In early childhood wheezing and asthma are more common in boys than girls.1 This difference has either disappeared or reversed by early adulthood,2 although the age at which the change occurs is unclear. We therefore measured the age and sex specific prevalence of self reported wheeze and diagnosed asthma in 11-16 year old children attending secondary schools in the Nottingham area.
Subjects, methods, and results
In 1996 we completed a prevalence survey of all pupils attending 44 secondary schools in a defined postcode area in and around Nottingham. Questionnaires about lifetime and current wheeze and asthma diagnosed by a doctor (Appendix) were distributed to pupils for self completion during school time. Data were collected on 27 826 pupils (over 80% of registered pupils) aged 11-16 years, 51% of whom were boys. Parental responses to the same questions were obtained for a 1 in 4 random subsample of 3894 pupils (59% response).
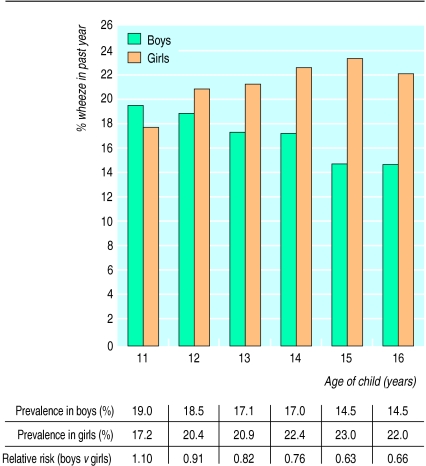
The self reported lifetime prevalence of wheeze was 30.1% (8317/27 632), with 19.0% (5253/27 668) of children reporting having wheezed in the past year. Of the children with current wheeze who also provided information on asthma 3527/5154 (68%) had had asthma diagnosed by a doctor at some time. Wheeze and diagnosed asthma were both significantly more prevalent in girls than boys (relative risk for wheeze in the past year 1.24 (95% confidence interval 1.18 to 1.30) (figure); full data are available in a table on our website). However, this sex difference was dependent on age. At 11 years wheeze was more common in boys, but thereafter the prevalence of wheeze decreased with age in boys (χ2 test for trend=23.2, P<0.0001) and increased with age in girls (χ2=20.4, P<0.0001), the sex reversal occurring at age 12. Inclusion of an age-sex interaction term in the multiple logistic regression model confirmed the significance of this effect (P<0.0001).
Analysis of parental responses gave lower prevalences of wheeze (lifetime 25.6% (994/3878), past year 16.4% (636/3880)). Sex reversal in wheezing (P<0.01 for age-sex interaction term) occurred at age 13, after which prevalence was relatively constant.
Comment
The male predominance of wheezing during the first decade of life is reversed around the time of puberty due to an increase in reported wheeze in girls and a fall in boys. A tendency for girls to overreport asthma symptoms and boys to deny them may have overestimated the size of the sex difference. However, the effect persisted when we used parental responses and also when we restricted the analysis to current wheezers with a doctor’s diagnosis of asthma, both of which measures may be less affected by sex. We found no difference in response rates among boys or girls of different ages except for slightly lower rates by both sexes in year 11, which would not have affected the overall trend. Parental response rates did fall with age, but this does not appear to have biased the estimates since the pattern of parent reported prevalence is similar to that of self reported prevalence. It therefore seems unlikely that the sex effect is solely the result of reporting or selection bias.
Hormonal changes occurring in early puberty could have a role in the changing prevalences. Troisi et al reported a positive dose-response relation between oestrogen use and the risk of adult onset asthma in women.3 Also there is evidence that girls are more likely than boys to develop asthma in adolescence,4 which could be hormone related or due to girls experiencing different exposures to triggers for wheeze at this age, such as smoking. The decrease in wheezing prevalence in boys may be related to the increase in size of airways, which are on average smaller than those of girls in infancy.5 The effects of hormones and other factors operating during early adolescence on measures of airway inflammation and other markers of asthma deserve further investigation.
Figure.
Self reported prevalence of wheeze in past year by age and sex
Acknowledgments
We thank Peter Housden, director of education; Julia Swan, assistant director; and Tony Dessent, senior assistant director of Nottinghamshire County Council for their support of the study and all the teaching and secretarial staff at the participating schools who made the survey work possible. We also thank Marilyn Antoniak, Andrea Goldsmith, Chris Smith, and Nicola Williamson for help with data collection.
Appendix: Respiratory questions asked
Have you ever had attacks of wheezing in the chest? (a noisy whistling sound from the chest, not the throat, causing tightness and breathlessness)
Have you had any wheezing attacks in the last year?
Have you ever been told by a doctor that you have asthma?
Footnotes
Funding: National Asthma Campaign and Department of Health.
Conflict of interest: None.
References
- 1.Luyt DK, Burton PR, Simpson H. Epidemiological study of wheeze, doctor diagnosed asthma, and cough in preschool children in Leicestershire. BMJ. 1993;306:1386–1390. doi: 10.1136/bmj.306.6889.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax. 1992;47:537–542. doi: 10.1136/thx.47.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med. 1995;152:1183–1188. doi: 10.1164/ajrccm.152.4.7551368. [DOI] [PubMed] [Google Scholar]
- 4.Schachter J, Higgins MW. Median age at onset of asthma and allergic rhinitis in Tecumseh, Michigan. J Allergy Clin Immunol. 1976;57:342–351. doi: 10.1016/0091-6749(76)90091-9. [DOI] [PubMed] [Google Scholar]
- 5.Tepper RS, Morgan WJ, Cota K, Wright A, Taussig LM. Physiological growth and development of the lung during the first year of life. Am Rev Respir Dis. 1986;134:513–519. doi: 10.1164/arrd.1986.134.3.513. [DOI] [PubMed] [Google Scholar]