Abstract
Improvements in pancreatic islet transplantation for treatment of diabetes are hindered by the absence of meaningful islet quality assessment methods. Oxygen consumption rate (OCR) has previously been used to assess the quality of organs and primary tissue for transplantation. In this study, we describe and characterize a stirred microchamber for measuring OCR with small quantities of islets. The device has a titanium body with a chamber volume of about 200 µL and is magnetically stirred and water jacketed for temperature control. Oxygen partial pressure (pO2) is measured by fluorescence quenching with a fiber optic probe, and OCR is determined from the linear decrease of pO2 with time. We demonstrate that measurements can be made rapidly and with high precision. Measurements with βTC3 cells and islets show that OCR is directly proportional to the number of viable cells in mixtures of live and dead cells and correlate linearly with membrane integrity measurements made with cells that have been cultured for 24 h under various stressful conditions.
Keywords: islets of Langerhans, islet viability, oxygen consumption rate, stirred microchamber, islet quality
Introduction
Recent improvement in the success of transplanting pancreatic islets of Langerhans for treatment of type 1 diabetes in humans (Shapiro et al., 2000; Kaufman and Lowe, 2003; Lakey et al., 2003; Sutherland, 2003; Hering et al., 2004; Hering 2005; Ricordi et al., 2005) has prompted interest for more widespread application, which will require standardized islet quality assessment methods to ensure in vivo efficacy (Knazek, 2002; Weber et al., 2002), particularly because islets are compromised during the steps from pancreas procurement to transplantation. Human organs are procured from brain dead donors (Bretzel et al., 1994; Contreras et al., 2003) and are exposed to periods of warm and cold ischemia (Papas et al., 2005; Paraskevas et al., 2000). Islets are further damaged by stressful mechanical and enzymatic procedures during isolation. Currently there are no reliable quantitative assays that are predictive of transplantation outcome (Ricordi et al., 2001; FDA, 2000, 2003), which has also impeded progress in improving procedures for pancreas procurement, storage, and transportation, as well as islet isolation, purification, culture, and shipment.
The structure of islets, which are spheroidal aggregates with an average diameter of about 150 µm containing roughly 1,600 cells (Colton et al., 2007), precludes use of most methods for enumeration, viability, and apoptosis assessment of single cells. Membrane integrity tests, currently used to assess fractional viability of islet preparations (Bank, 1987, 1988), rarely result in estimates of fractional viability below 80% and do not provide meaningful information for predicting transplantation outcome unless the preparation is grossly damaged (Colton et al., 2007).
We have proposed the use of oxygen consumption rate (OCR) measurements for quality assessment of islet preparations prior to transplantation (Papas et al., 2001b). OCR has previously been applied to assess organ quality in liver, heart, kidney, and skin transplantation (Steinlechner-Maran et al., 1997; Stubenitsky et al., 2000; Yang et al., 2000; Zhang et al., 2000; Ricciardi et al., 2001; Schwitalla et al., 2001; Martin et al., 2003), to assess the ability of preservation solutions or supplements, to maintain pancreas viability following cold storage (Leonhardt et al., 1990, 1993; Tytko et al., 1993), to estimate numbers of viable cells in continuous bioreactors (Jorjani and Ozturk, 1999), and to support stress and toxicity studies with cellular systems (Papas et al., 1999a,b; Hynes et al., 2003).
Since the first measurements with rodent islets (Hellerstrom, 1966, 1967), OCR has been used as a tool to understand glucose-stimulated insulin secretion in islets and β-cells (Hellerstrom, 1966, 1967; Hedeskov et al., 1972; Hellerstrom et al., 1980; Hutton and Malaisse, 1980; Welsh et al., 1982; Panten et al., 1986; Ohta et al., 1991; Erecinska et al., 1992; Matschinsky, 1996; Papas and Jarema, 1998). Because quantities of islets were limited, a very sensitive method using Cartesian-divers was originally developed and applied (Hellerstrom, 1966, 1967; Hedeskov et al., 1972; Welsh et al., 1982). Subsequent studies to measure OCR and insulin secretion employed perfusion systems (Hutton and Malaisse, 1980; Papas et al., 1999a,b), and continuously stirred chambers (Panten and Klein, 1982; Papas and Jarema, 1998) together with a Clark-type electrode. These systems were also recently employed to assess islet quality (Steurer et al., 1999) to a limited extent. Polarographic microelectrode oxygen partial pressure (pO2) sensors suitable for measurements with small sample sizes consumed oxygen, were unstable, and required frequent calibration. Large polarographic sensors were more stable (Papas et al., 1999a,b), but their size precluded measurements in small liquid volumes required due to the limitation in the number of islets available. The development of optical fiber sensors that rely on the effect of oxygen in altering the decay of phosphorescent or fluorescent intensity following irradiation made possible rapid, continuous, stable measurements of pO2, and have been applied in perfusion bioreactors (Dionne, 1989; Dionne et al., 1991; Sweet et al., 2002a, 2004), static culture devices (Arain et al., 1998; Guarino et al., 2004; Wang et al., 2005), and continuously stirred chambers (Colton et al., 2007; Papas et al., 2001). A perfusion bioreactor system employing optical measurements of pO2 (Sweet et al., 2002a,b, 2004) enabled continuous measurement of OCR, insulin secretion, and redox state of cytochromes in islets. Such systems are especially useful for long-term culture and for following the transient response after changes in environmental parameters. However, they are more complex and time consuming to use compared to the stirred chamber. Also they are less efficient when multiple samples or replicates need to be examined in a short period of time. Static culture devices have also been recently described, which allow for simple operation and large numbers of simultaneous measurements (Arain et al., 1998; Guarino et al., 2004; Wang et al., 2005). However, these devices at their present state suffer from issues related to reproducibility and accuracy that are described in detail elsewhere (Colton et al., 2007).
In this paper, we describe a stirred chamber device for OCR measurements that incorporates previous experience with such systems (Panten and Klein, 1982; Papas and Jarema, 1998). Emphasis was placed on minimizing chamber volume so that measurements could be made rapidly with a small number of islets. We report on the characteristics of the device, its use for in vitro OCR measurements with cells and islets, and its utility for assessing mixtures of live and dead cells and islets that have been subjected to stressful culture conditions. We show that the device is capable of making rapid, accurate, and precise OCR measurements for assessing islet viability.
Materials and Methods
Islet Isolation and Culture
Rat, porcine, and human islets were provided by the Islet Core at the Joslin Diabetes Center (Boston, MA). Rat islets were isolated from male Sprague-Dawley rats by using collagenase digestion/ficoll purification (Lacy and Kostianovsky, 1967; Gotoh et al., 1985). Standard collagenase/protease digestion methods were used for porcine (O’Neil et al., 2001) and human (Ricordi et al., 1988; Shapiro et al., 2000) islets. The islets were cultured in Petri dishes (various sizes, Falcon, Becton Dickinson, Bedford, MA) with a surface culture density of 20 islet equivalents (IE)/cm2 (or 0.35% surface coverage) and a medium depth of 3 mm (unless specified otherwise) in a 37°C incubator with 5% CO2. The culture medium was RPMI 1640 (Cellgro 10–040-CM, Mediatech, Herndon, VA), supplemented with 10% fetal bovine serum (FBS) (heat-inactivated, Cellgro 35-011-CV), 100 U/mL penicillin and 100 µg/mL streptomycin (Cellgro 30-002-CI, Mediatech), and 10 mM HEPES buffer (Gibco 09487, Invitrogen, Carlsbad, CA).
βTC3 Cell Culture
βTC3 is a mouse insulinoma cell line (obtained from Dr. Shimon Efrat, Albert Einstein College of Medicine, Bronx, NY) derived by introducing an SV40 T-antigen into embryonic cells of transgenic mice (Efrat et al., 1988). Cultures with passage numbers 32–40 were used. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Cellgro 10–013-CM, Mediatech) supplemented with 10% FBS (Cellgro), 100 U/mL penicillin and 100 µg/mL streptomycin (Cellgro), and 10 mM HEPES buffer (Gibco, Invitrogen) with a medium depth of 2 mm in a 37°C incubator with 5% CO2.
Islet Enumeration by Visual Counting
Two aliquots containing 300–500 islets with a diameter larger than 50 µm were examined under an inverted microscope equipped with an ocular micrometer. Islets with a diameter larger than 50 µm were categorized into size groups in 50 µm increments, converted to total volume according to the original procedure (Ricordi et al., 1990), and reported as the number of IE, defined as a spherical islet with a diameter of 150 µm having volume of 1.77 × 10−6 cm3.
DNA Content
DNA was measured by fluorospectrophotometry (Pisania et al., 2007a) using the CyQUANT Cell Proliferation Assay Kit (Molecular Probes, C-7026 Eugene, OR), which is based on the strong fluorescence enhancement of CyQUANT GR dye when bound to nucleic acids. The fluorescence intensity was linearly related to the amount of nucleic acids in the sample. Fluorescence was read at 480 nm excitation and 520 nm emission wavelength in a plate reader (Spectra MAX Gemini microplate spectrophotometer, Molecular Devices, Sunnyvale, CA).
Membrane Integrity Assays
Several vital staining dyes were used to assess membrane integrity.
Trypan Blue
A volume between 25 and 200 µL of cell suspension was diluted with 0.4% trypan blue solution (Sigma-Aldrich, St. Louis, MO) to a total cell concentration of about 5 × 105 cells/mL. The volume ratio of trypan blue to cell suspension was at least 1 to 1. A 12 µL sample was loaded onto a hemacytometer slide. Both stained cells and unstained cells were counted using 40× magnification with a Zeiss Photo 3 microscope. The fraction of cells with compromised membranes was the ratio of the number of cells stained with trypan blue divided by the total number (stained and unstained) of cells. With βTC3 cells heat-killed by incubation at 60°C for 1 h, 97% of cells immediately stained with trypan blue. With cells exposed to other stresses, such as anoxia, staining increased slowly with time and reached a steady value within 24 h (Oh et al., 2004).
7-Aminoactinomycin (7-AAD) Sequential Staining
This assay used fluorescent staining with 7-AAD (Molecular Probes, Eugene, OR), which binds with high affinity to nucleic acids but is not capable of penetrating intact cell membranes, so that only nuclei in cells with compromised membranes were labeled (Pisania et al., 2007b). A 100 µL aliquot containing about 5 × 106 cells/mL of culture medium was centrifuged, and the cells were resuspended in 100 µL of Dulbecco’s phosphate buffered saline (D-PBS, Invitrogen, Carlsbad, CA) and 5 µL of 1 mg/mL 7-AAD and incubated for 20 min at 4°C protected from light. After two washes with 1 mL of D-PBS, cells were disrupted by adding 50 µL of a lysis solution containing 1% Triton TX-100 and 0.1 M citric acid in D-PBS to 50 µL of the cell solution or islet suspension combined with vortex mixing for cells or shearing through a needle for islets. Labeled nuclei were then counted immediately in a flow cytometer (Guava Personal Cell Analysis (PCA) System, Guava Technologies, Hayward, CA) or stored on ice for less than 15 min before counting. A portion of the islet suspension was further stained with 7-AAD, thereby labeling all of the previously unlabelled nuclei, and the total number of nuclei was counted. The fraction of cells with compromised membranes was estimated as the ratio of the initially stained nuclei (first measurement) to the total number of nuclei (second measurement). If only the total number of cells was of interest, nuclei were stained with 7-AAD only after cell disruption and the data reported as the total number of cells.
MTT (1-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) Assay
The viability of cells and islets was assessed with MTT, a tetrazolium salt that is reduced to an insoluble purple formazan as a consequence of the redox state in live cells (Mosmann, 1983; Vistica et al., 1991; Berridge and Tan, 1993; Liu et al., 1997). One millimolar MTT working solution in phenol red-free medium was made fresh before the assay. Islets or cells were centrifuged, the supernatant was aspirated, and the cells resuspended in D-PBS with 20 mM glucose. A known number of cells in 100 µL of the suspension were transferred to each well of a 96-well plate, and 20 µL of 5 mg/mL MTT reagent (Molecular Probes) added to each well. The plate was incubated at 37°C in a shaker (Jitterbug Model 130,000, Boekel Scientific, Feasterville, PA). After 2–4 h, 100 µL of lysis solution, which included 9% sodium dodecyl sulfate (SDS L-6026, Sigma-Aldrich) in 0.01 M HCl, was added to each well, and the plate was incubated at 37°C in a humidified chamber for 18 h to dissolve the precipitates, after which optical density was read at 570 nm in a plate reader (SpectraMAX 250 microplate spectrophotometer, Molecular Devices). Extreme care was taken to ensure that test conditions were identical each time the assay was carried out.
Oxygen Consumption Rate (OCR)
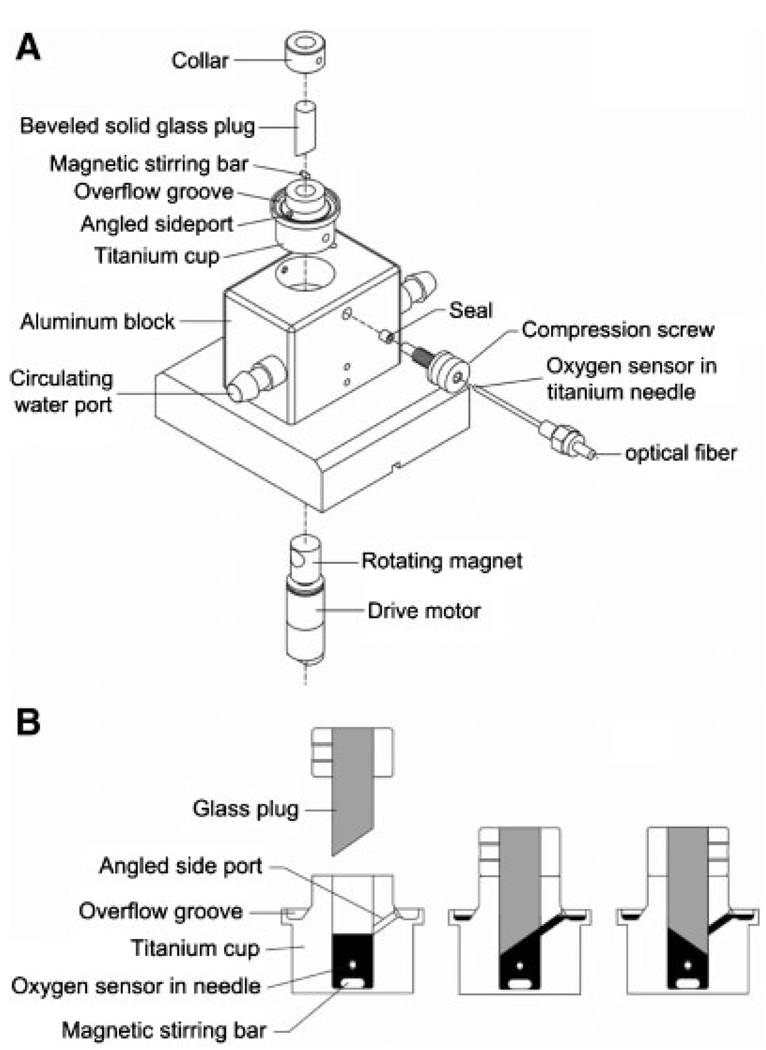
In collaboration with the manufacturer, we designed and tested a variety of stirred chambers. The final design used for all data presented here is now commercially available (Micro Oxygen Uptake System, FO/SYSZ- P250, Instech Laboratories, Plymouth Meeting, PA). The stirred chamber device (Figure 1) was water jacketed for temperature control and was stirred with a glass coated magnetic stirring bar. Titanium was used for the chamber body and for the oxygen probe jacket because the metal is oxygen impermeable, inert, has high thermal conductivity, and will not introduce artifactual drift arising from the surface oxidation. pO2 was measured with a fiber optic oxygen sensor calibrated at 0 and 160 mmHg. The fiber optic sensors were stable, capable of a long-lasting calibration, insensitive to stirring artifacts, and had virtually zero oxygen consumption. With the stirring bar in place, the chamber had a nominal volume of about 200 µL. Exact chamber volume, measured by filling it with water, then carefully removing and weighing the water, ranged from 184 to 219 µL for eight commercial devices. This variation reflected the custom fabrication of device components, especially the magnetic stirring bar. The stirrer speed was controlled by a potentiometer. The stirrer rotational rate (S) was measured with a stroboscope and was related to the potentiometer setting (P) by S = 44.7P–81 rpm. The potentiometer was normally set at “3” out of “10”, corresponding to a rotation rate of 53 rpm that was just enough to suspend the islets off the bottom surface. The oxygen leakage rate of the sealed chambers was zero but could be as high as 0.2 mmHg/min · mmHg if the sealing plug was chipped. Virtually 100% of the tissue added could be recovered from the chamber for use with further measurements.
Figure 1.
Schematic diagram of device for measuring oxygen consumption. A: Exploded view. The device consists of a titanium cup that sits in a water jacketed enclosure for temperature control. After addition of a glass-coated magnetic stirring bar (nominal length 5 mm, diameter 2 mm) into the opening in the cup, a transparent beveled glass plug is placed into the cup opening (diameter 6.4 mm). The void space remaining in the opening defines the chamber that contains the cell or tissue suspensions. Oxygen in the chamber is measured by fluorescence quenching following oxygen binding to a flourophor in a gel overlain by a silicone rubber film at the tip of an optical fiber. The fiber is held inside the chamber by a titanium jacket and elastomeric seal. A magnet attached to a rotating motor is paced within the enclosure in close proximity to the stirring bar. B: Cross section through the titanium cup showing the filling procedure. After the magnetic stirring bar is added, a volume of the cell or tissue suspension corresponding to the chamber volume plus 5–10 µL excess is placed in the chamber (left figure), the beveled plug is inserted, and any excess fluid is expelled through the angled side port and collected in a groone around the cap. When filling is complete, the beveled plug is rotated (right figure) to block access to the port and seal the chamber. If a bubble is observed from the top, the plug is removed, cells or islets are allowed to settle, additional medium is added, and the plug is reinserted as the bubble is washed through the angled side port.
For OCR measurements, islets were centrifuged for 2 min at 173g, the supernatant was removed by vacuuming with a Pasteur pipette, and each sample was resuspended in 250 µL of pre-warmed (37°C) DMEM (Mediatech, 10-013-CM) that contained 4.5 g/L glucose and 0.6 g/l l-glutamine supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 10 mM HEPES, and no added serum (so as to minimize bubble formation). The islet (or cell) suspension was added to the device, the chamber was sealed, and thermal equilibration at 37°C occurred in about 15 s. After a transient increase in pO2 resulting from the decreased oxygen solubility at the higher temperature, pO2 decreased with time. If the tissue viability, as reflected by OCR, did not change during the course of the experiment, and the minimum pO2 in the islet remained far above the Michaelis constant for oxygen consumption (<1 mm Hg), then the slope ΔpO2/Δt was constant. A typical measurement took about 20 min.
The OCR was calculated from:
| (1) |
where Vch is the chamber volume and α is the Bunsen solubility coefficient, taken to be 1.27 nmol/cm3 · mm Hg at 37°C (Avgoustiniatos, 2001). Data above 60 mmHg in the region yielding the steepest slope of pO2 versus time was fitted to a straight line using linear regression. OCR per cell was obtained by dividing both sides of Equation (1) by the number of cells nC in the chamber:
| (2) |
where the quantity nC/Vch is the cell concentration measured, for example, by nuclei counting. The quantity OCR/DNA can be calculated from Equation (2) if the denominator is replaced by DNA concentration in the chamber.
Statistics
All measurements were made with three or more replicates. Data is reported as mean ± SD.
Results
Characterization of the OCR Apparatus and Measurements
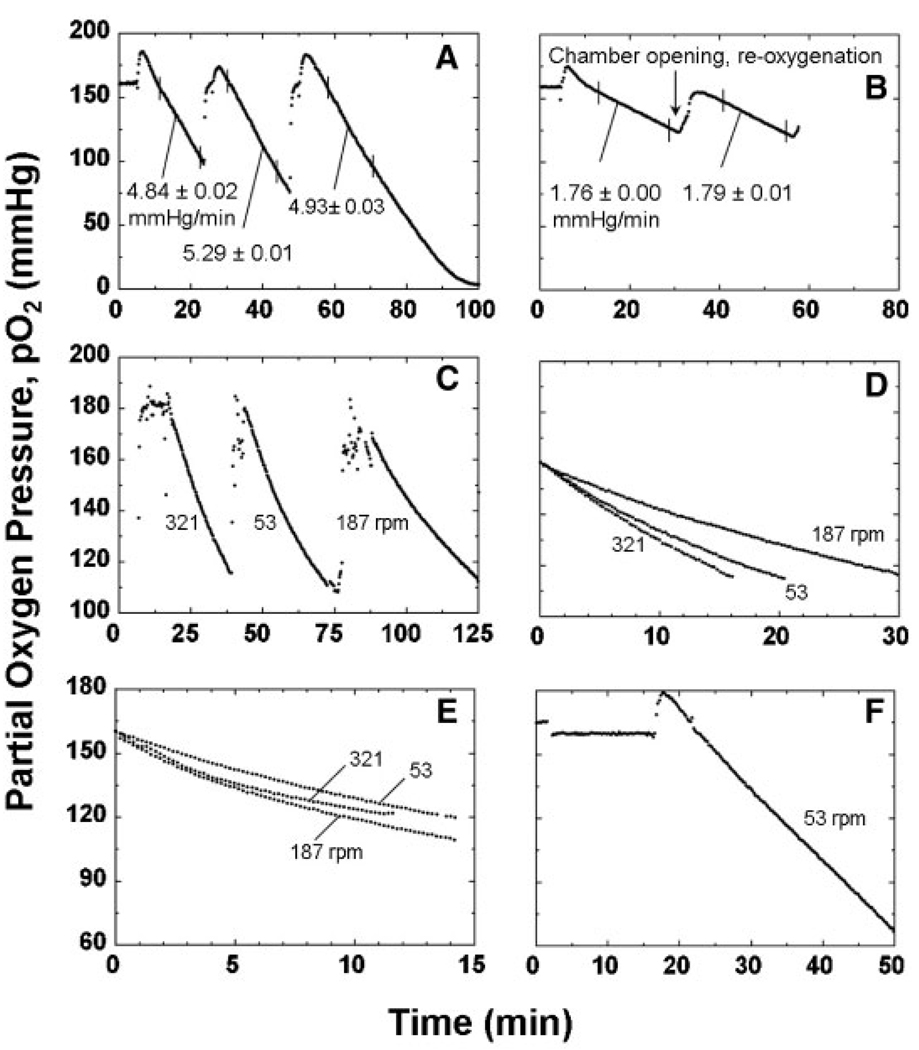
Data from a typical set of OCR measurements made with three different aliquots of rat islets originating from the same sample pool is shown in Figure 2A. The mean of the three slopes was 5.02 ± 0.24 mmHg/min, which corresponded to an OCR of 12.8 ± 0.6 nmol/min. The coefficient of variation (COV) was 0.6% or less for the estimate of the slope of the individual measurements and 4.8% for the triplicate measurements. The mean value of OCR/cell for these measurements was 3.82 ± 0.45 fmol/min · cell. The standard deviation estimate was obtained from propagation of error associated with both the slope and nuclei measurements. Curvature in the data of the third measurement at low pO2 reflected the presence of pO2 gradients within the islets and the reduction in OCR at low pO2 associated with Michaelis–Menten kinetics.
Figure 2.
Actual traces (individual data points) of measured pO2 versus time with rat islet samples. A: OCR measurements, each about 20–50 min with a stirring rotational rate of 53 rpm, were performed with a fresh aliquot obtained from the same islet preparation. The measured cell concentration of the islets in the chamber was 1.6 × 106 cells/mL, corresponding to an islet concentration of 1030 IE/mL. Data were fitted to a straight line in the steepest portion of the trace (indicated by vertical marks), yielding slopes listed n the figure. Points below a pO2 of 60 mmHg were not used in any of the slope estimates to ensure that all cells within the islets were exposed to a high enough pO2 so that OCR could be assumed constant throughout the islet. If the experiment is run long enough to allow pO2 to decrease to much lower values, curvature occurs in the plot of pO2 versus time, as shown in the third measurements, which reflects the interaction of intra-islet oxygen gradients and the decrease in OCR as the local pO2 approaches 0 mmHg. B: Two measurements performed with the same sample, the second one after re-oxygenation, with a stirrer rotational rate of 53 rpm and 1.7 × 106 islet cells/mL, corresponding to 1090 IE/mL. The mean of the slopes was 1.77 mmHg/min, corresponding to an OCR of 4.75 nmol/min and an OCR/cell of 1.33 fmol/min · cell. C: Sequential OCR measurements made 4–6 h after isolation with different samples from the same rat islet preparation at stirring speed settings of, “9,” “3,” and “6”, corresponding to rotational rates of 321, 53, and 187 rpm, respectively. D: Trace of each experiment shown in (C) with time adjusted so that pO2 = 0 at t = 0. E: pO2 versus adjusted time from OCR measurements performed with rat islets made within 10 min after isolation was completed at three stirring speed settings. F: Data from OCR measurement performed with islets from same preparation as used in (E) but made 4 h later.
The linearity of the pO2 profiles with time in the high pO2 regions of Figure 2A indicated that the OCR and the viability of islets in the chamber was constant for the duration of the measurement and was not affected by the experimental procedure. Similar behavior was observed when islets were retained within the chamber for successive measurements. Figure 2B shows data from one experiment in which two consecutive measurements were made with the same rat islets over a 60-min period. After the first measurement was ended, the beveled acrylic plug was removed while stirring was maintained, and the islet suspension in the chamber was allowed to equilibrate with ambient air, after which the plug was replaced. The second measurement was performed with the same sample after re-oxygenation. There was essentially no change in the slope between the first and second measurements and no indication of damage to the islets during the 60-min period in which the islet suspension was stirred. In other experiment with rat and human islets, there was no significant change in membrane integrity by 7-AAD sequential staining measured before or after 15 min stirring at 53 rpm. At much higher stirring speeds, a modest increase in the fraction of cells with compromised membrane integrity was observed with rat but not human islets.
In rare situations, the linearity displayed in Figure 2A and B was not maintained. Figure 2C and D show an example of data from OCR measurements with the rat islet samples from the same preparation, each carried out at a different stirrer speed setting. The profiles of pO2 versus time exhibited curvature, suggesting that islet cell death occurred within the chamber during measurement. The slope after 10 min divided by the initial value (at pO2 = 160 mmHg) ranged from 0.61 to 0.72. Consistent behavior was observed with membrane integrity measurements by 7-AAD sequential staining. The fraction of cells with impermeable membranes was 60% when assayed 4 h after isolation and decreased slowly with time in storage. The fraction ranged from 30 to 38% for the islets recovered from the chamber after OCR measurement. These data suggest that this particular preparation was extensively damaged during isolation and that the samples were further damaged by mechanical agitation during OCR measurement. However, neither the extent of curvature nor the decrease in membrane integrity correlated with the magnitude of the stirring speed.
All data with rat islets shown in Figure 2A – D were obtained 4 h after isolation. A limited number of measurements were made immediately (within 10 min) after isolation. Some of these revealed curvature in profiles of pO2 versus time, whereas the curvature disappeared when samples were tested 4 h later. An example is given in Figure 2E, which shows data from OCR measurements with rat islets from two different preparations carried out within 10 min after completion of isolation at three different stirring speeds. Immediately after isolation, plots of pO2 versus time exhibited significant curvature that was not affected by stirring speed. These data suggest that the decline in OCR with time reflected the presence in the chamber of dying cells that were damaged by the isolation process. OCR measurements performed with islets from the same preparations 4 h later produced a straight line with constant slope (Figure 2F), which suggests that, this phenomenon may be restricted to the period shortly after isolation in most preparations.
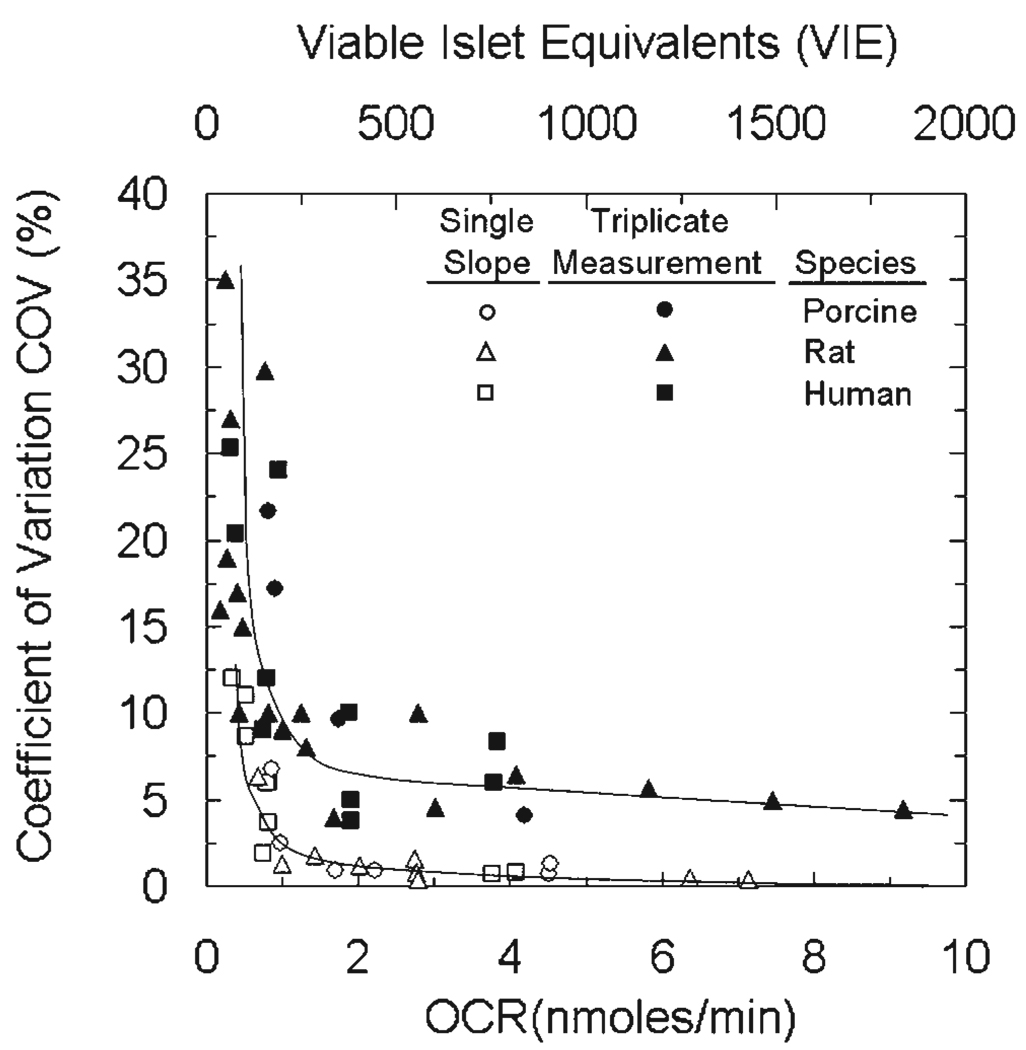
The precision of the OCR measurement in Figure 2A and B was typical throughout this study. The COV from a large number of experiments is plotted versus the measured OCR (bottom) or the corresponding number of viable IE (top) in Figure 3. The COV was calculated as the standard error of the estimate of the slope divided by the estimate of the slope for a single measurement and as the standard deviation divided by the mean of the slope for triplicate measurements conducted with different samples taken from the same islet preparation. Both sets of data followed the same trend for all three species of islets studied: the COV increased slowly with decreasing OCR or number of viable IE and then rapidly at very low OCR or number of islets. The COV was substantially larger for triplicate measurements as compared to the estimate of the slope for a single measurement; this behavior reflected sampling errors and possible heterogeneity in the OCR properties of the islets. Typically, the COV was about 10% with 250 viable IE and 6% with 500 viable IE.
Figure 3.
Precision of the OCR measurement. Coefficient of variation (COV) versus the OCR measured in the experiment (bottom) or the corresponding estimated number of viable islet equivalents (top) for measurements conducted with rat, porcine, and human islets in the OCR measurement apparatus. Data for single and triplicate measurements are shown. For illustrative purposes, the number of viable islet equivalents was calculated assuming (OCR/DNA)viable = 500 nmol/min · mg DNA, 6.5 pg DNA/cell, and 1,560 cells/IE (Pisania, 2007a).
OCR Correlation With Measures of Viability
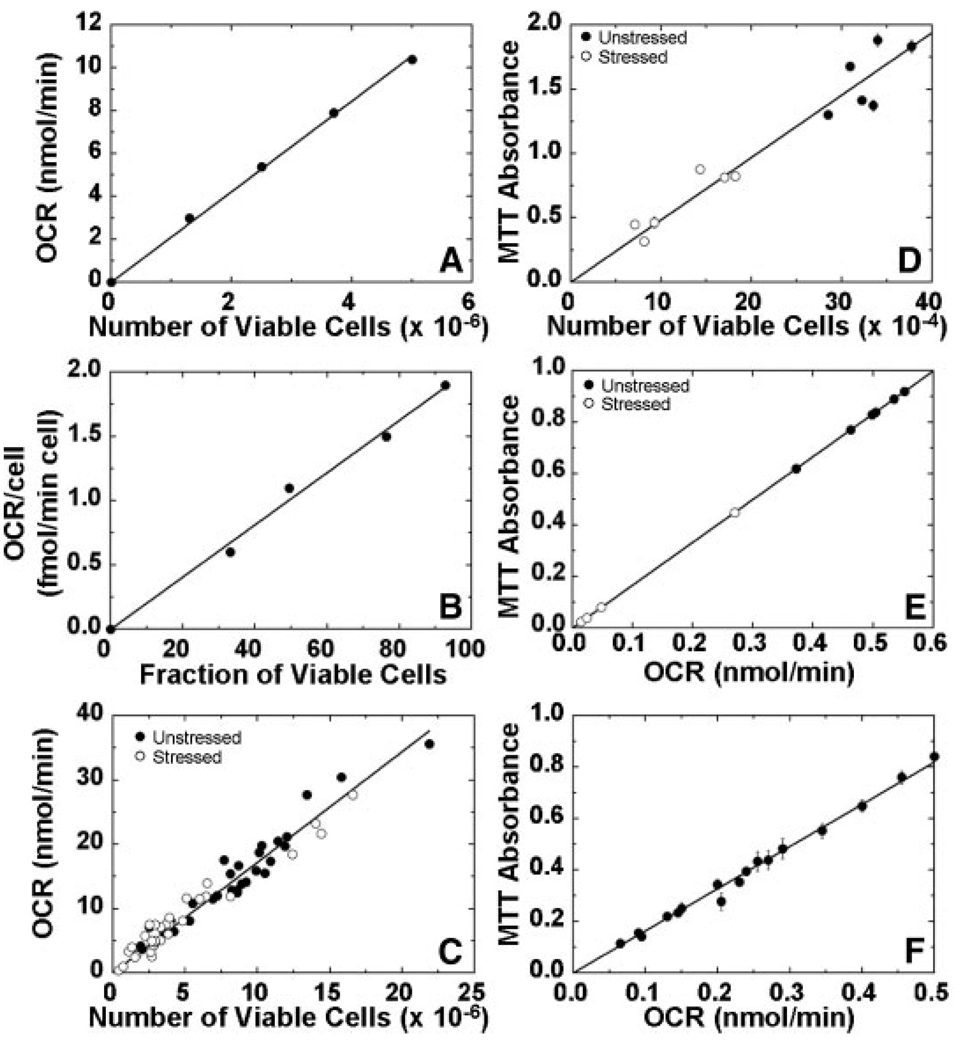
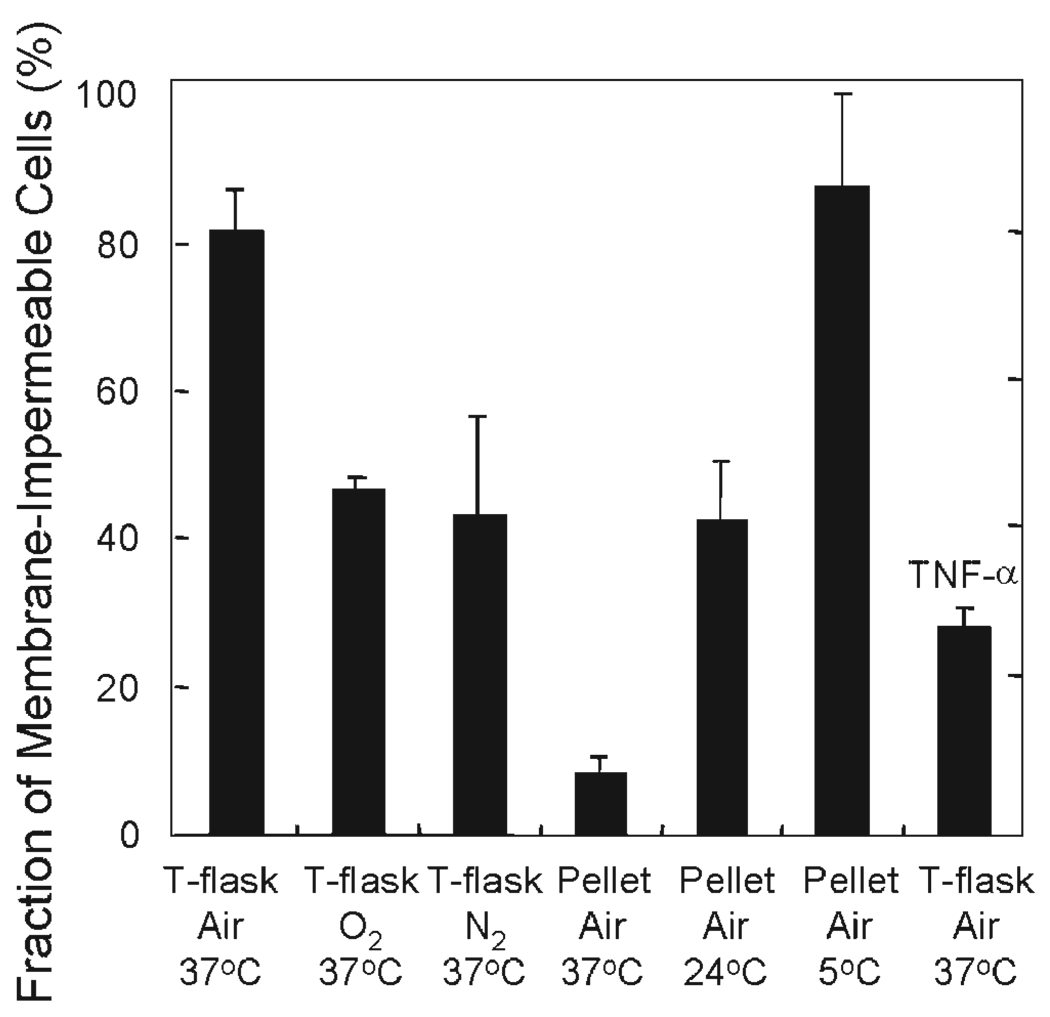
Experiments with cells and islets were used to explore the utility of OCR measurements for assessing the viability of mixtures of live and dead cells and of cells and islets that have been cultured under stressful conditions. As a first step, mixtures of healthy and heat killed βTC3 cells were prepared at predefined ratios. OCR was measured with healthy and heat killed cells as well as their mixtures, a common method for evaluating viability assays (Vandewalle et al., 1999). The number of viable cells was measured with trypan blue membrane integrity. Plots of OCR versus viable cell number and OCR/cell versus fraction viable cells were linear (Figures 4A and B). These data demonstrate the usefulness of OCR measurements for assessing viability with mixtures of live and dead cells. The experiments were extended to include exposure of cells to different types and magnitudes of stresses, including those that islets might be exposed to during isolation, culture, transportation, and transplantation (Papas et al., 2001), which can damage cells but do not kill all of them. The stresses were primarily hypoxia and anoxia, along with hyperoxia, nutrient depletion, and exposure to TNF-α, all for 24 h. Surface-attached cells cultured at 37°C with air and 5% CO2 (used in all experiments) were used as controls. Representative results for the effect of stressful culture conditions are shown in Figure 5. The fraction of cells that were membrane-impermeable after 24 h ranged from more than 85% for the control and for pellets cultured at 5°C to less than 10% for pellets cultured in air at 37°C. Surface attached culture (T-flask) under anoxia at 37°C resulted in a value of 40%; this higher level is consistent with the presence of an active glycolytic pathway in βTC3 cells (Papas et al., 1996, Papas et al., 2001). The much lower value in the pellet in air at 37°C indicates that some other stress in addition to severe hypoxia or anoxia, such as nutrient depletion or toxic waste buildup, must have been operative in the pellet.
Figure 4.
Dependence of OCR on cell viability. In (A, B, and C), viability was assessed by membrane integrity measurements with trypan blue. A: OCR as a function of the number of viable cells and (B) OCR/cell as a function of mixture composition for healthy, heat-treated, and mixtures of healthy and heat-treated βTC3 cells in ratios of 25/75, 50/50, and 75/25. Heat-killed cells were incubated at 60°C for 1 h. C: OCR and viable cell number from various batches of βTC3 cells cultured for 24 h with and without imposed stressful conditions. Aliquots for membrane integrity and corresponding OCR measurement were taken from the same sample. The solid line is the best fit of a line through the origin by linear regression (R2 = 0.983). The estimate of the slope was 1.72 ± 0.03 fmol/min · viable cell. D: Relationship between MTT absorbance and viable cell number. βTC3 cells from a single batch were cultured for 24 h under unstressed (control) or stressed conditions (TNF-α, 500 U/mL culture medium). E: MTT absorbance versus OCR for porcine islets from a single preparation cultured under normal and stressful conditions. Islets were cultured for 24 h in multi-well plates at 37°C with medium depths of 3 and 10 mm and with fractional islet surface coverage ranging from 0.4% (control) to 30%. At the end of the incubation period islets were removed from the corresponding wells for MTT and OCR measurements. F: MTT versus OCR for samples of roughly similar islet volume from 17 different rat islets preparations (R2 = 0.993). In (A) through (F), measurements were made in triplicate. Error bars indicate standard deviation and are contained within domain of symbol if not visible.
Figure 5.
Fraction of βTC3 cells that were membrane-impermeable after culture under normal and a variety of stressful conditions for 24 h. Approximately 2 × 107 βTC3 cells were used in each experiment. For surface-attached cultures, cells were cultured in T-75 flasks in a 37°C incubator at a plating density such that they would not be confluent after 24 h. Medium depth was 2 mm. For pelletized culture, cells were placed in 15 mL centrifuge tubes with 15 mL culture media and let settle by gravity, leading to cell depth of about 3 mm overlain by 11 cm of media. The tubes were sealed and cultured at temperatures of 5, 24, and 37°C. For hyperoxic and anoxic culture, flasks, and tubes were placed in sealed chambers within an incubator, and the sealed chambers were continuously flushed with premixed gases (95% O2 or 95% N2, balance CO2). At the end of the 24-h culture, cells in flasks were trypsinized. All cells from each condition were collected and samples for OCR and trypan blue staining were taken. Fraction of membrane-impermeable cells was determined as the number of cells that did not take up trypan blue divided by the total number of cells counted.
Data from a large number of 24 h culture experiments, such as those illustrated in Figure 5, are summarized in Figure 4C. Measured OCR is plotted versus the number of viable cells in the OCR chamber, which was quantified as the number of cells that excluded trypan blue. Data from cells cultured under both stressed and unstressed conditions clustered about a single straight line for the relationship between OCR and the number of viable cells. The slope of the best straight line through the origin was 1.72 ± 0.03 fmol/min · viable cells. This linear relationship demonstrates that OCR measurement is suitable for assessing viability of cells subjected to different types and levels of stress. This finding was further substantiated with a limited number of experiments using the MTT assay. A linear relationship between MTT absorbance and viable cell number was also observed in these experiments (Figure 4D).
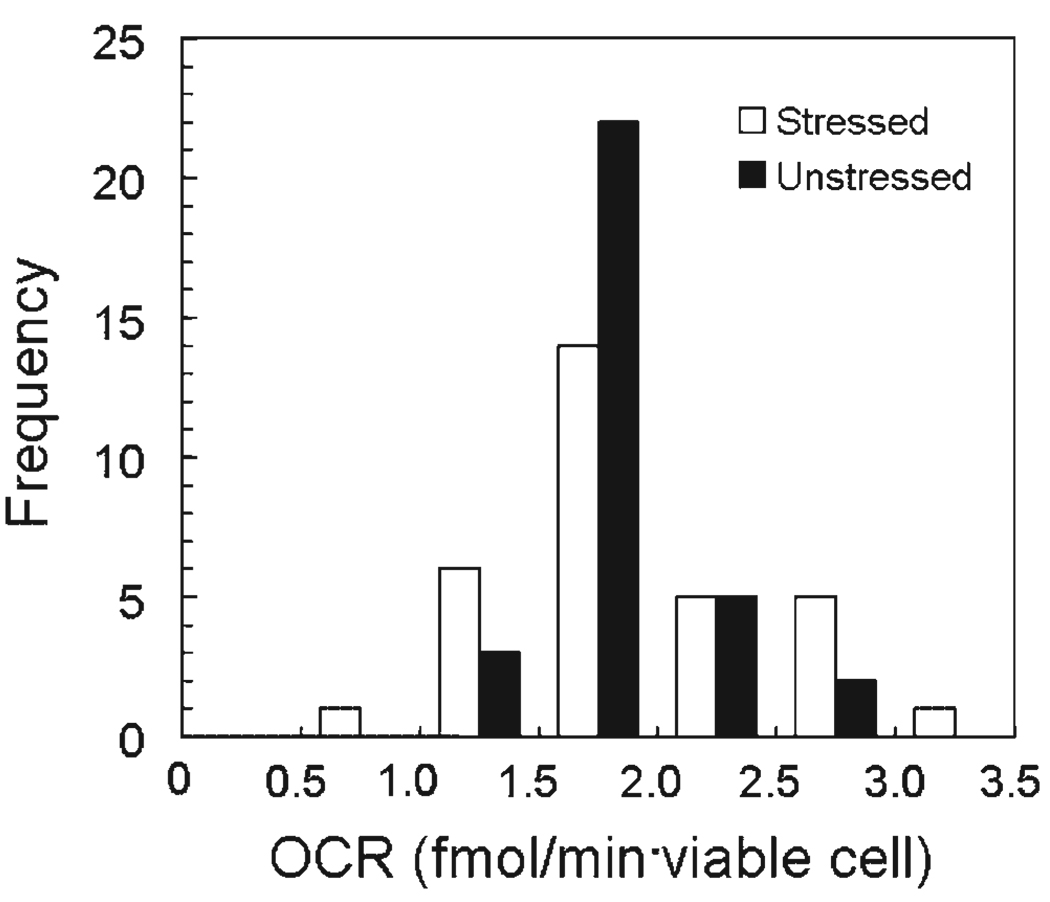
Figure 6 is the frequency distribution of the values obtained for OCR/viable cell for all the conditions examined in Figures 4C and 5. Open bars represent data obtained with the stressed cells and solid bars those obtained with unstressed cells. The values obtained with stressed cells covered a threefold range, whereas those obtained with unstressed cells covered a twofold range. The mean values of OCR for stressed and unstressed cells were not significantly different and the mean value for all measurements was 1.86 ± 0.45 fmol/min · viable cell (n = 64).
Figure 6.
Frequency distribution of OCR per viable cell for stressed and unstressed βTC3 cells cultured for 24 h under stressed and unstressed conditions (Figures 4C and 5). OCR per viable cell was estimated by dividing the measured OCR by the number of viable cells placed in the chamber at the time of the measurement as determined with a hemocytometer using trypan blue exclusion. Values of OCR/viable cell ranged between 1.46 and 2.81 (n = 33) for stressed and 0.94–2.99 fmol/min · viable cell (n = 31) for unstressed cells. Mean values were 1.88 ± 0.54 and 1.84 ± 0.34 fmol/min · viable cell (n = 31) for stressed and unstressed cells, respectively. Fractional viability by trypan blue exclusion averaged 0.95, and the OCR uncorrected for viability was 1.75 ± 0.32 fmol/min · cell. OCR values for stressed cells covered a wider range, but the mean values of OCR for stressed and unstressed cells were not significantly different. The mean value for all measurements was 1.86 ± 0.45 (n = 64).
In the experiments shown in Figures 4D and 5, the fraction of membrane-impermeable cells varied over a wide range as illustrated in Figure 4C The equivalence between OCR/viable cell estimates for both stressed and unstressed cells in Figure 6 suggests that membrane integrity measurements provided a valid estimate of the number of viable cells following 24 h culture under various conditions.
OCR Measurements With Islets
Analogous to the studies with βTC3 cells, experiments were carried out to examine the use of OCR measurements for assessing the viability of islets that have been cultured under stressful conditions. Experiments with stresses of the type used with βTC3 cells (e.g., Figure 5) led to much greater loss of viability in the case of both porcine and rat islets. For example, after 24 h in a pellet at 24°C, rat and porcine islets had OCRs relative to the control of about 15 and 5%, respectively. In contrast, βTC3 cells retained about 45% fractional viability under same culture conditions (Figure 5). This greater sensitivity of islets to hypoxic stress was consistent with there being a relatively inactive glycolytic pathway resulting from the low levels of lactate dehydrogenese in islets (Sekine et al., 1994). Because high-stress experiments of this type resulted in a very large decrease in viability, necessitating use of several thousand islets to get a measurable OCR, graded stresses associated with hypoxia in high-density islet culture were employed. Islets were cultured for 24 h in multi-well plates at 37°C with medium depths of 3 and 10 mm and with fractional islet surface coverages ranging from 0.4% (control) to 30%. Islet viability was measured with the MTT assay, which has been employed by others for islet viability measurement (Vistica et al., 1991; Janjic and Wollheim, 1992; Kumar et al., 1994; van de Loosdrecht et al., 1994; Marshal et al., 1995; Liu et al., 1997; Segu et al., 1998). Fractional viabilities, defined as the MTT absorbance of samples from the test culture relative to that of the control, in experiments with rat islets ranged from about 20 to 70%. In a similar series of high-density culture experiments with porcine islets from a single preparation, both OCR and MTT assays were performed. The data, which is cross-plotted in Figure 4E, demonstrates a linear relationship between OCR and MTT absorbance, thereby substantiating the usefulness of OCR measurements for evaluating the viability of islets subjected to differing amounts of stress. OCR measurements were made with a large number of rat islet preparations, each a mixture of islets from 6 to 20 animals. OCR and MTT measurements were made with a sample from each of 17 different rat preparations (Figure 4F), and the two parameters displayed excellent linear correlations of the entire range of measurements.
Discusssion
Improvements in islet transplantation are seriously hindered by the absence of quantitatively meaningful islet quality assessment methods. Current islet quality assessment methods are operator dependent, subjective, and not predictive of transplantation outcome in humans (Ricordi et al. 2001; FDA, 2000, 2003; Eckhard et al., 2004). In particular, there is need for reliable, practical, predictive assays of islet viability and function that will consistently define the potency of an islet preparation before transplantation (Shapiro and Ricordi, 2004). Currently, fractional viability is routinely estimated by membrane integrity tests, typically FDA/PI, even though the data is operator dependent and unreliable.
In this paper, we describe a novel stirred chamber device for rapidly measuring OCR of islet preparations, which is a measure of the total amount of viable tissue. Using the stirred chamber, we demonstrated that OCR measurements with rat islet samples could be reproducibly conducted in a short period of time (less then an hour for triplicates) with 500 viable IE or less with excellent precision (Figure 3). Measured OCR was in general unaffected by incubation in the OCR chamber with mechanical stirring (Figures 2A and B). In some cases, in particular with infrequent highly damaged islet preparations examined 4 h after isolation and with a few preparations tested immediately after isolation, islet viability decreased during the measurement itself, which was demonstrated by a continuous decline in the slope during the OCR measurement (Figures 2C – F) and by changes in membrane integrity measurements. The extreme fragility observed with a few preparations is consistent with observations using a perfusion chamber (Sweet et al., 2002a) that islets disintegrated and recovery from the system was incomplete when the enzymatic digestion during isolation was deemed to be “less than perfect”. The fragility of islets and their death in the OCR chamber may be an additional and useful indicator of their quality. The observation of curvature in the pO2 trace immediately after isolation but not when examined 4 h later suggests that some of the islets are dying as isolation is completed. This reduction in viability should to be studied in much greater detail, and the OCR chamber is especially useful for this purpose because individual measurements can be made in about 20 min.
Measurements with βTC3 cells demonstrated that the measured OCR is directly proportional to the number of viable cells in mixtures of live and dead cells and correlates linearly with membrane integrity measurements of fractional viability (Figures 4A – C and 5) and MTT viability measurements (Figure 4D) made with cells that have been cultured for 24 h under various stressful conditions. Estimates of OCR/viable cell obtained from all samples of stressed and unstressed cells did not produce a single value. Instead, the data yielded frequency distributions that covered similar ranges with mean values for stressed and unstressed cells that were not significantly different (Figure 6). This finding indicates that when a sample of cells was subjected to stress for 24 h, leading to the reduction in fractional viability for the sample (Figures 4C and 5), a fraction of the cells lost all viability. The remaining viable cells had essentially the same mean value of OCR/viable cell as the original unstressed sample. There was no evidence for a population of cells having a partial reduction of OCR/viable cell.
Measurements with islets similarly demonstrated that the measured OCR is sensitive to damage caused by stress and correlates linearly with MTT viability measurements (Figure 4E) made with porcine islets that had been cultured for 24 h under stressed and unstressed conditions and with samples from a large number of rat islet preparations (Figure 4F). Together with findings from measurements with βTC3 cells, the data indicates that the measured OCR is directly proportional to the number of viable cells in the sample of islets.
OCR measurements in this study were carried out primarily with highly purified (>95%) rat and porcine islet preparations. In human islet transplantation, current practice is to use preparations that have purities as low as 40% in order to maximize the number of islets transplanted. The presence of exocrine tissue in such impure preparations may limit the use of OCR measurements (or any other assay of tissue viability) in characterizing islet quality. Human islet preparations are produced in a high and a low purity fraction, both of which are subsequently mixed prior to transplantation. We hypothesize that islet characterization be conducted on the highly purified fraction, which contains the vast majority of islets in the preparation, since islet quality in the high purity fraction is expected to be representative of that in the entire preparation. This notion is supported by use of the highly purified islet fraction for transplantation in the nude mouse bioassay, which is considered the best assay in islet quality assessment and the only one that currently correlates with transplantation outcome in humans (Ricordi et al., 2001). Furthermore, this hypothesis has been tested and validated in diabetic mouse models with rat and human islets characterized by OCR and DNA measurements and transplanted under the kidney capsule (Papas et al., 2007a,b). Current work is directed towards extending these findings to transplantation in humans.
Acknowledgments
Contract grant sponsor: National Institutes of Health
Contract grant number: NCRR ICR U4Z 16606; ROI-DK063108-01A1
Contract grant sponsor: Juvenile Diabetes Research Foundation
This study was supported in part by grants from the NIH (NCRR ICR U4ZPR 16606 and ROI. KD063108 OIA) and the JDRF Center for Islet Transplantation at Harvard Medical School. We wish to thank Mike Loughnane (Instech Laboratories, Inc.) for helpful discussions and for help with the art work included in the manuscript. Helpful discussions and technical assistance with aspects of the work were provided by Dr Susan Bonner-Weir, John O’Neil, Jennifer Lock (Joslin Diabetes Center, Harvard Medical School), and Daryl Powers, Michael J. Rappel (MIT, Chemical Engineering).
References
- Arain S, Svenja W, Heinzle E, Gernot JT, Krause C, Klimant I. Sensing microplates with optodes: Influence of oxygen exchange between sample, air, and plate material. Biotechnol Bioeng. 1998;90:271–280. doi: 10.1002/bit.20348. [DOI] [PubMed] [Google Scholar]
- Avgoustiniatos ES. Ph.D. Thesis. Cambridge: Massachusetts Institute of Technology; 2001. Oxygen diffusion limitation in pancreatic islet culture and immunoisolation. [Google Scholar]
- Bank HL. Assessment of islet cell viability using fluorescent dyes. Diabetologia. 1987;30:812–816. doi: 10.1007/BF00275748. [DOI] [PubMed] [Google Scholar]
- Bank HL. Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cell Dev Biol. 1988;24:266–273. doi: 10.1007/BF02628826. [DOI] [PubMed] [Google Scholar]
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–482. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- Bretzel RG, Alejandro R, Hering BJ, van Suylichem PT, Ricordi C. Clinical islet transplantation: Guidelines for islet quality control. Transplant Proc. 1994;26:388–392. [PubMed] [Google Scholar]
- Colton CK, Papas KK, Pisania A, Rappel MJ, Powers DE, O’Neil JJ, Omer A, Weir G, Bonner-Weir S. Characterization of islet preparations. In: Halberstadt C, Emerich DF, editors. Cell transplantation from laboratory to clinic. New York: Elsevier, Inc; 2007. pp. 85–132. [Google Scholar]
- Contreras JL, Eckstein C, Smyth CA, Sellers MT, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Thompson AJ, Chaudry IH, Eckhoff DE. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003;52:2935–2943. doi: 10.2337/diabetes.52.12.2935. [DOI] [PubMed] [Google Scholar]
- Dionne KE. Ph.D. Thesis. Cambridge: Massachusetts Institute of Technology; 1989. Effect of hypoxia on insulin secretion and viability of pancreatic islet tissue. [Google Scholar]
- Dionne KE, Colton CK, Yarmush L. A microperifusion system with environmental controls for studying insulin secretion by pancreatic tissue. Biotechnol Prog. 1991;7:359–368. doi: 10.1021/bp00010a011. [DOI] [PubMed] [Google Scholar]
- Eckhard E, Brandhorst D, Winter D, Jaeger C, Jahr H, Bretzel RG, Brendel MD. The role of current product release criteria for identification of human islet preparations suitable for clinical transplantation. Transplantation Proc. 2004;36:1528–1531. doi: 10.1016/j.transproceed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Efrat S, Linde S, Kofod H, Spector D, Delannoy M, Grant S, Hanahan D, Baekkeskov S. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci USA. 1988;85:9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Bryla J, Michalik M, Meglasson MD, Nelson D. Energy metabolism in islets of langerhans. Biochim Biophys Acta. 1992;1101(3):273–295. doi: 10.1016/0005-2728(92)90084-f. [DOI] [PubMed] [Google Scholar]
- FDA. Biological Response Modifiers Advisory Committee Summary Minutes, Meeting no. 26, March, 20–21, 2000. 2000 ( http://www.fda.gov/ohrms/dockets/ac/00minutes/3604m1minutes.pdf).
- FDA. Biological Response Modifier Advisory Committee Summary Minutes, Meeting no. 36, Oct 9–10, 2003. 2003 ( http://www.fda.gov/ohrms/dockets/ac/cber03.html#BiologicalResponseModofiers).
- Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monako AF. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- Guarino RD, Dike LE, Haq TA, Rowley JA, Pitner JB, Timmins MR. Method for determining oxygen consumption rates of static cultures from microplate measurements of pericellular dissolved oxygen concentration. Biotechnol Bioeng. 2004;86:775–787. doi: 10.1002/bit.20072. [DOI] [PubMed] [Google Scholar]
- Hedeskov CJ, Hertz L, Nissen C. The effect of mannoheptulose on glucose-and pyruvate-stimulated oxygen uptake in normal mouse pancreatic islets. Biochim Biophys Acta. 1972;261:388–397. doi: 10.1016/0304-4165(72)90063-3. [DOI] [PubMed] [Google Scholar]
- Hellerstrom C. Oxygen consumption of isolated pancreatic islets of mice studied with the cartesian-diver micro-gasometer. Biochem J. 1966;98:7C–9C. doi: 10.1042/bj0980007c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstrom C. Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Endocrinology. 1967;81:105–112. doi: 10.1210/endo-81-1-105. [DOI] [PubMed] [Google Scholar]
- Hellerstrom C, Andersson A, Welsh M. Respiration of the pancreatic B-cell: Effects of glucose and 2-aminonorbornane-2-carboxylic acid. Horm Metab Res Suppl. 1980;10:37–43. [PubMed] [Google Scholar]
- Hering BJ. Achieving and maintaining insulin independence in human islet transplant recipients. Transplantation. 2005;79:1296–1298. doi: 10.1097/01.tp.0000157321.55375.86. [DOI] [PubMed] [Google Scholar]
- Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, Paraskevas S, Eckman PM, Sageshima J, Nakano M, Sawada T, Mat-sumoto I, Zhang HJ, Sutherland DER, Bluestone JA. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4:390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- Hutton JC, Malaisse WJ. Dynamics of O2 consumption in rat pancreatic islets. Diabetologia. 1980;18:395–405. doi: 10.1007/BF00276821. [DOI] [PubMed] [Google Scholar]
- Hynes J, Floyd S, Soini AE, O’Connor R, Papkovsky DB. Fluorescence-based cell viability screening assays using water-soluble oxygen probes. J Biomol Screen. 2003;8:264–272. doi: 10.1177/1087057103008003004. [DOI] [PubMed] [Google Scholar]
- Janjic D, Wollheim CB. Islet cell metabolism is reflected by the MTT (tetrazolium) colorimetric assay. Diabetologia. 1992;35:482–485. doi: 10.1007/BF02342448. [DOI] [PubMed] [Google Scholar]
- Jorjani P, Ozturk SS. Effects of cell density and temperature on oxygen consumption rate for different mammalian cell lines. Biotechnol Bioeng. 1999;64:349–356. doi: 10.1002/(sici)1097-0290(19990805)64:3<349::aid-bit11>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Kaufman DB, Lowe WL., Jr Clinical islet transplantation. Curr Diab Rep. 2003;3:344–350. doi: 10.1007/s11892-003-0028-7. [DOI] [PubMed] [Google Scholar]
- Knazek RA. The human pancreatic islet cell resource consortium. Diabetes Technol Ther. 2002;4:551–552. doi: 10.1089/152091502760306652. [DOI] [PubMed] [Google Scholar]
- Kumar P, Delfino V, McShane P, Gray DW, Morris PJ. Rapid assessment of islet cell viability by MTT assay after cold storage in different solutions. Transplant Proc. 1994;26:814. [PubMed] [Google Scholar]
- Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lakey JR, Burridge PW, Shapiro AM. Technical aspects of islet preparation and transplantation. Transpl Int. 2003;16:613–632. doi: 10.1007/s00147-003-0651-x. [DOI] [PubMed] [Google Scholar]
- Leonhardt U, Barthel M, Tytko A, Droge M, Siegel EG, Nebendahl K, Kohler H, Creutzfeldt W. Preservation of the porcine pancreas with HTK and Euro-Collins solution: Studies in a reperfusion system. Eur J Clin Invest. 1990;20:536–539. doi: 10.1111/j.1365-2362.1990.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Leonhardt U, Tytko A, Exner B, Barthel M, Stockmann F, Kohler H, Siegel EG, Nebendahl K, Creutzfeldt W. The effect of different solutions for organ preservation on immediate postischemic pancreatic function in vitro. Transplantation. 1993;55:11–14. doi: 10.1097/00007890-199301000-00003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- Marshal MJ, Goodwin CJ, Holt SJA. Critical assessment of the use of microculture trtrazolium assays to measure cell growth and function. Growth Regul. 1995;5:69–84. [PubMed] [Google Scholar]
- Martin J, Yerebakan C, Goebel H, Benk C, Krause M, Derjung G, Lutter G, Siegenthaler M, Beyersdorf F. Viability of the myocardium after twenty-four-hour heart conservation—A preliminary study. Thorac Cardiovasc Surg. 2003;51:196–203. doi: 10.1055/s-2003-42262. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Oh H, Livingston R, Smith K, Abrishamian-Gracia L. Comparative study of the time dependency of cell death assays. MURJ. 2004;11:53–62. [Google Scholar]
- Ohta M, Nelson D, Nelson J, Meglasson MD, Erecinska M. Relationships between energy level and insulin secretion in isolated rat islets of Langerhans. A study at various pH values. Biochem Pharmacol. 1991;42:593–598. doi: 10.1016/0006-2952(91)90322-v. [DOI] [PubMed] [Google Scholar]
- O’Neil JJ, Stegemann P, Nicholson DT, Gagnon KA, Solomon BA, Mullon CJ. The isolation and function of porcine islets from market weight pigs. Cell Transpl. 2001;10:235–246. doi: 10.3727/000000001783986792. [DOI] [PubMed] [Google Scholar]
- Panten U, Klein H. O2 consumption by isolated pancreatic islets, as measured in a microincubation system with a Clark-type electrode. Endocrinology. 1982;111:1595–1600. doi: 10.1210/endo-111-5-1595. [DOI] [PubMed] [Google Scholar]
- Panten U, Zunkler BJ, Scheit S, Kirchhoff K, Lenzen S. Regulation of energy metabolism in pancreatic islets by glucose and tolbutamide. Diabetologia. 1986;29:648–654. doi: 10.1007/BF00869265. [DOI] [PubMed] [Google Scholar]
- Papas KK, Jarema MAC. Glucose-stimulated insulin secretion is not obligatorily linked to an increase in O2 consumption in βHC9 cells. Am J Physiology. 1998;275:E1100–E1106. doi: 10.1152/ajpendo.1998.275.6.E1100. [DOI] [PubMed] [Google Scholar]
- Papas KK, Long RC, Jr, Constantinidis I, Sambanis A. Effects of oxygen on metabolic and secretory activities of βTC3 cells. Biochim Biophys Acta. 1996;1291:163–166. doi: 10.1016/0304-4165(96)00062-1. [DOI] [PubMed] [Google Scholar]
- Papas KK, Long RC, Jr, Sambanis A, Constantinidis I. Towards the development of a bioartificial pancreas: I. Effects of glucose on long-term entrapped βTC3 cell cultures. Biotechol Bioeng. 1999a;66:219–230. [PubMed] [Google Scholar]
- Papas KK, Long RC, Jr, Sambanis A, Constantinidis I. Towards the development of a bioartificial pancreas: II. Effects of oxgyen on long-term entrapped βTC3 cell cultures. Biotechnol Bioeng. 1999b;66:231–237. [PubMed] [Google Scholar]
- Papas KK, Colton CK, Gounarides JS, Roos ES, Jarema MAC, Shapiro AMJ, Cheng LL, Cline GW, Shulman GI, Wu H, Bonner-Weir S, Weir GC. NMR spectroscopy in b cell engineering and islet transplantation. Ann NY Acad Sci. 2001a;944:96–119. doi: 10.1111/j.1749-6632.2001.tb03826.x. [DOI] [PubMed] [Google Scholar]
- Papas KK, Wu H, Colton CK. Rapid islet quality assessment prior to transplantation. Cell Transplant. 2001b;10:519. [Google Scholar]
- Papas KK, Hering BJ, Gunther L, Rappel MJ, Colton CK, Avgoustiniatos ES. Pancreas oxygenation is limited during preservation with the two-layer method. Transplant Proc. 2005;37:3501–3504. doi: 10.1016/j.transproceed.2005.09.085. [DOI] [PubMed] [Google Scholar]
- Papas KK, Colton CK, Nelson RA, Rosack PR, Avgoustiniatos ES, Scott WE, II, Pisania A, Weir GC, Hering BJ. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007a;7(3):703–713. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas KK, Colton CK, Qipo A, Wu H, Auchincloss H, Weir GC, Koulmanda M. Islet oxygen consumption rate and DNA content are predictors of transplantation outcome. 2007b (submitted). [Google Scholar]
- Paraskevas S, Maysinger D, Wand R, Duguid WP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20:270–276. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- Pisania A, Papas KK, Powers DE, Rappel MJ, Bonner-Weir S, Weir GC, Colton CK. Enumeration of islet cells in islet preparations by nuclei counting and microscopic observations. Lab Invest. 2007a doi: 10.1038/labinvest.2010.125. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisania A, Papas KK, Rappel MJ, Powers DE, Weir GC, Colton CK. A quantitative membrane integrity test for islets of Langerhans. Lab Invest. 2007b (submitted). [Google Scholar]
- Ricciardi R, Foley DP, Quarfordt SH, Vittimberga FJ, Kim RD, Donohue SE, Wheeler SM, Anwaruddin S, Callery MR, Meyers WC. Hemodynamic and metabolic variables predict porcine ex vivo liver function. J Surg Res. 2001;96:114–119. doi: 10.1006/jsre.2000.6068. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;34:413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, Lake SP, London NJ, Socci C, Alejandro R. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Lakey JR, Hering BJ. Challenges toward standardization of islet isolation technology. Transplant Proc. 2001;33:1709. doi: 10.1016/s0041-1345(00)02651-8. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Inverardi L, Kenyon NS, Goss J, Bertuzzi F, Alejandro R. Requirements for success in clinical islet transplantation. Transplantation. 2005;79:1298–1300. doi: 10.1097/01.tp.0000157275.64874.84. [DOI] [PubMed] [Google Scholar]
- Schwitalla S, Heres F, Rohl FW, Pfau G, Kessling C. Intraoperative oxygen consumption and organ function in liver transplantation. Anaesthesiol Reanim. 2001;26:88–94. [PubMed] [Google Scholar]
- Segu VB, Li G, Metz SA. Use of a soluble tetrazolium compound to assay metabolic activation of intact beta cells. Metabolism. 1998;47:824–830. doi: 10.1016/s0026-0495(98)90120-2. [DOI] [PubMed] [Google Scholar]
- Sekine N, Cirulli V, Regazzi R, Brown LJ, Gine E, Tamarit-Rodriguez J, Girotti M, Marie S, MacDonald MJ, Wollheim CB, Rutter GA. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- Shapiro AMJ, Ricordi C. Unraveling the secret of single donor success in islet transplantation. Am J Transplant. 2004;4:295–298. doi: 10.1046/j.1600-6143.2003.00375.x. [DOI] [PubMed] [Google Scholar]
- Shapiro AMJ, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosup-pressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- Steinlechner-Maran R, Eberl T, Kunc M, Schrocksnadel H, Margreiter R, Gnaiger E. Respiratory defect as an early event in preservation-reoxygenation injury of endothelial cells. Transplantation. 1997;63:136–142. doi: 10.1097/00007890-199701150-00025. [DOI] [PubMed] [Google Scholar]
- Steurer W, Stadlmann S, Roberts K, Fischer M, Margreiter R, Gnaiger E. Quality assessment of isolated pancreatic rat islets by high-resolution respirometry. Transplant Proc. 1999;31:650. doi: 10.1016/s0041-1345(98)01600-5. [DOI] [PubMed] [Google Scholar]
- Stubenitsky BM, Booster MM, Brasile L, Green EM, Haisch CE, Singh HK, Jacobs RW, Kootstra G. II: Ex vivo viability testing of kidneys after postmortem warm ischemia. ASAIO J. 2000;46:62–64. doi: 10.1097/00002480-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Sutherland DE. Current status of beta-cell replacement therapy (pancreas and islet transplantation) for treatment of diabetes mellitus. Transplant Proc. 2003;35:1625–1627. doi: 10.1016/s0041-1345(03)00563-3. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Cook DL, Wiseman RW, Greenbaum CJ, Lernmark A, Matsu-moto S, Teague JC, Kronh K. A dynamic perifusion to maintain and assess isolated pancreatic islets. Diabetes Technol Ther. 2002a;4:67–76. doi: 10.1089/15209150252924111. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Khalil G, Wallen AR, Steedman M, Schenkman A, Reems JA, Kahn SE, Callis JB. Continuous measurement of oxygen consumption by pancreatic islets. Diabetes Technol Ther. 2002b;4:661–672. doi: 10.1089/152091502320798303. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Cook DL, DeJulio E, Wallen AR, Khalil G, Callis JB, Reems JA. Regulation of ATP/ADP in pancreatic islets. Diabetes. 2004;53:401–409. doi: 10.2337/diabetes.53.2.401. [DOI] [PubMed] [Google Scholar]
- Tytko A, Exner B, Schrock E, Barthel M, Siegel EG, Kohler H, Nebendahl K, Leonhardt U. Hydroxyethyl starch does not improve pancreas preservation with HTK. Langenbecks Arch Chir. 1993;378:82–85. doi: 10.1007/BF00202114. [DOI] [PubMed] [Google Scholar]
- van de Loosdrecht AA, Beelen RH, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MM. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods. 1994;174:311–320. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Vandewalle B, Douillard C, Kerr Conte J, Gmyr V, Riachi R, D’herbomez M, Pattou F, Lefebvre J. Human pancreatic islet quality control: Easy assessment of metabolic functions. Exp Clin Endocrinol Diabetes. 1999;107:214–219. doi: 10.1055/s-0029-1212101. [DOI] [PubMed] [Google Scholar]
- Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991;51:2515–2520. [PubMed] [Google Scholar]
- Wang W, Upshaw L, Strong DM, Robertson RP, Reems JA. Increased oxygen consumption rates in response to high glucose detected by a novel oxygen biosensor system in non-human primate and human islets. J Endocrinol. 2005;185:445–455. doi: 10.1677/joe.1.06092. [DOI] [PubMed] [Google Scholar]
- Weber DJ, McFarland RD, Irony I. Selected Food and Drug Administration review issues for regulation of allogeneic islets of Langerhans as somatic cell therapy. Transplantation. 2002;74:1816–1820. doi: 10.1097/00007890-200212270-00034. [DOI] [PubMed] [Google Scholar]
- Welsh M, Hellerstrom C, Andersson A. Respiration and insulin release in mouse pancreatic islets of mice. Effects of l-leucine and 2-ketoisocaproate in combination with d-glucose and l-glutamine. Bio-chim Biophys Acta. 1982;721:178–184. doi: 10.1016/0167-4889(82)90066-0. [DOI] [PubMed] [Google Scholar]
- Yang H, Jia XM, Acker JP, Lung G, McGann LE. Routine assessment of viability in split-thickness skin. J Burn Care Rehabil. 2000;21:99–104. doi: 10.1097/00004630-200021020-00004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ohkohchi N, Oikawa K, Sasaki K, Satomi S. Assessment of viability of the liver graft in different cardiac arrest models. Transplant Proc. 2000;32:2345–2347. doi: 10.1016/s0041-1345(00)01693-6. [DOI] [PubMed] [Google Scholar]