Abstract
Oxidative damage is a consistent finding in a number of central nervous system (CNS) disorders. Uric acid (UA) is a potent hydrophilic antioxidant that is modified by diet and drug. Several lines of evidence suggest that plasma UA may modulate outcomes in neurologic disease, but little attention has been paid to CNS levels of UA. Our objective was to test the hypothesis that cerebrospinal fluid (CSF) UA is determined by plasma UA, modified by blood-brain barrier (BBB) integrity and associated with rate of cognitive decline in Alzheimer’s disease (AD). Also, since UA and ascorbic acid may act as antioxidants for one another, we also explored a potential interaction between them in the brain. Thirty-two patients with mild to moderate AD (Mini-Mental Status Exam 19 ± 5) participated in a longitudinal biomarker study for one year involving standardized clinical assessments. CSF and blood were collected at baseline for UA, ascorbic acid, and albumin. Cognitive measures were collected at baseline and again one year later. CSF UA was independent of age, gender, and AD severity. CSF and plasma UA were positively correlated (r = 0.669, p = 0.001) and BBB impairment was associated with higher CSF levels of UA (p = 0.028). Neither plasma nor CSF UA reached significant association with rates of cognitive decline over 1 year. CSF UA and CSF ascorbic acid were positively correlated (r = 0.388, p = 0.001). The hypothesis that CSF UA is determined by plasma UA and BBB integrity is supported, as is the hypothesis that UA and ascorbic acid are associated in CSF but not plasma. Adequately powered prospective studies would help assess any role for UA in primary and secondary prevention of AD.
Keywords: Alzheimer’s disease, ascorbic acid, blood-brain barrier, cerebrospinal fluid, uric acid
INTRODUCTION
Oxidative stress has been implicated in a number of central nervous system (CNS) disorders and is an early feature of neurons in Alzheimer’s disease (AD) and the degeneration of dopamine producing cells in Parkinson’s disease (PD) [1,2]. In multiple sclerosis (MS), peroxynitrite and other free radicals can contribute to the inflammation and demyelination of axons [3]. Preventing oxidative damage may delay onset and improve the prognosis of these common CNS disorders.
Uric acid (UA) is an endogenously produced water-soluble antioxidant that is modified by both drug and diet [4–6]. UA accounts for over half of the free radical scavenging activity in human blood [7] by quenching superoxide and singlet oxygen, and protecting oxidation of vitamin C (ascorbic acid; AA) through the chelation of iron [8–10]. Others have shown the ability of AA to repair oxidized urate, which highlights a potential synergy for maintaining antioxidant capacity by these two antioxidants [11]. These qualities make UA an attractive CNS antioxidant because neurons are remarkably susceptible to oxidative stress, and AA is concentrated as high as 10,000 µM in neurons [12].
Epidemiological studies focused on PD have observed lower incidence and better prognosis with higher serum UA [13–16]. A postmortem study of substantia nigra from PD patients revealed depletion of UA content compared to age-matched controls [17]. Some studies in MS have reported lower serum UA in cases as compared to controls, and higher serum UA has been associated with delayed onset of the first neurologic episode, stressing some potential for a preventative role [18–20]. Other studies in MS suggest there may be therapeutic value in raising serum UA [21–23].
It is less clear whether UA is important to the development or progression of AD [24–29]. Cross-sectional studies of serum UA have identified lower concentrations in AD [27,28] and mild cognitive impairment (MCI) [27] patients compared to healthy controls, but another report found no difference [26]. A secondary analysis of MCI clinical trial data found that among placebo treated subjects, lower serum UA was associated with more rapid cognitive decline and higher incidence of AD over 3 years, but this observation was not seen in the other study arms [29].
Two small studies of CSF UA in AD are inconsistent, with one reporting higher [24] and the other lower concentrations [25] of CSF UA compared to controls.
CSF UA is consistently about ten times lower than serum levels, but very few have examined the relationship between CSF and plasma UA in AD [25]. We are unaware of any studies examining CSF UA prospectively in AD with simultaneous measures of blood-brain barrier (BBB) integrity and CSF ascorbic acid. Our primary goal was to measure brain UA and to identify the determinants and consequences of CSF UA in AD. We hypothesized that: 1) CSF UA is determined by plasma UA and modified by BBB integrity; 2) CSF UA is associated with rates of cognitive decline; and 3) since UA and AA may act as antioxidants for one another, we also explored whether any relationship exists between these two antioxidants.
METHODS
We analyzed baseline and 12 month CSF and plasma UA, AA, and serum albumin concentrations along with clinical risk factors and cognitive measures in 32 people with mild to moderate AD. Study participants were followed for cognitive change over 1 year.
Study participants
The sample population for this longitudinal biomarker study were patients in the NIA – Layton Center for Aging and Alzheimer’s Research at Oregon Health & Science University (OHSU) with a diagnosis of probable AD. Participants were diagnosed by National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association criteria [30] and Clinical Dementia Rating of 0.5 or 1.0, establishing mild to moderate AD. All patients from the biomarker study with available biochemical specimens were included, yielding 32 participants with mild to moderate probable AD. Informed consent was obtained in accord with the Institutional Review Board for Human Study at Oregon Health & Science University.
Data collection
Thirty-two elderly (10 females, mean age 71 ± 7 years) were assesed at baseline and 12 months. Clinical evaluation included medical history, physical exam, Mini-Mental Status Exam (MMSE) [31], Clinical Dementia Rating (CDR) [32], Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) [33], Hachinski ischemia score [34] as a measure of vascular burden, and Geriatric Depression Scale [35]. Lumbar CSF and peripheral blood were collected at baseline for analyses.
Biochemical assays
Lumbar punctures were performed in the morning under standardized conditions at L3–L4 or L4–L5 interspace. CSF samples were immediately aliquoted and snap frozen at −70°C until assayed, at which point samples had normal cell count and glucose levels. Blood samples collected at the same visit as lumbar puncture for CSF were analyzed for albumin, UA, and AA. Briefly, UA and AA were done by paired-ion reversed-phase HPLC coupled with electro-chemical detection. Fifty microliters of plasma was mixed with an equal volume of cold 5% (wt/vol) metaphosphoric acid and centrifuged to remove the precipitated proteins. Fifty microliters of the supernatant was mixed with 15 µl of cold 2.58 M K2HPO4 buffer (pH 9.8) followed by the addition of 185 µl of HPLC eluant (see below). Forty microliters of this mixture was immediately chromatographed on an LC18DB column [25 cm × 4.6 mm (i.d.)] (Supelco) preceded by a guard column [2 cm × 4.6 mm (i.d.)] containing the same material. The eluant, delivered at a flow rate of 1.0 ml/min, consisted of 40 mM sodium acetate, 0.54 mM Na2EDTA, 1.5 mM dodecyltriethylammonium phosphate, and 7.5% (vol/vol) methanol, taken to pH 4.75 with glacial acetic acid. The eluate was analyzed with an LC 4B amperometric electrochemical detector equipped with a glassy-carbon working electrode and an Ag/AgCl reference electrode (Bioanalytical Systems, West Lafayette, IN). The applied potential was +0.5 V, with a sensitivity setting of 500 nÅ for urate and 50 nÅ for ascorbate. Urate and ascorbate eluted as single peaks with retention times of 5.8 and 11.5 min, respectively [36]. CSF and serum albumin were quantified to calculate CSF Albumin Index as a validated marker of BBB integrity [37,38]. All biochemical analyses were performed and the results recorded by staff blinded to subject’s clinical information.
Statistical methods
Our primary hypotheses were that CSF UA was determined by plasma UA, BBB integrity (CSF Albumin Index), and CSF AA and associated with rates of cognitive decline in AD. Means and SD were generated for baseline study characteristics. Pearson correlation coefficients were used to confirm UA and AA independence from age, gender, and AD severity. Regression analysis was used to examine any relationship between the predictors (plasma UA, CSF Albumin Index, and CSF AA) and response (CSF UA) and CSF UA with rates of disease progression over one year (change in MMSE, ADAS-cog, CDR-sum of boxes). Independent t-test compared the mean difference in CSF UA by a priori category of BBB integrity (intact, CSF AI < 9.0; impaired, CSF AI ≥ 9.0). All statistical significance was two-tailed with α set at 0.05. SPSS version 17 and Macintosh were the software and hardware for the analysis (Chicago, IL).
RESULTS
Study population
The mean age of the sample was 71.0 ± 7.0 years. Thirty-one percent of the study participants were women. Mean CSF UA was about 10 fold lower than plasma levels. Conversely, AA was higher in CSF (~3:1). The mean CSF Albumin Index (BBB integrity marker) was 7.5 ± 3.8 and eight of the thirty-two subjects had BBB impairment (CSF AI ≥ 9.0). Baseline MMSE was 19 ± 5 and cognitive decline was remarkable over the year of follow-up (Table 1).
Table 1.
Study population characteristics (n = 32)*
| Age, years | 71 (7) |
| Female (%) | 10 (31) |
| CSF uric acid (µM) | 17.7 (7) |
| Plasma uric acid (µM) | 172.3 (51) |
| CSF ascorbic acid (µM) | 129 (52) |
| Plasma ascorbic acid (µM) | 41 (30) |
| CSF albumin (µM) | 30.5 (16) |
| Serum albumin (µM) | 4052.5 (371.3) |
| CSF Albumin Index | 7.5 (3.8) |
| Mini-Mental State Exam | 19 (5) |
| Clinical Dementia Rating – sum of box | 5.9 (1.4) |
| Alzheimer’s Disease Assessment Scale – cognitive subscale | 24 (10) |
| Annual change in Mini Mental State Exam | 3.3 (3.8) |
| Annual change in AD Assessment Scale – cognitive subscale | 8.9 (9.1) |
| Annual change in Clinical Dementia Rating – sum of boxes | 6.8 (2.7) |
Mean (SD) unless otherwise denoted.
Relationship between CSF UA and established risk factors for AD progression
There were no significant correlations between CSF UA and age, gender, or disease severity (Table 2).
Table 2.
Correlation coefficients generated between CSF and plasma antioxidants with age, gender, baseline disease severity and rates of cognitive change in Alzheimer’s disease
| Baseline cognitive status | Annual change in cognition (Δ) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | MMSE | ADAS | CDR | MMSE Δ | ADAS Δ | CDR Δ | ||
| CSF uric acid (µM) | r | 0.289 | −0.268 | 0.195 | −0.089 | −0.118 | 0.257 | 0.105 | 0.144 |
| Sig. | 0.108 | 0.138 | 0.286 | 0.630 | 0.518 | 0.155 | 0.576 | 0.431 | |
| Plasma uric acid (µM) | r | 0.113 | −0.161 | 0.122 | −0.151 | −0.101 | 0.223 | 0.193 | 0.048 |
| Sig. | 0.538 | 0.380 | 0.507 | 0.410 | 0.584 | 0.221 | 0.298 | 0.795 | |
| CSF ascorbic acid (µM) | r | −0.080 | 0.094 | −0.091 | 0.265 | 0.189 | 0.232 | 0.048 | 0.255 |
| Sig. | 0.663 | 0.611 | 0.621 | 0.143 | 0.301 | 0.200 | 0.799 | 0.159 | |
| Plasma ascorbic acid (µM) | r | 0.105 | 0.259 | −0.060 | 0.123 | 0.081 | 0.166 | 0.151 | 0.204 |
| Sig. | 0.566 | 0.153 | 0.744 | 0.502 | 0.660 | 0.365 | 0.417 | 0.262 | |
CSF, cerebrospinal fluid; MMSE, Mini Mental State Exam; ADAS, Alzheimer’s Disease Assessment Scale−cognitive subscale; CDR, Clinical Dementia Rating sum of box score.
Consequences of UA in AD
We did observe cognitive and functional decline in our population (Table 1), but CSF or plasma UA did not explain the annual decline (correlation analysis, Table 2; regression coefficient data not shown).
Determinants of CSF UA in AD: Plasma UA, BBB integrity, and CSF AA
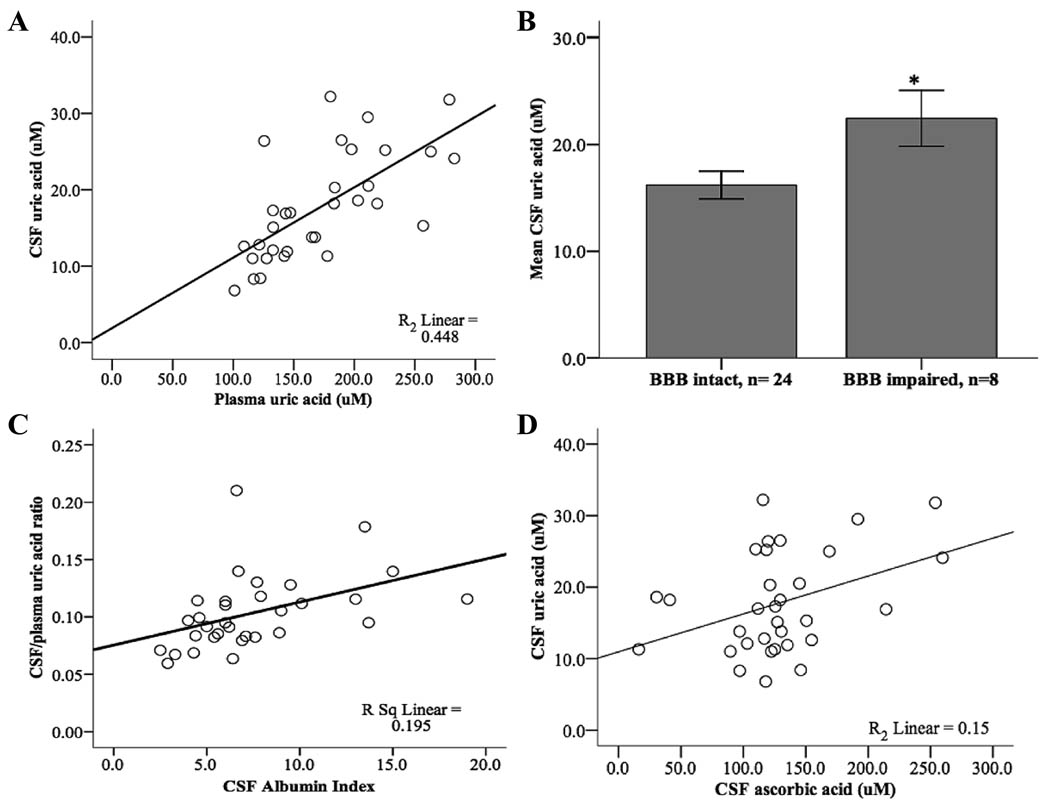
Each µmol/L increase in plasma UA was associated with about a 5% increase in CSF UA (β = 0.092 µmol/L, p < 0.001, 95% CI = 0.054–0.130, Fig. 1A). BBB impairment was defined a priori as CSF Albumin Index of 9.0 or greater and was identified in 25% (8/32) of the sample. CSF UA was 6.2 µmol/L higher in subjects with BBB impairment (p = 0.028, Fig. 1C). Regression analysis demonstrated a continuous and positive linear association between CSF-to-plasma UA ratio and CSF Albumin Index (β = 0.004, p = 0.011, Fig. 1D). Each µmol/L increase in CSF AA was also associated with about 6% higher CSF UA content (β = 0.053 µmol/L, p = 0.028, 95% CI = 0.006–0.100, Fig. 1B). Plasma UA and AA had a positive correlation initially (r = 0.463, p = 0.008), but after careful examination of the data, it was apparent that this association was driven by an outlying plasma AA value greater than 2 SD from the norm (163 µM). After excluding this outlier, plasma UA and AA were not correlated (r = 0.293, p = 0.110, n = 31).
Fig. 1.
Association between uric acid, ascorbic acid, and blood-brain barrier integrity in AD.A) Plasma UA versus CSF UA,r = 0.669, p = 0.001; B) Mean difference in CSF uric acid between BBB intact (CSF Albumin Index < 9.0) and BBB impaired (CSF Albumin Index ≥ 9.0) was 6.2 µmol/L (p = 0.028); Standard error bars set at 1.0; C) CSF Albumin Index versus CSF-to-plasma UA, r = 0.442, p = 0.011; D) CSF AA versus CSF UA, r = 0.388, p = 0.001.
DISCUSSION
These data show that CSF UA is determined by plasma UA and BBB integrity, and may be modified by CSF AA. Although CSF UA did not predict the rate of AD progression over 1 year, a role for UA in modifying the rate of neurodegeneration in AD cannot be excluded with certainty, given the relatively small sample size and short duration of follow-up.
Although older reports indicate that the human brain has no xanthine oxidase enzyme [39], more recent studies have established the presence of this enzyme in brain, so that the brain has the capacity to generate uric acid in situ. However, the positive correlation between plasma and CSF UA and the 10 fold higher plasma UA level compared to CSF content suggests that this purine metabolite is manufactured peripherally, and that access to the brain is limited by the BBB. Other studies have proposed CSF-to-plasma UA ratio as a marker of BBB integrity, similar to CSF Albumin Index since both UA and albumin are generated outside of the CNS [40]. Our positive correlation between CSF-to-plasma UA ratio and CSF Albumin Index supports this view.
We were unable to identify any relationship between UA and AA in plasma with confidence, but we did in the CSF. Synergy between these two antioxidants has been demonstrated as an apparent mechanism for maintaining antioxidant capacity of serum. One study suggests UA as an antioxidant for AA and another experiment demonstrated a reducing or “repairing” of oxidized UA by AA [9,11]. The correlation between CSF UA and AA is a new finding to our knowledge and raises the possibility that these antioxidants may also interact in the brain. The possibility that UA might influence AA levels in the brain is intriguing but requires confirmation.
To our knowledge this is the largest prospective study of CSF UA in AD, but sample size remains a limitation to making firm conclusions regarding the effect of UA upon rate of progression in AD. Other larger studies have suggested that plasma UA may modify the rate of progression from MCI to AD [29], so further study of this relationship may be in order. However, it appears from the present study that CSF levels are not more informative than plasma levels. Pharmacologic means for modulating plasma UA are well established, so this question has clear implications for clinical trial strategies.
ACKNOWLEDGMENTS
NCCAM Career Development Award K23 AT004777 (GLB), NCCAM P01 AT002034 (BF), VA Advanced Research Development Award, Dana Foundation (JFQ), NIA P30 AG08017 (JAK)
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=200).
REFERENCES
- 1.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53 Suppl 3:S26–S36. doi: 10.1002/ana.10483. discussion S36-28. [DOI] [PubMed] [Google Scholar]
- 2.Moreira PI, Honda K, Zhu X, Nunomura A, Casadesus G, Smith MA, Perry G. Brain and brawn: parallels in oxidative strength. Neurology. 2006;66:S97–S101. doi: 10.1212/01.wnl.0000192307.15103.83. [DOI] [PubMed] [Google Scholar]
- 3.Hooper DC, Scott GS, Zborek A, Mikheeva T, Kean RB, Koprowski H, Spitsin SV. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. FASEB J. 2000;14:691–698. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- 4.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52:283–289. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Qi L, Qiao N, Choi HK, Curhan G, Tucker KL, Ascherio A. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. 2007;50:306–312. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson’s disease risk in men. Am J Epidemiol. 2008;167:831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sevanian A, Davies KJ, Hochstein P. Serum urate as an antioxidant for ascorbic acid. Am J Clin Nutr. 1991;54:1129S–1134S. doi: 10.1093/ajcn/54.6.1129s. [DOI] [PubMed] [Google Scholar]
- 10.Waugh WH. Inhibition of iron-catalyzed oxidations by attainable uric acid and ascorbic acid levels: therapeutic implications for Alzheimer’s disease and late cognitive impairment. Gerontology. 2008;54:238–243. doi: 10.1159/000122618. [DOI] [PubMed] [Google Scholar]
- 11.Maples KR, Mason RP. Free radical metabolite of uric acid. J Biol Chem. 1988;263:1709–1712. [PubMed] [Google Scholar]
- 12.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 13.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol. 1996;144:480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 14.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 15.Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarzschild MA, Schwid SR, Marek K, Watts A, Lang AE, Oakes D, Shoulson I, Ascherio A, Hyson C, Gorbold E, Rudolph A, Kieburtz K, Fahn S, Gauger L, Goetz C, Seibyl J, Forrest M, Ondrasik J. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65:716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church WH, Ward VL. Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res Bull. 1994;33:419–425. doi: 10.1016/0361-9230(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 18.Rentzos M, Nikolaou C, Anagnostouli M, Rombos A, Tsakanikas K, Economou M, Dimitrakopoulos A, Karouli M, Vassilopoulos D. Serum uric acid and multiple sclerosis. Clin Neurol Neurosurg. 2006;108:527–531. doi: 10.1016/j.clineuro.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Toncev G, Milicic B, Toncev S, Samardzic G. Serum uric acid levels in multiple sclerosis patients correlate with activity of disease and blood-brain barrier dysfunction. Eur J Neurol. 2002;9:221–226. doi: 10.1046/j.1468-1331.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 20.Spitsin S, Hooper DC, Mikheeva T, Koprowski H. Uric acid levels in patients with multiple sclerosis: analysis in mono- and dizygotic twins. Mult Scler. 2001;7:165–166. doi: 10.1177/135245850100700305. [DOI] [PubMed] [Google Scholar]
- 21.Toncev G. Therapeutic value of serum uric acid levels increasing in the treatment of multiple sclerosis. Vojnosanit Pregl. 2006;63:879–882. doi: 10.2298/vsp0610879t. [DOI] [PubMed] [Google Scholar]
- 22.Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott GS, Hooper DC. The role of uric acid in protection against peroxynitrite-mediated pathology. Med Hypotheses. 2001;56:95–100. doi: 10.1054/mehy.2000.1118. [DOI] [PubMed] [Google Scholar]
- 24.Degrell I, Niklasson F. Purine metabolites in the CSF in presenile and senile dementia of Alzheimer type, and in multi infarct dementia. Arch Gerontol Geriatr. 1988;7:173–178. doi: 10.1016/0167-4943(88)90029-5. [DOI] [PubMed] [Google Scholar]
- 25.Tohgi H, Abe T, Takahashi S, Kikuchi T. The urate and xanthine concentrations in the cerebrospinal fluid in patients with vascular dementia of the Binswanger type, Alzheimer type dementia, and Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1993;6:119–126. doi: 10.1007/BF02261005. [DOI] [PubMed] [Google Scholar]
- 26.Polidori MC, Mecocci P. Plasma susceptibility to free radical-induced antioxidant consumption and lipid peroxidation is increased in very old subjects with Alzheimer disease. J Alzheimers Dis. 2002;4:517–522. doi: 10.3233/jad-2002-4608. [DOI] [PubMed] [Google Scholar]
- 27.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 28.Kim TS, Pae CU, Yoon SJ, Jang WY, Lee NJ, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:344–348. doi: 10.1002/gps.1469. [DOI] [PubMed] [Google Scholar]
- 29.Irizarry MC, Raman R, Schwarzschild MA, Becerra LM, Thomas RG, Peterson RC, Ascherio A, Aisen PS. Plasma urate and progression of mild cognitive impairment. Neurodegener Dis. 2009;6:23–28. doi: 10.1159/000170883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Dooneief G, Marder K, Tang MX, Stern Y. The Clinical Dementia Rating scale: community-based validation of “profound” and “terminal” stages. Neurology. 1996;46:1746–1749. doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- 33.Rosen WGMR, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 34.Moroney JT, Bagiella E, Desmond DW, Hachinski VC, Molsa PK, Gustafson L, Brun A, Fischer P, Erkinjuntti T, Rosen W, Paik MC, Tatemichi TK. Meta-analysis of the Hachinski Ischemic Score in pathologically verified dementias. Neurology. 1997;49:1096–1105. doi: 10.1212/wnl.49.4.1096. [DOI] [PubMed] [Google Scholar]
- 35.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 36.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 38.Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Khalidi UA, Chaglassian TH. The species distribution of xanthine oxidase. Biochem J. 1965;97:318–320. doi: 10.1042/bj0970318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niklasson F, Hetta J, Degrell I. Hypoxanthine, xanthine, urate and creatinine concentration gradients in cerebrospinal fluid. Ups J Med Sci. 1988;93:225–232. doi: 10.3109/03009738809178548. [DOI] [PubMed] [Google Scholar]