species. In addition, we utilized bidirectional tracers, and combinations of anterograde and retrograde tracers to re-examine which pIIIPG motoneurons receive input from the OPt, and so are part of the pupillary light reflex pathway in macaques. The procedures used to inject and reveal these tracers have largely been described previously (Chen and May, 2000, 2007; Erichsen and May, 2002).
RESULTS
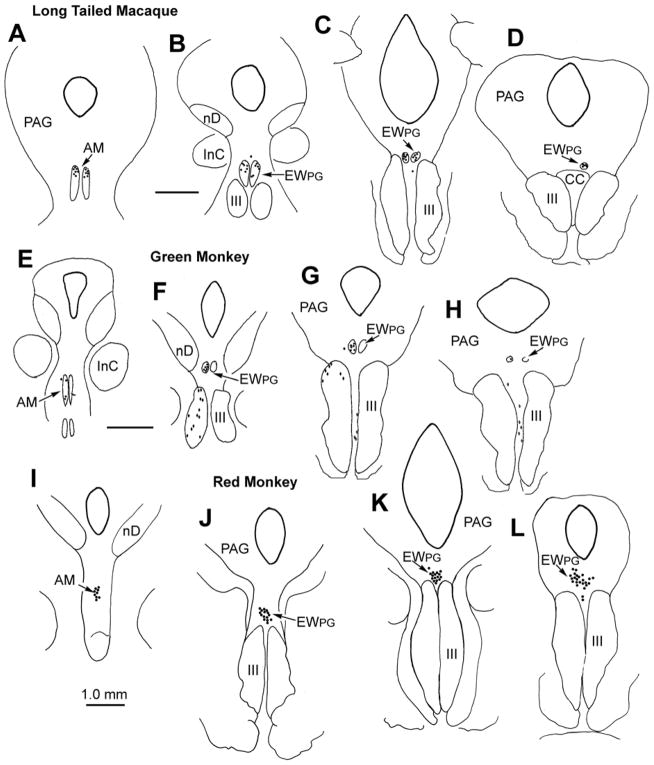
The distributions of trans-synaptically labeled preganglionic motoneurons (pIIIPG) in three different species of monkey are shown in figure 1. In each case, tracer which crossed the synapses of the ciliary ganglion was revealed by use of tetramethylbenzidene (TMB). Injections of wheat germ agglutinin conjugated horseradish peroxidase (WGA-HRP) into either the aqueous (right) or vitreous (left) of long tailed macaque monkeys (Macaca fascicularis) resulted in the labeling of pIIIPG motoneurons in a portion of AM (Fig. 1A), and in the rest of the EWPG. (Fig. 1B-D). The labeled cells lay dorsal to III, and ended above the caudal central subdivision (CC). Although occasional cells were found outside the borders of EWPG (Fig. 1B&C), there was no consistent subdivision of the labeled motoneurons into separate columns. Similar results were observed in rhesus monkeys (M. mulatta). Evidence from an African green monkey (Cercopithecus aethiops), which received an injection of WGA-HRP in combination with HRP into the left vitreal chamber is shown in figure 1E-H. Once again, the trans-synaptically labeled pIIIPG motoneurons were located in AM (E), and in a column dorsal to III that stretched through EWPG (Fig. 1F-H). The inclusion of HRP in the injection mixture caused some spread to the extraocular muscles, which labeled motoneurons in and between III. However, once again, the segregation of the pIIIPG motoneurons into separate subdivisions was not evident. The results from a red monkey (Erythrocebus patas) that received WGA-HRP injections into the aqueous (left) or vitreous (right) are illustrated in figure 1I-L. As in the other monkeys, trans-synaptically labeled pIIIPG motoneurons were located in both AM (Fig. 1I) and EWPG (Fig. 1J-L). However, the cells in this species did not form paired columns. Instead, they formed a single midline column that began rostrally in AM, and extended caudally above III. While occasional outliers were present, there was no consistent subdivision of this population into separate columns.
Figure 1.
Pattern of trans-synaptic retrograde label (dots) observed in a rostrocaudal series of sections from three species of monkey following injections of WGA-HRP into the anterior (A-D right & I-L left) and vitreal chambers (A-H left & I-L right) of the eye. Diamonds in E-H represent directly labeled motoneurons. Figure abbreviations: AM - anteromedian nucleus, BC -brachium conjunctivum, CC - caudal central subdivision of III, EWPG - preganglionic Edinger-Westphal nucleus, III - oculomotor nucleus, IIIn - oculomotor nerve, InC - interstitial nucleus of Cajal, lvc - lateral visceral column, MD - mediodorsal nucleus, MRF- midbrain reticular formation, nD - nucleus of Darkschewitsch, nOT - nucleus of the optic tract, nPC - nucleus of the posterior commissure, OPt - olivary pretectal nucleus, PAG - periaqueductal gray, Pt -pretectum, Pul - pulvinar, R - red nucleus, SOA - supraoculomotor area, VP - ventroposterior nucleus.
We then examined the input of the pretectum onto the EWPG population by making an injection of biocytin into the pretectum of a macaque monkey (M. fascicularis) in which we had also placed WGA-HRP into the ciliary ganglion. The pretectum was approached on the left side by aspirating the cerebral cortex overlying the midbrain. The ciliary ganglion was approached on the right side by retracting the anterior border of the temporalis muscle to reveal the temporal bone over the lateral wall of the orbit. This bone and the underlying lateral rectus muscle were removed to allow access to the region behind the globe. The ciliary ganglion was then isolated and penetrated by pins with WGA-HRP crystalized on their tips. The tissue was reacted using a dual tracer protocol in which WGA-HRP in the retrogradely labeled neurons was stained brown, and biocytin in the anterogradely labeled axons was stained black (Chen and May, 2007).
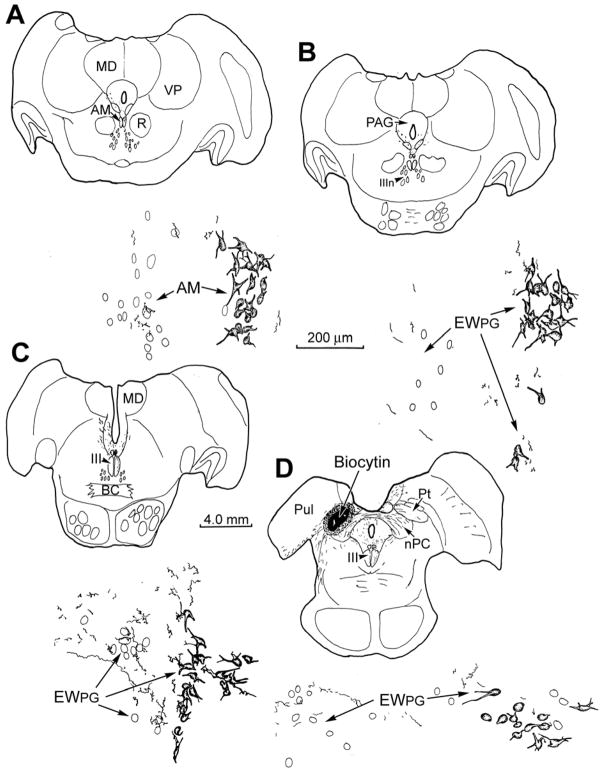
The resulting injection site involved portions of all the nuclei in the pretectum (Fig. 2D), but included all the OPt, which is believed to be the source of the luminance signal that drives pupillary preganglionic motoneurons. Anterogradely labeled axons extended ipsilaterally from this injection site through the periaqueductal gray (PAG) towards the pIII region (Fig. 2D). In addition, labeled axons crossed in the posterior commissure and curved into the contralateral pIII region. On both sides, these axons extended rostrally (Fig. 2C-A). As shown in the high magnification illustrations (Fig. 2), retrogradely labeled preganglionic motoneurons were present on the right side, ipsilateral to the injected ciliary ganglion. There was no consistent division of these labeled neurons into different columns, although occasional outliers were present (Fig. 2B). Biocytin labeled pretectal axons arborized bilaterally within EWPG (Fig. 2B-D), and even in AM (Fig. 2A). They were seen both amongst the counterstained neurons (left) and retrogradely labeled ones (right). The distribution of terminals was not the same along the course of the pIIIPG population; it was densest ventromedially, in middle third of its rostrocaudal extent. It was in this region that close associations between the anterogradely labeled boutons and the somata and proximal dendrites of the retrogradely labeled pIIIPG motoneurons were most evident. Examples of these close associations (arrowheads) are shown in figure 3A. The biocytin labeled pretectal axons displayed terminal arbor sprays with en passant and terminal boutons. These boutons were located adjacent to the proximal dendrites and somata of WGA-HRP labeled pIIIPG motoneurons. Due to the limited dendritic filling, contacts with distal dendrites could not be defined. The synaptic contact suggested by these close associations was confirmed ultrastructurally.
Figure 2.
Pattern of anterograde labeling in axons following a biocytin injection of the pretectum (D), with respect to unlabeled (left) and retrogradely labeled (right) preganglionic motoneurons. The later were labeled following WGA-HRP injection of the right ciliary ganglion. The rostrocaudal level of each illustration is indicated by the accompanying charting.
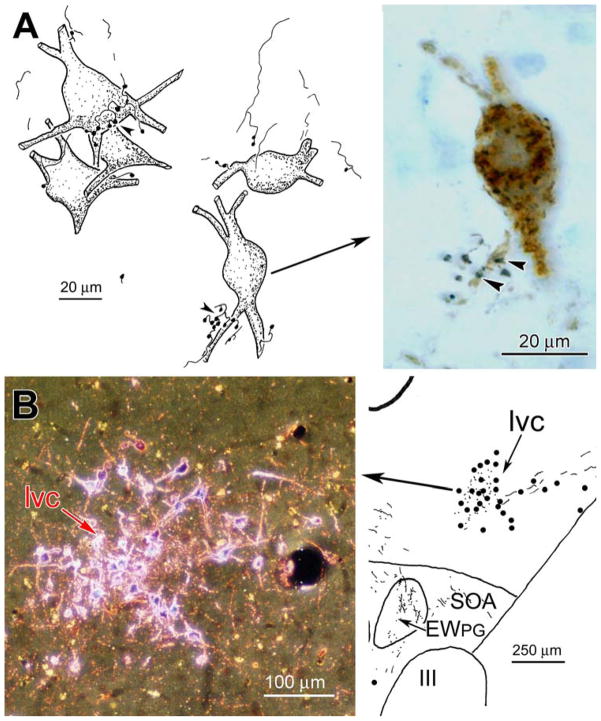
Figure 3.
A: Close associations (arrowheads) between biocytin labeled pretectal axonal boutons and WGA-HRP labeled preganglionic motoneurons. B: WGA-HRP labeled neurons and terminals in the lateral visceral cell column (lvc) following an injection in the pretectum (see Fig. 4). Photomicrograph in B taken with crossed polarizers.
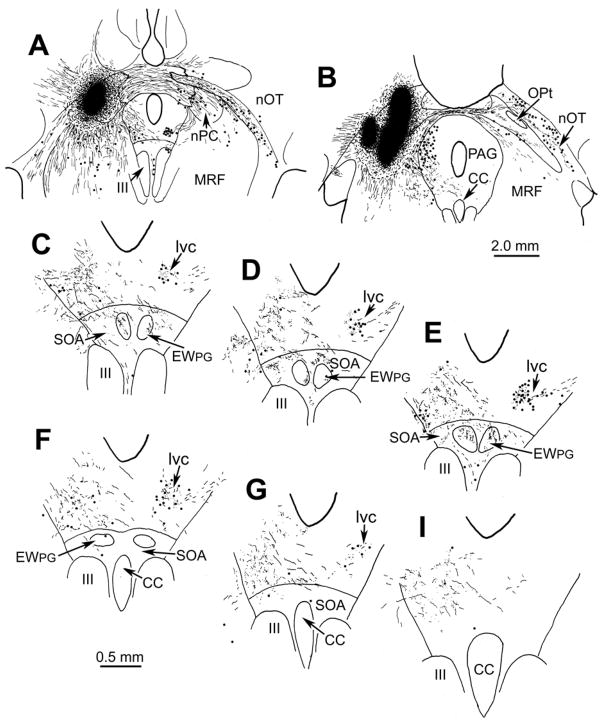
Those cells contacted by pretectal inputs represent the pupillary component of the pIIIPG population. In contrast to previous reports, they did not lie in the lvc. In fact, no such subdivision was observed amongst pIIIPG motoneurons. However, evidence for a set of neurons that may represent the previously described lvc came from another experiment in which the macaque (M. fascicularis) pretectum was injected with WGA-HRP, and the tissue was reacted using TMB histochemistry. The injection shown in figure 4A&B involved portions of all the pretectal nuclei except the medial, and completely filled the OPt. As with the biocytin injection (Fig. 2), labeled axons extended ipsilaterally and, by way of the posterior commissure, contralaterally. Note that the contralateral OPt was largely free of anterograde and retrograde label, but numerous labeled cells (dots), as well as labeled terminals (stipple), were present in the nucleus of the optic tract (nOT) (Fig. 4B). Labeled axonal arbors were present within the confines of AM (not illustrated) and the EWPG (Fig. 4C-F) on both sides of the midline. It appeared that most of the axons supplying this input arrived via the ipsilateral path. In addition, neurons that were retrogradely labeled with WGA-HRP (dots) were observed contralaterally, in a circular cell group located dorsolateral to EWPG (Fig. 4C-G). We believe this structure is equivalent to the one termed the lateral visceral column (lvc) in previous studies (Kourouyan and Horton, 1997; Baleydier et al., 1990; Büttner-Ennever et al., 1996), and so have continued to use this term to identify it. The lvc appears to be connected to the contralateral pretectum by way of the posterior commissure (Fig. 4B). Punctate labeling, believed to represent labeled terminals (stipple), was also present amongst these cells, and the same area displayed labeled terminals on the ipsilateral side, as well (Fig. 4C-G). The pattern of contralateral labeling in lvc is further illustrated in figure 3B. These lvc cells were slightly smaller than the pIIIPG motoneurons in EWPG (Fig. 2&3A).
Figure 4.
The pattern of retrograde cell (dots) and anterograde terminal (stipple) label is shown in a rostrocaudal series of illustrations of the pIII region (C-I) following an injection of WGA-HRP into the pretectum (A&B). Note the label in lvc and EWPG.
DISCUSSION
The results of these experiments illustrate several important points: 1. The perioculomotor preganglionic (pIIIPG) population in the primate EWPG is organized into a unitary nucleus, without subdivisions. 2. The projection of the pretectum onto primate pIIIPG motoneurons is concentrated ventromedially, in the middle third of the rostrocaudal extent of EWPG. 3. The structure previously considered to be the pupillary subdivision, the lateral visceral column (lvc), actually contains non-motoneurons that project back upon the pretectum.
We did not find evidence that EWPG was separated into subdivisions or “columns” in the primate species investigated here (fig. 1). These species inhabit a variety of different niches: macaques are semiterrestrial jungle dwellers, green monkeys are arboreal jungle dwellers, and red monkeys roam the plains. Thus, they differ both in the patterns of close and distance work they undertake, and the luminance changes they encounter. Nevertheless, they all exhibited a unitary pIIIPG population, which agrees with our previous finding in the prosimian Galago (Sun and May, 1993) and the organization in man (Horn et al., 2008). However, some species differences were observed. The EWPG of the red monkey was organized as a single, midline nucleus, instead of paired columns. This feature was also observed in the Galago (Sun and May, 1993). Evidence for subdivision of the pIIIPG population largely comes from a single study (Burde and Williams, 1989), and was not observed by Akert and colleagues (1980), with the exception of the extension of the pIIIPG motoneuron column into AM. We have observed occasional outliers in this population. Perhaps these cells were designated as the visceral columns and nucleus of Perlia in the previous study. It is not surprising that some variation may be present, given that non-primate species do not confine the pIIIPG population to EW, and instead scatter these cells dorsal, ventral and rostral to III (Erichsen and May, 2002; May et al., 2007). However, it appears that amongst mammals, possession of an EWPG made up of a consolidated preganglionic population is a striking primate feature. The fact that a well defined EWPG is also present in birds (Gamlin et al., 1984) may indicate that a propensity for close work in highly visual species can lead to consolidation of pIIIPG motoneurons into a unitary nucleus.
There is considerable evidence that the pathway subserving the light reflex is relayed by way of the OPt (for review see Loewenfeld, 1993). The OPt primarily contains wide-field luminance units (Clarke et al., 2003; Pong and Fuchs, 2000) that represent the primary source of pretectal input to the pIII region (Steiger and Büttner-Ennever, 1979). Presumably, OPt supplies information on luminance levels to the pIIIPG motoneurons that control the sphincter pupillae muscle. Here we have identified a population of motoneurons projecting to the ciliary ganglion that display close associations with boutons labeled anterogradely following pretectal injections (Fig. 2&3A). There are reports that vergence units may also be present in the pretectum (Mays et al., 1986), so we can not be sure that the labeled axons are purely pupil-related. However, in light of the preponderant OPt projection observed by Steiger and Büttner-Ennever (1979), it seems likely that most of the motoneurons with pretectal inputs are part of the light reflex circuit. The ventromedial location of these motoneurons is also in agreement with the distibution of pupil-related units recorded in marmosets (Clarke et al., 1985). The bilateral terminal distribution in EWPG following pretectal injections (Fig. 2&4), presumably represents an anatomical substrate for balanced direct and consensual pupillary responses.
Previous anterograde studies of the primate pretectal projections reported terminals in a distinctive nucleus in the ventrolateral PAG, which was termed the lvc (Baleydier et al., 1990; Büttner-Ennever et al., 1996), and was believed to represent a component of EWPG (Burde and Williams, 1989). Trans-synaptic tracer experiments suggested that this nucleus receives retinal input by way of the pretectum (Kourouyan and Horton, 1997). The present study indicates the lvc is not part of EWPG, but is instead a satellite nucleus that receives bilateral pretectal input and projects back upon the contralateral pretectum, by way of the posterior commissure. Based on the findings of Kourouyan and Horton (1997), it must be supplied with visual sensory input from either the OPt or the nOT. The function of this cell group is unknown. However, it is noteworthy that unlike the bird organization where the OPt are interconnected to balance activity levels (Gamlin et al., 1984), there is no evidence of connections between the macaque OPt, even though the nOT are heavily interconnected (Fig. 4). Perhaps this nucleus acts to balance the luminance activity in the two OPt. It will be interesting to determine whether it also projects to EWPG.
Acknowledgments
This work was made possible by NIH Grants EY07166 and EY014263 to PJM.
References
- Akert K, Glicksman MA, Lang W, Grob P, Huber A. The Edinger-Westphal nucleus in the monkey. a retrograde tracer study. Brain Research. 1980;184:491–498. doi: 10.1016/0006-8993(80)90816-1. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Magnin M, Cooper HM. Macaque accessory optic system: II. Connections with the pretectum. J Comp Neurol. 1990;302:405–416. doi: 10.1002/cne.903020216. [DOI] [PubMed] [Google Scholar]
- Burde RM. Disparate visceral neuronal pools subserve spinal cord and ciliary ganglion in the monkey: a double labeling approach. Brain Research. 1988;440:177–180. doi: 10.1016/0006-8993(88)91173-0. [DOI] [PubMed] [Google Scholar]
- Burde RM, Williams F. Parasympathetic nuclei. Brain Research. 1989;498:371–375. doi: 10.1016/0006-8993(89)91119-0. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AKE, Reisine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. J Comp Neurol. 1996;366:348–359. doi: 10.1002/(SICI)1096-9861(19960304)366:2<348::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. The feedback circuit connecting the superior colliculus and central mesencephalic reticular formation: a direct morphological demonstration. Exp Brain Res. 2000;131:10–21. doi: 10.1007/s002219900280. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. Premotor circuits controlling eyelid movements in conjunction with vertical saccades in the cat: II. Interstitial nucleus of Cajal. J Comp Neurol. 2007;500:676–692. doi: 10.1002/cne.21203. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Coimbra CJP, Alessio ML. Distribution of parasympathetic motoneurons in the oculomotor complex innervating the ciliary ganglion in the marmoset (Callithrix jacchus) Acta Anat. 1985;121:53–58. doi: 10.1159/000145942. [DOI] [PubMed] [Google Scholar]
- Clarke RJ, Zhang HY, Gamlin PDR. Primate pupillary light reflex: receptive field characteristics of pretectal luminance neurons. J Neurophysiol. 2003;89:3168–3178. doi: 10.1152/jn.01130.2002. [DOI] [PubMed] [Google Scholar]
- Erichsen JT, May PJ. The pupillary and ciliary components of the cat Edinger-Westphal nucleus: a transsynaptic transport investigation. Visual Neurosci. 2002;19:15–29. doi: 10.1017/s0952523801191029. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, Reiner A, Erichsen JT, Karten HI, Cohen DP. The neural substrate for the pupillary light reflex in the pigeon (Columbia liva) J Comp Neurol. 1984;226:523–543. doi: 10.1002/cne.902260407. [DOI] [PubMed] [Google Scholar]
- Horn AJ, Eberhorn A, Härtig W, Ardeleanu P, Messoudi A, Büttner-Ennever JA. Perioculomotor cell groups in monkey and man defined by their histochemical and functional properties: a reappraisal of the Edinger-Westphal nucleus. J Comp Neurol. 2008 doi: 10.1002/cne.21598. (In Press) [DOI] [PubMed] [Google Scholar]
- Kourouyan HD, Horton JC. Transneuronal retinal input to the primate Edinger-Westphal nucleus. J Comp Neurol. 1997;381:68–80. doi: 10.1002/(sici)1096-9861(19970428)381:1<68::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Li M, Arimura A. The activation of urocortin immunoreactive neurons in the Edinger-Westphal nucleus following acute pain stress in rats. Stress. 2001;4:85–90. doi: 10.3109/10253890109115724. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. The Pupil- Anatomy, Physiology and Clinical Applications. Wayne State University Press; Detroit: 1993. [Google Scholar]
- Loewy AD, Saper CB, Yamodis D. Re-evaluation of the efferent projections of the Edinger-Westphal nucleus in the cat. Brain Res. 1978;141:153–159. doi: 10.1016/0006-8993(78)90624-8. [DOI] [PubMed] [Google Scholar]
- Maciewicz R, Phipps BS, Foote WE, Aronin N, DiFiglia M. The distribution of substance P-containing neurons in the cat Edinger-Westphal nucleus: relationship to efferent projection systems. Brain Research. 1983;270:217–230. doi: 10.1016/0006-8993(83)90595-4. [DOI] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008 doi: 10.1002/cne.21514. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays LE, Porter JD, Gamlin PDR, Tello CA. Neuronal control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986;56:1007–1021. doi: 10.1152/jn.1986.56.4.1007. [DOI] [PubMed] [Google Scholar]
- Pong M, Fuchs AF. Characteristics of the pupillary light reflex in the macaque monkey: discharge patterns of pretectal neurons. J Neurophysiol. 2000;84:964–974. doi: 10.1152/jn.2000.84.2.964. [DOI] [PubMed] [Google Scholar]
- Steiger HJ, Büttner-Ennever JA. Oculomotor nucleus afferents in the monkey demonstrated with horseradish peroxidase. Brain Research. 1979;160:1–15. doi: 10.1016/0006-8993(79)90596-1. [DOI] [PubMed] [Google Scholar]
- Sun W, May PJ. Organization of the extraocular and preganglionic motoneurons supplying the orbit in the lesser galago. Anat Rec. 1993;237:89–103. doi: 10.1002/ar.1092370109. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt JC, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AW, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]