Abstract
A gaze-related region in the caudal midbrain tegementum, termed the central mesencephalic reticular formation (cMRF), has been designated on electrophysiological grounds in monkeys. In macaques, the cMRF correlates with an area in which reticulotectal neurons overlap with tectoreticular terminals. We examined whether a region with the same anatomical characteristics exists in cats by injecting biotinylated dextran amine into their superior colliculi. These injections showed that a cat cMRF is present. Not only do labeled tectoreticular axons overlap the distribution of labeled reticulotectal neurons, these elements also show numerous close boutonal associations, suggestive of synaptic contact. Thus, the presence of a cMRF that supplies gaze-related feedback to the superior colliculus may be a common vertebrate feature. We then investigated whether cMRF connections indicate a role in the head movement component of gaze changes. Cervical spinal cord injections in both the cat and monkey retrogradely labeled neurons in the ipsilateral, medial cMRF. In addition, they provided evidence for a spinoreticular projection that terminates in this same portion of the cMRF, and in some cases contributes boutons that are closely associated with reticulospinal neurons. Injection of the physiologically defined, macaque cMRF demonstrated that this spinoreticular projection originates in the cervical ventral horn, indicating it may provide the cMRF with an efference copy signal. Thus, the cat and monkey cMRFs have a subregion which is reciprocally connected with the ipsilateral spinal cord. This pattern suggests the medial cMRF may play a role in modulating the activity of antagonist neck muscles during horizontal gaze changes.
Keywords: Oculomotor, Gaze, Reticulospinal, Head Movement, Superior Colliculus
Introduction
The possibility that portions of the caudal midbrain reticular formation (MRF) might be involved in controlling gaze changes first received detailed electrophysiological support from experiments in which electrical stimulation of a portion of the MRF in macaque monkeys resulted in horizontal saccades (Cohen et al., 1985; 1986). These experiments supported earlier stimulation studies (Bender and Shanzer, 1964), and anatomical studies that indicated the midbrain reticular formation is interconnected with the superior colliculus (cat: Edwards, 1975; Edwards et al., 1979; Graham, 1977; monkey: Cohen and Büttner-Ennever, 1984; Harting, 1977). Cohen and colleagues termed this oculomotor region the central mesencephalic reticular formation (cMRF). It has been distinguished from more rostral regions of the midbrain, including the interstitial nucleus of Cajal (InC), the rostral midbrain reticular formation adjacent to the InC (piMRF), and the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), whose main role lies in controlling the vertical components of conjugate eye movements (Büttner et al., 1977; Crawford et al., 1991; Fukushima et al., 1995; King and Fuchs, 1979; Waitzman et al., 2000b). Recordings from neurons within the macaque cMRF have confirmed the relationship of their firing to saccades (Cromer and Waitzman, 2006; Handel and Glimcher, 1997; Waitzman et al., 1996). Furthermore, intracellular investigation in spider monkeys has shown that activity in a subset of the cMRF neurons is tightly coupled to activity in the intermediate layers of the superior colliculus (SC), and that the axons of this same population target the SC (Moschovakis et al., 1988b). Reversible cMRF lesions disrupted accurate saccadic eye movements. They also caused changes in head orientation, suggesting that this region may modulate the head component of a gaze change (Waitzman et al., 2000a). The latter possibility was directly investigated by recording from monkeys under head-free conditions (Pathmanathan et al., 2006a&b). These studies indicated that the discharges of a subset of cMRF gaze-related neurons have latencies that correlate better with the head component of the gaze change than the eye component. Furthermore, anatomical evidence suggests that that the MRF contains neurons projecting to the rostral cervical spinal cord, the region where head turns are organized (Castiglioni et al., 1978; Robinson et al., 1994).
Investigations of the physiologically defined cMRF have been confined to studies done in primates, with the notable exception of experiments done in the gold fish, which indicate the fish midbrain is tied to generation of gaze changes that employ body movement, and is intimately interconnected with the optic tectum (Luque et al., 2005; 2006; 2007). This lends credence to the supposition that a cMRF that plays a role in gaze may be a general vertebrate feature. However, other evidence to support this contention, even in non-primate mammals, is generally lacking. This is due to the fact that the cMRF has not been defined in non-primate mammalian species; although it is very likely that portions of the cuneiform nucleus, as investigated by Edwards (1975) in the cat, correlate with the cMRF.
We decided to take advantage of results from an earlier primate study, which indicated that a region in the caudal MRF that is reciprocally connected with the SC correlates well with the physiologically defined cMRF, to establish whether a region similar to the primate cMRF exists in cats (Chen and May, 2000). Consequently, in the present series of experiments, we first injected the cat SC with a bidirectional tracer to establish the existence and borders of this cMRF area. Next, we made tracer injections into the upper cervical spinal cord to examine whether this same region has a pattern of connections that would suggest the cMRF of cats is involved in modulating the head component of gaze changes. Finally, we investigated whether the pattern of connections that we noted in the cat was also present in primates, by making injections of tracers into the spinal cord and cMRF of macaque monkeys. Portions of this study have been reported in brief, but this represents the first detailed presentation and analysis of our findings (May et al., 2001; 2002).
Materials and Methods
The experiments reported here utilized cats (Felix domesticus) (n = 8) and monkeys (Macaca fascicularis and mulatta) (n = 6). Adult cats of both sexes were used. The monkeys were young adult, or adult males. All work was done in strict accordance with NIH policies on the care and use of animals, including the “Principles of Laboratory Care” (NIH publication # 86-23, 1985). The procedures used were approved by the Animal Care and Use Committees of the relevant institutions.
In the cat experiments, animals were sedated with isoflurane gas (1–3 %), and a catheter was placed in a forelimb vein. They were then anesthetized with sodium pentobarbital (up to 35 mg/kg, iv), and placed in a stereotaxic head holder. Reflexes were monitored periodically throughout the surgical procedure to ensure appropriate analgesia, and the anesthetic level was supplemented as necessary. Atropine sulfate (0.05 mg/kg, im) was given to control airway secretions, and dexamethasone (2.5 mg/kg, iv) was given to preclude edema. Core body temperature was monitored and maintained within normal limits. The superior colliculus (n = 3) was approached by aspirating the overlying cortex following a craniotomy. The tracer injected into the colliculus was a 10% solution of the bidirectional tracer, biotinylated dextran amine (BDA) (Molecular Probes) in dH 2O. It was injected by use of a 10 μl Hamilton syringe oriented in a micromanipulator so that the tip was angled up 10° in the rostrocaudal plane. One to three injections of 0.1 μl of tracer were made at a depth of 1.0–1.5 mm beneath the tectal surface. The aspiration defect was filled with moist gelfoam, and the overlying muscle and skin were reapproximated, and then stabilized with sutures. In other animals, the cervical spinal cord (n = 5) was approached by first tipping the cat nose down 30°, and then making a midline incision from the nuchal crest to the spinous process of C2. The muscles on the back of the skull were dissected along the midline and retracted laterally to reveal the atlanto-occipital membrane. This membrane was then incised to reveal the dorsal surface of the medulla, and upper spinal cord, which had been pulled forward by flexing the head. The view was enlarged by removing a portion of the vertebral arch of C1. One or two injections of 10 % BDA (0.1–0.2 μl) were made into the spinal cord at a depth of 2.0–3.0 mm by use of a 10 μl Hamilton syringe held at an angle of 20°, tip up, in the parasagittal plane. Alternatively, 0.02–0.04 μl of an aqueous solution containing the bidirectional tracers wheat germ agglutinin congugated horseradish peroxidase (WGA-HRP) (Sigma) (2.0 %) and horseradish peroxidase (Sigma) (15 %) was injected at 1–2 sites by use of a 1.0 μl Hamilton syringe. Following the injections, gelfilm was used to repair the defect in the atlanto-occipital membrane. The muscles and skin were then reapproximated in layers along the midline and stabilized with sutures. In all cases, the wound area was infused with Sensorcaine (0.5– 1.0 ml), and the animals received analgesics (Buprenex, 0.01mg/kg/12 h), once they recovered from the anesthesia.
Monkeys that received spinal cord injections (n = 4) were sedated with ketamine HCl (10 mg/kg, im), and anesthetized with isoflurane (1–3 %) via an endotracheal airway. An intravenous line was used to supply sterile Ringers solution for fluid support. Atropine sulfate (0.05 mg/kg, im) was given to control airway secretions, and dexamethasone (2.5 mg/kg, iv) was given to preclude edema. Pulse, respiratory rate, temperature and respiratory gasses were monitored and maintained within normal limits. The animals were placed within a stereotaxic head holder, which was tilted 30° nose-down to provide easier access to the rostral spinal cord. The surgical approach and injection procedures were essentially the same as utilized to inject WGA-HRP/HRP into the cat cervical spinal cord. Postoperative sensorcaine (sc) and buprenex (im) were used as in the cats. The cMRF was injected in two additional monkeys. In one animal, 0.3 μl of 10 % BDA was placed in the cMRF by stereotaxis. The animal was prepared for surgery as described above for the spinal cord injections, and the left superior colliculus was approached as described in the cat procedures. The tracer was injected along two tracks made into the right cMRF by angling the Hamilton syringe 30° in the horizontal plane in order to avoid the superior colliculus. The muscle and skin were reapposed and sutured, as described above. The other animal received an injection of fluorescent tracers under physiological guidance. The surgical methods used to place the recording chamber on this animal’s head, eye coils on its sclera, and to physiologically characterize the cMRF have been described in detail elsewhere (Pathmanathan et al., 2006a&b). Once the borders of the cMRF had been thoroughly characterized, an injection of retrograde fluorescent tracers. Specifically, the tracer Fast Blue (Sigma), 4.0 % in sterile saline, was introduced into the middle of the cMRF on the left side. The tracer (2.475 μl) was injected from a stainless steel cannula (32 gauge, thin wall) placed in the cMRF through a preset guide tube. A second injection consisting of 1.3 μl of 10 % Fluororuby (Molecular Probes) in sterile saline was made just caudal to the border of the physiologically defined cMRF on the right side. These solutions were injected by use of a picospritzer (WPI).
The following survival times were used in these experiments: BDA (2–3 weeks), WGA-HRP/HRP (2 days), Fast Blue/Fluororuby (3 weeks). At the completion of the survival period needed for effective transport, the animals were sedated with ketamine HCl (10 mg/kg, im) and deeply anesthetized with sodium pentobarbital (50–70 mg/kg, ip or iv). They were then transfused with 0.1 M, pH 7.2 phosphate buffered saline (PBS), followed by a fixative solution that consisted of 1.0 % paraformaldehyde and 1.25 % glutaraldehyde in 0.1 M, pH 7.2 phosphate buffer (PB), in the case of the BDA or WGA-HRP/HRP injections, and 4.0 % paraformaldehyde in 0.1 M, pH 7.2 PB, in the case of the fluorescent tracer injections. The brains were blocked in the frontal plane and postfixed for 1 h (mixed aldehydes fixation) or overnight (4 % paraformaldehyde fixation). The brains fixed with mixed aldehydes were cut coronally on a vibratome at a thickness of 100 μm. The paraformaldehyde fixed brain was equilibrated in 30 % sucrose as a cryoprotectant, frozen and sectioned at 80 μm on a sliding microtome in the frontal plane. In most cases, the spinal cords were cut longitudinally.
To visualize the BDA or HRP, an ordered series of sections were reacted using standard procedures, as previously described (Perkins et al., 2006). Specifically, the BDA was localized by incubation in Avidin-HRP (Vector) 1:500 in 0.1 M, pH 7.2 PB with 0.05 % triton X-100 at 4° C for 18 hours. This was followed by reaction in a solution of 5 % diaminobenzedine (DAB) in 0.1 M, pH 7.2 PB with 0.05 % cobalt chloride and 0.05 % nickel ammonium chloride through the addition of 0.011% H2O2. The sections were then rinsed in and mounted out of the same buffer. To visualize the transported HRP, sections were incubated in a 0.005% tetramethylbenzedine (TMB) solution of 0.1 M, pH 6.0 PB with 0.245 % ammonium molybdate, 0.25 % ethanol and 0.011 % H2O2 at 4° C for 18 h. They were then stabilized in 5.0 % ammonium molybdate in 0.1 M, pH 6.0 PB. Finally, they were rinsed in and mounted out of 0.1 M, pH 6.0 PB. In both cases, the sections were counterstained in cresyl violet, dehydrated in a graded series of ethanols, and cleared in toluene before being coverslipped under Permount (Fisher). A series of sections from the brain containing the fluorescent tracer was mounted out of PB, dehydrated in ethanol, cleared in toluene and coverslipped under Cytoseal 60 (VWR), a non-fluorescing medium. An adjacent series of sections was counterstained with cresyl violet to allow designation of cytoarchitectural boundaries.
Sections containing the chromagens, TMB and DAB, were charted using an Olympus BH-2 microscope equipped with a drawing tube. Photomicrographs were taken using a black and white (CoolSnap 2) or a color (Nikon DXM1220F) digital camera mounted on a Nikon fluorescence photomicroscope (Eclipse E600). Images were obtained using Metamorph software, which allowed up to 15 z-axis focal planes to be combined into a single picture using the “stack arithmetic summation” feature. They were composed, converted to gray scale images, and adjusted for brightness and contrast to match the viewed image, by use of Photoshop software. Sections with fluorescent markers were charted through the use of a fluorescence photomicroscope (Leica) equipped with digital stage position sensors and employing a Datametrics software/hardware package. Photomicrographs of the fluorescent material were obtained with the same equipment and software procedures as described above.
Results
Cat Collicular Injections
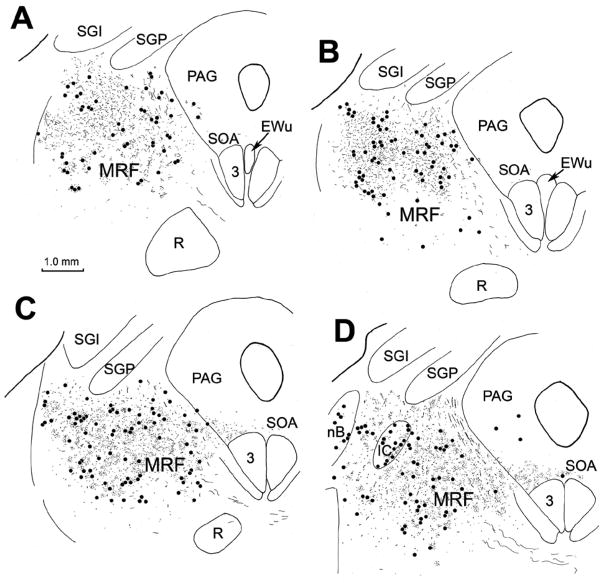
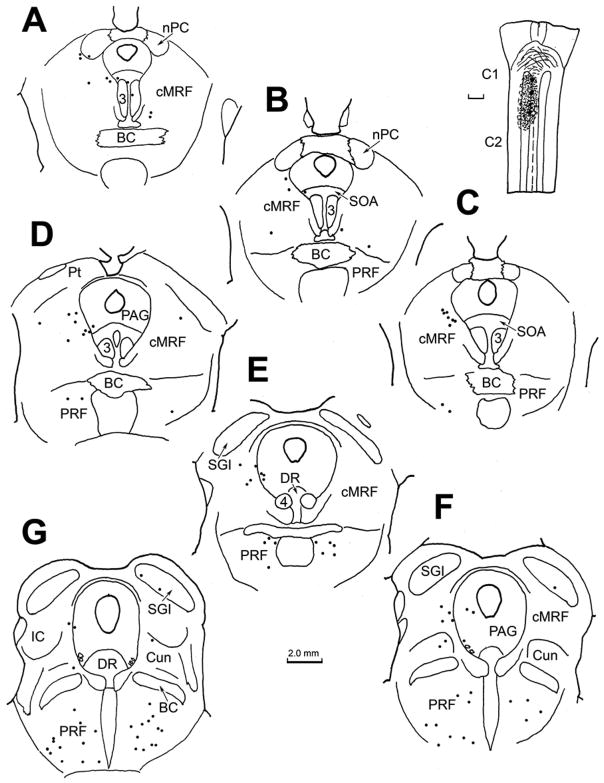
An example of the pattern of retrograde labeling observed following a BDA injection of the cat superior colliculus is illustrated in figure 1. In this case, the BDA injection was located in the middle of the SC (Fig. 1C–F). With the exception of a slight involvement of the periaqueductal gray, the injected tracer did not extend outside the borders of the SC. Ipsilaterally, retrogradely labeled cells (black dots) were present in nucleus of the posterior commissure, and extended ventrolaterally into the midbrain reticular formation to occupy the area lateral to the interstitial nucleus of Cajal (InC) (Fig. 1A–C). More caudally, the retrogradely labeled neurons were located in the midbrain reticular formation (MRF) immediately beneath the SC, and extended ventrally to the level of the oculomotor nucleus (Fig. 1D&E). Note the presence of a level in which there was a relative absence of labeled cells, in between these two MRF groups (Fig. 1C). Caudally, as the inferior colliculus appears (Fig. 1F), retrogradely labeled neurons were found in the reticular formation ventral and dorsal to it. A few cells occupied the cuneiform nucleus caudal to this point (Fig. 1G&H). Numerous retrogradely labeled neurons were also present on the contralateral side in the caudal MRF (Fig. 1D&E).
Figure 1.
Chartings of the distribution of retrogradely labeled neurons (dots) in the rostral brainstem following a BDA injection into the superior colliculus (C–F) in the cat. A rostral to caudal series of frontal sections is shown in this and other chartings. The core of the injection site, and most of the surrounding corona (stipple) were contained within the superior colliculus and involved all layers. Retrogradely labeled neurons are located in the contralateral intermediate gray layer (SGI) (C–G). Labeled neurons are also present ipsilaterally in the nucleus of the posterior commissure (nPC)(A&B), bilaterally in the dorsal portion of the midbrain reticular formation (MRF) (A–F) and bilaterally in the rostral pole of the inferior colliculus (IC)(F&G), and the cuneiform nucleus (G&H).
The pattern of anterograde labeling observed following BDA injections of the SC, including ipsilateral descending projections and the crossed descending projection by way of the predorsal bundle, was similar to that reported in earlier studies (Graham, 1977; Huerta and Harting, 1982). The patterns of retrograde cell and anterograde terminal label within the caudal half of the ipsilateral MRF are indicated in figure 2. Numerous retrogradely labeled reticulotectal neurons (dots) were distributed from just beneath the layers of the SC to the level of the red nucleus.
Figure 2.
Detailed chartings of the ipsilateral midbrain reticular formation showing the distribution of retrogradely labeled neurons (dots) and anterogradely labeled terminals (stipple) following the BDA injection of the superior colliculus illustrated in figure 1. These sections are a rostral to caudal series lying between the levels shown in figure 1C and 1F. Note the extensive overlap between the terminal field and labeled reticulotectal neurons.
These neurons were distributed across the mediolateral extent of the MRF. Anterogradely labeled terminal arbors (stipple) were also present in this same region. Like the retrograde label, the density of the anterograde label tapered off ventrally. Thicker axons belonging to the predorsal bundle projection passed in a ventromedial direction within the medial third of the region. Sparse terminal label was also present among the retrogradely labeled cells found in this area contralaterally (not illustrated) and in the supraoculomotor area (Fig. 2C&D). It should be noted that a similar overlap of labeled terminals and cells was observed medially, in the rostral MRF (not illustrated).
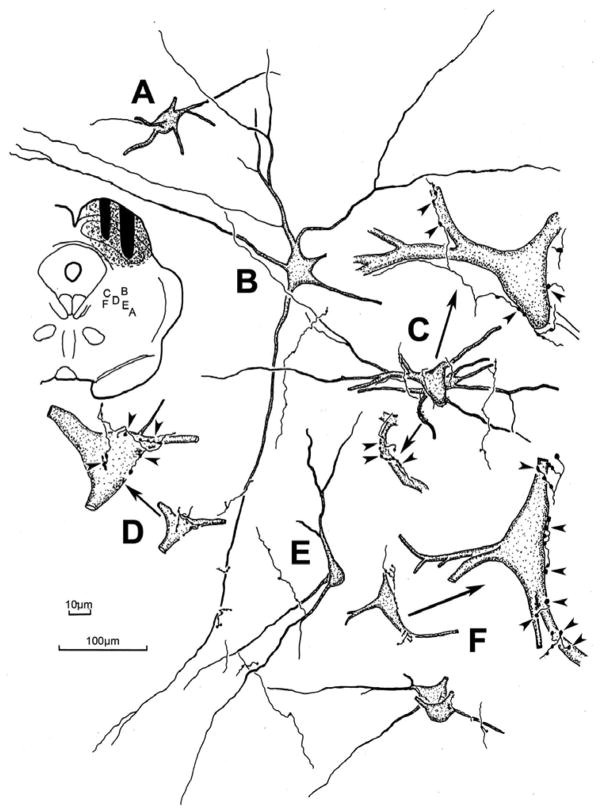
Figure 3 shows the morphology of the retrogradely labeled reticulotectal neurons found in the ipsilateral midbrain reticular formation. The case shown had a large injection that involved nearly all of the SC. Most of the labeled neurons displayed multipolar somata with 3–6 poorly branched, radiating dendrites. BDA also anterogradely labeled tectoreticular axons within the ipsilateral MRF. These axons showed numerous en passant boutons, as well as short branches with en passant and terminal boutons of various sizes. Close associations between these terminal boutons and the somata (Fig. 3C,D&F), as well as the dendrites (Fig. 3B,C, E&F) of retrogradely labeled reticulotectal neurons were common in this area. Individual axons often made numerous presumptive contacts with the same neuron (Fig. 3C,D&F enlargements). The characteristics of these close associations are further demonstrated in the photomicrographs shown in figure 4. BDA labeled tectoreticular axons were often observed extending along the somata and dendrites of these labeled cells, and displayed a number of boutons that lay in close association with both these elements (Fig. 4A–D). This suggests that individual axonal inputs would provide a major synaptic drive to particular cells. In other cases, the axon just passed by a labeled neuron and exhibited one or two boutons in close association with its dendrites (Fig. 4G&H). This type of axon may provide a small modulatory input to several neurons. Terminals were also present in the neuropil, which had no relation to retrogradely labeled neurons (Fig. 4G&H), or lay beside countersatined somata (Fig. 4F), suggesting there may be other target populations in the midbrain reticular formation besides the reticulotectal neurons. A minor population of finer axons and boutons was present (Fig. 4E), suggesting either that the output of colliculus is heterogeneous, or that an additional source of input to the MRF had been inadvertently labeled.
Figure 3.
The morphology of the labeled reticulotectal neurons and associated labeled tectoreticular axons in the cat. The BDA injection site and location of the illustrated examples is shown in the charting in the upper left. The labeled neurons were multipolar in shape and, in some cases (C,D&F), displayed numerous close associations between the labeled boutons of individual axons and a labeled cell. Other examples (A,B&E) showed few or no close associations. Arrows in C,D&F point to higher magnification drawings of examples with close associations (arrowheads). 100μm scale bar for cells A–F; 10 μm scale bar for enlargements of C,D&F.
Figure 4.
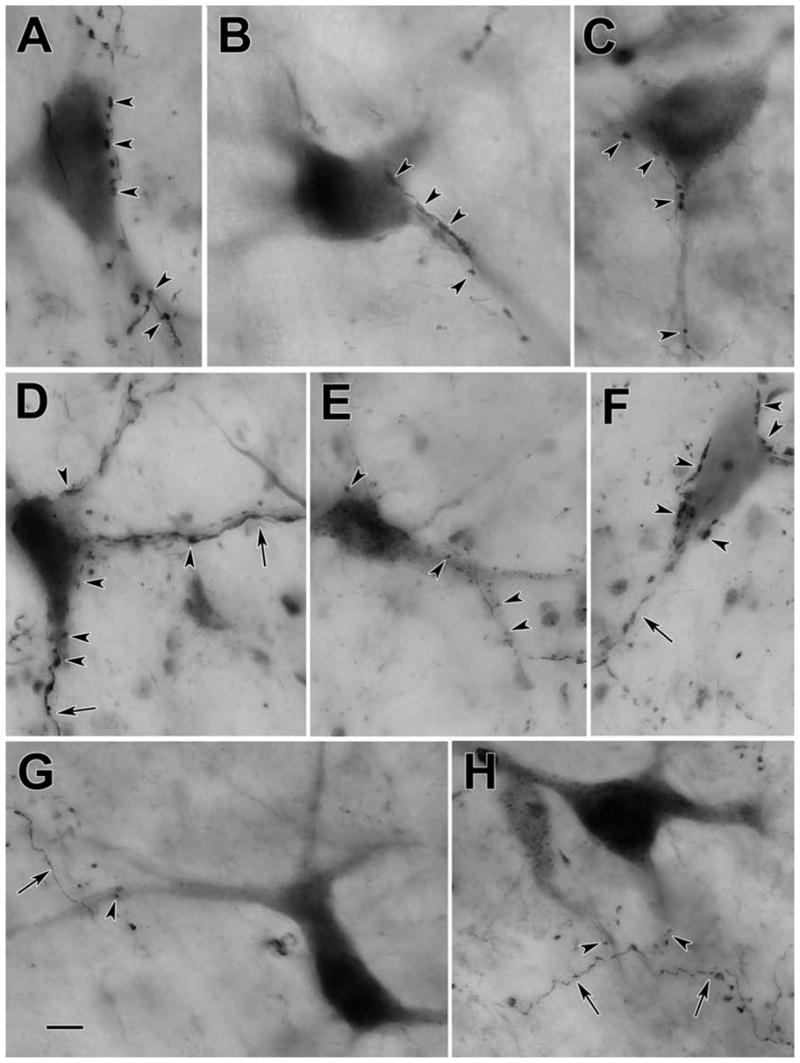
Photomicrographs of BDA labeled reticulotectal neurons (A–E,G&H) and tectoreticular axons (A–H) in the cat. Numerous close associations between labeled boutons and cells (arrowheads) were observed in many cases (A–E). However, in other cases, only a few such associations were observed (G&H). Labeled axons (arrows) also displayed terminal boutons located in the neuropil that had no association with labeled elements (G&H) or with counterstained, unlabeled somata (F). Scale bar = 10 μm. The number of z-axis planes combined to make plate A–C=1, D=8, E=4, F=5, G=9, H=7.
Based on the close similarities between the pattern of labeling observed in the caudal MRF in the cat, as shown in the previous figures, and that observed in the monkey (Chen and May, 2000), we concluded that this region caudal to the InC was likely to be equivalent to the primate cMRF. Consequently we will term this caudal region the feline cMRF for the rest of this report.
Cat Spinal Cord Injections
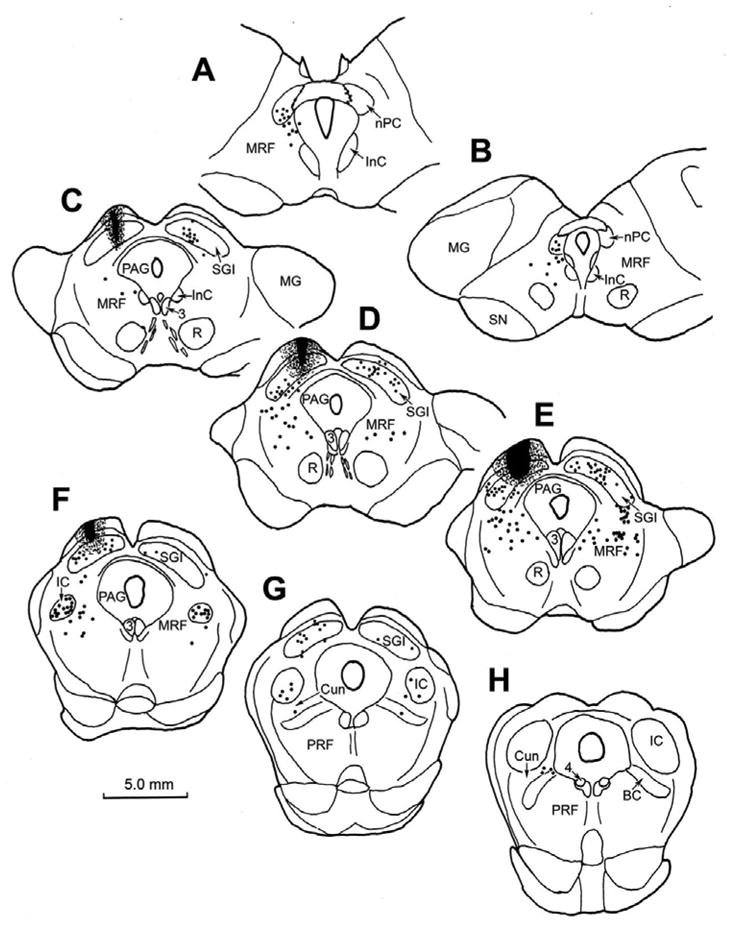
We then investigated the connectivity between the cMRF and the rostral cervical spinal cord. The pattern of retrograde labeling observed in the cat midbrain following an injection of BDA on the left side at the level of C1 is shown in figure 5 (upper right). As expected, numerous retrogradely labeled neurons were present in the intermediate and deep layers of the contralateral superior colliculus (Fig. 5D–G) and the contralateral red nucleus (Fig. 5A–D). At rostral levels of the midbrain (Fig. 5A–C), retrogradely labeled neurons were observed bilaterally in the nucleus of the posterior commissure, and ipsilaterally in the InC. Labeled reticulospinal neurons were found bilaterally, with an ipsilateral predominance, within the medial half of the MRF, lateral to the InC and ventral to the nucleus of the posterior commissure (Fig. 5A–C). At more caudal levels (Fig. 5D–F), the retrogradely labeled reticulospinal population continued to be distributed in the medial half of the cMRF, lateral to the oculomotor nucleus, and in some cases extending into the adjacent periaqueductal gray. The distribution was overwhelmingly ipsilateral, especially at more caudal levels (Fig. 5E&F). Labeled cells were also present in the ipsilateral cuneiform nucleus and pontine reticular formation (Fig. 5G&H).
Figure 5.
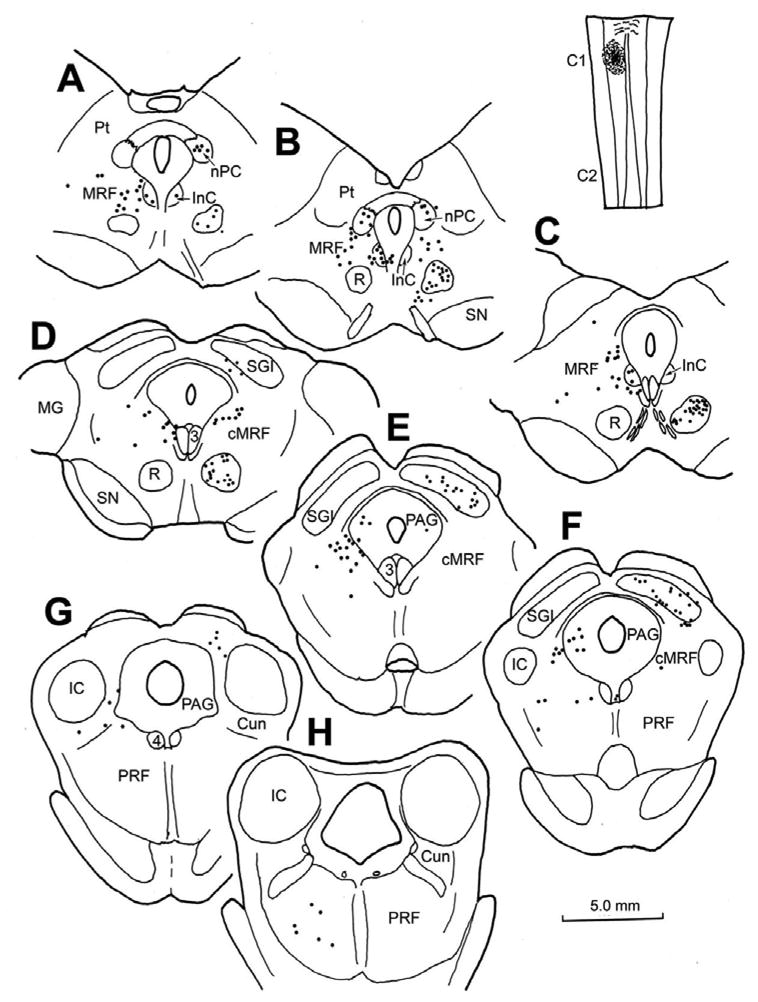
Chartings demonstrating the distribution of retrogradely labeled neurons following an injection of BDA into the spinal cord of a cat at the level of C1. The injection site is illustrated in a longitudinal section in the upper right. Retrogradely labeled neurons (dots) were observed in the contralateral red nucleus (A–D) and intermediate gray layer of the superior colliculus (D–G). Rostrally, labeled neurons were also present bilaterally in the nucleus of the posterior commissure (A&B) and interstitial nucleus of Cajal (A–C). Retrogradely labeled neurons were found in both the midbrain and pontine reticular formation. In the rostral MRF, the labeled cells were bilaterally distributed (A–C), but were primarily ipsilaterally distributed in the medial half of the cMRF (D–F) and in the pontine reticular formation (F–H).
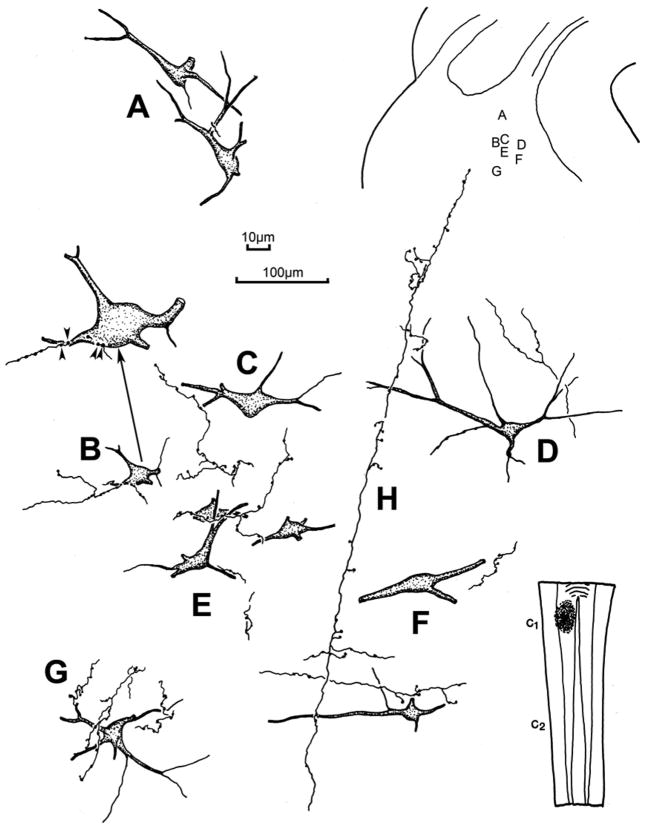
Examples of the retrogradely labeled reticulospinal neurons located in the cMRF of this case are illustrated in figure 6. These sparsely branched, multipolar neurons exhibited 3–5 primary dendrites. Anterogradely labeled spinoreticular axonal arbors were also observed in the ipsilateral cMRF of these cases. A few labeled axonal arbors were also present contralaterally (not illustrated), but these may represent collaterals of the heavily labeled predorsal bundle axons. The axonal arbors were more common medially, and displayed numerous boutons (Fig. 6H). Their main axons tended to run dorsoventrally, with mediolaterally oriented branches. In most cases, individual axons had a fairly limited number of contacts with any individual labeled neuron, and the majority of the observed boutons were located in the neuropil, with no obvious relationship to a labeled neuron (Fig. 6A,C,F&H). When close associations were present, most of these interactions were with the dendrites of the labeled cells (Fig. 6D,E&G), and axosomatic relationships were less common (Fig. 6B). The varied nature of the close associations is further shown in figure 7. Both the thicker, dorsoventrally oriented main fibers of spinoreticular axons and their thinner branches display numerous boutons (Fig. 7A). The axons and boutons varied in size, and a few examples of fine axons were present (Fig. 7F). Most of the observed terminal cell associations were on dendrites (Fig. 7B,C,E&F), although axosomatic associations were occasionally present (Fig. 7B&F). Some labeled reticulospinal cells did not display any associations, even when their dendrites were extensively labeled (Fig. 7D), In contrast, a few labeled neurons displayed extensive interactions like those seen following collicular injections (Fig. 7E).
Figure 6.
The morphology of the labeled reticulospinal neurons and associated labeled spinoreticular axons in the cMRF of the cat. The BDA injection site is illustrated in the lower right and the location of the illustrated examples is shown in the upper right. The labeled neurons were multipolar in shape and, in some cases (B,E&G), displayed numerous close associations between the labeled boutons of an individual axon and a labeled cell. Other examples showed only a few (C–F) or no (A) close associations. Arrow points to a higher magnification drawing of an example with close associations (arrowheads) in B. The spinoreticular main axons (H) often had long dorsoventral courses, with mediolaterally running branches. Axon H was associated with neurons D&F. 100μm scale bar for cells A–G; 10 μm scale bar for enlargement of B.
Figure 7.
Photomicrographs of BDA labeled reticulospinal neurons and spinoreticular axons in the cat. Close associations (arrowheads) between labeled axonal boutons and labeled cells were observed in many cases (A–C,E&F), although well labeled neurons sometimes showed no such contacts (D). Only rarely did the same axon provide numerous boutons to the same cell (E). Bouton size was variable (Compare E&F). As indicated by arrows, the cell in A is shown at higher magnification in B. Scale bar = 50 μm in D and 20 μm in F. Scale for A=D; B,C&E=F. The number of z-axis planes combined to make plate A=15, B=10, C=1, D=15, E=1, F=10.
Monkey Injections
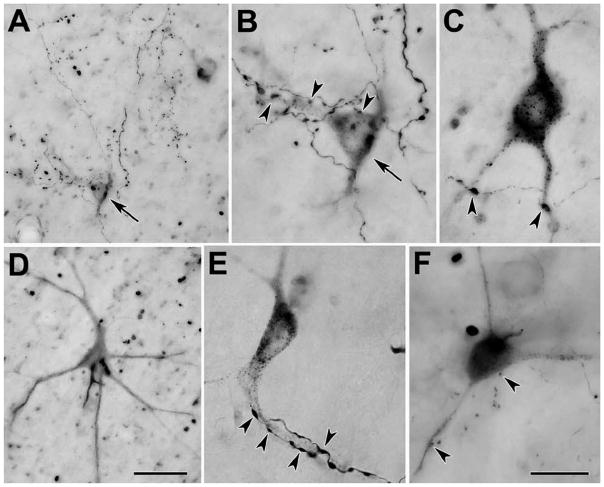
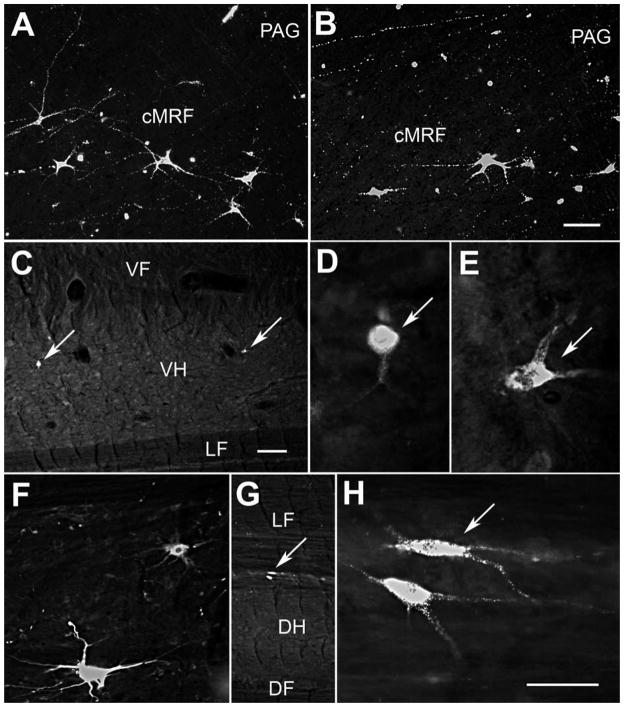
The distribution of retrogradely labeled reticulospinal neurons in the macaque cMRF is illustrated in figure 8. The WGA-HRP/HRP injection site included most of the spinal gray on the left side between C1 and C2 (Fig. 8, upper right). Within the cMRF, which extends from the level of the nPC to the level of the inferior colliculus, nearly all the retrogradely labeled neurons were located ipsilaterally (Fig. 8A–F). Most were distributed within the medial half of the cMRF, and in the adjacent periaqueductal gray. Labeled reticulospinal neurons were also present bilaterally in the rostral pontine reticular formation (Fig. 8D–G). Photomicrographs taken using crossed polarizers show these cMRF cells to be multipolar neurons, with relatively few dendrites (Fig. 9A&B). They tend to be clustered near the border with the periaqueductal gray. Labeled axons were also present in the field, which might constitute spinoreticular axons like those observed in the cat.
Figure 8.
Chartings demonstrating the distribution of retrogradely labeled neurons following an injection of BDA into the spinal cord of a macaque at the level of C1. The injection site is illustrated in a longitudinal section in the upper right. Retrogradely labeled neurons (dots) were found in both the midbrain and pontine reticular formation. The labeled cells were primarily ipsilaterally distributed in the cMRF (A–F) and bilaterally distributed in the pontine reticular formation (C–G). The cMRF cells were mainly located medially, and some labeled cells were found in the adjacent periaqueductal gray. The scale bars indicate equivalent distances.
Figure 9.
Photomicrographs of retrogradely labeled neurons in the cMRF (A&B) and spinal cord (C–H) of the macaque. A&B: WGA-HRP labeled reticulospinal neurons with multipolar morphologies were distributed in the cMRF adjacent to the periaqueductal gray, as demonstrated with crossed polarization. Labeled axons were also apparent. C–E: Two Fast Blue labeled spinoreticular neurons (arrows) in the ventral horn of the cervical spinal are shown at lower (C) and higher magnifications (D&E). F–H: Fluororuby labeled neurons in the dorsal horn of the cervical cord labeled following an injection of the cuneiform nucleus are shown at lower (F) and higher magnifications (G&H). (G shows the cell in F.) Scale bar = 100 μm in B and 200 μm in C and 50 μm in G. Scale for A=B; C=F; D,E&H=G.
To test whether a spinoreticular projection to the cMRF is present in the macaque, an injection of Fast Blue was made into the core of the physiologically defined cMRF, as illustrated in figure 10(A–C). The tracer was present on the left side, at and caudal to the level of the nPC, where the injection site occupies the middle third of the cMRF. Retrogradely labeled spinoreticular neurons were observed to form a column within the ipsilateral ventral horn (Fig. 10E2&3). This column stretched from C1 through C3, and was located just dorsal to the pools of motoneurons that supply the neck musculature. For comparison, an injection of Fluororuby was made just caudal to the physiologically defined right cMRF of the same animal. As seen in figure 10(D), this injection lay within the cuneiform nucleus. Retrograde labeling with this tracer was observed primarily ipsilaterally within the spinal cord and spinal trigeminal nucleus. Thus, most of the spinal cells labeled with Fluororuby were located dorsal to the neurons labeled with Fast Blue that projected to the cMRF (compare Fig. 10F1 to Fig 10E2 & 3). Examples of the Fast Blue labeled spinoreticular neurons are shown in figure 9(C–E). These relatively small neurons were scattered within the ventral horn. The labeling of the Fluororuby cells was relatively more conspicuous (Fig. 9F–G). Most of these cells were located in the dorsal horn (Fig. 9H), and the immediately adjacent white matter (Fig. 9F&G).
Figure 10.
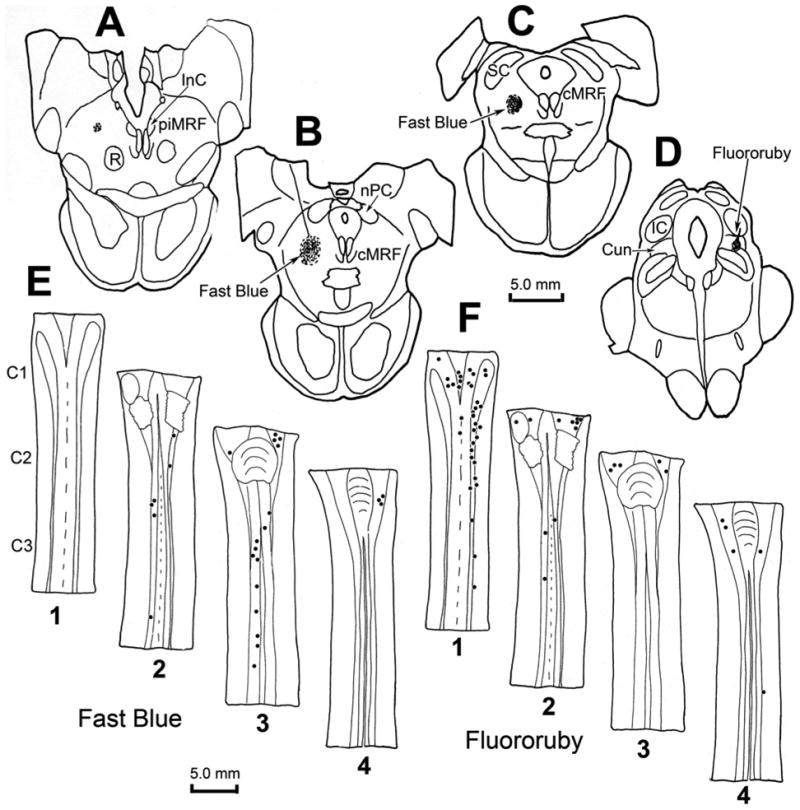
Chartings demonstrating the distribution of retrogradely labeled neurons (dots) in the cervical spinal cord following injections of the cMRF (left) and cuneiform nucleus (right) of a macaque. The distributions of the cells are shown in two dorsal (D) to ventral (V) chartings of the same series of horizontal sections through the upper cervical spinal cord (E1–4 and F1–4), that were made for each fluorophore. Fast Blue, injected into the physiologically defined left cMRF (A–C) retrogradely labeled neurons primarily in the ipsilateral ventral horn of the spinal cord (E2&3). Fluororuby, injected into the right cuneiform nucleus (D), retrogradely labeled neurons primarily in the ipsilateral dorsal horn (F1).
For purposes of comparison, the pattern of reticulospinal projections and spinoreticular neurons within the upper cervical spinal cord were explored following a BDA injection into the cMRF (Fig. 11). Since the MRF was approached from the contralateral side, diagonal tracks passed through the midbrain (Fig. 11A–C). The injection site involved the cMRF, particularly the medial portion where reticulospinal neurons were previously observed (Fig. 8). However, there was also tracer spread into the central gray, the dorsolateral corner of the oral pontine reticular formation and basilar pontine gray, and the middle cerebellar peduncle. The terminal field observed in the ipsilateral spinal cord at C1 is largely confined to the ventral horn (Fig. 11D&E). More widespread labeling was observed contralaterally, but this was assumed to be due to spread of the injection site outside the confines of the cMRF, and so is not illustrated here. Terminal arbors were densest in the more lateral aspect of the ventral horn. Most of these terminals were not located within the motoneuron pools of Rexed’s lamina IX, although some were (Fig. 11D3).
Figure 11.
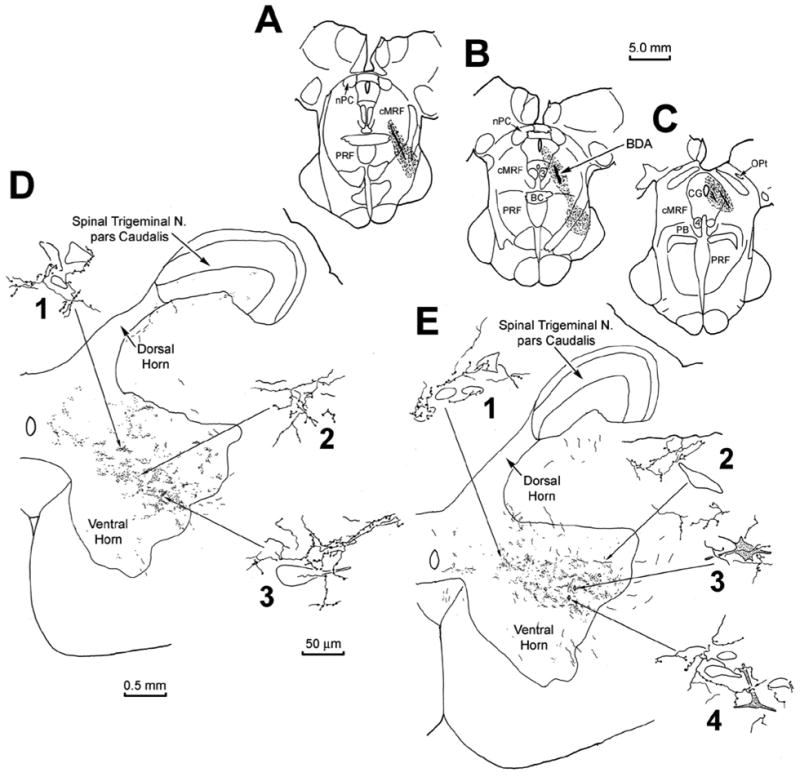
The pattern of labeling in C1 following an injection of BDA centered in the cMRF of a monkey (A–B). Anterograde terminal label was present in the middle of the ipsilateral ventral horn (D&E). Labeled terminal arbors showed close relationships with medium sized (D1, E1&2), and large (D3) counterstained somata in the ventral horn. Retrogradely labeled spinoreticular neurons (stipple) were also observed in the ventral horn (E, diamonds). These often displayed close associations with BDA labeled reticulospinal arbors (E3&4).
Instead, these terminal arbors were often observed in the immediate vicinity of small to medium sized, counterstained somata (Fig. 11D1, E1&2). A small number of retrogradely labeled neurons were also encountered in the ventral horn (Fig. 11E3&4). These spinoreticular neurons were multipolar in shape, with 3–4 primary dendrites. They were found within the BDA labeled terminal field, and were often seen to have close associations with the boutons of the BDA labeled, reticulospinal axonal arbors.
Discussion
The results of these experiments demonstrate a number of points (Fig. 12). First, there is a region within the caudal midbrain reticular formation (MRF) of the cat in which tectoreticular terminals and reticulotectal cells overlap. As this region of overlap is similar to that seen in the cMRF of the monkey (Chen and May, 2000), we propose to designate it as the feline cMRF. Second, within the cat cMRF, as in the monkey, tectoreticular terminals appear to directly contact reticulotectal neurons, suggesting the presence of a monosynaptic feedback circuit. Third, the cMRFs of both the cat and the monkey contain neurons that project to the upper levels of the cervical spinal cord, suggesting this midbrain region may play a role in modulating the head component of a gaze change. The fact that in both the cat and monkey, these neurons are largely restricted to the medial cMRF, suggests this subregion may be specialized for head control. These same reticulospinal neurons also appear to receive direct input from the cervical spinal cord. Since the cells of origin for this pathway, as observed in the monkey, are located in the ventral horn, the information carried by this projection may represent an efference copy signal for the head, feeding back up to the cMRF. Greater knowledge of these pathways may help explain the complexities observed for gaze-related head movements (Fuller, 1992; Stahl, 2001).
Figure 12.
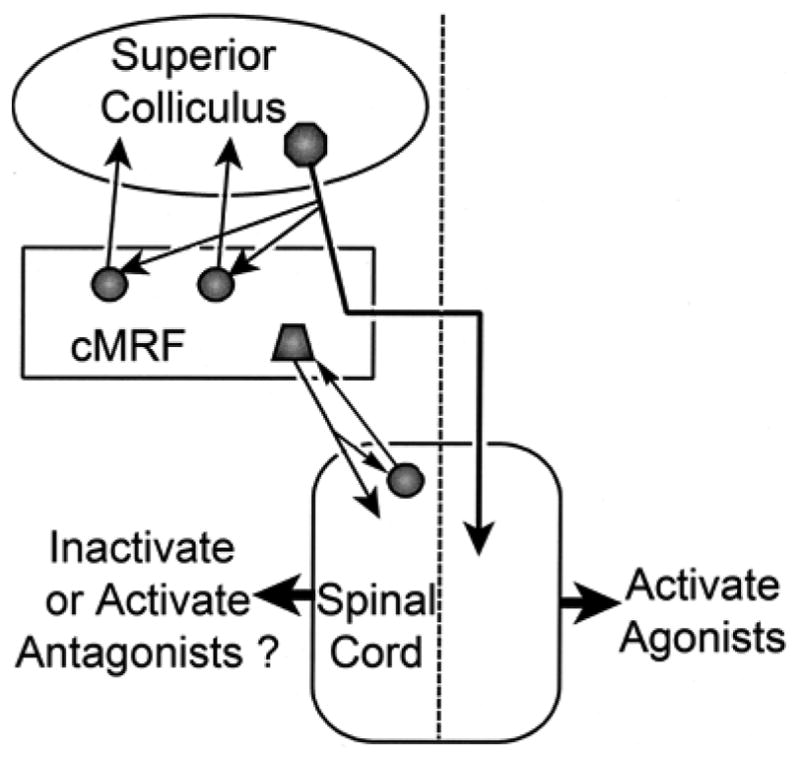
Schematic showing the connections demonstrated in this study, and their possible influence on the neck muscles responsible for gaze-related head movements. Dashed line indicates midline.
Beginning with Olszewski and Baxter (1954), previous authorities have designated a nucleus termed the cuneiform in portions of the MRF (Appell and Behan, 1990; Castiglioni et al., 1978; Edwards, 1975; Huerta and Harting, 1982; Robinson et al., 1994). There is general agreement that the cuneiform nucleus begins caudally as a rectilinear region beneath the inferior colliculus. However, there is considerable difference of opinion on the extent to which this nucleus extends rostrally into the MRF, with some authors extending it throughout the MRF, and other confining it to the caudal portion of the MRF. It is restricted to the medial half of the MRF by many authorities. The term cMRF was coined to identify a physiologically defined region in macaques in which horizontally directed saccades could be elicited (Cohen et al., 1985; 1986). This physiologically defined region was demonstrated to receive topographically organized collicular input (Cohen and Büttner-Ennever, 1984). We have recently suggested that it can be anatomically defined as the area of the caudal MRF where the distribution of reticulotectal neurons overlaps the distribution of tectoreticular terminals (Chen and May, 2000). In the present study, we have shown that a region with the same connectional characteristics is present in the cat. In comparing the distribution of cMRF reticulotectal cells to the previously suggested borders of the cuneiform nucleus, it is clear that this population of cMRF neurons extends across the entire mediolateral extent of the MRF, and thus does not respect the boundaries of the cuneiform nucleus in either cat or monkey. We have consequently chosen to restrict the term cuneiform nucleus to the rectilinear nucleus found beneath the inferior colliculus, in line with recent atlases (Paxinos et al., 2000). We will reserve the term “cMRF” to designate the caudal region of the MRF where the reticulotectal neurons overlap with the tectoreticular terminals. In support of this dichotomy, we have shown here that the spinoreticular projection to the cMRF derives from the ventral horn, while the projection to the cuneiform nucleus derives from the dorsal horn (Fig. 10).
These neuroanatomical results must be interpreted within the technical constraints imposed by tracer techniques, in particular, the possibility of labeling axons of passage and the need to confine tracers to the target area. With respect to the retrograde labeling of reticulotectal neurons following collicular injections in the cat, the precision of the injection sites, and the relative lack of axons of passage in this structure provide little opportunity for these problems. However, it can not be specified whether the labeled cMRF neurons projected to the ipsilateral and/or contralateral colliculus, as the decussating axons pass contralaterally via the collicular commissure (Moschovakis et al., 1988b). The fact that intracellular staining of neurons projecting in the predorsal bundle has demonstrated axonal arbors within the cMRF supports the contention that the anterogradely labeled axon arbors found in the cMRF after collicular injections are of tectal origin (cat: Grantyn and Grantyn, 1982; Moschovakis and Karabelas, 1985; monkey: Moschovakis et al., 1988a). The injections of the upper cervical spinal cord in the cat and monkey were well localized to the upper cervical segments. Still, the possibility that axons passing either to or from lower levels of the cord may have been involved can not be ruled out until experiments tracing the downstream connections of the cMRF are completed. The injections made in the cMRF of the monkey may have inadvertently labeled axons of other spinal projections. However, the anterograde labeling of terminal arbors following cord injections presented here certainly supports the contention that spinoreticular terminations are present. The BDA injection into the cMRF (Fig. 11) spread into other regions containing reticulospinal neurons, specifically the oral pontine reticular formation (Fig. 8). Consequently, we must assume that the terminal field it produced combines elements of the mesencephalic and pontine reticulospinal projection, and we will not emphasize these data in the Discussion. We can not determine which cervical muscle groups are targeted by this projection because muscle cervical motoneuron pools overlap, and their dendrites spread throughout the ventral horn (Gordon and Richmond, 1991; Rose, 1981).
Connections of the cMRF and Superior Colliculus
Edwards and colleagues (1979) reported the presence of midbrain reticulotectal neurons, but the present study represents the first detailed examination of the bilateral distribution of these neurons in the feline MRF. The presence of reticulotectal neurons in the caudal MRF of the cat suggests that this area is homologous with the cMRF of the macaque (Chen and May, 2000). Furthermore the existence of similar reticulotectal neurons in the fish, suggests that the presence of a gaze-related area in the caudal MRF is a general feature of vertebrates (Luque et al., 2005). The bilateral nature of this population is in agreement with the findings from intracellular staining of squirrel monkey cMRF neurons, which revealed that a portion of these cells have axons that terminate bilateral in the intermediate gray layer (Moschovakis et al., 1988b). Numerous retrogradely labeled neurons were also present in the rostral MRF, between the nucleus of the posterior commissure and the interstitial nucleus of Cajal. These probably represent a population whose firing is more closely related to the vertical components of the gaze change, which was designated the piMRF by Waitzman and associates (2000a&b). We will concentrate here on the cMRF population. The specific distribution pattern of the cMRF reticulotectal population appears to differ between the cat and macaque. In the macaque, these neurons distribute as a dense band in the middle of the dorsoventral extent of the MRF, while in the cat, the labeled cells have a more dispersed pattern in the portion of the MRF located immediately beneath the SC. However, this difference in appearance may primarily be due to the fact that a frontal section through the cat’s brainstem is closer to an axial cut, than in the highly inclined macaque brainstem. Indeed, at the caudal end of the monkey cMRF, the distribution of retrogradely labeled neurons beneath the SC resembles the pattern observed here in the cat (Chen and May, 2000; see also Moschovakis et al., 1988b).
The other feature that supports the contention that the cat and macaque cMRF are similar structures is the presence of numerous close associations between individual retrogradely labeled reticulotectal neurons and anterogradely labeled tectoreticular axonal boutons (Fig. 3&4). While ultrastructural examination is required to prove synaptic connections, this extensive relationship strongly suggests the activity of these reticulotectal neurons is dominated by a discrete collicular input, i.e. it is a driver input (Sherman and Guillery, 1998). Indeed, Moschovakis and colleagues (1988b) noted that neither the latency of the first spike nor the latency of the high frequency burst recorded in cMRF reticulotectal (reticular long lead burst) neurons could be differentiated from these features in the tectobulbospinal (tectal long lead burst) neurons of spider monkeys, suggesting the two are tightly coupled. Additional physiological studies in macaques reveal a subset of cMRF neurons whose receptive field characteristics bear a close resemblance to those observed within the intermediate layer of the SC (Cromer and Waitzman, 2006; Pathmanathan et al., 2006a&b). Specifically, this subset of cMRF neurons has well defined receptive field borders (“closed fields”) and their activation is best correlated with the amplitude of the saccade. The connections demonstrated here form a feedback circuit (Fig. 12), but the exact nature and purpose of this feedback is unknown. At least a portion of the cells participating in this projection are GABAergic (Appell and Behan, 1990), so it is possible that the action of the cMRF on the superior colliculus may lead to decreased tectal neuron firing.
The occasional presence of numerous boutons in association with unlabeled, counterstained somata in the cMRF suggests that not all of the neurons whose physiological characteristics closely resemble collicular neurons project back upon the SC. In addition, reticulotectal neurons were encountered which received only a few, or no, presumptive contacts from labeled tectoreticular axons. It is possible that other contacts were present, but were not observable due to incomplete filling of the dendritic tree of the reticulotectal neurons. Nevertheless, based on the distribution of terminals, these cMRF cells presumably are less heavily influenced by the physiological characteristics of the collicular input than those whose somata and proximal dendrites are associated with multiple boutons. This suggests it is possible that the SC may also receive feedback from cMRF neurons whose physiological characteristics differ from the collicular output, such as cells whose firing characteristics are modulated with respect to the dynamic aspects of a saccade (Crommer and Waitzman, 2006).
Connections of the cMRF and Cervical Spinal Cord
Injections of tracer into the upper cervical spinal cord of cats and monkeys in the present experiments revealed a similar pattern of retrograde labeling. In both species, reticulospinal neurons were present in the cMRF and were largely confined to its medial aspect (Fig. 12). In both species, the vast majority of these neurons were located ipsilaterally. This pattern of label in the cMRF correlates well with that seen previously, and ascribed either to the MRF or cuneiform nucleus (cat: Cowie and Holstege, 1992; Holstege and Cowie, 1989; monkey: Castiglioni et al., 1978; Nudo and Masterton, 1988; Robinson et al., 1994; Satoda et al., 2002). Electrophysiological examination of the monkey cMRF has revealed cells with post-saccadic activity, whose firing appears to correlate with gaze-related head movements. However, reconstructions of the location of these units did not reveal a more medial location with respect to units with saccade-related activity (Pathmanathan et al., 2006a). We have preliminary evidence indicating that wider regions of the cMRF are involved in modulating head movement, but they project to medullary regions controlling head movements (Cowie and Robinson, 1994; Cowie et al. 1994; May et al., 2005; Quessy and Freedman, 2004). The rostral region of the MRF also contained labeled reticulospinal neurons. These had a bilateral distribution, and appeared to be continuous with labeled cells in the interstitial nucleus of Cajal. This pattern of reticulospinal cell distribution in the rostral MRF was similar to that reported previously (cat: Cowie and Holstege, 1992; Holstge and Cowie, 1989; Spence and Saint-Cyr, 1988; Zuk et al., 1983; monkey: Castiglioni et al., 1978; Nudo and Masterton, 1988). Furthermore, Satoda and colleagues (2002) have shown that the rostral MRF cells project more medially within the ventral horn, while the caudal neurons project more laterally, supporting the idea of subdividing the rostral and caudal MRF populations. The bilateral distribution of the rostral MRF neurons may correlate with their proposed function in vertical gaze, which requires co-contraction of agonist muscles (e.g., flexors) and inhibition of antagonists muscles (e.g., extensors) on both sides of the neck (cat: Isa and Naito, 1994; Fukishima, 1987; Fukushima et al., 1979; monkey: Waitzman et al., 2000a&b; Waitzman et al., 2002). In contrast, horizontal gaze requires activation of muscles on one side of the neck, with inhibition of muscles on the other side of the neck (Corneil et al., 2001; Thomson et al., 1996). Thus, the fact the cMRF seems to play a particular role in horizontal gaze (Cohen et al., 1985; 1986; Waitzman et al., 1996) may explain why the cMRF projection to the spinal cord is not bilateral. In fact, this connectional difference may suggest that the rostral border of the cMRF should perhaps be more precisely placed at the point where the ipsilateral reticulopinal distribution becomes a bilateral one (Fig. 5D).
The question arises as to why the cMRF projects primarily ipsilaterally, while other descending pathways, such as the tectospinal projection, are primarily contralateral in nature (Harting, 1977; May and Porter, 1992; Fig. 12). One possibility is that this projection subserves the avoidance, as opposed to orientation, aspects of collicular function, for these tectal projections are believed to be ipsilateral in nature (Ellard and Goodale, 1986; Buckenham and Yeomans, 1993). In fact, it has been suggested that in fish, the cMRF neurons receive inputs from non-decussating tectal axons (Luque et al., 2007). However, the axons providing a crossed brainstem projection from the superior colliculus clearly contribute numerous terminals to the ipsilateral cMRF before decussating (cat: Grantyn and Grantyn, 1982; Moschovakis and Karabelas, 1985; monkey: Moschovakis et al., 1988), stimulation of this region produces contraversive eye movements (Cohen et al., 1985; 1986), and the firing of cells in this area correlates with eye and head movements toward the contralateral side (Waitzman et al., 1996; Pathmanathan et al., 2006a&b). Thus, we must consider what the role of this ipsilateral reticulospinal projection might be in contraversive orienting movements of the head. One possibility is that it is either directly or indirectly inhibitory in nature, and that it eliminates the tone in antagonist muscles to allow the agonist muscles activated by the crossed tectospinal projection to quickly and effectively promote the gaze change (Fig. 12). In fact, suppression of antagonist activity is generally observed with lateral head turns (Corneil et al., 2001; Richmond et al., 1992; Roucoux et al., 1989). In initial investigations, electrical stimulation of the cMRF in head free monkeys elicited little head movement, in agreement with an inhibitory role for the ipsilaterally directed cMRF reticulospinal neurons (Waitzman et al., 2002). Moreover, inactivation of the cMRF led to a contraversive head roll, not the ipsilateral head turn that might be expected if this structure provided agonist activity for contraversive gaze movements (Waitzman et al., 2000a). Alternatively, a late excitatory drive that activates antagonist muscles might serve to stop the gaze movement at the correct point (Fig. 12). The greater momentum of the head, as opposed to the eyes, may require such braking actions. The evidence for this is somewhat equivocal, as EMG activity in antagonist neck muscles has not been observed in trained gaze movements and untrained head turns (Corneil et al., 2001; Richmond et al., 1992), although it has been reported in higher velocity, volitional head turns (Roucoux et al., 1989). Electrical stimulation of the superior colliculus evokes suppression followed later by activity in antagonist muscles (Corneil et al. 2002a&b). The cMRF reticulospinal neurons appear to lie within the tectoreticular terminal field (Compare Fig. 2 & 5), and so would be in a position to produce the changes in activity recorded following collicular stimulation.
A projection from the cervical spinal cord back upon the cMRF was revealed in cat and monkey by the present experiments (Figs.10–12). To the best of our knowledge, spinomesencephalic projections to the cMRF from the ventral horn have not been specifically reported. However, most examinations of ascending projections to the midbrain have concentrated on somatosensory projections that arise in the dorsal horn and terminate, primarily contralaterally, in the periaqueductal gray and superior colliculus, adjacent to the current zone of interest (Blomqvist and Wiberg, 1985; Wiberg et al., 1987). The fact the cells of origin for the cMRF projection are located within the ventral horn suggests that they are not carrying proprioceptive information about neck position, but are instead likely to carry some form of efference copy information related to current activity in neck motoneuron pools. Our data further suggest that a reciprocal loop is present, with reticulospinal neurons receiving spinoreticular input, and visa-versa. This type of feedback of information to reticulospinal neurons may contribute to the effects of head position on gaze evoked EMG activity (Corneil et al., 2002b).
Acknowledgments
We gratefully acknowledge the technical assistance of Olga Golanov, MD and Jennifer Cotton in aspects of this study. We are deeply indebted to Frances JR Richmond, PhD for advice and encouragement at the outset of this study.
Grant Sponsor: National Eye Institute; Grant numbers EY014263 (to SW & PJM) and EY09481 (to DMW).
Abbreviations
- BC
brachium conjunctivum
- cMRF
central mesencephalic reticular formation
- Cun
cuneiform nucleus
- C1
first segment of the cervical spinal cord
- C2
second segment of the cervical spinal cord
- C3
third segment of the cervical spinal cord
- DF
dorsal funiculus
- DH
dorsal horn
- DR
dorsal raphe
- EWU
urocortin containing Edinger Westphal nucleus
- IC
inferior colliculus
- InC
interstitial nucleus of Cajal
- LF
lateral funiculus
- MG
medial geniculate nucleus
- MRF
midbrain reticular formation
- nB
nucleus of the brachium of the inferior colliculus
- nPC
nucleus of the posterior commissure
- PAG
periaqueductal gray
- piMRF
peri InC mesencephalic reticular formation
- PRF
pontine reticular formation
- Pt
pretectum
- SN
substantia nigra
- R
red nucleus
- SC
superior colliculus
- SGI
intermediate gray layer
- SGP
deep gray layer
- SOA
supraoculomotor area
- Vc
spinal trigeminal nucleus pars caudalis
- VF
ventral funiculus
- VH
ventral horn
- 3
oculomotor nucleus
- 4
trochlear nucleus
References
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol. 1990;302:143–158. doi: 10.1002/cne.903020111. [DOI] [PubMed] [Google Scholar]
- Bender MB, Shanzer S. Oculomotor pathways defined by electrical stimulation and lesions in the brain stem of the monkey. In: Bender MB, editor. The Oculomotor System. New York: Harper and Row; 1964. pp. 81–140. [Google Scholar]
- Blomqvist A, Wiberg M. Some aspects of the anatomy of somatosensory projections to the cat midbrain. In: Rowe MH, Willis WD, editors. Development, Organization, and Processing in Somatosensory Pathways. NewYork: Alan R. Liss, Inc; 1985. pp. 215–222. [Google Scholar]
- Buckenham KE, Yeomans JS. An uncrossed tectopontine pathway mediates ipsiversive circling. Behav Brain Res. 1993;54:11–22. doi: 10.1016/0166-4328(93)90044-q. [DOI] [PubMed] [Google Scholar]
- Büttner U, Büttner-Ennever JA, Henn V. Vertical eye movement related unit activity in the rostral mesencephalic reticular formation of the monkey. Brain Res. 1977;139:239–252. doi: 10.1016/0006-8993(77)90273-6. [DOI] [PubMed] [Google Scholar]
- Castiglioni AJ, Gallaway MC, Coulter JD. Spinal projections from the midbrain in monkey. J Comp Neurol. 1978;178:329–346. doi: 10.1002/cne.901780208. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. The feedback circuit connecting the superior colliculus and the central mesencephalic reticular formation: a direct morphological demonstration. Exp Brain Res. 2000;131:10–21. doi: 10.1007/s002219900280. [DOI] [PubMed] [Google Scholar]
- Cohen B, Büttner-Ennever JA. Projections from the superior colliculus to a region of the central mesencephalic reticular formation (cMRF) associated with horizontal saccadic eye movements. Exp Brain Res. 1984;57:167–176. doi: 10.1007/BF00231143. [DOI] [PubMed] [Google Scholar]
- Cohen B, Matsuo V, Fradin J, Raphan T. Horizontal saccades induced by stimulation of the central mesencephalic reticular formation. Exp Brain Res. 1985;57:605–616. doi: 10.1007/BF00237847. [DOI] [PubMed] [Google Scholar]
- Cohen B, Waitzman DM, Büttner-Ennever JA, Matsuo V. In: Horizontal saccades and the central mesencephalic reticular formation. Freund H-J, Büttner U, Cohen B, Noth J, editors. Amsterdam: Elsevier Science Publishers BV; 1986. pp. 243–256. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Richmond FJR, Loeb GE, Munoz D. Neck muscles in the rhesus monkey. II. Electromyographic patterns of activation underlying postures and movements. J Neurophysiol. 2001;86:1729–1749. doi: 10.1152/jn.2001.86.4.1729. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol. 2002a;88:1980–1999. doi: 10.1152/jn.2002.88.4.1980. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of the monkey superior colliculus. II. Gaze initiation and volitional head movements. J Neurophysiol. 2002b;88:2000–2018. doi: 10.1152/jn.2002.88.4.2000. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Holstege G. Dorsal mesencephalic projections to pons, medulla, and spinal cord in the cat: Limbic and non-limbic components. J Comp Neurol. 1992;319:536–559. doi: 10.1002/cne.903190406. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol. 1994;72:2648–2664. doi: 10.1152/jn.1994.72.6.2648. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Smith MK, Robinson DL. Subcortical contributions to head movements in macaques. II. Connections of a medial pontomedullary head-movement region. J Neurophysiol. 1994;72:2665–2682. doi: 10.1152/jn.1994.72.6.2665. [DOI] [PubMed] [Google Scholar]
- Crawford JD, Cadera W, Vilis T. Generation of torsional and vertical eye position signals by the interstitial nucleus of Cajal. Science. 1991;252:1551–1553. doi: 10.1126/science.2047862. [DOI] [PubMed] [Google Scholar]
- Cromer JA, Waitzman DM. Neurones associated with saccade metrics in the monkey central mesencephalic reticular formation. J Physiol. 2006;570:507–523. doi: 10.1113/jphysiol.2005.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SB. Autoradiographic studies of the projections of the midbrain reticular formation: descending projections of nucleus cuneiformis. J Comp Neurol. 1975;161:341–358. doi: 10.1002/cne.901610306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol. 1979;184:309–330. doi: 10.1002/cne.901840207. [DOI] [PubMed] [Google Scholar]
- Ellard CG, Goodale MA. The role of the predorsal bundle in head and body movements elicited by electrical stimulation of the superior colliculus in the Mongolian gerbil. Exp Brain Res. 1986;64:421–433. doi: 10.1007/BF00340479. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Hirai N, Rapoport S. Direct excitation of neck flexor motoneurons by the interstitiospinal tract. Brain Res. 1979;160:358–362. doi: 10.1016/0006-8993(79)90432-3. [DOI] [PubMed] [Google Scholar]
- Fukushima K. The interstitial nucleus of Cajal and its role in the control of movements of head and eyes. Prog Neurobiol. 1987;29:107–192. doi: 10.1016/0301-0082(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Ohashi T, Fukushima J, Kaneko CRS. Discharge characteristics of vestibular and saccade neurons in the rostral midbrain of alert cats. J Neurophysiol. 1995;73:2129–2143. doi: 10.1152/jn.1995.73.6.2129. [DOI] [PubMed] [Google Scholar]
- Fuller JH. Head movement propensity. Exp Brain Res. 1992;92:152–164. doi: 10.1007/BF00230391. [DOI] [PubMed] [Google Scholar]
- Gordon DC, Richmond FRJ. Distribution of motoneurons supplying dorsal suboccipital and intervertebral muscles in the cat neck. J Comp Neurol. 1991;304:343–356. doi: 10.1002/cne.903040302. [DOI] [PubMed] [Google Scholar]
- Graham J. An autoradiographic study of the efferent connections of the superior colliculus in the cat. J Comp Neurol. 1977;173:629–654. doi: 10.1002/cne.901730403. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Grantyn R. Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res. 1982;46:243–256. doi: 10.1007/BF00237182. [DOI] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Response properties of saccade-related burst neurons in the central mesencephalic reticular formation. J Neurophysiol. 1997;78:2164–2175. doi: 10.1152/jn.1997.78.4.2164. [DOI] [PubMed] [Google Scholar]
- Harting JK. Descending pathways from the superior colliculus: and autoradiographic analysis in the rhesus monkey (Macaca mulatta) J Comp Neurol. 1977;173:583–612. doi: 10.1002/cne.901730311. [DOI] [PubMed] [Google Scholar]
- Holstege G, Cowie RJ. Projections from the rostral mesencephalic reticular formation to the spinal cord. Exp Brain Res. 1989;75:265–279. doi: 10.1007/BF00247933. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. Tectal control of spinal cord activity: Neuroanatomical demonstration of pathways connecting the superior colliculus with the cervical spinal cord grey. Prog Brain Res. 1982;57:293–328. doi: 10.1016/s0079-6123(08)64135-7. [DOI] [PubMed] [Google Scholar]
- Isa T, Naito K. Activity of neurons in Forel’s field H during orienting head movements in alert head-free cats. Exp Brain Res. 1994;100:187–199. doi: 10.1007/BF00227190. [DOI] [PubMed] [Google Scholar]
- King WM, Fuchs AF. Reticular control of vertical saccadic eye movements by mesencephalic burst neurons. J Neurophysiol. 1979;42:861–876. doi: 10.1152/jn.1979.42.3.861. [DOI] [PubMed] [Google Scholar]
- Luque MA, Perez-Perez MP, Huerrero L, Torres B. Involvement of the optic tectum and mesencephalic reticular formation in the generation of saccadic eye movements in goldfish. Brain Res Rev. 2005;49:388–397. doi: 10.1016/j.brainresrev.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Luque MA, Pérez-Pérez MP, Herrero L, Waitzman DM, Torres B. Eye movements evoked by electrical microstimulation of the mesencephalic reticular formation in goldfish. Neurosci. 2006;137:1051–1073. doi: 10.1016/j.neuroscience.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Luque MA, Perez-Perez MP, Herrero L, Torres B. Connections of eye-saccade-related areas within mesencephalic reticular formation with optic tectum in goldfish. J Comp Neurol. 2007;500:6–19. doi: 10.1002/cne.21104. [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD. The laminar distribution of macaque tectobulbar and tectospinal neurons. Vis Neurosci. 1992;8:257–276. doi: 10.1017/s0952523800002911. [DOI] [PubMed] [Google Scholar]
- May PJ, Perkins E, Zhou L, Warren S. Macaque Central Mesencephalic Reticular Formation Connections Underlying Collicular Control of Gaze. Soc Neurosci Abst. 2005:31. [Google Scholar]
- May PJ, Richmond FRJ, Waitzman DA. The macaque cMRF reticulospinal head control pathway. Soc Neurosci Abst. 2001;27:1073. [Google Scholar]
- May PJ, Warren S, Chen B, Richmond FJR, Olivier E. Midbrain reticular formation circuitry subserving gaze in the cat. Neurobiology of Eye Movements. In: Kaminski HJ, Leigh J, editors. Annal NY Acad Sci. Vol. 956. 2002. pp. 405–408. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB. Observations on the somatodendritic morphology and axon trajectory of intracellularly HRP-labeled efferent neurons located in the deeper layers of the superior colliculus of the cat. J Comp Neurol. 1985;239:276–308. doi: 10.1002/cne.902390304. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. I. Morphological classification of efferent neurons. J Neurophysiol. 1988a;60:232–262. doi: 10.1152/jn.1988.60.1.232. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. II. Morphological identity of presaccadic neurons. J Neurophysiol. 1988b;60:263–302. doi: 10.1152/jn.1988.60.1.263. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord: A comparative study of 22 mammals. J Comp Neurol. 1988;277:53–79. doi: 10.1002/cne.902770105. [DOI] [PubMed] [Google Scholar]
- Olszewski J, Baxter D. Cytoarchitecture of the Human Brain Stem. Philadelphia: J.B. Lippincott; 1954. [Google Scholar]
- Pathmanathan JS, Presnell R, Cromer JA, Waitzman DM. Spatial characteristics of neurons in the central mesencephalic reticular formation (cMRF) of head-unrestrained monkeys. Exp Brain Res. 2006a;168:455–470. doi: 10.1007/s00221-005-0104-0. [DOI] [PubMed] [Google Scholar]
- Pathmanathan JS, Cromer JA, Cullen KE, Waitzman DM. Temporal characteristics of neurons in the central mesencephalic reticular formation of head unrestrained monkeys. Exp Brain Res. 2006b;168:471–492. doi: 10.1007/s00221-005-0105-z. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2000. [Google Scholar]
- Perkins E, Wang Y, Warren S, Lin RC-S, May PJ. Projections of somatosensory cortex and frontal eye field onto incertotectal neurons in the cat. Anat Rec. 2006;288:1310–1329. doi: 10.1002/ar.a.20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quessy S, Freedman E. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis. I. Characteristics of evoked head movements. Exp Brain Res. 2004;156:342–356. doi: 10.1007/s00221-003-1787-8. [DOI] [PubMed] [Google Scholar]
- Richmond FJR, Thomson DB, Loeb GE. Electromyographic studies of neck muscles in intact cat. I. Patterns of recruitment underlying posture and movement during behaviors. Exp Brain Res. 1992;88:41–58. doi: 10.1007/BF02259127. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: Brainstem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Rose PK. Distribution of dendrites from biventer cervicis and complexus motoneurons stained intracellularly with horseradich peroxidase in the adult cat. J Comp Neurol. 1981;197:395–409. doi: 10.1002/cne.901970304. [DOI] [PubMed] [Google Scholar]
- Roucoux A, Crommelinck M, Decostre M-F. Neck muscle activity in eye-head movements. In: Allum JHJ, Hulliger M, editors. Prog Brain Res. Vol. 80. 1989. pp. 351–362. [DOI] [PubMed] [Google Scholar]
- Satoda T, Matsumoto H, Zhou L, Rose PK, Richmond FJR. Mesencephalic projections to the first cervical segment in the cat. Exp Brain Res. 2002;144:397–413. doi: 10.1007/s00221-002-1047-3. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SJ, Saint-Cyr JA. Comparative topography of projections from the mesodiencephalic junction to the inferior olive, vestibular nuclei and upper cervical cord in the cat. J Comp Neurol. 1988;268:357–374. doi: 10.1002/cne.902680306. [DOI] [PubMed] [Google Scholar]
- Stahl JS. Adaptive plasticity of head movement propensity. Exp Brain Res. 2001;139:201–208. doi: 10.1007/s002210100749. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Premotor correlates of integrated feedback control for eye-head gaze shifts. J Neurosci. 2006;26:4922–4929. doi: 10.1523/JNEUROSCI.4099-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DB, Loeb GE, Richmond FJR. Effects of neck posture on patterns of activation of feline neck muscles during horizontal rotation. Exp Brain Res. 1996;100:392–400. doi: 10.1007/BF00229139. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Pathmanathan J, Presnell R, Ayers A, DePalma S. Contributions of the superior colliculus and mesencephalic reticular formation to gaze control. In: Kaminski HJ, Leigh RJ, editors. Neurobiology of Eye Movements from Molecules to Behavior, Ann NY Acad Sci. Vol. 956. 2002. pp. 111–129. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, Cohen B. Central mesencephalic reticular formation (cMRF) neurons discharging before and during eye movements. J Neurophysiol. 1996;75:1546–1572. doi: 10.1152/jn.1996.75.4.1546. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, DePalma-Bowles S, Ayers AS. Effects of reversible inactivation of the primate mesencephalic reticular formation. I. Hypermetric goal-directed saccades. J Neurophysiol. 2000a;83:2260–2284. doi: 10.1152/jn.2000.83.4.2260. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, DePalma-Bowles S, Ayers AS. Effects of reversible inactivation of the primate mesencephalic reticular formation. II. Hypometric vertical saccades. J Neurophysiol. 2000b;83:2285–2299. doi: 10.1152/jn.2000.83.4.2285. [DOI] [PubMed] [Google Scholar]
- Wiberg M, Westman J, Blomqvist A. Somatosensory projection to the mesencephalon: an anatomical study in the monkey. J Comp Neurol. 1987;264:92–117. doi: 10.1002/cne.902640108. [DOI] [PubMed] [Google Scholar]
- Zuk A, Rutherford JG, Gwyn DG. Projections from the interstitial nucleus of Cajal to the inferior olive and to the spinal cord in cat: a retrograde fluorescent double-labeling study. Neurosci Lett. 1983;38:95–101. doi: 10.1016/0304-3940(83)90023-x. [DOI] [PubMed] [Google Scholar]