Abstract
Cellulose acetate phthalate (CAP; cellulose acetate 1,2-benzenedicarboxylate) is a common polymeric oral tablet coating. CAP is also a vaginal microbicide candidate that potently inhibits HIV-1 proliferation. This paper describes the development of a precise, stability-indicating gel permeation chromatography (GPC) assay for CAP. During accelerated stability studies monitored by separate reversed-phase high-performance liquid chromatography (RP-HPLC) and GPC analyses, an apparent loss of mass balance was observed. This deficit was corrected by recalculating the response factor (RF) for each degraded sample, proportional to the fraction of phthalate remaining bound to the polymeric CAP. The correction factor enabled CAP and the degradation product phthalic acid (PA) to be quantitated by a single GPC analysis. The chromatographic approach taken here could potentially apply to any polymer containing degradable chromophores.
Keywords: Cellulose acetate phthalate, gel permeation chromatography (GPC), reversed-phase HPLC, stability studies
1. Introduction
Cellulose acetate phthalate (CAP, Fig. 1A) has been used for decades as a pH-sensitive pill coating for orally-administered drugs such as aspirin [1]. CAP is listed as a delayed action acid-resistant enteric coating excipient in the National Formulary (NF) Inactive Ingredients Database (IIG) under the name Cellacefate, permissible at up to 70 mg per tablet. CAP has not yet been approved as an active pharmaceutical ingredient (API) in a pharmaceutical drug product, suggesting that validated, quantitative analytical methods to measure CAP stability in storage have not been developed.
Fig. 1.
Structures of cellulose acetate phthalate (CAP), phthalic acid, and phthalic anhydride.
Recently, CAP was shown to possess potent antiviral activity [2, 3]. CAP is effective against herpes virus [2], SIV [4], a wide range of HIV clades and subtypes[5], and pathogenic bacteria [6], yet it does not inhibit the growth of bacterial species representative of the protective normal flora including lactobacilli [3]. CAP is inexpensive [7], water dispersible [7], and non-toxic [4]. Beads containing CAP are stable during storage [8, 9, 10, 11, 12]. The high average molecular weight (~60,000) of CAP and the development of CAP drug products in micronized form limits its diffusion away from the area of application, requiring no separate applicator device [7].
CAP contains phthalate and acetate moieties ester-linked at the oxygen atoms of the glucose subunits of the CAP cellulose backbone (Fig. 1A), yielding phthalic acid (PA; Fig. 1B) and acetic acid (AA) as hydrolysis products upon storage. The susceptibility of CAP to humidity and hydrolysis has been observed, spurring the development of special packaging that limits exposure of CAP powder and formulations to atmospheric moisture and underscoring the crucial importance of stability studies to the development of any drug product containing CAP as an API.
During CAP sample analysis, chromatographic peaks are quantitated by comparison with the UV response factor (RF = peak area/concentration) of the corresponding peak in the calibration standard. A decrease of detector signal intensity proportional to the loss of phthalate groups hydrolyzed from the cellulose backbone was expected. However, as phthalate chromophores are lost from the cellulose chain, the levels of CAP exhibit a greater decline than anticipated. If the RF of intact CAP standard is used at later stability time points, a GPC assay monitored by a UV detector would underestimate CAP levels in the samples. Therefore, A GPC assay was developed to determine CAP accurately through adjustment of its original (T=0) RF in proportion to the relative decrease in bound phthalate (BP).
In order to simulate CAP degradation throughout the range of concentrations relevant to a stability study, CAP was subjected to forced thermal degradation at 100 °C. A separate RP-HPLC assay was developed and validated to determine PA and AA. The RP-HPLC assay was intended to analyze PA and AA at each stability time point. However, low AA levels combined with acceptable precision of PA quantitation by GPC presented the opportunity to eliminate the RP-HPLC assay from stability time points, greatly simplifying the overall assay.
2. Experimental
2.1. Materials
USP grade Acetic acid (AA), ACS grade PA, and ACS grade phthalic anhydride, were obtained from Sigma-Aldrich (St. Louis, MO). HPLC grade water, methanol, acetone, phosphoric acid, and reagent grade ethanol were obtained from EMD Chemicals (Gibbstown, NJ). Monobasic sodium phosphate, sulfuric acid, and unpreserved tetrahydrofuran (THF) optima (ACS grade) were obtained from Fisher (Fair Lawn, NJ). Glycerin was obtained from Spectrum (Gardena, CA).
Aquateric aqueous enteric coating reference standard, consisting of 70% CAP with a polyoxyethylene–polyoxypropylene block copolymer and distilled acetylated monoglycerides [13], were obtained from FMC BioPolymer (Philadelphia, PA). Polystyrene standards were obtained from Phenomenex (Torrance, CA). Syringe-tip filters were obtained from Pall (East Hills, NY).
Both RP-HPLC and GPC analyses were performed on an Agilent 1100 system (Agilent Technologies, Wilmington, DE) consisting of a diode-array detector (DAD), refrigerated autosampler, quaternary pump, micro vacuum degasser, and thermostat-controlled column compartment. Acquired data were processed using Agilent ChemStation, Version 8, Rev. B.01.03. The GPC column was the Phenomenex (Torrance, CA) Phenogel GPC/SEC, 1 kDa to 75 kDa molecular weight range, 5μ particle size, 1000 Å pore size, 300 × 7.8 mm ID, with Phenomenex 5μ Linear/Mixed, 50 × 7.8 mm GPC guard column. The RP-HPLC column was the Phenomenex Luna C18 (2) column, 5 μ particle size, 150 × 4.00 mm with C18 guard column, 4 mm × 3.0 mm.
2.2. Solvents and solutions
The RP-HPLC Mobile Phase A consisted of phosphate buffer (pH 3.0; 25 mM) pH-adjusted with phosphoric acid. The RP-HPLC Mobile Phase B (MPB) was methanol. The GPC mobile phase was THF. All mobile phases were filtered through 0.45-μm nylon filters. Acetic acid (0.0975 mg/mL) and PA (0.130 mg/mL) RP-HPLC standards were dissolved in water and filtered through a 0.45 mm nylon syringe-tip filter, discarding the first 1.5 mL of filtrate. The next 1 mL of filtrate was added to an autosampler vial and analyzed. GPC calibration and check standards were prepared to a final concentration of 1.3 mg/mL by first dispersing in 2 mL of a 1:1 glycerin:ethanol dispersion solution, then diluting to 10 mL final volume with THF. Polystyrene 13 to 104 kDa standard solutions (Phenomenex Part ALO-2762) were prepared in THF according to the manufacturer’s instructions and analyzed as external column quality controls.
2.3. Chromatographic analysis
The RP-HPLC analysis parameters were: 25°C column temperature, 1.0 mL/minute flow rate, 25 μL injection volume, 208 nm detection until 6 min, and 275 nm detection thereafter. The gradient timetable was (min/%MPB): 0/1, 4/1, 4.5/20, 16.5/20, 17.5/55, 24.5/55, 25/1, 35/1. The isocratic GPC analysis parameters were as follows: 40 °C column temperature, 0.8 mL/minute flow rate, 20 μL injection volume, 280 nm detection, and 25 minute run time. Autosamplers were at room temperature for both methods. External calibration and check controls were applied as per International Committee for Harmonization (ICH) guidelines. Peak areas were expressed in units of either milliabsorbance units*seconds (mAU*s) or millivolts*seconds (mV*s). Using Equation 2 (see Section 2.8), CAP, PA, and AA were quantitated against the average area of the respective calibration standard. The RF of the 14.9-minute degradation peak (Fig. 2) was calculated by comparison to the average area of the PA standards. This 14.9 minute peak was subsequently shown to be phthalic anhydride (Fig. 1C) by co-chromatography with phthalic anhydride standard (Fig. 3).
Fig. 2.
24-hour forced degradation of CAP at 100 °C assayed by GPC
Fig. 3.
Gel permeation chromatography control injections are shown with no x-axis offset. The polystyrene 104 kDa size marker control ran at approximately the same retention time as CAP. The acetone control at 15.5 minutes retention time marks the lower size limit that can be resolved using the GPC method. Acetic acid was not retained by GPC. A PA standard injection is shown for comparison. Inset: Acetic acid and phthalic acid calibration standard injections as analyzed by the RP-HPLC method.
2.4. Validation
The HPLC methods were validated per ICH guidelines at a Phase I level. The GPC method without RF adjustment was validated to determine CAP drug substance. The RP-HPLC method was validated to determine PA and AA, as pure substances and in a gel formulation. The validated procedure for the dissolution and analysis of standard solutions was identical to the procedure followed in the forced degradation study. The GPC injection precision was 0.3% RSD for CAP. The RP-HPLC injection precision was 0.1% RSD for PA and 0.3% for AA. Both methods were sufficiently accurate, linear, specific, and stability-indicating to support Phase I clinical studies.
2.5. Forced degradation study
CAP was weighed into glass vials. The 0-hour control was stored at Controlled Room Temperature (CRT) in a desiccator. Other samples were placed in a 100 °C oven and taken out at 1, 2, 4, 8, 16, and 24-hour time points. Successive samples removed from the oven were allowed to equilibrate to room temperature and then were stored in a desiccator, prior to analysis. With increasing incubation time in the oven, samples became increasingly rigid, necessitating pulverization by mortar and pestle. For GPC analysis, approximately 18.6 mg of each pulverized sample was weighed into 10-mL volumetric flasks, dissolved, and diluted to volume with THF, then centrifuged at 2500 × g for 10 minutes. The supernatants were transferred into autosampler vials for analysis. For RP-HPLC analysis, approximately 14.3 mg of each pulverized sample was weighed into a 5-mL volumetric flask. In order to fully dissolve these samples, 250 μL of THF was added to each sample, followed by vortex mixing, shaking on a wrist-action shaker for 1 hour, and storage at CRT overnight. The samples were separately diluted to volume with water, vigorously mixed by vortex, centrifuged at 2500 × g for 30 min, and filtered through a 0.45-μm filter prior to analysis.
2.6. Base hydrolysis of CAP
For each forced degradation sample and a T = 0 CRT control, approximately 14.3 mg of CAP sample was combined with 2 mL of 2M sodium hydroxide solution in a 5-mL volumetric flask and vigorously mixed by vortex to dissolve. The T=0 sample was immediately neutralized by the addition of 3 mL of 1 M sulfuric acid solution, which was added drop-wise while stirring. Each hydrolysis mixture except the T=0 time point was placed at 105 °C for 2 hours. After equilibration to CRT, 3 mL of 1 M sulfuric acid solution was added drop-wise while stirring. Stirring continued for at least 10 minutes. Each solution was syringe-filtered through a 0.45μm nylon filter into an autosampler vial for analysis by RP-HPLC.
2.7. Data analysis
The corrected CAP percent of label claim (% LC) was calculated using Equations 1 through 9 (see Section 2.8). Uncorrected CAP, PA, and AA % LC values were calculated with Equations 2, 8, and 9, using the RF and potency values for their respective standards. The 14.9-minute degradation product % LC was initially calculated against the PA RF and, once it was identified, subsequently calculated against the phthalic anhydride RF. Total phthalate (TP) % LC was determined as the PA content quantitated by RP-HPLC after base hydrolysis. Mass balance was calculated as the sum of % LC values.
2.8. Theoretical
Abbreviations: BP = Bound Phthalate; TP = Total Phthalate; PA = Phthalic Acid; DV = Dilution Volume; std = CAP Aquateric standard; RF refers to the CAP response factor.
Given:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
RFsample is the RF corrected for the amount of BP present in each sample with its stability time point used in place of the RFstd. The amount of CAP present in the sample by weight is then given as:
| (8) |
The %w/w is expressed as a % LC of the CAP Aquateric standard, factoring in the % potency of the material as determined by the certificate of analysis:
| (9) |
3. Results
3.1. The GPC and RP-HPLC analyses of the forced degradation samples were precise
The chromatographic analysis of the forced degradation experiment consisted of a single GPC chromatographic sequence and a single RP-HPLC sequence. The GPC injection precision was 0.3% RSD for CAP. In the forced degradation study, method precision was calculated as percent relative standard deviation (% RSD) by analyzing 6 preparations of the 24 hour, 100 °C-degraded sample (Table 1). CAP and a peak with a retention time (RT) of 14.9 minutes, later identified as phthalic anhydride, (Fig. 2) were determined by GPC. PA was measured by both GPC and RP-HPLC. AA was measured by RP-HPLC. TP was measured at each degradation time point as the PA peak in a hydrolysate of CAP by RP-HPLC. Precision was less than 3.0% for all analytes (Table 2), and all area responses of CAP, PA, and AA values fell within the validated linearity ranges for those analytes.
Table 1.
Recovery of CAP and free phthalic acid (PA) from forced degradation studies as a % label claim (LC) of the 70% CAP Aquateric standard.
| Time at 100 °C (Hours) | CAP By GPC | PA By RP-HPLC | Uncorrected Mass Balance (PA by RP-HPLC) | PA By GPC | Uncorrected Mass Balance (PA By GPC) | Corrected Mass Balance (PA by RP-HPLC) |
|---|---|---|---|---|---|---|
| 0 | 96.4 | 3.00 | 99.4 | 2.66 | 99.0 | 99.8 |
| 1 | 91.2 | 4.29 | 95.5 | 3.36 | 94.6 | 101.0 |
| 2 | 90.2 | 5.33 | 95.5 | 4.25 | 94.5 | 103.0 |
| 4 | 84.6 | 6.88 | 91.5 | 5.40 | 90.0 | 103.5 |
| 8 | 76.6 | 8.49 | 85.1 | 6.75 | 83.3 | 102.0 |
| 16 | 70.5 | 10.3 | 80.8 | 8.09 | 78.6 | 103.3 |
| 24 | 65.3 | 11.2 | 76.6 | 9.00 | 74.3 | 101.4 |
| Mean % LC | 102.0 | |||||
| % RSD | 1.3 | |||||
PA was calculated by GPC and RP-HPLC. Mass balance is CAP+PA. CAP values are uncorrected. Each value shown is the mean of four injections: two replicate injections of each of two replicate preparations. Corrected Mass Balance values were calculated by Equation 7.
Table 2.
Precision resulting from the 24-hour 100 °C forced degradation time point as a % LC of the 70% CAP Aquateric standard.
| Replicate Preparation | CAP by GPC |
Phthalic Anhydride By GPC | Free PA |
TP By RP-HPLC (After Base Hydrolysis) | ||
|---|---|---|---|---|---|---|
| Un-Corrected | Corrected By Equation 7 | By RP-HPLC | By GPC | |||
| 1 | 66.0 | 89.1 | 2.65 | 11.2 | 9.07 | 35.1 |
| 2 | 64.6 | 87.3 | 2.61 | 11.3 | 9.00 | 34.8 |
| 3 | 65.3 | 88.2 | 2.60 | 11.8 | 9.00 | 34.7 |
| 4 | 65.1 | 88.0 | 2.57 | 10.9 | 8.98 | 34.8 |
| 5 | 65.6 | 88.5 | 2.65 | 11.3 | 9.00 | 34.8 |
| 6 | 65.3 | 88.2 | 2.63 | 11.1 | 8.95 | 35.6 |
| Mean % LC | 65.3 | 88.2 | 2.62 | 11.2 | 9.0 | 35.0 |
| % RSD | 0.7 | 0.7 | 1.2 | 2.7 | 0.4 | 0.9 |
The reported %LC value for each of the 6 preparations shown was calculated from the mean of 2 injections. CAP is calculated both with and without the correction factor shown in Equation 7. TP %LC was determined as the PA peak analyzed by RP-HPLC after base hydrolysis. Each of the six replicate sample preparations shown is itself the mean of two replicate injections except for TP.
3.2. The uncorrected CAP RF decreased more than expected
GPC chromatograms are shown in Fig. 2, and controls for that same run are shown in Fig. 3. The CAP standard eluted as a broad peak between 7 to 13 minutes RT, consistent with a size distribution of varying cellulose chain lengths. PA eluted as a sharp, symmetrical peak at 14.2 minutes RT. Over the 24-hour course of the forced degradation experiment, several compounds other than CAP and PA were detected by GPC. Degradation products emerged at 13.1 minutes and 14.9 minutes RT. The 14.9 minute peak was quantitated using the PA RF as 2.69% LC at 24 hours. Acetic acid measured by six replicate RP-HPLC injections was 2.24% LC at 24 hours (data not shown).
The uncorrected mass balance was defined as CAP determined by GPC plus PA determined by RP-HPLC, which was 65.3 % LC at the 24 hour time point (Tables 1 and 2). The collective % LC of the other observed peaks did not account for the shortfall.
3.3. PA by RP-HPLC was approximately equal to the sum of PA and phthalic anhydride by GPC
The GPC assay was validated to quantitate CAP and PA, and the RP-HPLC assay was validated to quantitate PA and AA. However, the PA %LC values obtained from the GPC assay versus the RP-HPLC assays differed significantly (Fig. 4 and Table 2). At the 24 hour time point, the PA values were 9.0% for GPC and 11.2% for RP-HPLC. Although additional confirmation with spiked standards was not performed, the 2.2% difference could be accounted for by the area of the 14.9-minute phthalic anhydride GPC peak, if it coelutes with the PA peak in the RP-HPLC analysis.
Fig. 4.
Bound Phthalate (BP) calculated at each stability time point using Equation 1. Measured quantities are shown as solid lines and calculated quantities are dashed lines. Total phthalate (TP) was measured by RP-HPLC after hydrolysis, and phthalic acid (PA) by RP-HPLC was subtracted from it to give BP. PA + phthalic anhydride by GPC is closer to the RP-HPLC PA peak than PA by GPC is to PA by RP-HPLC, suggesting that PA and phthalic anhydride coelute in the RP-HPLC assay.
3.4. A CAP RF correction factor was derived based on a direct relationship between the RF of the CAP Aquateric standard and the apparent RF of the degraded samples
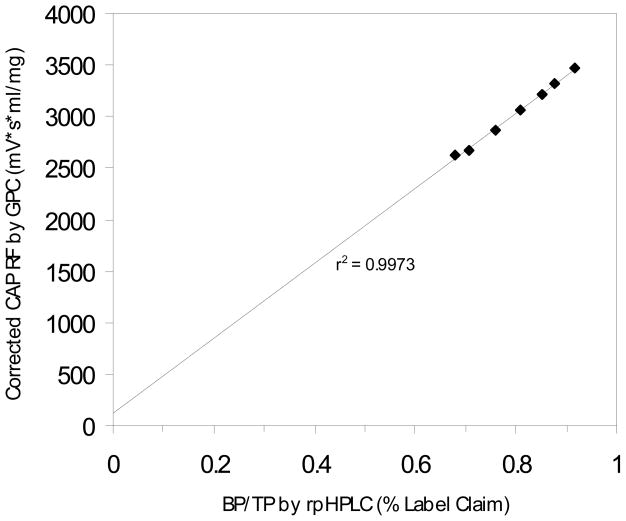
Base hydrolysis was conducted on the Aquateric CAP standard and on the heat-degraded samples at each degradation time point. As a control to ensure no PA adhered to cellulose, a THF extraction conducted after the hydrolysis step gave PA and BP results equal to the results of the hydrolysis followed by aqueous extraction, as described in Section 2.4 (data not shown). TP was defined as the area of the phthalic acid peak obtained in the RP-HPLC assay after base hydrolysis of CAP. BP was then calculated at each time point using Equation 1. Comparing the post-hydrolysis BP values with the observed CAP RF values, the correlation shown in Equation 2 became apparent between RF and BP for both samples and standards. Substituting Equation 3 into Equation 4 and canceling the percentages yielded a correction factor, Equation 5. This relationship was strongly supported by the linearity (r2=0.9973) of the plot of the quotient of BP/TP against the corrected CAP RF by GPC (as shown in Fig. 5). The presence of TP terms in Equation 5 implies that hydrolysis followed by RP-HPLC analysis would be necessary for each analysis. However, Fig. 4 shows that TP remained constant for all time points. As expressed in Equation 6, TP is constant for all standards and samples, implying that hydrolysis followed by RP-HPLC analysis need only be conducted once per lot. Substituting Equation 6 into Equation 5 cancels the TP term, simplifying the expression to the useful form of the CAP RF correction factor, Equation 7.
Fig. 5.
Linearity of the CAP RF correction factor. A linear relationship between the corrected CAP RF and BP/TP is predicted by Equation 5.
3.5. The RF correction factor simplified the analysis of CAP to a single GPC run
Because Equation 7 is valid, acid hydrolysis followed by the RP-HPLC quantitation of hydrolysate needs to be conducted only once per lot with Aquateric reference standard to determine the constant TP. In addition, because the forced degradation results demonstrate that PA is quantifiable by GPC, the RP-HPLC assay initially deemed necessary to determine PA is superfluous. Utilizing the PA response and Equation 1, both CAP and BP are calculable from the GPC run.
3.6. Applying the CAP RF correction restored mass balance
As is shown in Table 1, the mass balance declined as the forced degradation experiment progressed to 76.6% at the 24-hour time point. Applying the correction factor, mass balance was restored close to 100% at each of the 7 time points, with a range of 99.80 to 103.47%, mean of 101.97%, and RSD of 1.32%. Table 1 effectively demonstrates the validated precision of the corrected mass balance calculation over its relevant linear range.
4. Discussion
A search of the literature revealed no closely related GPC technique with a pharmaceutical application, possibly because polysaccharides of any derivation are rarely proposed as APIs. Published techniques for CAP are limited to chemical titrations to determine cellacefate [15] and a semiquantitative method measuring a complex between CAP and ruthenium red [16]. The GPC method presented herein for CAP was more precise than comparable published methods of substituted cellulose determination. In one study, fifteen commercial grades of hydroxypropylmethylcellulose phthalate of 36,900–62,000 molecular weight were quantified by GPC with multi-angle laser light scattering detector (MALLS), giving precision values ranging from 1.7–6.5% RSD [17]. A GPC comparison of the evaporative light scattering (ELS) and refractive index (RI) detection of hydroxypropylmethylcellulose acetate succinate (HPMCAS) gave method precisions of 2.2–2.5% for ELS and 2.4–4.5% for RI [18]. A report of a gradient elution liquid chromatographic method for HPMCAS with ELS detection gave 1.2% RSD [19] and the same authors reported intra-day precision of less than 2% and inter-day precision of less than 5% in the determination of HPMCAS by size exclusion chromatography [20]. By comparison, the validated precision of the GPC method for CAP was 0.5% RSD, and the precision shown in Table 2 for CAP in the forced degradation study was 0.7% RSD. In addition to their precision, the GPC and RP-HPLC assays were stability-indicating and representative of the expected extent of degradation. The value of 9.0% PA ± 0.4% RSD hydrolyzed after 24 hours in closed scintillation vials at 100 °C (Table 1; GPC) was within experimental error of the 9.1% PA ± 1.1% RSD hydrolyzed in a published stability study of CAP stored 6 months in open containers at 40°C/75% relative humidity [9].
CAP decomposes by hydrolysis of phthalate and acetate esters to give phthalic and acetic acids. During this process the RF of the decomposed CAP is reduced relative to original CAP because there is less bound phthalate. The initial presumption was that if, for example, 20% of the phthalate ester groups in CAP were hydrolyzed, then the UV signal absorbance of CAP would also be reduced by 20%. This direct ratio was not observed.
According to the Beer-Lambert law, the decline of absorbance of a pure substance is proportional to the decline in the molar absorptivity given by the extinction coefficient, ε. Determining the varying values of absorptivity (ε) of the CAP distribution would require the purification of fractions of the CAP GPC peak followed by the analysis of each by UV spectrophotometry. By contrast, utilization of the empirical RF circumvents the formal investigation of absorptivity variation. The methods utilized in this report circumvent the direct calculation of ε. However, the decline in RF is consistent with a change in ε proportional to BP/TP during heat degradation.
Another possible explanation for the decline in RF is that degradation products spectrally dissimilar to CAP and PA were generated during the course of forced degradation. The most significant degradation peak present in the GPC analysis eluted at 14.9 minutes retention time. The DAD spectrum of this peak showed a region of absorbance red-shifted from the 280 nm local maximum of CAP and PA (Fig. 6). The spectrum of the unknown degradation product matched precisely with that of phthalic anhydride, the reactant that esterifies with the cellulose acetate backbone to form CAP, during CAP synthesis. The reverse reaction of phthalate to phthalate anhydride is evidently favored in a dry incubator at 100 °C. The 14.9-minute peak was positively identified as phthalic anhydride in a subsequent forced degradation experiment and by co-chromatography, comparing the retention time of the degraded sample with a phthalic anhydride standard. Phthalic anhydride, PA, and CAP were heat-degraded for 24 hours as described earlier. Pure PA and phthalic anhydride were almost completely stable under these conditions.
Fig. 6.
Normalized UV spectra acquired by the DAD of an Agilent 1100 during GPC analysis. Triangles: CAP. Squares: PA. Diamonds: Phthalic anhydride. For comparison purposes, absorbance values are shown as a fraction of the maxima of 574 mAU at 210 nm for CAP, 203 mAU at 210 nm for PA, and 59.0 nm at 212 nm for phthalic anhydride. Inset: Spectral data to scale.
It is unlikely that both of the sensitive and robust HPLC methods employed would fail to resolve degradation products containing a chromophore such as phthalate or acetate. The linear relationship shown in Fig. 5 suggests that any potential, undetected degradation products coeluting under the CAP peak are insignificant. Degradants with ε sufficient to affect the mass balance would also maintain the linearity (Fig. 5) if produced in proportion to the decline in PA. Unmodified polysaccharides or glucose monomers lack chromophores with significant absorption at the UV wavelengths monitored. However, given the initial degree of saturation of phthalate and acetate resulting in a 35% TP value (Table 2), the statistical probability of a detectable loss of mass balance due to unmodified and undetected sugars and polysaccharides is vanishingly small.
UV spectra in THF were collected by the diode array detector in 2 nm increments as a part of the method validations and the forced degradation study (Fig. 6). CAP and PA spectra of a degraded sample were nearly superimposable, with local absorption maxima at 210, 228, and 276 nm for CAP and 210, 226, and 276 nm for PA. The spectral differences between PA and CAP, though slight, support the explanation that variations in molar absorptivity underlie the observed variations in RF between the standard and the degraded samples measured at 280 nm. The AA peak had a single absorbance maximum of 210 nm, while phthalic anhydride exhibited a local maximum at 212 nm, an inflection point at approximately 244 nm and a local maximum at 290 nm.
Unlike the spectrum of the PA standard, the absorbance of phthalic anhydride is closer to a local minimum than a local maximum at 280 nm. The relatively minor contribution of this peak to the overall mass balance, reported in Table 2 as 2.62% at 24 hours using PA RF, indicates that it is of minor importance in the overall mass balance shortfall. The overestimation of PA by RP-HPLC compared to GPC suggests that phthalic anhydride coeluted with PA by RP-HPLC.
5. Conclusion
The work presented here will save time and resources in any future CAP method development. Using GPC for PA, the expense of rpHPLC is reduced to one run per lot of Aquateric standard. The empirical RF correction procedure will also facilitate future stability studies of CAP or other substitute polymers in combination with other candidate microbicides, since sources of error will be increasingly difficult to isolate in formulations with two or more API’s [21]. The mass balance shortfall noticed in the forced degradation of pure CAP might be missed in studies with multiple API and degradant peaks. Finally, the approach presented here could potentially apply to any analysis of the stability of polymer-linked chromophores.
Acknowledgments
The authors wish to thank Mr. Chang Min Kim, Ms. Nancy Tesche and Mr. Dominic Manansala for their technical expertise, Dr. Simon Yeh for helpful comments and editing, and Ms. Coral Campbell for her assistance in editing and formatting this manuscript. This work was supported by National Institutes of Health Grants PO1 HD41761 and U19 HD048957 (ARN Program Director).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin SY, Kawashima Y. Pharm Res. 1987;4:70–74. doi: 10.1023/a:1016442330193. [DOI] [PubMed] [Google Scholar]
- 2.Gyotoku T, Aurelian L, Neurath AR. Antiviral Chem Chemother. 1999;10:327–332. doi: 10.1177/095632029901000604. [DOI] [PubMed] [Google Scholar]
- 3.Neurath AR. AIDS Patient Care STDS. 2000;14:215–9. doi: 10.1089/108729100317830. [DOI] [PubMed] [Google Scholar]
- 4.Manson KH, Wyand MS, Miller C, Neurath AR. Antimicrob Agents Chemother. 2000;44:3199–202. doi: 10.1128/aac.44.11.3199-3202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu H, Zhao Q, Wallace G, Liu S, He Y, Shattock R, Neurath AR, Jiang BS. AIDS Res Hum Retroviruses. 2006;22:411–8. doi: 10.1089/aid.2006.22.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neurath AR, Strick N, Li YY, Lin K, Jiang S. Biologicals. 1999;27:11–21. doi: 10.1006/biol.1998.0169. [DOI] [PubMed] [Google Scholar]
- 7.Neurath AR, Strick N, Li YY. BMC Infect Dis. 2003;3:27. doi: 10.1186/1471-2334-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Williams RO., 3rd Eur J Pharm Sci. 2002;17:31–41. doi: 10.1016/s0928-0987(02)00131-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Williams RO., 3rd Eur J Pharm Biopharm. 2002;53:167–73. doi: 10.1016/s0939-6411(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 10.Williams RO, 3rd, Liu J. Pharm Dev Technol. 2001;6:607–19. doi: 10.1081/pdt-120000298. [DOI] [PubMed] [Google Scholar]
- 11.Roxin P, Karlsson A, Singh SK. Drug Dev Ind Pharm. 1998;24:1025–41. doi: 10.3109/03639049809089946. [DOI] [PubMed] [Google Scholar]
- 12.Crawford RR, Esmerian OK. J Pharm Sci. 1971;60:312–4. doi: 10.1002/jps.2600600238. [DOI] [PubMed] [Google Scholar]
- 13.Neurath AR, Lia Y, Mandeville R, Richard L, Antimicrob J. Chemother. 2000;45:713–714. doi: 10.1093/jac/45.5.713. [DOI] [PubMed] [Google Scholar]
- 14.Williams RO, 3rd, Liu J. Eur J Pharm Biopharm. 2000;49:243–52. doi: 10.1016/s0939-6411(00)00065-5. [DOI] [PubMed] [Google Scholar]
- 15.USP-NF (United States Pharmacopeia 28/National Formulary 23) United States Pharmacopeial Convention, Inc; Rockville: 2005. p. 2982. [Google Scholar]
- 16.Neurath AR, Strick N. Anal Biochem. 2001:288. 102–4. doi: 10.1006/abio.2000.4890. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, Seburg R, Tsai E. J Pharm Biomed Anal. 2006;40:1089–1096. doi: 10.1016/j.jpba.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Rashan J, Jr, Chen R. J Pharm Biomed Anal. 2007;44:23–28. doi: 10.1016/j.jpba.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Fukasawa M, Obara S. Chem Pharm Bull. 2004;51:1304–1306. doi: 10.1248/cpb.51.1304. [DOI] [PubMed] [Google Scholar]
- 20.Fukasawa M, Obara S. Chem Pharm Bull. 2004;52:1391–1393. doi: 10.1248/cpb.52.1391. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Lu H, Neurath AR, Jiang S. Antimicrob Agents Chemother. 2005;49:1830–1836. doi: 10.1128/AAC.49.5.1830-1836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]