SUMMARY
Inflammatory cytokines have been proposed to regulate epithelial homeostasis during intestinal inflammation. We report here that interferon-γ (IFN-γ) regulates the crucial homeostatic functions of cell proliferation and apoptosis through serine-threonine protein kinase AKT-β-catenin and Wingless-Int (Wnt)-β-catenin signaling pathways. Short-term exposure of intestinal epithelial cells to IFN-γ resulted in activation of β-catenin through AKT, followed by induction of the secreted Wnt inhibitor Dkk1. Consequently, we observed an increase in Dkk1-mediated apoptosis upon extended IFN-γ treatment, and reduced proliferation through depletion of the Wnt co-receptor LRP6. These effects were enhanced by tumor necrosis factor-α (TNF)-α, suggesting synergism between the two cytokines. Consistent with these results, colitis in vivo was associated with decreased β-catenin-T-cell factor (TCF) signaling, loss of plasma membrane-associated LRP6, and reduced epithelial cell proliferation. Proliferation was partially restored in IFN-γ - deficient mice. Thus, we propose that IFN-γ regulates intestinal epithelial homeostasis by sequential regulation of converging β-catenin signaling pathways.
INTRODUCTION
Self-renewal of the intestinal epithelium is tightly regulated by interacting intracellular signaling pathways, which control stem cell proliferation and cell differentiation (Crosnier et al., 2006). In particular, Wingless-Int (Wnt)-β-catenin signaling has emerged as a key regulator of enterocyte proliferation and survival, and mutations in this pathway are strongly associated with the development of intestinal cancer (de Lau et al., 2007; Logan and Nusse, 2004; Pinto and Clevers, 2005). Interestingly, development of colorectal cancer has also been linked to chronic inflammatory conditions of the intestine such as ulcerative colitis, which is thought to result from accumulating mutations due to ongoing crypt hyper-proliferation and tissue repair (Feagins et al., 2009).
A key feature of such intestinal inflammation is a persistently increased expression of mucosal cytokines, in association with altered epithelial homeostasis, particularly as the disease progresses from acute to chronic phase. Most notably, decreased epithelial proliferation is observed in the early stages of colitis, whereas increased crypt epithelial turn-over is seen during chronic inflammation (Renes et al., 2002; Serafini et al., 1981). How the inflammatory milieu contributes to these opposing effects on epithelial cell proliferation is not understood. However, there is mounting evidence that cytokines play important roles in regulating intestinal epithelial homeostasis during inflammation. For example, (interleukin-6) IL-6 and IL-22 have recently been shown to promote epithelial proliferation and carcinogenesis through activation of Signal Transducer and Activator of Transcription-3 (STAT3) (Grivennikov et al., 2009; Pickert et al., 2009). Conversely, two major pro-inflammatory cytokines, interferon-γ (IFN-γ) and tumor necrosis factor-α TNF-α), are known to negatively regulate the barrier properties and self-renewal of the intestinal epithelium, thus modulating epithelial homeostasis and exacerbating mucosal inflammation (Bruewer et al., 2006; Capaldo and Nusrat, 2009; Kaiser and Polk, 1997; Ruemmele et al., 1998).
We now report that IFN-γ, in synergy with TNF-α, exerts a bi-phasic effect on intestinal epithelial cell proliferation and apoptosis, by sequential modulation of the serine-threonine protein kinase AKT-β-catenin and Wnt-β-catenin signaling pathways. At the onset of inflammation, IFN-γ activated β-catenin through phosphoinositide-3 kinase (PI3K) and AKT, which in turn facilitated the induction of the secreted Wnt antagonist Dkk1 in the colonic mucosa. Consequently, we observed that degradation of the Dkk1-low-density lipoprotein receptor-related protein 6 (LRP6) ligand-receptor-complex inhibited epithelial cell proliferation and promoted apoptosis, despite continued AKT-β-catenin activation. Thus, the extended activation of AKT resulted in a shift from an early pro-proliferative to a delayed anti-proliferative phenotype, both in tissue culture and in an animal model of acute intestinal inflammation. These results demonstrate that the pro-inflammatory cytokines IFN-γ and TNF-α are key regulators of β-catenin signaling and epithelial homeostasis during intestinal mucosal inflammation.
RESULTS
Prolonged intestinal inflammation inhibits IEC proliferation and promotes cell death
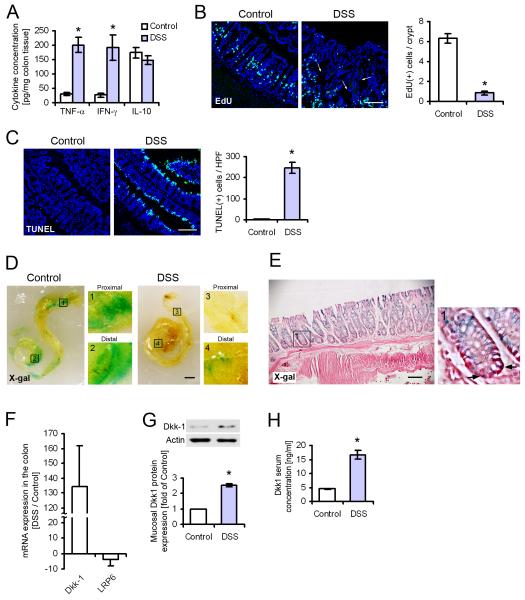
Extended exposure of intestinal epithelial cells (IEC) to pro-inflammatory cytokines, as seen in human inflammatory bowel disease and animal models of intestinal inflammation, dysregulates epithelial homeostasis and exacerbates disease progression. To study the homeostasis of the intestinal epithelium during inflammation in vivo, we used the murine dextran sulfate sodium (DSS) colitis model of acute epithelial injury and mucosal inflammation. We established a baseline for these studies by treating animals with DSS for 7 days, which resulted in clinical symptoms resembling the active phase of human inflammatory bowel disease, including bloody diarrhea, mucosal ulceration, and extensive crypt loss. Because intestinal inflammation is associated with high mucosal concentrations of inflammatory cytokines, whole colon tissue samples were screened for a panel of 22 chemokines and cytokines known to regulate inflammation and epithelial homeostasis (Figure 1A; Figure S1A and B). The pro-inflammatory cytokines IFN-γ and TNF-α were found to be among the most highly induced mediators in the inflamed tissue (6.6-fold and 7.1-fold of healthy control, respectively); in contrast, the concentration of anti-inflammatory interleukin-10 (IL-10) was unchanged compared to control animals at this time point. Both IFN-γ and TNF-α are known to regulate IEC proliferation and apoptosis (Kaiser and Polk, 1997; Ruemmele et al., 1998), and we observed that after 7 days of DSS treatment, the overall number of proliferating IEC was substantially reduced (Figure 1B). At the same time, the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells at the luminal interface was greatly increased compared to untreated control animals, indicating massive cell death in response to DSS treatment (Figure 1C). Because β-catenin is a key molecule in the regulation of intestinal homeostasis, we next examined the effect of prolonged inflammation on this pathway using β-catenin-T-cell factor (TCF) reporter (BAT-gal) mice (Figure 1D). Largely uniform X-Gal staining was seen in the entire colon of unchallenged control animals. In contrast, almost no staining was observed in the colon of mice treated with DSS for 7 days, indicating that β-catenin signaling is greatly reduced in the inflamed intestine. Microscopic analysis of tissue sections confirmed that the reporter was exclusively expressed in epithelial cells (Figure 1E); in contrast, no X-gal staining was observed in the submucosa and muscularis mucosa. Previous reports indicate that the potent Wnt inhibitor Dkk1 is induced by inflammatory cytokines, and regulates inflammation in vivo (Diarra et al., 2007; Gollob et al., 2005). We therefore measured the transcription of Dkk1, and of the Dkk-Wnt co-receptor LRP6 by real-time RT-PCR of mRNA from colonic samples (Figure 1F). We found that Dkk1 mRNA was dramatically enhanced after 7 days of DSS treatment. In contrast, LRP6 mRNA was markedly down-regulated in the inflamed tissue. To confirm that Dkk1 expression was induced at the protein level, and to demonstrate the possibility of Dkk1 signaling in intestinal epithelial cells, we analyzed epithelial cell lysates from healthy and DSS-treated mice by immunoblot analysis, and serum samples from both groups by ELISA (Figure 1G and H, respectively). Consistent with our PCR data, we found that Dkk1 protein was substantially increased during inflammation in both tissue and serum. Taken together, these results suggest that inflammatory cytokines are associated with attenuated IEC proliferation and increased apoptosis, which may be facilitated by inhibition of the Wnt-β-catenin pathway during prolonged inflammation.
Figure 1.
Reduced epithelial cell proliferation during intestinal inflammation is associated with high mucosal concentrations of inflammatory cytokines and reduced β-catenin signaling. (A) Whole colon cytokine concentrations in healthy and inflamed mice (7 days of DSS treatment) were determined using a multiplex protein array (see also Figure S1). TNF-α and IFN-γ were among the most highly induced cytokines after 7 days of DSS treatment, whereas the anti-inflammatory IL-10 was unchanged. (B) Proliferation in healthy and inflamed colon epithelium was determined by EdU incorporation for 2 hours. Inflammation substantially reduced the number of proliferating enterocytes per crypt. Arrows indicate the crypt bottom in the inflamed tissue. Scale bar, 100μm. (C) Cell death in the same tissue as above was determined by TUNEL staining. A dramatic increase of dead epithelial cells at the crypt surface was observed in response to DSS treatment. Scale bar, 100μm. (D) Whole colons from BAT-gal mice were stained with X-gal (blue) to reveal regions of active β-catenin-TCF signaling. Almost no staining was seen after 7 days of DSS challenge. Magnified images show highlighted areas in the proximal and distal colon. Images are representative of 3 animals per group. Scale bar, 2mm. (E) Frozen sections from control animals were stained with X-gal, and then counter-stained with eosin (magenta). X-gal-positive nuclei of epithelial cells are indicated with arrows in the magnified panel on the right. Scale bar, 100μm. (F) mRNA was isolated from whole colon tissue, and analyzed by real-time RT-PCR. Transcription of Dkk1 and the Dkk-Wnt co-receptor LRP6 were considerably changed during DSS colitis. (G) Dkk1 protein levels in mucosal epithelial cells were substantially increased after 7 days of DSS challenge. (H) Dkk1 concentration in serum from the peripheral blood was determined by ELISA. Protein expression was increased after DSS colitis. Data in all graphs in this figure are represented as mean ± s.e.m. * P < 0.001 vs control (Student’s t-test).
IFN-γ and TNF-α synergistically regulate proliferation and apoptosis of IEC in vitro
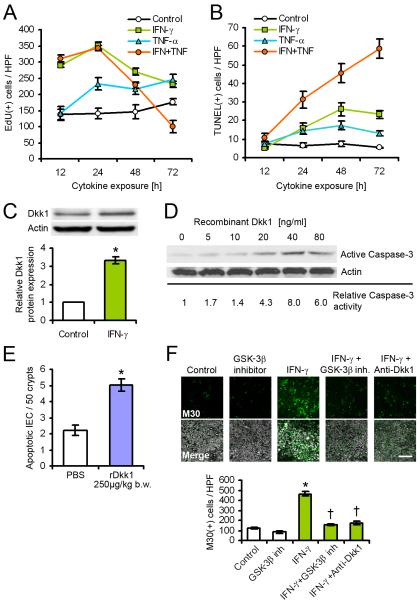
To study the kinetics of IEC regulation by inflammatory cytokines, we treated model intestinal epithelial T84 cells with recombinant IFN-γ and TNF-α, and determined proliferation and apoptosis at consecutive time points. We found that during the entire observation period (72h), IFN-γ strongly increased the proliferation of IEC with a peak at 24h, as indicated by EdU incorporation (Figure 2A). Similarly, extended TNF-α treatment enhanced proliferation, although this effect was delayed (24h) and not as dramatic as with IFN-γ. Interestingly, co-treatment with IFN-γ and TNF-α showed an early increase in proliferation that peaked at 12-24 h, and a subsequent drop in the number of EdU-positive cells at 48-72h. Because both proliferation and apoptosis are required to maintain epithelial homeostasis, we next assessed the effect of inflammatory cytokines on IEC apoptosis (Figure 2B). As previously reported (Ruemmele et al., 1998), IFN-γ alone substantially increased apoptosis compared to untreated control from 24-72h, as indicated by TUNEL staining. Additionally, co-treatment with IFN-γ and TNF-α showed a synergistic effect that dramatically enhanced cell death, with increasing amounts of TUNEL-positive cells over the entire observation period. Our in vivo data show that Dkk1 expression is strongly enhanced during intestinal inflammation. Thus, to investigate the role of Dkk1 in vitro, we treated T84 cells with IFN-γ, and determined Dkk1 protein levels after 72h (Figure 2C). In agreement with the in vivo results, cytokine treatment strongly induced Dkk1 expression. In addition, we found that exposure of IEC to recombinant Dkk1 (rDkk1) dose-dependently induced apoptosis, as indicated by Caspase-3 cleavage (Figure 2D and (Koch et al., 2009)). To confirm the pro-apoptotic potential of Dkk1 in vivo, healthy mice received a single intraperitoneal injection of rDkk1 or PBS, and the number of apoptotic IEC was determined by cleaved (active) Caspase-3 staining after 16 hours (Figure 2E). Consistent with the in vitro data, we observed that rDkk1 increased apoptosis in the large intestine. We therefore hypothesized that IFN-γ-induced apoptosis may be mediated by inhibition of Wnt-β-catenin signaling through Dkk1. Indeed, treatment of IEC with either a functional Dkk1 antibody, or an inhibitor of the downstream effector kinase GSK-3β, efficiently prevented apoptosis of T84 cells in the presence of IFN-γ (Figure 2F), compared to untreated control. DMSO carrier and IgG isotype controls were run separately, and yielded results comparable to no treatment control (data not shown). In summary, these results indicate that inflammatory cytokines regulate IEC proliferation and apoptosis through induction of Dkk1.
Figure 2.
Regulation of epithelial cell proliferation and apoptosis by inflammatory cytokines is associated with inhibition of Wnt signaling. (A) Proliferation of T84 intestinal epithelial cells was determined by EdU incorporation for 2 hours. IFN-γ elicited a biphasic response after extended treatment, which was potentiated by TNF-α. (B) Apoptosis of T84 cells was determined by TUNEL staining. IFN-γ and TNF-α synergistically enhanced cell death over the observation period. (C) Dkk1 protein expression in T84 cells was induced by IFN-γ treatment for 72 hours. (D) Recombinant Dkk1 dose-dependently induced Caspase-3 activation in T84 cells after 72 hours. (E) Mice were treated with recombinant Dkk1 (rDkk1) or PBS for 16 hours, and apoptosis was determined by active Caspase-3 staining. The graph shows results from >1000 crypts of two mice per group. (F) IFN-γ-induced apoptosis in T84 cells after 72 hours was inhibited by co-treatment with GSK-3β inhibitor AR-A014418, or a functional Dkk1 antibody. Apoptosis was assessed by M30 staining. Scale bar, 100μm. Data in all graphs in this figure are represented as mean ± s.e.m. * P < 0.001 vs control (Student’s t-test); † P < 0.01 vs IFN-γ (ANOVA with Dunnett’s post-test).
IFN-γ and TNF-α regulate AKT-β-catenin and Wnt-β-catenin signaling in a temporally distinct fashion
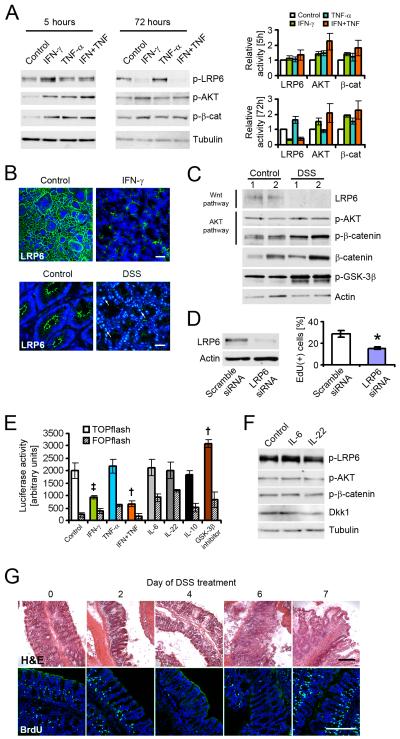
Our results suggested that inflammatory cytokines elicit a bi-phasic proliferative and apoptotic response in intestinal epithelial cells. To explore the underlying signaling pathways, T84 cells were treated with cytokines for 5, 24, or 72h, and the activity of major signaling molecules involved in Wnt and AKT signaling were then determined by immunoblot (Figure 3A; Figure S2A). In agreement with previous findings (Kaur et al., 2008a), AKT was rapidly and persistently activated by IFN-γ, as shown by increased phosphorylation of AKT residue Thr308 at all time points. This effect was facilitated through PI3K (Nguyen et al., 2001), as there was complete loss of IFN-γ-induced AKT activation in the presence of PI3K inhibitor LY294002 (Figure S2B). Interestingly, wheras TNF-α alone had a limited effect on AKT activity, co-treatment with IFN-γ enhanced AKT phosphorylation at 5h and 72h of cytokine exposure. Consequently, phosphorylation of β-catenin by AKT at residue Ser552, which indicates transcriptional activation of the protein (He et al., 2007), was increased in cells treated with both cytokines in all experiments (Figure 3A). In contrast, phosphorylation of LRP6 at residue Ser1490 indicative of active Wnt signaling (Tamai et al., 2004) was decreased as early as 24h in the presence of IFN-γ and TNF-α (Figure S2A). This coincided with increased expression of Dkk1 at this time point. After 72h, almost no LRP6 activity was observed when cells were treated with IFN-γ, and this effect was enhanced after co-treatment with TNF-α. In contrast, no changes in the protein expression of the Dkk1 receptor Kremen-1 were observed in these experiments (Figure S2A), or during inflammation in vivo (data not shown). Because the reduction of LRP6 activity may be caused be a loss of total protein, the effect of IFN-γ treatment in vitro and inflammation in vivo on LRP6 expression was investigated by immunofluorescence microscopy (Figure 3B). LRP6 was almost completely absent from T84 cells treated with IFN-γ for 72h. This was mediated by Dkk1, rather than a direct cytokine effect, as evidenced by a striking loss of LRP6 in intestinal epithelial cells after 1 hour in the presence of recombinant Dkk1, but not IFN-γ (Figure S2C). Likewise, little LRP6 was detectable in the lateral membrane of colonic enterocytes from mice treated with DSS for 7 days, although we observed increased nuclear LRP6 staining consistent with endodomain shedding (Mi and Johnson, 2007). To confirm these observations, IEC lysates from healthy or inflamed tissue were analyzed by immunoblot (Figure 3C). In agreement with the data shown in Figure 3B, LRP6 protein was virtually undetectable in animals treated with DSS for 7 days, whereas AKT and β-catenin activity were increased. Interestingly, although the AKT phosphorylation target GSK-3β was more inactive in the inflamed tissue, we did not observe substantial changes in total amounts of β-catenin protein. Furthermore, β-catenin expression or activation did not correlate with GSK-3β activity. These observations suggest that although GSK-3β may be a key molecule in regulating chronic inflammation, β-catenin protein stability is of secondary importance at the time points investigated here. We next asked if loss of LRP6 is sufficient to inhibit IEC proliferation. Indeed, downregulation of LRP6 in Caco-2 cells using siRNA caused a substantial reduction of EdU incorporation (Figure 3D). We additionally investigated the effect of IFN-γ using a β-catenin-TCF reporter assay (TOPflash), and included IL-6 and IL-22, which have been shown to regulate epithelial cell proliferation through STAT3 (Grivennikov et al., 2009; Pickert et al., 2009) (Figure 3E). SK-CO15 model intestinal epithelial cells were transfected with the reporter plasmid, and treated with cytokines for 24h. IFN-γ, but not IL-6, IL-22, or IL-10 substantially inhibited TCF activity. TNF-α, which by itself had no discernible effect, synergistically increased the influence of IFN-γ The effect of IL-6 and IL-22 on AKT and Wnt signaling after 24h was additionally investigated by immunoblot (Figure 3F), with no apparent change in the activity of LRP6, AKT, and β-catenin, or the expression of Dkk1. Taken together, these data suggest that at early time points, IFN-γ and TNF-α promote IEC proliferation by activating AKT-β-catenin signaling, but inhibit proliferation after prolonged exposure by inhibition of Wnt-β-catenin through Dkk1 upregulation. To investigate if this temporal regulation could be observed in vivo, mice were challenged with DSS, and epithelial turn-over on subsequent days was determined by 5-bromo-2-deoxyuridine (BrdU) incorporation (Figure 3G, and Figure S2D). We observed a slight increase in BrdU-positive cells on days 1-3, but an almost complete absence of proliferating enterocytes on days 4-6. Interestingly, while there was very little proliferation in most of the epithelium at the later time points, there was an increase in IEC proliferation on day 7, and a dramatic increase in BrdU incorporation in crypts adjacent to mucosal ulcers (fig S2D). We additionally investigated cell death in these experiments by TUNEL staining (Figure S2E). No DNA fragmentation was observed on days 0-4; in contrast, pronounced TUNEL staining was seen on days 5-7, coinciding with marked histological damage illustrated in figure 3G and Figure S2D. Taken together, these data suggest that IFN-γ and TNF-α elicit a bi-phasic effect on IEC proliferation, by sequential regulation of β-catenin signaling pathways.
Figure 3.
Inflammatory cytokines differentially regulate AKT and Wnt signaling pathways. (A) Activity of LRP6 (phosphorylation of Ser1490), AKT (phosphorylation of Thr308), and β-catenin (phosphorylation of Ser552) in T84 cells was analyzed after 5 and 72 hours. IFN-γ activated AKT-β-catenin signaling, but inhibited Wnt activity. TNF-α partially amplified this effect. (B) Upper panels, immunofluorescence microscopy revealed a loss of LRP6 protein in T84 cells after treatment with IFN-γ for 72 hours. Scale bar, 50μm. Lower panels, DSS challenge in vivo for 7 days resulted in a loss of LRP6 from the apical and lateral membrane of colonic enterocytes. Arrows highlight nuclear LRP6 staining. Scale bar, 20μm. (C) Loss of LRP6 after DSS treatment was confirmed by immunoblot of mucosal epithelial cells. Additionally, inflammation increased the activity of AKT and β-catenin. Samples were taken from two animals per group. (D) LRP6 was targeted in Caco-2 cells using specific siRNA. Loss of LRP6 caused a substantial drop in cell proliferation, as indicated by EdU incorporation for 1 hour. (E) β-catenin transcriptional activation was assessed using a TOPflash assay in SK-CO15 intestinal epithelial cells. Cells were treated as indicated for 24 hours. (F) Immunoblot analysis revealed no effect of IL-6 and IL-22 on Wnt and AKT signaling after 24 hours of treatment. Blots are representative of three experiments. (G) Temporal regulation of enterocyte proliferation during DSS colitis was determined by BrdU incorporation for 1 hour. Corresponding H&E stainings demonstrate the severity of colitis. The lowest number of BrdU-positive epithelial cells was observed on day 4. Images are representative of three animals per time point. Scale bars, 200μm. Data in all graphs in this figure are represented as mean ± s.e.m. * P < 0.01 vs control (Student’s t-test); † P < 0.05, ‡ P = 0.06 vs control (ANOVA with Dunnett’s post-test). For additional information please see also Figure S2 in the supplemental material.
Inflammatory cytokines regulate converging β-catenin signaling pathways
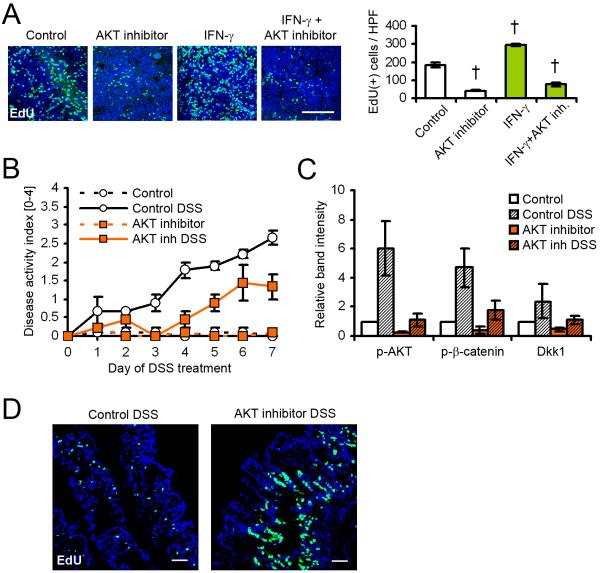
To further evaluate the contribution of AKT signaling to the increased IEC proliferation after short-term cytokine treatment, T84 cells were treated with IFN-γ and the AKT inhibitor triciribine for 24h (Figure 4A). As can be seen, AKT inhibition dramatically decreased the proliferation of IEC in the absence of cytokine compared to DMSO control, and almost completely inhibited IFN-γ-induced proliferation, suggesting a critical role for AKT signaling in intestinal epithelial homeostasis. The potency of the AKT inhibitor, as well as its effect on β-catenin phosphorylation, was investigated by immunoblot (Figure S3A). AKT and β-catenin phosphorylation on residues Thr308 and Ser552, respectively, was efficiently inhibited in the presence of the inhibitor after 4 hours, compared to DMSO control. In the same experiment, we also observed that AKT inhibition reduced the expression of IFN-γ-induced Dkk1. Because these results indicated that Dkk1 expression is, in part, controlled by AKT, we investigated the effect of AKT inhibition in vivo (Figure 4B-D). Mice receiving DSS and triciribine developed substantially milder colitis, as indicated by reduced disease activity index (Figure 4B), and decreased colon weight/length ratios (data not shown). Consistent with the cell culture data, AKT inhibition reduced AKT and β-catenin phosphorylation, and consequently, decreased Dkk1 expression (Figure 4C). Finally, 5-ethynyl-2-deoxyuridine (EdU) incorporation revealed strongly increased crypt cell proliferation in animals treated with the AKT inhibitor during DSS colitis (Figure 4D). Taken together, these in vivo and in vitro data suggest the following model of cytokine-mediated regulation of IEC proliferation and apoptosis: In the healthy intestinal epithelium, tissue homeostasis is maintained by integrated outside-to-inside signaling pathways, including the AKT and Wnt pathway. At the onset of inflammation, exposure of intestinal epithelial cells to inflammatory cytokines, primarily IFN-γ and TNF-α promotes proliferation by activation of β-catenin signaling through PI3K-AKT (Scoville et al., 2008). Prolonged inflammation thus results in the induction of β-catenin-TCF target genes, including the secreted Wnt inhibitor Dkk1. Consequently, the accumulation of Dkk1 in the intestinal mucosa leads to inhibition of Wnt-β-catenin signaling by internalization and degradation of LRP6 (Mao et al., 2002; Niehrs, 2006), resulting in a shift from an early pro-proliferative to a delayed pro-apoptotic phenotype with reduced proliferation. This effect may be exacerbated by additional secretion of Dkk1 from non-epithelial cells, such as infiltrating leukocytes and platelets (Figure S3B) (Voorzanger-Rousselot et al., 2009), and the inhibition of GSK-3β by AKT. Thus, inflammatory cytokines inhibit IEC proliferation during sustained intestinal inflammation by inhibiting the Wnt-β-catenin signaling pathway.
Figure 4.
Epithelial cell proliferation and Dkk1 expression are controlled by AKT. (A) T84 cell proliferation in the presence of AKT inhibitor triciribine and IFN-γ was determined by EdU incorporation for 2 hours following treatment for 22 hours. Inhibition of AKT signaling attenuated IFN-γ-induced proliferation. Scale bar, 100μm. (B) AKT inhibition ameliorated intestinal inflammation in vivo. The graph shows the results from three mice per group. (C) Immunoblot analysis of mucosal lysates showed strongly inhibited AKT and β-catenin activity, as well as Dkk1 expression, in mice receiving DSS and AKT inhibitor. (D) EdU incorporation for 2 hours revealed increased crypt cell proliferation in animals treated with triciribine. Scale bars, 50μm. Data in all graphs in this figure are represented as mean ± s.e.m. † P < 0.01 vs control (ANOVA with Dunnett’s post-test). For additional information please see also Figure S3 in the supplemental material.
Loss of IFN-γ decreases intestinal inflammation in vivo
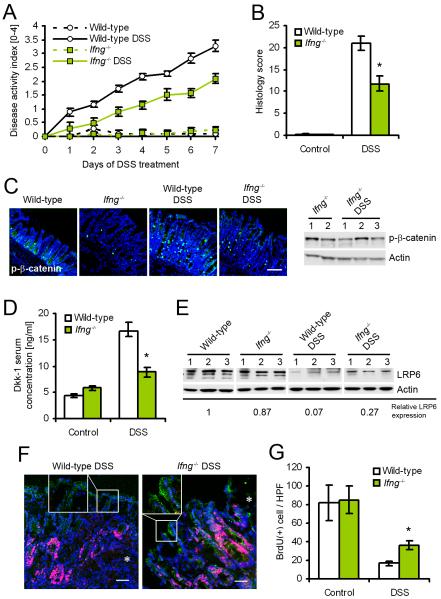
To test the model, we challenged IFN-γ-deficient (Ifng−/−) mice and wild-type controls with DSS for 7 days. We hypothesized that IFN-γ may exacerbate intestinal inflammation in vivo by regulating AKT-β-catenin and Wnt-β-catenin signaling, and conversely, loss of IFN-γ may ameliorate disease progression. We determined that in the DSS colitis model, IFN-γ is primarily secreted by CD8+ T cells and NK cells at the onset of inflammation (24h post challenge) (Figure S4A). Additional limited expression of IFN-γ was observed in a subset of dendritic cells and neutrophils, consistent with previous reports (Chan et al., 2006; Yin and Ferguson, 2009). In contrast, after 7d of DSS treatment, IFN-γ is strongly expressed in most lymphocytes (Figure S4B), as shown in a recent study on viral infection of mucosal epithelial cells (Nakanishi et al., 2009). As reported previously (Ito et al., 2006), inflammation was substantially decreased in Ifng−/− mice, as indicated by a reduced combined disease activity score (Figure 5A) and blinded histology scores (Figure 5B). We hypothesized that loss of IFN-γ decreases the activity of AKT-β-catenin signaling, and consequently, reduces the induction of Dkk1 during inflammation. Indeed, DSS treatment of wild-type mice for 7 days greatly increased the number of phospho-β-catenin Ser552 staining in enterocytes (Figure 5C). In contrast, considerably less phospho-β-catenin staining was observed in Ifng−/− mice, which was confirmed by immunoblot analysis of epithelial cell lysates. Additionally, there were substantially reduced concentrations of Dkk1 in the sera of Ifng−/− mice compared to wild-type animals after DSS challenge (Figure 5D). Consequently, we observed a partial rescue of LRP6 protein levels in the Ifng−/− mice after 7 days of DSS treatment, compared to wild-type animals (Figure 5E). To exclude the possibility that increased LRP6 protein levels might simply be the result of decreased inflammation in Ifng−/− mice, we investigated LRP6 expression and IEC proliferation in histological samples with matched disease activity (Figure 5F, and Figure S4C). LRP6 expression in the lateral membrane of enterocytes was strongly enhanced both in acutely ulcerated areas and non-inflamed tissue of Ifng−/− mice, compared to wild-type controls. This coincided with an extension of the proliferative zone, as indicated by Ki67 staining. Finally, BrdU incorporation assay after 7 days of DSS treatment revealed a partial rescue of enterocyte proliferation in Ifng−/− mice (Figure 5G), but no detectable difference in TUNEL-positive cells (data not shown). Taken together, these data suggest that loss of IFN-γ protects against intestinal inflammation by preventing the activation of AKT-β-catenin signaling, and consequently the inhibition of Wnt-β-catenin by induction of Dkk1. This conclusion was further supported by the observation that treatment of Ifng−/− mice with recombinant Dkk1 reduced IEC proliferation and exacerbated DSS colitis (data not shown), consistent with previous reports (Kuhnert et al., 2004; Pinto et al., 2003). In summary, these results show that reduced intestinal inflammation in Ifng−/− mice is associated with decreased Dkk1 expression, and consequently increased IEC proliferation.
Figure 5.
IFN-γ-deficient mice are protected from DSS colitis, which coincides with increased Wnt signaling and proliferation. (A) Combined disease activity index of wild-type and IFN-γ-deficient mice (Ifng−/−) during DSS colitis. Ifng−/− animals showed a substantially ameliorated course of disease over the entire observation period. (B) Histological scoring after 7 days of DSS challenge revealed greatly reduced severity of disease in Ifng−/− mice. (C) Absence of IFN-γ inhibited the activation of β-catenin (phosphorylation of Ser552) after DSS challenge, as shown by immunofluorescence microscopy (left) and immunoblot (right). (D) Induction of Dkk1 during inflammation was substantially reduced in Ifng−/− mice, as shown by ELISA of serum from the peripheral blood. (E) Immunoblot of mucosal epithelial cells from three mice per group revealed a partial rescue of LRP6 expression in Ifng−/− mice. (F) Immunofluorescence staining of LRP6 (green) and Ki67 (red) revealed increased lateral membrane association of LRP6 and an extension of the proliferative crypt compartment in histologically comparable tissue from Ifng−/− mice. Asterisks indicate the margin of mucosal ulcers. (G) Proliferation of colonic enterocytes, as shown by BrdU incorporation for 1 hour, was partially rescued in Ifng−/− mice after DSS treatment for 7 days. Data in all graphs in this figure are represented as mean ± s.e.m. * P < 0.01 vs wild-type (Student’s t-test). For additional information please see also Figure S4 in the supplemental material.
DISCUSSION
Cytokines such as IFN-γ and TNF-α are critical mediators of inflammatory disorders. We and others have previously shown that IFN-γ contributes to the epithelial barrier defect associated with inflammatory bowel disease, by disassembling tight junctions and reducing the rate of intestinal epithelial cell migration (Gunzel et al., 2006; Tong et al., 2005; Utech et al., 2005; Wang et al., 2005). Furthermore, it is well-established that both cytokines regulate intestinal epithelial cell proliferation and apoptosis in vitro and in vivo (Kaiser and Polk, 1997; Ruemmele et al., 1998; Seno et al., 2009; Turner, 2006).
In this report, we demonstrate that IFN-γ exacerbates intestinal inflammation in vivo by temporally distinct regulation of converging β-catenin signaling pathways. Using cell culture and mouse colitis models, we show that cytokine exposure leads to an early and sustained activation of AKT-β-catenin, which is likely initiated by phosphorylation of PI3K by IFN-γ receptor-associated Jak1 and 2 kinases (Kaur et al., 2008b; Lekmine et al., 2004), and results in increased IEC proliferation. This effect is mainly mediated by IFN-γ, and potentiated by TNF-α, which by itself does not appreciably affect epithelial homeostasis. This study demonstrates that both cytokines co-operate to promote proliferation through AKT-β-catenin, although synergy of IFN-γ of TNF-α has been reported in other contexts, such as nuclear factor (NF)-κB signaling (Fish et al., 1999; Ganster et al., 2005; Robinson et al., 2003; Schmitz et al., 1999). Conversely, we observed that extended exposure to inflammatory cytokines inhibits epithelial cell proliferation and promotes IEC apoptosis, despite continued AKT-β-catenin signaling. We propose that this is achieved by induction of the Wnt inhibitor Dkk1, which is a direct transcriptional target of β-catenin-TCF (Niida et al., 2004). Indeed, in agreement with previous studies (Diarra et al., 2007; Gollob et al., 2005), we observed that Dkk1 expression is dramatically up-regulated upon cytokine exposure in vitro and in vivo. Effective Wnt inhibition in epithelial cells during inflammation was indicated by an almost complete loss of the Wnt-Dkk1 co-receptor LRP6, as the current model of Dkk1 signaling postulates internalization and degradation of the ligand-receptor complex (Niehrs, 2006). Accordingly, inhibition of Dkk1 in vivo has been shown to be beneficial in inflammatory arthritis (Diarra et al., 2007), and we observed that Dkk1 hypomorphic doubleridge mice (MacDonald et al., 2004) are protected from DSS colitis, and demonstrate accelerated mucosal wound healing after DSS withdrawal (unpublished data).
Active Wnt-β-catenin signaling is required for intestinal epithelial homeostasis, and aberrant Wnt signaling has been shown to be strongly associated with the development of intestinal cancers (Barker et al., 2009; de Lau et al., 2007). However, we show here that prolonged cytokine exposure inhibits the Wnt pathway and TCF-mediated gene transcription. In light of this observation, it is interesting to note that we and others observed that crypts adjacent to ulcers show a high rate of proliferation (Renes et al., 2002; Seno et al., 2009), suggesting that these “hot spots” may be the origins of colorectal cancer seen in long-standing inflammatory bowel disease. In this context, it is also noteworthy that Dkk1 in the inflamed tissue may be derived from sources other than epithelial cells, including inflammatory macrophages (Diarra et al., 2007), platelets (Voorzanger-Rousselot et al., 2009), and neutrophils (Figure S3B). This suggests that inflammatory cell infiltrates actively inhibit epithelial cells proliferation by secretion of Dkk1. Indeed, a recent report shows that treatment of mice with R-spondin1, an antagonist of Dkk1, ameliorated DSS colitis, which was associated with increased IEC proliferation (Zhao et al., 2007).
Consistent with our model and a previous report (Ito et al., 2006), IFN-γ cytokine-deficient mice exhibited a less severe progression of experimental colitis. This coincided with reduced release of Dkk1, and higher rates of proliferation in the intestinal epithelium. It is not surprising that such animals are not completely protected from intestinal disease, for a number of reasons. For example, Dkk1 may also be induced by TNF-α (Diarra et al., 2007), or other inflammatory mediators that have not been investigated in this study. Furthermore, other factors contributing to disease manifestation and severity, including tissue damage caused by invading neutrophils, may not be affected by IFN-γ. A most interesting observation is that IEC proliferation during inflammation exhibits a multi-phasic phenotype, with high proliferation in the beginning, followed by a period with almost no proliferation, and a late recovery phase despite continued cytokine exposure. A similar pattern of proliferation has in fact been observed in patients with inflammatory bowel disease, and it has been proposed that increased proliferation during active colitis is responsible for pre-neoplastic lesions (Serafini et al., 1981). We propose that these temporally discrete changes in epithelial turn-over rates are facilitated by alternating integrated responses in β-catenin signaling. Alternatively, there is evidence from in vitro studies showing that extended treatment of intestinal epithelial cell lines with IFN-γ and TNF-α can change their response from growth arrest to high proliferation (Seidelin et al., 2004), an effect which may be facilitated by changes in MAP kinase signaling or cytokine receptor presentation (Kaiser et al., 1999; Pimentel-Muinos and Seed, 1999). Additionally, it has been proposed that cytokine secretion itself may fluctuate over time (Nakamura et al., 1992), thereby regulating proliferation and apoptosis during different stages of inflammation. Finally, other cytokines such as IL-6 and IL-22 have been shown to promote intestinal epithelial cell proliferation and carcinogenesis through STAT3 (Grivennikov et al., 2009; Pickert et al., 2009). Although we show here that these cytokines do not regulate β-catenin signaling, at least in the time frame of our experiments, it is possible that during chronic colitis, crypt hyper-proliferation and remodeling are driven by both STAT and β-catenin signaling.
In summary, we show that IFN-γ and TNF-α synergistically regulate epithelial homeostasis during intestinal inflammation, by differential control of AKT-β-catenin and Wnt-β-catenin signaling pathways. These observations provide new insight into the signaling pathways governing intestinal epithelial cell turn-over, and likely have important implications for the pathology of inflammatory bowel disease, and inflammation-associated tumorigenesis.
EXPERIMENTAL PROCEDURES
Antibodies and reagents
Primary antibodies were obtained from the following sources: BD Pharmingen (CD4, CD8, CD11b, CD11c, Ly6G, NK1.1, IFN-γ), Cell Signaling (LRP6, phospho-LRP6 Ser1490, phospho-AKT Thr308), Novus Biologicals (anti-human Dkk1), R&D Systems (anti-mouse Dkk1), Roche Pharmaceuticals (M30), Santa Cruz (Kremen-1), and Sigma-Aldrich (Actin, Caspase-3, tubulin). The antibody against phospho-β-catenin Ser552 was produced as described previously (He et al., 2007). Secondary antibodies were purchased from Invitrogen and Jackson ImmunoResearch. The functional inhibitory Dkk1 antibody was obtained from R&D Systems, and used at a concentration of 20μg/ml. Recombinant mouse Dkk1 carrying the Strep-tag (Schmidt and Skerra, 2007) was prepared by refolding of inclusion bodies expressed in E. coli, followed by purification via streptavidin affinity chromatography and gel filtration. Recombinant human IFN-γ was provided by Genentech, and used at a concentration of 100U/ml. Recombinant human TNF-α was purchased from BioVision, and used at a concentration of 50ng/ml. Recombinant human IL-6, IL-10, and IL-22 were bought from BioLegend, and used at a concentration of 100ng/ml. LRP6 and scrambled siRNA SMARTpools were obtained from Dharmacon, and transfected using TransIT siQUEST (Mirus Bio). Primers for real-time RT-PCR were purchased from IDT (sequences are as follows: Dkk1 forward 5′-TAC AAT GAT GGC TCT CTG CAG CCT-3′; Dkk1 reverse 5′-GCA GGT TCT TGA TCG CGT TGG AAT-3′; LRP6 forward 5′-AAG CAG TGA TCT CTC AGG TGC CAA-3′; LRP6 reverse 5′-ATT CGA GCC TGG ACC TTG GTT CTT-3′). AKT inhibitor triciribine, PI3K inhibitor LY294002, and GSK-3β inhibitor AR-A014418 were obtained from Calbiochem, and used according to the supplier’s recommendation.
Cell culture
Intestinal epithelial T84 cells were grown in 1:1 DMEM and modified Ham’s F-12 medium, and SK-CO15 and Caco-2 cell were grown in DMEM with 10% fetal calf serum and antibiotics. Cells were maintained in a humidified incubator with 5% CO2. For functional studies, cells were seeded onto collagen-coated, permeable filters (Costar) or glass cover slips.
Animal experiments
All procedures using animals were reviewed and approved by the Emory University Institutional Animal Care and Use Committee (IACUC), and were performed according to NIH criteria. β-catenin-TCF reporter mice (BAT-gal) (Maretto et al., 2003), IFN-γ cytokine-deficient mice, and matching control mice with a C57Bl/6J genetic background were obtained from The Jackson Laboratories. Animals were housed in a standard day and night cycle, with free access to food and water. Experimental colitis was induced by addition of 3% wt/vol dextran sulfate sodium (DSS; US Biologicals) to the drinking water. Disease activity was monitored by daily assessment of a combined disease activity index (Cooper et al., 1993). Histological specimens were analyzed in blinded fashion by a trained surgical GI pathologist (A.N.), and scored as described previously (Dieleman et al., 1998). Detailed information on disease activity and histological scoring, as well as assessment of additional clinical parameters from experiments with IFN−/− mice, can be found in the supplementary table S1, and Figure S4D-F). Intestinal epithelial cells were isolated from washed colon segments by repeated incubation with 50mM EDTA (Sigma-Aldrich) in HBSS. mRNA was isolated from whole colon samples by mechanical destruction of snap-frozen tissue specimens into IsolRNA Lysis Reagent (5 Prime), followed by phenol and chloroform extraction. Serum Dkk1 concentrations were determined by ELISA using antibodies from R&D Systems, with recombinant mouse Dkk1 as standard. β-galactosidase expression in BAT-gal mice was visualized by 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-gal) staining, essentially as described previously (Byrne et al., 1994). Briefly, tissue specimens were fixed with 3.7% wt/vol paraformaldehyde (PFA), incubated with X-gal staining solution overnight, and then post-fixed in PFA before further processing. Intestinal epithelial cell apoptosis was induced by i.p. injection of 250μg/kg body weight recombinant mouse Dkk1, with PBS as control. AKT was inhibited by daily i.p. injection of 2mg/kg body weight triciribine, with DMSO as control.
Assessment of proliferation and apoptosis
Proliferation was determined in vivo by intraperitoneal injection of 100μg 5-ethynyl-2-deoxyuridine (EdU, Invitrogen) or 50mg/kg body weight 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich). EdU incorporation was determined using a Click-iT EdU Cell Proliferation Kit (Invitrogen), according to the supplier’s instructions. BrdU was detected following EtOH and glycine fixation of 5μm frozen sections of intestinal mucosa. Specimens were digested with 0.05% trypsin, and antigen was retrieved by denaturation with 4M HCl. Neutralized slides were then incubated with a fluorophore-labeled anti-BrdU antibody (Roche Pharmaceuticals). Proliferation in vitro was assessed by EdU incorporation. Apoptosis in vivo was determined by staining with an antibody against cleaved (active) Caspase-3 (R&D Systems). Cell death was determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), using an In Situ Cell Death Detection Kit (Roche Pharmaceuticals), according to the supplier’s guidelines.
Immunoblot
Samples were resuspended in Ripa lysis buffer (150mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 50mM Tris pH 8.0) containing protease and phosphatase inhibitors (Sigma), sonicated, and cleared by centrifugation. Protein concentration was determined using a BCA protein assay, and samples were boiled in SDS sample buffer with 50mM dithiothreitol. Equal amounts of protein were separated by SDS-PAGE, and transferred onto nitrocellulose membranes. Membranes were blocked for 1h with 5% wt/vol dry milk or BSA in Tris-buffered saline containing 0.1% Tween-20, and incubated with primary antibodies in blocking buffer overnight at 4°C.
Immunofluorescence microscopy
Cells grown on cover-slips or transparent filter inserts were fixed and permeabilized with either 100% methanol or ethanol at −20°C for 20 minutes, or PFA 3.7% wt/vol for 10 minutes followed by 0.5% vol/vol Triton X-100 for 5 minutes. Tissue sections were fixed with PFA, then permeabilized with 100% ethanol. Samples were then blocked with 2% wt/vol BSA for 1h, and incubated with primary antibodies overnight at 4°C. Following incubation with fluorophore-labeled secondary antibodies for 1h, nuclei were stained with ToPro-3 iodide (Molecular Probes), and cover-slips were mounted in p-phenylene. Images were taken on an LSM 510 confocal microscope (Zeiss) with Plan-NEOFLUAR 100x/1.3 oil, 40x/1.3 oil, and 20x/0.5 dry objectives, using software supplied by the vendor.
In vivo cytokine screen
Whole colon samples were homogenized into Hanks’ buffer containing 0.1% Triton X-100, as well as protease and phosphatase inhibitors. Cytokines were detected using a customized MILLIPLEX MAP Mouse Cytokine/Chemokine Panel (Millipore), according to the supplier’s guidelines.
Statistics
Dunnett’s post-test following one-way ANOVA, or two-tailed Student’s t-test was used to analyze the data. P < 0.05 was considered statistically significant. Results are displayed as mean ± standard error of the means (s.e.m.).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank K. den Beste and A. Akyildiz for expert technical assistance, and J. Brazil for support with cell isolation. A.S. wishes to thank C. Niehrs (DKFZ, Heidelberg, Germany) for kindly providing the mouse Dkk1 cDNA.
Supported by grants from the National Institutes of Health (DK72564, DK61379, and DK79392 to C.A.P.), (DK53202, DK55679, and DK59888 to A.N.), DK64399 (NIH DDRC tissue culture and morphology grant), the Crohn’s and Colitis Foundation of America (fellowship award to P.N., S.K., and C.T.C.), and the German Research Foundation (Deutsche Forschungsgemeinschaft, Bonn, Germany - La 2359/1-1 to M.G.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- Byrne C, Tainsky M, Fuchs E. Programming gene expression in developing epidermis. Development. 1994;120:2369–2383. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–491. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- Fish SM, Proujansky R, Reenstra WW. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–198. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganster RW, Guo Z, Shao L, Geller DA. Differential effects of TNF-alpha and IFN-gamma on gene transcription mediated by NF-kappaB-Stat1 interactions. J Interferon Cytokine Res. 2005;25:707–719. doi: 10.1089/jir.2005.25.707. [DOI] [PubMed] [Google Scholar]
- Gollob JA, Sciambi CJ, Huang Z, Dressman HK. Gene expression changes and signaling events associated with the direct antimelanoma effect of IFN-gamma. Cancer Res. 2005;65:8869–8877. doi: 10.1158/0008-5472.CAN-05-1387. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzel D, Florian P, Richter JF, Troeger H, Schulzke JD, Fromm M, Gitter AH. Restitution of single-cell defects in the mouse colon epithelium differs from that of cultured cells. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1496–1507. doi: 10.1152/ajpregu.00470.2005. [DOI] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Kita M, Ueda Y, Iwakura Y, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–338. doi: 10.1111/j.1365-2249.2006.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser GC, Polk DB. Tumor necrosis factor alpha regulates proliferation in a mouse intestinal cell line. Gastroenterology. 1997;112:1231–1240. doi: 10.1016/s0016-5085(97)70135-5. [DOI] [PubMed] [Google Scholar]
- Kaiser GC, Yan F, Polk DB. Conversion of TNF alpha from antiproliferative to proliferative ligand in mouse intestinal epithelial cells by regulating mitogen-activated protein kinase. Exp Cell Res. 1999;249:349–358. doi: 10.1006/excr.1999.4488. [DOI] [PubMed] [Google Scholar]
- Kaur S, Sassano A, Dolniak B, Joshi S, Majchrzak-Kita B, Baker DP, Hay N, Fish EN, Platanias LC. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc Natl Acad Sci U S A. 2008a;105:4808–4813. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Sassano A, Joseph AM, Majchrzak-Kita B, Eklund EA, Verma A, Brachmann SM, Fish EN, Platanias LC. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J Immunol. 2008b;181:7316–7323. doi: 10.4049/jimmunol.181.10.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Capaldo CT, Samarin S, Nava P, Neumaier I, Skerra A, Sacks DB, Parkos CA, Nusrat A. Dkk-1 inhibits intestinal epithelial cell migration by attenuating directional polarization of leading edge cells. Mol Biol Cell. 2009;20:4816–4825. doi: 10.1091/mbc.E09-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekmine F, Sassano A, Uddin S, Smith J, Majchrzak B, Brachmann SM, Hay N, Fish EN, Platanias LC. Interferon-gamma engages the p70 S6 kinase to regulate phosphorylation of the 40S S6 ribosomal protein. Exp Cell Res. 2004;295:173–182. doi: 10.1016/j.yexcr.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi K, Johnson GV. Regulated proteolytic processing of LRP6 results in release of its intracellular domain. J Neurochem. 2007;101:517–529. doi: 10.1111/j.1471-4159.2007.04447.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Saito H, Kasanuki J, Tamura Y, Yoshida S. Cytokine production in patients with inflammatory bowel disease. Gut. 1992;33:933–937. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J Biol Chem. 2001;276:33361–33368. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel-Muinos FX, Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783–793. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
- Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306:357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renes IB, Verburg M, Van Nispen DJ, Taminiau JA, Buller HA, Dekker J, Einerhand AW. Epithelial proliferation, cell death, and gene expression in experimental colitis: alterations in carbonic anhydrase I, mucin MUC2, and trefoil factor 3 expression. Int J Colorectal Dis. 2002;17:317–326. doi: 10.1007/s00384-002-0409-4. [DOI] [PubMed] [Google Scholar]
- Robinson CM, Shirey KA, Carlin JM. Synergistic transcriptional activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2003;23:413–421. doi: 10.1089/107999003322277829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruemmele FM, Gurbindo C, Mansour AM, Marchand R, Levy E, Seidman EG. Effects of interferon gamma on growth, apoptosis, and MHC class II expression of immature rat intestinal crypt (IEC-6) cells. J Cell Physiol. 1998;176:120–126. doi: 10.1002/(SICI)1097-4652(199807)176:1<120::AID-JCP14>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Schmidt TG, Skerra A. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat Protoc. 2007;2:1528–1535. doi: 10.1038/nprot.2007.209. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112(Pt 1):137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Seidelin JB, Jaattela M, Nielsen OH. Continuous interferon-gamma or tumor necrosis factor-alpha exposure of enterocytes attenuates cell death responses. Cytokine. 2004;27:113–119. doi: 10.1016/j.cyto.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini EP, Kirk AP, Chambers TJ. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981;22:648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tong Q, Vassilieva EV, Ivanov AI, Wang Z, Brown GT, Parkos CA, Nusrat A. Interferon-gamma inhibits T84 epithelial cell migration by redirecting transcytosis of beta1 integrin from the migrating leading edge. J Immunol. 2005;175:4030–4038. doi: 10.4049/jimmunol.175.6.4030. [DOI] [PubMed] [Google Scholar]
- Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorzanger-Rousselot N, Goehrig D, Facon T, Clezardin P, Garnero P. Platelet is a major contributor to circulating levels of Dickkopf-1: clinical implications in patients with multiple myeloma. Br J Haematol. 2009;145:264–266. doi: 10.1111/j.1365-2141.2009.07587.x. [DOI] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Ferguson TA. Identification of an IFN-gamma-producing neutrophil early in the response to Listeria monocytogenes. J Immunol. 2009;182:7069–7073. doi: 10.4049/jimmunol.0802410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, de Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, Kim KA, Liu Y, Levy MD, Berg DJ, et al. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.