Abstract
The control of synapse formation and shape is important for establishing complex brain circuitry. New evidence now suggests that a component of a synaptic ion channel complex has an unexpected role in these processes.
The synapse is the fundamental unit of connectivity and communication between neurons; trillions exist within the mammalian brain where they establish the precise circuitry that gives rise to complex thought and movement. It is hypothesized that synapse morphology, strength, and synaptic partner choice can all be modulated to promote learning and memory. Numerous protein complexes localize to the synapse and are required for its function. One important complex, the voltage-gated calcium channel (VGCC), permits calcium entry into the cell following membrane depolarization, allowing for calcium-dependent release of neurotransmitter from presynaptic termini [1, 2]. VGCCs consist of an α1 subunit, which forms the pore through which calcium enters, and several accessory subunits, one of which is the α2δ subunit [3]. α2δ associates with α1 and regulates its membrane trafficking, current load and voltage dependence [4, 5]. α2δ is translated as a single polypeptide, and then cleaved into two peptides, α2 and δ, which remain linked by disulfide bonds [6]. α2 is entirely extracellular, while δ is predicted to consist of a short transmembrane segment (Figure 1A). A puzzling aspect of α2δ biology is its large, highly conserved extracellular domain, which includes protein-protein interaction motifs not required for association with α1 [7]. These motifs suggest that α2δ may bind other proteins and have functions beyond its association with α1. Recent work of two research groups [8, 9] now suggests that α2δ may control synapse formation and morphology, and, importantly, that this role is independent of VGCC regulation.
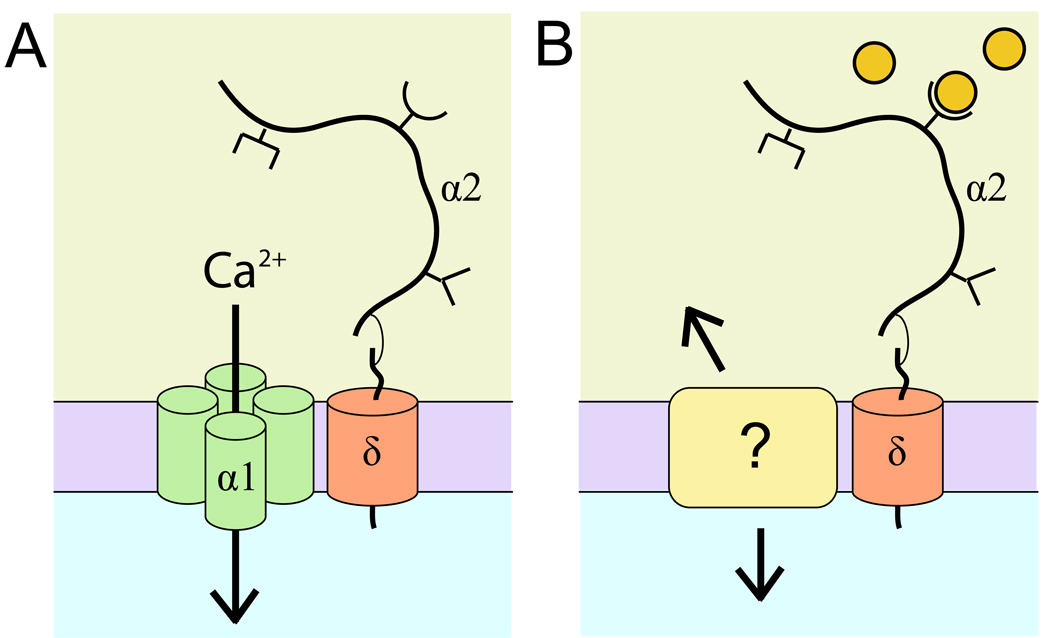
Figure 1. α2δ regulates synapse formation and shape by a novel pathway.
(A) α2δ associates with α1 to regulate the properties of voltage-gated calcium channels. α2δ consists of two domains, the extracellular α2 domain and the transmembrane domain δ, which are linked by disulfide bonds. Various protein interaction motifs are found on α2. (B) α2δ regulates synapse formation and shape independently of voltage-gated calcium channels: a possible model. Thrombospondins, extracellular circles, bind to the VWF interaction motif of α2, causing a conformational change in α2δ. This signal is propagated into the cell and perhaps to the extracellular space through another unidentified transmembrane complex.
In the fruit fly Drosophila melanogaster, neuromuscular junction synapses have a characteristic, rounded ‘bouton’ morphology. Kurshan, Schwarz, and colleagues [8] showed that in animals carrying mutations in the α2δ-3 gene, motor axons reach their correct muscle targets, form active zones, and align correctly with postsynaptic receptors. However, boutons never form, suggesting that synaptic morphogenesis does not occur correctly. Bouton formation requires the extracellular α2 peptide but not δ, suggesting that α2δ-3 may interact with an extracellular ligand to mediate its effects. Surprisingly, boutons formed normally in animals carrying mutations in the major α1 channel of the Drosophila neuromuscular junction. This finding suggests that α2δ-3 regulates synaptic morphology independently of its roles in modulating VGCCs.
How might α2δ-3 promote synaptic morphogenesis? A clue may be provided from recent studies of synaptogenesis in rodents by Eroglu, Barres, and colleagues [9]. Synapse formation was traditionally thought to be governed exclusively by the partnering neurons. However, previous studies from the Barres group showed that a second cell type found within the nervous system, glia, may also play a role in synaptogenesis. Usually thought of as passive bystanders with supportive roles, astrocytic glia were shown to induce synapse formation between mammalian neurons in culture [10]. They did this in part by secreting thrombospondins: large, extracellular matrix proteins [11]. In an effort to find the neuronal receptor for thrombospondins, Eroglu et al. [9] realized that thrombospondins had been previously shown to bind extracellular protein-protein interaction motifs known as Von Willebrand Factor (VWF) repeats [12]. Since α2 contains these repeats, they made an educated guess that α2δ may be the thrombospondin receptor. This guess paid off handsomely: the authors demonstrated that the α2δ-1 protein was required for thrombospondin induced synaptogenesis in cultured neurons, and that thrombospondins are associated with α2δ-1 in brain lysates. The EGF-like domain of thrombospondin was specifically required for synapse induction, and bound to the VWF motif of α2. Furthermore, as in the Kurshan et al. study, the synaptogenic properties of α2δ-1 were independent of its roles in regulating VGCCs: neither chemical inhibition of α1 channels nor over-expression of α1 affected synapse number.
Together, these studies suggest that α2δ may act as a cell surface receptor, regulating synapse formation and shape independently of the α1 channel (Figure 2B). Although it is unknown whether thrombospondins in Drosophila bind to α2δ, evidence from the nematode C. elegans suggests that there may be conservation of the ligand across species. Bacaj et al. [13] identified a thrombospondin-like protein, FIG-1, expressed by glia of sensory organs and which regulates properties of their associated sensory neurons. Two α2δ-like proteins are found in C. elegans, one of which is expressed in the sensory neurons that require FIG-1 [14]. Further studies are needed to determine whether this α2δ is indeed a FIG-1/thrombospondin receptor. Interestingly, unlike mammalian thrombospondin-induced synaptogenesis, FIG-1 exerts at least some of its roles independently of the EGF-like domains [13], perhaps indicating that mammalian thrombospondins have other functions yet to be elucidated.
The finding of a new role for α2δ proteins is intriguing, and hints at the existence of a novel signaling pathway that regulates synapse formation and morphology. Is α2δ required to specify partner identity at the synapse, or does this protein control more global aspects of synaptogenesis common to many or all synapses? Although this remains an open question, some observations may favor the latter model. First, the α2δ thrombospondin receptor localizes to synaptic sites of many neurons [9]. Second, while thrombospondin levels are high in the embryonic mammalian brain at the time most synapses form and are reduced in adults when synapses are generally stable [11], expression is observed throughout the brain. Third, Eroglu et al. show that thrombospondin-mediated synaptogenesis via α2δ is required for experience dependent plasticity in the mouse barrel cortex. Perturbation of α2δ affected the broad synaptic pattern of the responsive cortical structures in the rodent brain when animals were deprived of sensory inputs by whisker trimming [9]. Fourth, in thrombospondin knockout animals, global changes in synaptic patterning were disrupted [11]. These observations suggest the tantalizing model that secretion of thrombospondins by glia may define periods when and regions where synaptogenesis can occur.
Kurshan et al. suggest that α2δ may exert its effects at the synapse through rearrangements of the neuronal cytoskeleton [8]. However, the events that follow engagement of α2δ by its ligand are still unclear. Because δ has only a short intracellular domain, which is dispensable for regulating synapse formation and shape [8, 9], it is likely that another transmembrane protein associates with α2δ and is required to transmit signals into the cell (Figure 2B). It is also unclear whether other α2δ-type proteins have similar roles to the ones described in the studies of Eroglu et al. and Kurshan et al. If the α2δ signaling pathway proves to be conserved across species, as work in C. elegans and Drosophila may indicate, then these questions might be amenable to study in these genetically tractable organisms. The message for the time being, however, is that α2δ is a protein of many hats, reminding us that, sometimes, old proteins can reveal new tricks.
References
- 1.Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- 2.Young SM, Jr, Neher E. Synaptotagmin has an essential function in synaptic vesicle positioning for synchronous release in addition to its role as a calcium sensor. Neuron. 2009;63:482–496. doi: 10.1016/j.neuron.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Current opinion in neurobiology. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 4.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel alpha 2 delta subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 5.Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated delta peptides. The Journal of biological chemistry. 1991;266:3287–3293. [PubMed] [Google Scholar]
- 7.Wolf M, Eberhart A, Glossmann H, Striessnig J, Grigorieff N. Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. Journal of molecular biology. 2003;332:171–182. doi: 10.1016/s0022-2836(03)00899-4. [DOI] [PubMed] [Google Scholar]
- 8.Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha(2)delta-3 is required for synaptic morphogenesis independent of its Ca(2+)-channel functions. Nature neuroscience. 2009 doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science (New York, N.Y. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 11.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Pluskota E, Stenina OI, Krukovets I, Szpak D, Topol EJ, Plow EF. Mechanism and effect of thrombospondin-4 polymorphisms on neutrophil function. Blood. 2005;106:3970–3978. doi: 10.1182/blood-2005-03-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science (New York, N.Y. 2008;322:744–747. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frokjaer-Jensen C, Kindt KS, Kerr RA, Suzuki H, Melnik-Martinez K, Gerstbreih B, Driscol M, Schafer WR. Effects of voltage-gated calcium channel subunit genes on calcium influx in cultured C. elegans mechanosensory neurons. Journal of neurobiology. 2006;66:1125–1139. doi: 10.1002/neu.20261. [DOI] [PubMed] [Google Scholar]