Abstract
The auditory brainstem response (ABR) is an evoked potential response of auditory activity in the auditory nerve and subsequent fiber tracts and nuclei within the auditory brainstem pathways. The threshold, amplitude, and latency analysis of the ABR provides information on the peripheral hearing status and the integrity of brainstem pathways. In this study, we compared the threshold, amplitude, and latency of ABRs recorded from 149 mice of 10 commonly used inbred strains (BALB/cJ, C3HeB/FeJ, C3H/HeJ, CAST/EiJ, CBA/CaJ, CBA/J, FVB/ NJ, MRL/MpJ, NZB/BlNJ, and SJL/J) using clicks of different intensities. The ABR thresholds of these strains ranged from 32 to 43 dB SPL. The amplitude of both waves I and IV of ABRs, which increased monotonically with click intensity in most strains, differed significantly among different strains at each intensity tested. Moreover, the amplitude of both waves was inversely correlated with the body weight of each strain at most intensities tested. In general, the amplitude of wave IV was smaller than that of wave I resulting in the IV/I amplitude ratio of <1.0 in all strains. The peak latency of both waves I and IV decreased significantly with click intensity in each strain. However, this intensity-dependent decrease was greater for wave IV than for wave I such that the wave I–IV inter-peak latency also decreased significantly with increasing intensity. I–IV inter-peak latencies for MRL/MpJ, C3HeB/FeJ, NZB/BlNJ, and C3H/HeJ strains are longer than FVB/NJ, SJL/J, or CAST/EiJ. This work is the first step to study the genetic basis underlying strain-related differences in auditory pathway.
Keywords: Mouse, Inbred strain, Auditory brainstem response, Inter-peak latency, Amplitude
1. Introduction
The auditory brainstem response (ABR) is an evoked potential measurement of auditory activity in the auditory nerve and subsequent fiber tracts and nuclei within the auditory brainstem pathways. The threshold, amplitude, and latency analysis of the ABR provides information on the peripheral hearing status and the integrity of brainstem pathways. Therefore, the measurement of ABR has become a useful and practical procedure for the determination of hearing levels in animals and young children (Arnold, 2000; Musiek et al., 1994; Parham et al., 2001). In mice, the ABR threshold has been used successfully to estimate audiometric thresholds in hearing and genetic research (Davis et al., 2002; Duan and Canlon, 1996; Erway et al., 1996; Erway et al., 1993; Hirose and Liberman, 2003; Money et al., 1995; Munemoto et al., 1998; Rosowski et al., 2003; Szymko-Bennett et al., 2003). A PubMed search using key words of mouse, ABR, and threshold has revealed 80 of these studies since 1985.
The successful use of ABR threshold for assessment of the hearing sensitivity of mice has lead to strain characterization (Zheng et al., 1999), gene localization (Galambos and Hecox, 1978; Henry, 2004; Hirose and Liberman, 2003; Huang and Buchwald, 1978; Hunter and Willott, 1987; Ikeda et al., 2002; Ingham et al., 1998; Jimenez et al., 1999; Johnson et al., 2001), and gene identification (Ikeda et al., 2002; Johnson et al., 2000, 2001, 2003) since 1997. Moreover, the measurement of ABR threshold has proven essential in identifying modifier genes (Ikeda et al., 2002; Johnson and Zheng, 2002; Johnson et al., 2000, 2001; Noben-Trauth et al., 1997, 2003; Zheng and Johnson, 2001). However, previous studies have shown that, while some hearing impairments were characterized by significant changes in all ABR parameters such as the threshold, amplitude, and latency, others were associated with the abnormalities in the amplitude and latency but not the threshold (Evans et al., 1983; Fujiyoshi et al., 1994; Kanzaki et al., 1985). For example, Kanzaki et al. (1985) have shown that the peak latency of later ABR waves for the shiverer mice was prolonged with increased inter-peak latency although the threshold was normal. To further assess the hearing sensitivity with ABR, we have studied the ABR threshold as well as the variation in amplitude and latency of ABR components as a function of stimulus intensity in 10 different inbred strains of mice. These mouse strains include BALB/cJ, C3HeB/FeJ, C3H/ HeJ, CAST/EiJ, CBA/CaJ, CBA/J, FVB/NJ, MRL/MpJ, NZB/BlNJ, and SJL/J. Because these strains are commonly used and readily available, they have become increasingly important for medical research since the gene mutations on these strain backgrounds provide models for a variety of human disorders. To our knowledge, the amplitude and latency of ABR components determined at different intensities have only been reported for CBA/CaJ and CBA/J mice among these strains (Burkard et al., 2001; Hunter and Willott, 1987).
Millions of mice are produced annually at the Jackson Laboratory. These mice belong to nearly 2800 strains of genetically defined and modified strains, including standard inbred strains, recombinant inbred strains, congenic inbred strains, and inbred strains carrying both spontaneous and induced mutations. The Neuroscience Mutagenesis Facility at the Jackson Laboratory has undertaken a large scale auditory screening project, which is specifically designed to provide novel murine genetic models for human deafness. The data reported in this study provide a reliable reference for evaluating mouse hearing in terms of amplitude and latency of ABR components. The strain differences in ABR parameters reported in this study suggest genetic determinants, which may lead to gene identification and novel mechanism discovery for the future studies.
2. Results
As described above, we used clicks and tone bursts to study the ABR threshold as well as the variation in amplitude and latency of ABR components as a function of stimulus intensity in 10 different inbred strains of mice. Because of the large amount of data, we mainly present ABR results obtained using click stimulation in this report. To address the issue of age-related hearing loss (AHL), which is characterized by a gradual decrease in sensitivity in the high to low frequency direction, thresholds obtained with tone bursts of different frequencies were also included.
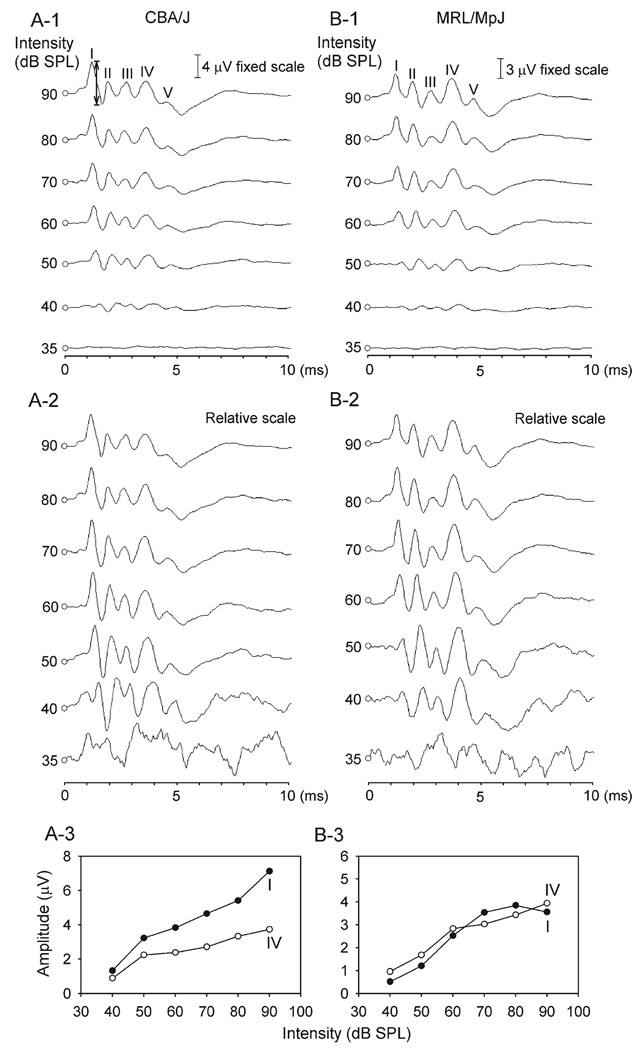
The top panel of Fig. 1 shows the family of ABR patterns of one CBA/J and one MRL/MpJ mouse determined with clicks of different intensities. These ABRs typically consisted of five vertical positive waves (labeled I, II, III, IV, and V, respectively in Figs. 1A-1, B-1) occurring within 6 ms post-stimulus. To determine the ABR threshold, these waves were also displayed in a relative scale (e.g. individual wave was normalized to the maximal wave within each ABR pattern). As shown in the middle panel of Fig. 1, normalized waves were more easily recognized than those displayed using the fixed scale, especially at threshold (Figs. 1A-2, B-2). According to the criterion described in the Experimental procedures, the ABR thresholds for these two mice were both estimated to be 40 dB SPL. The amplitude of individual ABR waves was measured as the difference between the positive peak and the following negative trough (shown by the arrow in Fig. 1A-1). As shown in the bottom panel of Fig. 1, the amplitude of waves I and IV for these two mice either increased monotonically with click intensity (Figs. 1A-3, filled or unfilled circles; B-3, unfilled circles) or increased with intensity to a maximum and decreased thereafter (Figs. 1B-3, filled circles).
Fig. 1.
(A-1, B-1) ABR patterns of two mice determined with clicks of different intensities in a fixed scale. Major wave components are labeled I through V in the 90 dB SPL examples. The strain names are shown at the top of each plot. (A-2, B-2) ABRs of these two mice displayed in a relative scale (e.g. individual wave was normalized to the maximal wave within each ABR pattern). (A-3, B-3) Amplitude–intensity functions of waves I and IV. For CBA/J mice, the amplitude of both waves increased monotonically with increasing intensity (A-3, filled and unfilled circles). However, while the amplitude of wave IV in MRL/MpJ mice increased monotonically with intensity (B-3, unfilled circles), that of wave I increased with intensity to a maximum and decreased thereafter (B-3, filled circles).
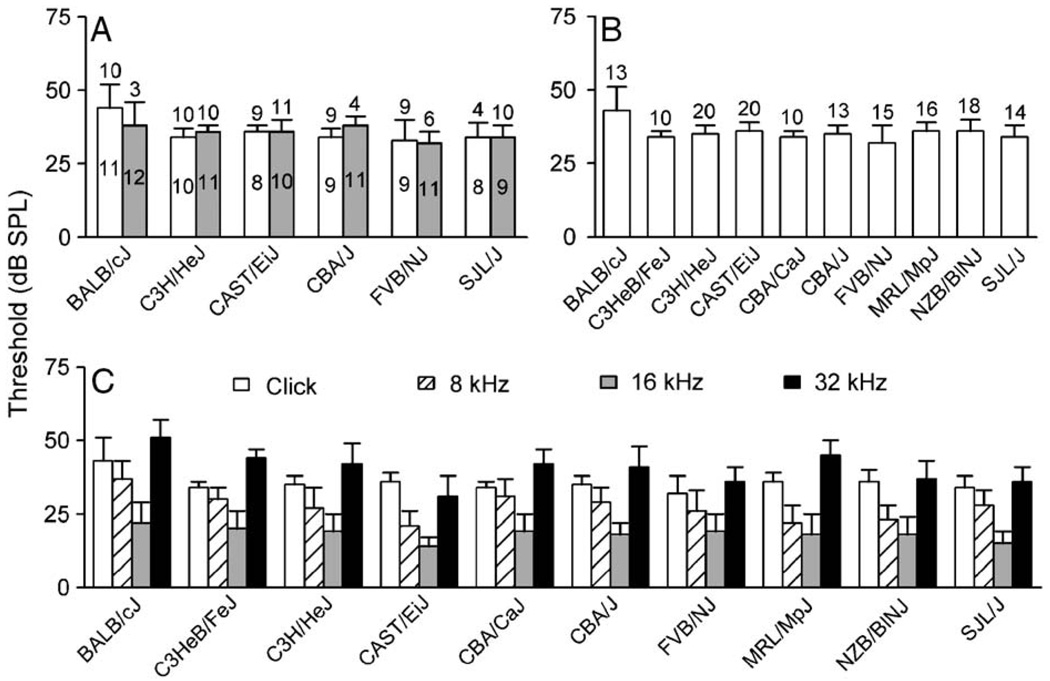
As described earlier, ABRs were measured for all mice at 8–12 weeks of age (Table 1). The ABR thresholds were determined for four mouse strains (e.g. C3HeB/FeJ, CBA/CaJ, MRL/MpJ, and NZB/BlNJ) in which the age difference was less than 2 days within each strain. To determine if the ABR threshold varied with age in the other six mouse strains that had an age difference as large as 1–2 weeks, we compared the average ABR threshold obtained with clicks for these strains (Fig. 2A). A Student’s t test did not reveal any significant age difference for each mouse strain (all P values >0.1). This observation is consistent with a previous study (Zheng et al., 1999).
Table 1.
Strain names, age, and number of mice tested in each strain
| Strains | Average age (weeks) | Number |
|---|---|---|
| BALB/cJ | 11.2 (11–12) | 13 |
| C3HeB/FeJ | 8 | 10 |
| C3H/HeJ | 10.5 (10–11) | 20 |
| CAST/EiJ | 9.1 (8–10) | 20 |
| CBA/CaJ | 9 | 10 |
| CBA/J | 9.6 (9–11) | 13 |
| FVB/NJ | 9.8 (9–11) | 15 |
| MRL/MpJ | 9 | 16 |
| NZB/BlNJ | 9 | 18 |
| SJL/J | 8.7 (8–9) | 14 |
Numbers in parenthesis represent the age range.
Fig. 2.
(A) Average ABR thresholds determined with clicks for six mouse strains in which the age difference was as large as 1–2 weeks. Note that the data for C3HeB/FeJ, CBA/CaJ, MRL/MpJ, and NZB/BlNJ were not included since they were collected at an age that differed by no more than 2 days within each strain. The number inside each bar indicates the age in weeks after birth. The number of mice tested and half a standard deviation is also shown at top each bar. (B) The combined average ARB threshold determined with clicks for each mouse strain. (C) The average ARB threshold determined with tone bursts of different frequencies for each mouse strain.
Because there was no age difference in these ABR data, we combined all data for each mouse strain. As shown in Fig. 2B, the average ABR thresholds determined with clicks ranged from 32 to 43 dB SPL, with the lowest thresholds for FVB/NJ mice and the highest thresholds for BALB/cJ mice. One-way ANOVA showed that there was significant difference of ABR thresholds in different strains [F(9,139) = 6.25, P < 0.0001]. This difference was due to the higher thresholds of BALB/cJ mice than those of other strains (Student–Newman–Keuls multiple comparison post-test, all P values <0.001). When determined with tone bursts, however, ABR thresholds varied significantly depending on the stimulus frequency. In general, thresholds at 32 kHz were the highest and those at 16 kHz were the lowest (Fig. 2C). Two-way ANOVA revealed main effects for frequency [F(2,417) = 553.08] and strain [F(9,417) = 20.01], and the frequency × strain interaction [F(18,417) = 3.33] for threshold values were significant for all strains studied (all P values <0.001). The result is in agreement with a previous study (Zheng et al., 1999).
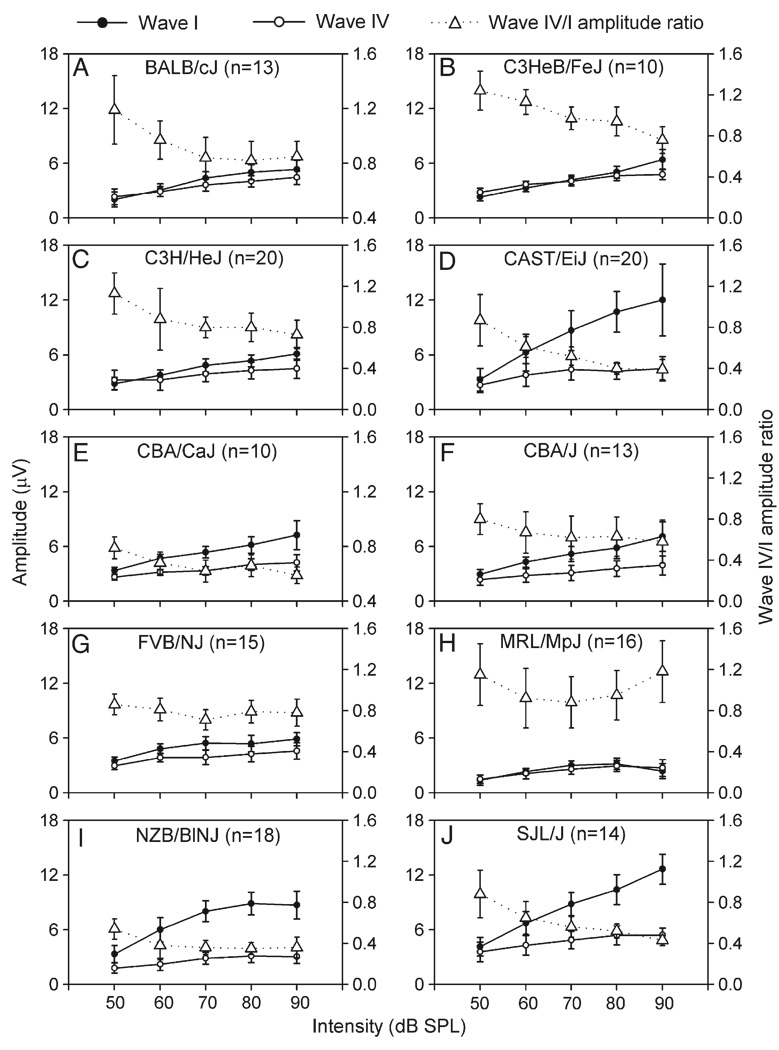
Fig. 3 shows the average amplitude of waves I and IV across click intensities for each strain. It is clear that, in most strains, the amplitude of both waves monotonically increased with increasing intensity. In some strains, however, the amplitude increased with intensity to a maximum and then leveled off or decreased with higher intensity (Figs. 3H, I, filled and unfilled circles). Over the intensity range of 50 to 90 dB, the increase in amplitude averaged from 0.99 (MRL/MpJ mice) to 8.73 µV (CAST/EiJ mice) for wave I and from 1.24 (MRL/MpJ mice) to 2.12 µV (BALB/cJ mice) for wave IV. Two-way ANOVA revealed that main effects of intensity [F(4,693) = 272 for wave I and F (4,693) = 88.18 for wave IV] and strain [F(9,693) = 184.8 for wave I and F(9,693) = 54.72 for wave IV] on the amplitude were highly significant for both waves (all P values <0.001). In general, wave IV had smaller amplitude than wave I (Fig. 3, unfilled vs. filled circles) such that the wave IV/I amplitude ratio was smaller than 1.0 at most intensities tested in all strains (Fig. 3, triangles with dotted line). As shown in Fig. 3, this amplitude ratio typically decreased with increasing intensity over the range of 50 to 90 dB in most strains (Figs. 3A–F, I, J). The results of two-way ANOVA also revealed significant main effects of intensity [F(4,693) = 70.4, P < 0.001] and strains [F(9,693) = 124.12, P < 0.001], and the intensity × strain interaction [F(36,693) = 3.66, P < 0.001] on the amplitude ratio.
Fig. 3.
Average amplitude of waves I (filled circles) and IV (unfilled circles) of ABRs determined with clicks of different intensities in different mouse strains (refer to left ordinate). The average wave IV/I amplitude ratio at each intensity (triangles with dotted line) is also shown within each plot (refer to right ordinate). Each vertical bar represents the standard deviation at each point. n, number of mice.
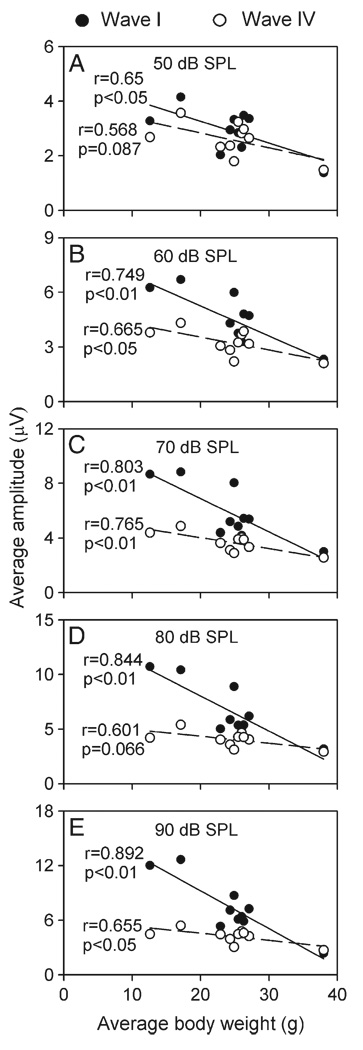
To determine how the amplitude of ABR waves in different strains might be related to their body weight, we plotted the average amplitude of waves I and IV determined at different click intensities against the average body weight of each strain (Fig. 4). Linear regression analyses revealed that the average amplitude of wave I decreased significantly with body weight (Fig. 4, filled circles, all P values <0.05). The average amplitude of wave IV also decreased with body weight although the significant correlation between the average amplitude and the body weight was found at 60, 70, and 90 dB SPL (Figs. 4B, C, E, unfilled circles, all P values <0.05) but not at 50 and 80 dB SPL (Figs. 4A, D, unfilled circles, both P values >0.05).
Fig. 4.
Scatter plots showing the average amplitude of waves I (filled circles) and IV (unfilled circles) of ABRs in relation to the average body weight of mouse strains. The linear regression line (solid line for wave I and dashed line for wave IV), correlation coefficient (r), and significant intensity (p) are shown within each plot.
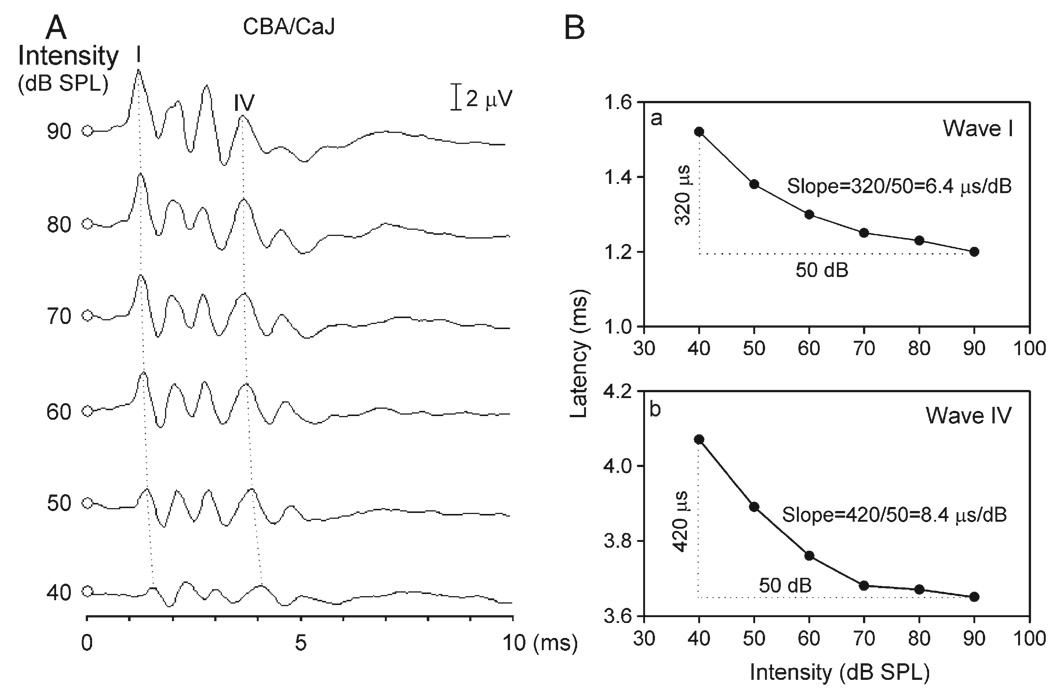
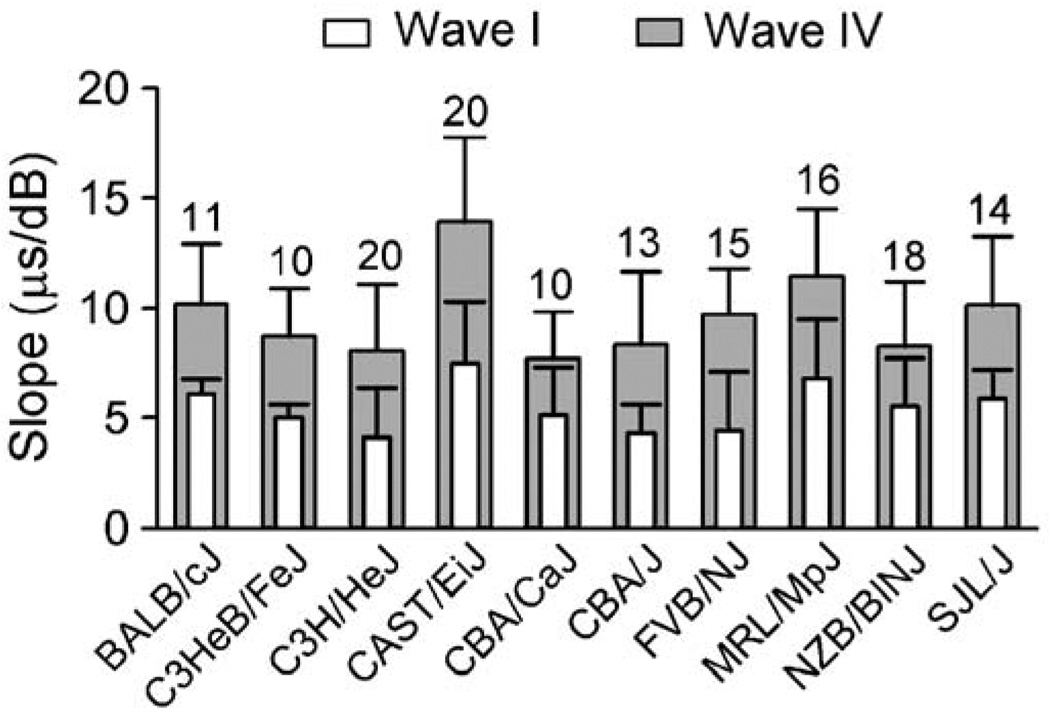
Peak latency is the interval between the delivery of the acoustic stimulus and the peak of the specified wave. Fig. 5A shows the effect of click intensity on the peak latency of both waves I and IV for one CBA/CaJ mouse. This effect was characterized by decreasing peak latency with increasing intensity for both waves (Fig. 5A, dotted lines). For both waves I and IV, we calculated the slope of each latency–intensity function by dividing the change in peak latency by the intensity difference (Fig. 5Ba, b). As shown in Fig. 5B, the slope of latency–intensity function of this CBA/CaJ mouse was 6.4 µs/dB for wave I and 8.4 µs/dB for wave IV. The average slope of latency–intensity function for wave I (Fig. 6, unfilled bars) ranged from 4.1 (C3H/HeJ mice) to 7.5 µs/dB (CAST/EiJ mice) while that for wave IV (Fig. 6, filled bars) ranged from 7.7 (CBA/ CaJ mice) to 14.0 µs/dB (CAST/EiJ mice). Two-way ANOVA revealed that main effects of waves [F(1,274) = 179.95] and strains [F(9,278) = 11.29] on slope of the latency–intensity function were significant (all P values <0.001). The effects were further supported by a Student’s t test to compare the slope of waves I and IV for each strain, and one-way ANOVA to compare the slope of different strains for each wave. A Student’s t test showed that the slope of latency–intensity function was significantly larger for wave IV than for wave I in all strains studied (BALB/cJ, df = 10, t = 5.16; C3HeB/FeJ, df = 9, t = 5.64; C3H/HeJ, df = 19, t = 4.88; CAST/EiJ, df = 19, t = 6.03; CBA/CaJ, df = 9, t = 2.75; CBA/J, df = 12, t = 4.32; FVB/NJ, df = 14, t = 5.62; MRL/MpJ, df = 15, t = 4.72; NZB/BlNJ, df = 17, t = 3.24; SJL/J, df = 13, t = 5.04, all P values <0.05). One-way ANOVA also showed significant differences in the slope of both waves among all strains studied [F(9,137) = 5.77 for wave I and F(9,137) = 8.7 for wave IV, both P values <0.0001].
Fig. 5.
(A) ABR patterns of a CBA/CaJ mouse determined with clicks of different intensities. Note that the peak latency of both waves I and IV decreased with increasing click intensity (dotted lines). (B) Latency–intensity functions of waves I (a) and IV (b). The slope of latency–intensity function was defined as the amount of change in peak latency per decibel (see text for details).
Fig. 6.
Average slope of latency–intensity function of waves I (unfilled bars) and IV (filled bars) of ABRs in different mouse strains. The number of mice tested in each strain and half a standard deviation is shown atop each bar.
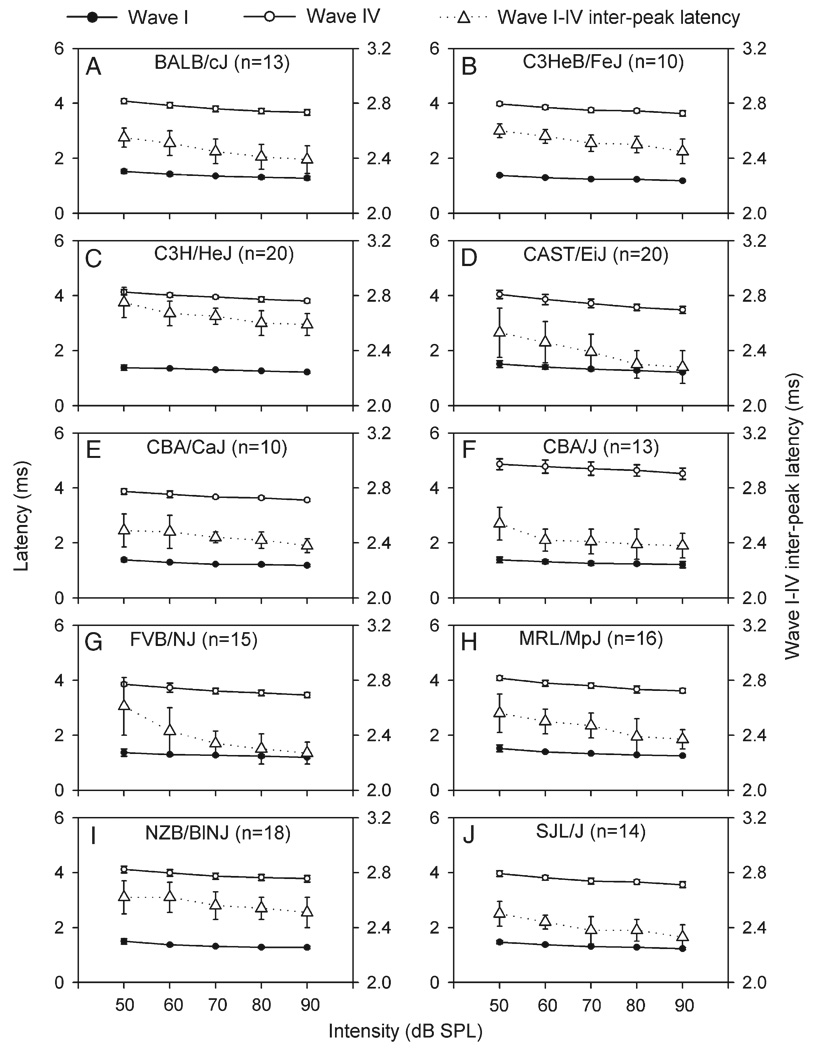
Fig. 7 plots the average peak latency of waves I and IV of different strains across the click intensity (Fig. 7, filled and unfilled circles). A two-way ANOVA showed a significant decrease in peak latency for both waves with increasing intensity [significant main effects of intensity, F(4,693) = 288.09 for wave I and F(4,693) = 269.97 for wave IV, both P values <0.001]. Since the decrease in peak latency with increasing intensity was greater for wave IV than for wave I (Fig. 5B and Fig. 6, unfilled vs. filled bars), the wave I–IV inter-peak latency also significantly decreased with increasing intensity, as shown in Fig. 7 (triangles with dotted line). Within the intensity range of 50 to 90 dB, the minimal decrease in average inter-peak latency was 0.11 ms for CBA/ CaJ mice (from 2.49 to 2.38 ms) and NZB/BlNJ mice (from 2.62 to 2.51 ms), while the maximal decrease was 0.34 ms for FVB/ NJ mice (from 2.61 to 2.27 ms). For any given click intensity, there were significant differences of these values in different strains. All these observations were confirmed by two-way ANOVA which revealed significant main effects of intensity [F(4,693) = 63.01, P < 0.001] (most significant at 70 dB) and strains [F(9,693) = 57.25, P < 0.001] on the inter-peak latency. Student’s t tests confirmed that at 70 dB, I–IV inter-peak latencies for MRL/MpJ, C3HeB/FeJ, NZB/BlNJ, and C3H/HeJ strains are significantly longer than FVB/NJ, SJL/J, and CAST/ EiJ (all P values <0.01).
Fig. 7.
Average peak latency of waves I (filled circles) and IV (unfilled circles) of ABRs determined with clicks of different intensities in different mouse strains (refer to left ordinate). The average wave I–IV inter-peak latency at each intensity (triangles with dotted line) is also shown within each plot (refer to right ordinate). Each vertical bar represents the standard deviation at each point. n, number of mice.
3. Discussion
As reported in previous studies (Chuu et al., 2001; Fujiyoshi et al., 1994; Hunter and Willott, 1987; Parham et al., 2001; Zheng et al., 1999), the ABR patterns of mice typically consisted of five vertical positive waves (Figs. 1A-1, B-1). Although the precise origins of ABR waves are not yet well defined, it is generally agreed that, in animal models such as cats, ferrets, non-human primates, and rodents, the first wave represents activities from the auditory nerve while the later waves represent neural transmission within the central auditory system. However, waves I and II are both generated predominantly by the auditory nerve in humans, due to an unusually long eighth nerve (Markand, 1994; Moller, 1994; Moller and Jannetta, 1985; Parham et al., 2001). Therefore, the first four waves of ABR in animal models are considered approximately equivalent to human waves I–V. Because wave I originates peripherally but wave V is of central origin, and especially, wave V of ABR in humans is the most robust wave with less within- and between-subject variability for amplitude and latency compared to the other waves, the wave V/I amplitude ratio and the wave I–V inter-peak latency have proven to be important parameters for otoneurological assessment in humans (Arnold, 2000). For this reason, we have specifically examined waves I and IV in this study to compare the amplitude and latency of ABR components among different mouse strains (Dammeijer et al., 2002).
Previous studies have shown that 2 (e.g. BALB/cJ and MRL/ MpJ) of 10 strains used in the present study exhibit AHL at later ages (Jimenez et al., 1999; Ralls, 1967; Willott et al., 1998; Zheng et al., 1999). When evaluated by click- and tone-pip-elicited ABR threshold, for example, BALB/cJ and MRL/MpJ mice exhibited late onset hearing loss at an age of 30 weeks or older (Zheng et al., 1999). In this study, ABRs were measured for all mice at 8–12 weeks of age (Table 1). We did find that high frequency thresholds of BALB/cJ and MRL/MpJ mice appeared to be higher than those of other strains (Fig. 2C). Click-elicited ABR thresholds for both strains have less than 10 dB elevation, which has little influence on ABR pattern. Thus, we included these two strains in ABR pattern analysis.
When determined with clicks of different intensities, the amplitude of waves I and IV in most mouse strains increased monotonically with increasing intensity (Fig. 3). This observation is similar to what has been reported in other animal species commonly used in evoked potential studies (Backoff and Caspary, 1994; Ingham et al., 1998; Overbeck and Church, 1992). We showed that there were significant differences in the amplitude of both waves among different mouse strains (Fig. 3). Moreover, the amplitude of both waves was inversely correlated with the body weight at most intensities tested (Fig. 4). This observation is likely due to the different head size relative to body weight. It has been indicated that small head size with small body weight would cause the recording electrodes to be closer to the generators resulting in larger amplitude than those with large body weight (Merzenich et al., 1983). On the other hand, the amount of fat between electrodes and generators might also contribute to the observed amplitude-body weight relationship. Previous study suggests that other factors such as the number of neural element firing and neural synchronicity within the generators are also the principal contributors to the amplitude of ABR waves (Merzenich et al., 1983).
Because the amplitude of ABR waves can be affected by many factors such as body temperature, electrode impedance and location, levels of physiological noise, recording procedures, and equipment characteristics, the amplitude may not be a good indicator of hearing level. However, because all waves are similarly affected by many of these variables, relative amplitude has proven to be useful (Starr and Achor, 1975). For example, it has been reported that auditory deficits associated with retrocochlear pathology in humans may cause a decrease in wave V amplitude and thus a decrease in the wave V/I amplitude ratio (Arnold, 2000). In this study, the wave IV had a smaller amplitude than wave I at most intensities tested such that the IV/I amplitude ratio was smaller than 1.0 (Fig. 3). Merzenich et al. (1983) investigated the amplitude of individual ABR waves in different species including mice, rats, guinea pigs, cats, and humans under optimal and corresponding recording conditions. They found that the first two waves of ABRs in mice had relatively larger amplitude than later ones; similar to our present findings. For rats and guinea pigs, waves II and III became relatively prominent. However, the prominent ones shifted to wave IV in cats and wave IV–V complex in humans. They suggested that the species-specific difference in amplitude of individual ABR waves might involve complex considerations such as the evolution of central nervous system, generator size and orientation, response characteristics of neurons within a generator, neural conduction velocity, and conduction path length.
When combined with ABR threshold, the slope of the latency–intensity function, which represents the degree of shift of individual ABR waves with increasing click intensity, is another useful parameter to estimate the hearing sensitivity. This parameter may be influenced by the type of hearing loss (conductive, mixed, sensorineural or retrocochlear) and the audiometric configuration. In this study, the slope of latency–intensity function of wave I ranged from 4.1 to 7.5 µs/dB while that of wave IV ranged from 7.7 to 14.0 µs/dB (Fig. 6). Burkard and his colleagues reported that the slope of latency–intensity functions of waves I and V were ~8 to 9 µs/dB in gerbils but were ~13 to 16 µs/dB in rats when examined under click stimulation conditions (Burkard and Voigt, 1989; Burkard et al., 1990). Other studies have reported that the slope of latency–intensity functions of waves I through IV were ~14 to 16 µs/dB in cats (Fullerton et al., 1987; Huang and Buchwald, 1978). In humans, the slope of latency–intensity function of wave V and other ABR waves was ~40 µs/dB (Burkard and Hecox, 1983; Galambos and Hecox, 1978). Compared with all these previous studies, we conclude that the slope of latency–intensity function of mice ABR waves is similar to that of gerbils, somewhat less than those of rats and cats, but substantially less than that of humans.
The wave I–IV inter-peak latency represents the time required for neural impulses to conduct through the auditory brainstem. When combined with frequency-specific threshold data, an abnormality of this value can be used to differentiate peripheral hearing loss from retrocochlear dysfunction. Retrocochlear lesions may slow neural conduction velocity and increase the inter-peak latency. In contrast, when click stimuli are used, a high-frequency hearing loss may prolong wave I more than later waves producing a decrease of the inter-peak latency. However, inter-peak latencies are influenced by numerous technical and subject-related factors, and the effects of cochlear damage on ABR inter-peak latencies can vary depending on the configuration and degree of threshold loss and the nature of the lesion (e.g. extent of inner vs. outer hair cell loss, spiral ganglion cell loss, damage to the stria vascularis). In this study, we observed that the peak latency of wave IV decreased in a greater degree with click intensity than wave I resulting in a decrease of wave I–IV inter-peak latency with increasing intensity (Fig. 6 and Fig. 7). We also found that there were strain differences of inter-peak latency, although the difference between the strain with the longest inter-peak latency and the strain with the shortest inter-peak latency was only ~0.25 to 0.32 ms at any given click intensity. The result is consistent with a previous study which reported significant strain differences for the inter-peak latency in C57BL/6, BDFL, and NZB/W mice at the age of 3–4 months (Church and Shucard, 1988). It has been suggested that the inter-peak latency is determined by a number of physiological processes including the synaptic delay and neural conduction velocity within the brainstem pathway, as well as anatomical factors such as head size (Arnold, 2000; Don et al., 1998). However, the mechanism underlying the observed mouse strain difference in inter-peak latency of ABRs remains to be studied.
Genetic mapping and positional cloning approaches offer the promise to identify genes that underlie the parameter differences of ABR between inbred strains. A successful example of using strain differences in ABR to identify a gene using its mutations or allele variances is provided by late onset hearing loss in C57BL/6J, which was used to map the ahl locus and identify Cdh23 as the mutated gene. By ABR evaluation, Erway et al. (1993) reported late onset hearing loss in C57BL/6J mice in 1993. Because CAST/EiJ mice have very good hearing even at very advanced age, Johnson et al. (1997), using a linkage cross between C57BL/6J and CAST/EiJ mice, mapped the first ahl locus on mouse chromosome 10 in 1997. Zheng and Johnson (2001) reported that the ahl/mdfw locus interacts epistatically with the deaf waddler (dfw) mutation and that the ahl locus is a major contributor to AHL in 12 inbred strains. By fine mapping, positional cloning, and candidate gene testing approaches, Noben-Trauth et al. (2003) reported that a hypomorphic 753A allele of Cdh23 gene causes in-frame skipping of exon 7 in many inbred strains associated with susceptibility to AHL. By similar approaches, for example, mapping underlying genes by a linkage cross between C3H/HeJ and CAST/ EiJ mice may (or can be expected to) advance our understanding of the molecular mechanisms that cause the parameter differences of ABR between these two strains. As demonstrated in results, C3H/HeJ mice have longer wave I–IV inter-peak latency than CAST/EiJ mice (Figs. 7C, D), which suggests a genetically determined variation that affects the auditory pathway including auditory conduction and synaptic process. Because I–IV inter-peak latencies for MRL/MpJ, C3HeB/FeJ, NZB/BlNJ, and C3H/HeJ strains are longer than those of FVB/NJ, SJL/J, or CAST/EiJ, we can make linkage crosses between them to map the underlying genes.
In summary, we compared the amplitude and latency of waves I and IV of ABRs determined with clicks of different intensities in 10 mouse strains. We showed that the amplitude of both waves I and IV was inversely correlated with the body weight of each strain at most intensities tested. In general, the amplitude of wave IV was smaller than that of wave I resulting in the IV/I amplitude ratio of <1.0. The peak latency of both waves decreased with click intensity. However, this intensity-dependent decrease was greater for wave IV than for wave I such that the wave I–IV inter-peak latency also decreased significantly with increasing intensity. I–IV inter-peak latencies for MRL/MpJ, C3HeB/ FeJ, NZB/BlNJ, and C3H/HeJ strains are longer than those of FVB/NJ, SJL/J, or CAST/EiJ. These data provide important baseline data for hearing assessment in mice. The significant differences in ABR parameters among different strains reported in this study may also lead to gene identification and novel mechanism discovery.
4. Experimental procedures
4.1. Animals
A total of 149 mice from 10 different inbred strains were used in this study. The strain names, age, and the number of mice tested in each strain are shown in Table 1. These mice were produced within the production facilities of the Jackson Laboratory. Prior to ABR recording, animals were anesthetized by intraperitoneal injection with Avertin (tribromoethanol stabilized in tertiary amyl hydrate) given at a dose of 5 mg tribromoethanol/10 g body weight. An otoscopic examination was then performed on each animal. Only those animals with clear external and middle ears were used for ABR recording. The care and use of the animals described in this study were approved by the Animal Care and Use Committee of The Jackson Laboratory (Grant # DC62108). The Jackson Laboratory is fully accredited by the Accreditation of Laboratory Animal Care.
4.2. ABR procedure
The stimulus presentation, ABR acquisition, equipment control, and data management were coordinated using the computerized Intelligent Hearing Systems (IHS, Miami, FL) with the Smart-EP Windows USB 3.62 version software. A pair of high frequency transducers (Mike Ravicz, Somerville, MA) was coupled with the IHS system to generate specific acoustic stimuli (clicks or tone bursts of different frequencies). The output from the high frequency transducers was channeled through 10 mm long by 3 mm diameter plastic tubes into the animal’s ear canals. The acoustic stimuli were calibrated with a Bruel and Kjaer Nexus conditioning amplifier, Type 2690, and 1/4 inch calibrated condenser microphone. Calibrations were made with reference to the programmed output for 70 dB sound pressure levels (SPL re: 20 µPa). The IHS click stimulus had substantial energy in the 2–8 kHz range (e.g. filtering frequencies above and below this decreased the SPL by less than 5 dB).
Details of the ABR recording methods have been described previously (Zheng et al., 1999). Briefly, the anesthetized animals were placed in a sound-attenuating chamber (Acoustic Systems, Austin, TX). Their body temperature, monitored by a rectal probe, was maintained at 37–38 °C by placing them on an isothermal pad (Deltaphase, model 39 dp, Braintree Scientific Inc., Massachusetts) during testing and recovery from anesthesia. Monitoring the body temperature in numerous mice under this condition showed that the body temperature dropped by only 0.5–1.0 °C over a 15-min ABR testing period. Sub-dermal needles were used as electrodes for recording (Model F-E2, Astro-Med Inc., Rhode Island). The active electrode was inserted at the vertex, the reference electrode ventrolateral to the left ear, and the ground electrode ventrolateral to the right ear.
Alternating click stimuli of 50 µs duration and tone bursts with 3 ms duration (1.5 ms rise–fall time with no plateau) of 8, 16, and 32 kHz were respectively routinely presented to the left ear of the animal. ABRs were band-pass filtered below 100 Hz and above 3000 Hz and amplified. The amplified responses were then averaged by a computer and displayed on the computer screen. Since a previous study demonstrated no consistent left–right ear ABR asymmetry (Zheng et al., 1999) for the mouse strains used in this study, we recorded ABRs from the left ear only, to increase the efficiency of data acquisition.
ABR threshold was obtained for each animal by reducing the stimulus intensity in 10 dB steps and finally in 5 dB steps to identify the lowest intensity at which an ABR wave I was detectable. This was done by comparing the ABR patterns with two or three suprathreshold ABRs displayed successively on the computer screen. After the ABR threshold was determined, a family of ABR patterns was then obtained for each stimulus at different intensities above the threshold. The ABR waveforms were typically averages of 512 stimuli presented at the rate of 19.1 per second for each stimulus condition. These extensive ABR data were stored digitally on disks for later offline measurements and analysis of amplitude and latency of ABR components.
4.3. Data analysis
The data obtained from these mouse strains under different stimulus conditions were compared statistically using oneway or two-way analysis of variance (one-way or two-way ANOVA) or a Student’s t test (two-tailed, paired or unpaired), with alpha level of 0.05. Alpha was adjusted, if necessary, according to the Bonferroni procedure. Since gender difference of ABR parameters has been previously reported in rats (Church et al., 1984), humans (McClelland and McCrea, 1979; Michalewski et al., 1980), and recently mice (Henry, 2004), we first processed the data obtained from male and female mice separately. However, later statistic analysis did not reveal any significant gender difference for all of these data; consistent with the previous studies (Hunter and Willott, 1987; Zheng et al., 1999). Therefore, all data were combined together for this report.
Acknowledgments
The work was supported by grants MH067670, NSFC30440080, DC005846, and a grant from the Research Board of University of Missouri-Columbia (URB-03-057). We thank Melissa L Berry for critical review of the manuscript. We thank Dr. Weidong Zhang for statistical assistance.
Abbreviations
- ABR
auditory brainstem response
- AHL
age-related hearing loss
- SPL
sound pressure level
REFERENCES
- Arnold SA. The auditory brain stem responses. In: Roeser RJ, Valente M, Hosford-Dunn H, editors. Auditory: Diagnosis. New York: Thieme Medical Publishers, Inc; 2000. pp. 451–470. [Google Scholar]
- Backoff PM, Caspary DM. Age-related changes in auditory brainstem responses in Fischer 344 rats: effects of rate and intensity. Hear. Res. 1994;73:163–172. doi: 10.1016/0378-5955(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Burkard R, Hecox K. The effect of broadband noise on the human brainstem auditory evoked response: I. Rate and intensity effects. J. Acoust. Soc. Am. 1983;74:1204–1213. doi: 10.1121/1.390024. [DOI] [PubMed] [Google Scholar]
- Burkard R, Voigt HF. Stimulus dependencies of the gerbil brain-stem auditory-evoked response (BAER): I. Effects of click intensity, rate, and polarity. J. Acoust. Soc. Am. 1989;85:2514–2525. doi: 10.1121/1.397746. [DOI] [PubMed] [Google Scholar]
- Burkard R, Feldman M, Voigt HF. Brainstem auditory-evoked response in the rat. Normative studies, with observations concerning the effects of ossicular disruption. Audiology. 1990;29:146–162. doi: 10.3109/00206099009072847. [DOI] [PubMed] [Google Scholar]
- Burkard R, Durand B, Secor C, McFadden SL. Auditory brainstem responses in CBA mice and in mice with deletion of the RAB3A gene. In: Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. Boca Raton, FL: CRC Press; 2001. pp. 603–615. [Google Scholar]
- Church MW, Shucard DW. The mouse brainstem auditory evoked potential as functions of strain and aging. Abstr. -Soc. Neurosci. 1988;14:800. [Google Scholar]
- Church MW, Williams HL, Holloway JA. Brain-stem auditory evoked potentials in the rat: effects of gender, stimulus characteristics and ethanol sedation. Electroencephalogr. Clin. Neurophysiol. 1984;59:328–339. doi: 10.1016/0168-5597(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Chuu JJ, Hsu CJ, Lin-Shiau SY. Abnormal auditory brainstem responses for mice treated with mercurial compounds: involvement of excessive nitric oxide. Toxicology. 2001;162:11–22. doi: 10.1016/s0300-483x(01)00348-1. [DOI] [PubMed] [Google Scholar]
- Dammeijer PFM, Schlundt Bodien QCM, Chenault MN, Manni JJ, Anteunis LJC. Effects of early auditory deprivation and stimulation on auditory brainstem responses in the rat. Acta Otol. 2002;122:703–708. [PubMed] [Google Scholar]
- Davis RR, Murphy WJ, Snawder JE, Striley CA, Henderson D, Khan A, Krieg EF. Susceptibility to the ototoxic properties of toluene is species specific. Hear. Res. 2002;166:24–32. doi: 10.1016/s0378-5955(02)00280-0. [DOI] [PubMed] [Google Scholar]
- Don M, Ponton CW, Eggermont JJ, Kwong B. The effects of sensory hearing loss on cochlear filter times estimated from auditory brainstem response latencies. J. Acoust. Soc. Am. 1998;104:2280–2289. doi: 10.1121/1.423741. [DOI] [PubMed] [Google Scholar]
- Duan ML, Canlon B. Forward masking is dependent on inner hair cell activity. Audiol. Neuro-Otol. 1996;1:320–327. doi: 10.1159/000259216. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear. Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Erway LC, Shiau YW, Davis RR, Krieg EF. Genetics of age-related hearing loss in mice: III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hear. Res. 1996;93:181–187. doi: 10.1016/0378-5955(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Webster DB, Cullen JK., Jr Auditory brainstem responses in neonatally sound deprived CBA/J mice. Hear. Res. 1983;10:269–277. doi: 10.1016/0378-5955(83)90092-8. [DOI] [PubMed] [Google Scholar]
- Fullerton BC, Levine RA, Hosford-Dunn HL, Kiang NY. Comparison of cat and human brain-stem auditory evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1987;66:547–570. doi: 10.1016/0013-4694(87)90102-7. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi T, Hood L, Yoo TJ. Restoration of brain stem auditory-evoked potentials by gene transfer in shiverer mice. Ann. Otol., Rhinol., Laryngol. 1994;103:449–456. doi: 10.1177/000348949410300606. [DOI] [PubMed] [Google Scholar]
- Galambos R, Hecox KE. Clinical applications of the auditory brain stem response. Otolaryngol. Clin. North Am. 1978;11:709–722. [PubMed] [Google Scholar]
- Henry KR. Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hear. Res. 2004;190:141–148. doi: 10.1016/S0378-5955(03)00401-5. [DOI] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CM, Buchwald JS. Factors that affect the amplitudes and latencies of the vertex short latency acoustic responses in the cat. Electroencephalogr. Clin. Neurophysiol. 1978;44:179–186. doi: 10.1016/0013-4694(78)90264-x. [DOI] [PubMed] [Google Scholar]
- Hunter KP, Willott JF. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hear. Res. 1987;30:207–218. doi: 10.1016/0378-5955(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Zheng QY, Zuberi AR, Johnson KR, Naggert JK, Nishina PM. Microtubule-associated protein 1A is a modifier of tubby hearing (moth1) Nat. Genet. 2002;30:401–405. doi: 10.1038/ng838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham NJ, Thornton SK, Comis SD, Withington DJ. The auditory brainstem response of aged guinea pigs. Acta Otolaryngol. 1998;118:673–680. doi: 10.1080/00016489850183160. [DOI] [PubMed] [Google Scholar]
- Jimenez AM, Stagner BB, Martin GK, Lonsbury-Martin BL. Age-related loss of distortion product otoacoustic emissions in four mouse strains. Hear. Res. 1999;138:91–105. doi: 10.1016/s0378-5955(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY. Ahl2, a second locus affecting age-related hearing loss in mice. Genomics. 2002;80:461–464. [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/ 6J mice. Hear. Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Bykhovskaya Y, Spirina O, Fischel-Ghodsian N. A nuclear-mitochondrial DNA interaction affecting hearing impairment in mice. Nat. Genet. 2001;27:191–194. doi: 10.1038/84831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Chang B, Zheng QY. Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum. Mol. Genet. 2003;12:3075–3086. doi: 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki J, Mikoshiba K, Tsukada Y. Auditory brain stem response in neuropathological mutant mice (shiverer and reeler) J. Otorhinolaryngol. Relat. Spec. 1985;47:294–298. doi: 10.1159/000275788. [DOI] [PubMed] [Google Scholar]
- Markand ON. Brainstem auditory evoked potentials. J. Clin. Neurophysiol. 1994;11:319–342. doi: 10.1097/00004691-199405000-00004. [DOI] [PubMed] [Google Scholar]
- McClelland RJ, McCrea RS. Intersubject variability of the auditory-evoked brain stem potentials. Audiology. 1979;18:462–471. doi: 10.3109/00206097909072637. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Garid JN, Vivion M. Animals. In: Moore EJ, editor. Bases of Auditory Brain-Stem Evoked Responses. New York: Grune and Stratton; 1983. pp. 391–412. [Google Scholar]
- Michalewski HJ, Thompson LW, Patterson JV, Bowman TE, Litzelman D. Sex differences in the amplitudes and latencies of the human auditory brain stem potential. Electroencephalogr. Clin. Neurophysiol. 1980;48:351–356. doi: 10.1016/0013-4694(80)90271-0. [DOI] [PubMed] [Google Scholar]
- Moller AR. Auditory neurophysiology. J. Clin. Neurophysiol. 1994;11:284–308. doi: 10.1097/00004691-199405000-00002. [DOI] [PubMed] [Google Scholar]
- Moller AR, Jannetta PJ. Neural generators of the auditory brain stem response. In: Jacobson JT, editor. The Auditory Brain Stem Response. San Diego, CA: College-Hill Press, Inc; 1985. [Google Scholar]
- Money MK, Pippin GW, Weaver KE, Kirsch JP, Webster DB. Auditory brainstem responses of CBA/J mice with neonatal conductive hearing losses and treatment with GM1 ganglioside. Hear. Res. 1995;87:104–113. doi: 10.1016/0378-5955(95)00083-g. [DOI] [PubMed] [Google Scholar]
- Munemoto Y, Kuriyama H, Doi T, Sato K, Matsumoto A, Sugatani J, Cho H, Komeda M, Altschuler RA, Kitajiri M, Mishina M, Yamashita T. Auditory pathway and auditory brainstem response in mice lacking NMDA receptor epsilon 1 and epsilon 4 subunits. Neurosci. Lett. 1998;251:101–104. doi: 10.1016/s0304-3940(98)00509-6. [DOI] [PubMed] [Google Scholar]
- Musiek FE, Borenstein SP, Hall III JW, Schwaber MK. Auditory brainstem response: threshold estimation and auditory screening. In: Katz J, editor. Handbook of Clinical Audiology. Baltimore: Williams and Wilkins; 1994. pp. 351–374. [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR, Nishina PM. mdfw: a deafness susceptibility locus that interacts with deaf waddler (dfw) Genomics. 1997;44:266–272. doi: 10.1006/geno.1997.4869. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat. Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeck GW, Church MW. Effects of tone burst frequency and intensity on the auditory brainstem response (ABR) from albino and pigmented rats. Hear. Res. 1992;59:129–137. doi: 10.1016/0378-5955(92)90110-9. [DOI] [PubMed] [Google Scholar]
- Parham K, Sun XM, Kim DO. Noninvasive assessment of auditory function in mice: auditory brainstem response and distortion product otoacoustic emissions. In: Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. Boca Raton, FL: CRC Press; 2001. pp. 37–58. [Google Scholar]
- Ralls K. Auditory sensitivity in mice: Peromyscus and Mus musculus. Anim. Behav. 1967;15:123–128. doi: 10.1016/s0003-3472(67)80022-8. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Brinsko KM, Tempel BI, Kujawa SG. The aging of the middle ear in 129S6/SvEvTac and CBA/CaJ mice: measurements of umbo velocity, hearing function, and the incidence of pathology. J. Assoc. Res. Otolaryngol. 2003;4:371–383. doi: 10.1007/s10162-002-3047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Achor J. Auditory brain stem responses in neurological disease. Arch. Neurol. 1975;32:761–768. doi: 10.1001/archneur.1975.00490530083009. [DOI] [PubMed] [Google Scholar]
- Szymko-Bennett YM, Kurima K, Olsen B, Seegmiller R, Griffith AJ. Auditory function associated with Col11a1 haploinsufficiency in chondrodysplasia (cho) mice. Hear. Res. 2003;175:178–182. doi: 10.1016/s0378-5955(02)00736-0. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG, Carlson S, Ding D, Seegers Bross L, Falls WA. The BALB/c mouse as an animal model for progressive sensorineural hearing loss. Hear. Res. 1998;115:162–174. doi: 10.1016/s0378-5955(97)00189-5. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR. Hearing loss associated with the modifier of deaf waddler (mdfw) locus corresponds with age-related hearing loss in 12 inbred strains of mice. Hear. Res. 2001;154:45–53. doi: 10.1016/s0378-5955(01)00215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]