Abstract
The potential role of phonological complexity in destabilizing the speech motor systems of adults who stutter was explored by assessing the performance of 17 adults who stutter and 17 matched control participants on a nonword repetition task. The nonwords varied in length and phonological complexity. Behavioral results revealed no differences between the stuttering and normally fluent groups on accuracy of nonword repetition. In contrast, dramatic differences between groups were observed in the kinematic data. Indices of the consistency of inter-articulator coordination revealed that adults who stutter were much less consistent in their coordinative patterns over repeated productions. With increasing length and complexity of the nonwords, between-group differences in coordinative consistency were more pronounced. Coordination consistency measures revealed that adults who stutter (but not normally fluent adults) showed within-session practice effects; their coordinative consistency improved in five later compared to five earlier productions. Adults who stutter produced the nonwords at a slower rate, but both groups showed increased rates of production on the later trials, indicating a practice effect for duration for both groups. We conclude that, though the adults who stutter performed behaviorally with the same accuracy as normally fluent adults, the nonword repetition task reveals remarkable differences in the speech motor dynamics underlying fluent speech production in adults who stutter compared to their normally fluent peers. These results support a multifactorial, dynamic model of stuttering in which linguistic complexity and utterance length are factors that contribute to the probability of breakdown of the speech motor system.
Keywords: Stuttering, Phonological processes, Motor learning, Speech motor control, oral motor coordination
Introduction
The most obvious and primary symptom of stuttering is the intermittent failure of the nervous system to generate appropriate command signals to the muscles whose activity must be dynamically controlled for fluent speech to be produced. One of the most perplexing aspects of stuttering is that these failures of the speech motor system typically occur within surrounding intervals of speech that is perceived as normally fluent. A critical question is: what causes these intermittent failures in the control signals in speech motor systems of individuals who stutter? We have proposed that the probability of occurrence of these intermittent failures is determined by many factors outside the motor system, including linguistic, cognitive, and emotional factors (Smith, 1999; Smith & Kelly, 1997). In our dynamic, multifactorial view of stuttering, perceptible disfluencies are not isolated, segmentable events, but are rather visible reflections of an underlying, continuous stream of dynamically interacting factors. Our model therefore predicts that the fluent speech of individuals who stutter will show signs of instability and inconsistency not characteristic of normally fluent control participants, signs of a system that is susceptible to disruption. We also propose that, as multileveled task demands increase, instabilities in the operation of speech motor systems of adults and children who stutter are more likely to be observed.
The results of kinematic studies have provided some evidence that adults who stutter, when producing perceptibly fluent speech, show differences in articulatory movement parameters and/or more variable timing of events within and between speech subsystems (Zimmermann, 1980; DeNil, 1995; McClean, Kroll, & Loftus, 1990; McClean, Levandowski, & Cord, 1994). However, studies also have reported movement parameters of adults who stutter that fall within the normal range (e.g., Smith & Kleinow, 2000; van Lieshout, Peters, & Starkweather, 1993; van Lieshout, Hulstijn, & Peters, 1996 a,b). Related to the present hypothesis, van Lieshout and his colleagues (van Lieshout et al., 1993; van Lieshout et al., 1996 a,b) tested the hypotheses that adults who stutter, even when producing fluent speech, differ substantially in speech motor plan assembly and production processes and that these differences are exacerbated by increased utterance length and linguistic complexity. Their results, however, which included response latencies and intersystem relative timing measures, e.g. timing between events in respiratory and articulatory systems, demonstrated significant overlap between performance measures of stuttering and nonstuttering adults, with only subtle timing differences related to overall slower performance in adults who stutter. Thus, their findings were generally negative with regard to the motor plan assembly hypothesis, and the predicted effects of utterance length and complexity were not observed.
Using a different approach, we also have explored the hypothesis that increasing utterance length and complexity produces greater evidence of instabilities in the speech motor output of young children and individuals who stutter. In a study of normally fluent children and adults, we (Maner, Smith, & Grayson, 2000) investigated whether consistency in speech motor output was affected by the length and/or syntactic complexity of the utterance to be spoken. Normally fluent adults and children produced a simple phrase in isolation (“buy Bobby a puppy”) and in a set of sentences in which that phrase was embedded (e.g., “You buy Bobby a puppy now if he wants one.”). The 5-year-olds, but not young adults, showed an increase in lower lip movement variability for the phrase when it was spoken in the context of the more complex and longer sentences. In a follow-up experiment on adults who stutter (Kleinow & Smith, 2000), we found that articulatory movement variability of adults who stutter was affected in the same way as in the young children in the Maner et al. (2000) study. Variability of repeated productions was higher when the phrase was produced as part of a longer, more complex utterance. Note that in this study we used the entire movement signal for the six syllable phrase “buy Bobby a puppy” in the analysis. We interpreted the results of the study of stuttering adults to support a basic tenet of our model: Linguistic factors affect the operation of the speech motor system in adults who stutter, and that this increase in movement variability with increased overall sentence length and syntactic complexity was a sign of a speech motor control system that is more susceptible to disruption. All of the sentences analyzed in this study were spoken fluently, so that this observed increased instability in the motor output for the adults who stutter was not perceptible.
The present experiment was designed to determine whether increased utterance length and phonological complexity in a novel nonword production task will reveal additional, atypical characteristics of the fluent speech of adults who stutter. Considering the potential effects of phonological factors in speech motor output in stuttering is an important step, because three recent theoretical accounts of stuttering (though they differ in significant ways in their details) posit that slowed and/or faulty phonological encoding is the core deficit in stuttering from which all “downstream” motor problems flow (Howell, 2007; Perkins, Kent, & Curlee, 1991; Postma & Kolk, 1993). As in Levelt and colleagues' model of spoken language production (Levelt, Roelofs, & Meyer, 1999), these theories of stuttering posit phonological processing stages that operate prior to and independently of motor planning and execution stages. In other words, in the context of Levelt et al.'s well known model of normal language production, these three theories generally propose that the motor breakdowns seen in stuttering are a “downstream” result of faulty or slowed input from the higher level networks involved in translating abstract phonological words via a phonetic encoding process to motor programs.
As noted above, our multifactorial, dynamic view of the etiology of stuttering (Smith, 1999; Smith & Kelly, 1997) is quite different from these phonological encoding theories in that linguistic factors are not the only, nor the primary factors capable of contributing to the onset and maintenance of stuttering. In addition, in our discussions of factors involved in stuttering and normal language production, we have emphasized language/motor interactions (Smith & Goffman, 2004) rather than viewing motor planning and execution as independent from prior language processing stages. We have argued that language and motor processes do not operate independently, as evidenced by numerous studies demonstrating that the linguistic goals of the speaker affect the details of speech motor output in normally developing children, children with specific language impairment, adults who stutter, and normal adults (see review in Smith & Goffman, 2004). Support for the idea that language processing and motor planning/execution are interactive also comes from a recent fMRI study of two-syllable nonword production in normal adults in which syllable frequency and phonological complexity was varied (Riecker, Brendel, Ziegler, Erb, & Ackermann, 2008). They found that producing nonwords with higher syllable onset complexity (CCV vs CV) resulted in higher activations of areas involved in speech motor planning and execution. Riecker et al. also noted that the production of CCV syllables is typically more complex motorically, and thus it is not possible to disambiguate phonological and motor contributions to the observed results. In summary, we hypothesize that increasing the phonological complexity of an utterance increases both linguistic and motor processing demands, and because these processes interact differently in stuttering vs. nonstuttering speakers, greater phonological/motoric challenges will reveal instabilities in the speech motor output of adults who stutter that are not observed in normal speakers.
In the present experiment we use a nonword repetition task to examine the abilities of adults who stutter to decode novel phonetic sequences, engage the phonological loop, to generate a new motor plan, and finally to produce the speech sequence. We also examine potential differences in stuttering and nonstuttering adults in speech motor learning processes over repeated trials. Nonword repetition tests have been widely employed in studies of young children, and deficits on this task are a marker for specific language impairment (see review in Graf Estes, Evans, & Else-Quest, 2007). Two recent studies of children who stutter revealed that their nonword repetition abilities lag those of their normally developing peers (Anderson, Wagovich, & Hall, 2006; Hakim & Ratner, 2004). Nonword production abilities of adults who stutter have received little attention. Ludlow, Siren, and Zikria (1997) reported a pilot investigation of learning two extremely complex, four-syllable nonwords (e.g., abisthwoychleet) in five adults who stutter. Both adults who stutter and normally fluent controls exhibited a practice effect within the experimental session, measured as an increase in the percent phonemes correct, but the effects of practice were different for the two groups. Adults who stutter showed significantly fewer phonemes correct on the later trials. Ludlow et al. (1997) suggested this was evidence of a phonological encoding deficit which affected the abilities of adults who stutter to learn novel phonological sequences.
Namasivayam & van Lieshout (2008) examined within-session practice effects and across-session motor learning effects for novel nonword production in five adults who stutter and five normally fluent controls.1 They asked participants to repeat successively the two syllable nonword /bapi/ at normal and fast rates, so this design was somewhat different than that used in traditional nonword repetition experiments. Upper lip, lower lip, and jaw movements were recorded and used to compute measures of movement amplitude and duration, indices of cycle to cycle variability (the “cyclic STI”), and inter-articulator coupling stability. Between group differences were not observed, but the trends suggested that adults who stutter had lower cycle-to-cycle movement stability and more variable interarticulator coupling, and that adults who stutter showed less robust practice and motor learning effects.
In summary, from our earlier study (Kleinow & Smith, 2000) there is evidence that increasing the length and syntactic complexity of utterances differentially affects speech motor dynamics in the fluent speech in adults who stutter. With respect to increasing phonological complexity, we have behavioral data from Ludlow et al. (1997) that adults who stutter do not learn complex novel nonwords with the same accuracy as their normally fluent peers, as measured by differences in the percent phonemes correct in later trials. In addition, there has been mounting evidence that adults who stutter are not as proficient in learning novel finger tapping and syllable sequences (e.g., Smits-Bandstra, De Nil, & Saint-Cyr, 2006), providing growing evidence of a more general motor learning deficit in stuttering. Earlier studies also have suggested differences in adults who stutter and normally fluent controls in the rate of improving motor performance as a result of practice (Ludlow et al., 1997; Smits-Bandstra et al., 2006; Namasivayam & van Lieshout, 2008). Within this context, then, the present study was designed to assess whether adults who stutter show greater differences in speech motor performance measures in the face of increasing phonological processing and speech motor planning and execution demands, and whether adults who stutter differ from their normally fluent peers in their response to practice when they produce novel nonwords over repeated trials.
We employ a paradigm that we used in an earlier study of normally fluent 9-10 year-old children and young adults (Walsh, Smith, & Weber-Fox, 2006). Participants hear a novel nonword, then repeat it in a carrier phrase. A block of five nonwords of increasing phonological complexity was presented with the nonwords pseudo-randomized within each block until each word was produced at least 10 times correctly. In the Walsh et al. (2006) study we found that the children improved in interarticulator coordination consistency over the course of the experimental session, demonstrating short-term practice effects. The young adults did not show this effect, rather they were likely at ceiling performance on the early trials, and no improvement in coordinative consistency was observed. Both children and adults increased rate of production on the later trials. We also found that the largest learning effects were observed when the children produced the longer, more complex nonword sequences.
The purposes of the present investigation are (1) to use a standardized nonword repetition task (Dollaghan & Campbell, 1998) to assess at a behavioral level the accuracy of nonword repetition in a group of adults who stutter; (2) to use kinematic measures to detect any differences in articulatory coordinative stability and speech rate in a novel nonword production task between adults who stutter and normally fluent controls; and (3) to determine if practice in producing novel nonwords over the course of the experimental session results in changes in coordinative consistency and/or speech rate in either group of speakers. Within our multifactorial, dynamic account of stuttering, we predict that analyses of the oral coordination patterns of adults who stutter producing nonwords fluently over repeated trials will reveal evidence of an underlying production system that operates differently compared to that of normally fluent speakers.
Method
Participants
Participants were 17 adults who stutter and 17 normally fluent adults matched for age (+/- 2 years, range 18-45 years), sex, and education (+/- 1 year, range high school to 6 years postgraduate). Each group included 12 males and 5 females. Participants performed within appropriate limits on the Test of Adolescent and Adult Language (TOAL-3; Hammill, Brown, Larsen, & Wiederholt, 1994) and the Oral Speech Mechanism Screening Evaluation-Revised (OSMSE-R St. Louis & Ruscello, 1987). Each participant passed a binaural hearing screening. All participants spoke American English as their first and primary language. Prospective participants were excluded if they failed any portion of the screening assessments, wore orthodonture, were taking medications expected to affect motor or cognitive performance, or reported positive history of any neurological or speech/language disorder other than stuttering.
Nineteen participants who stutter completed the Stuttering Severity Instrument (SSI-3). Two potential stuttering participants obtained severity ratings of moderate/severe and severe on the SSI, but they could not produce the novel nonwords fluently during the experimental session. Therefore they were not included in the present report, and all results are reported for the remaining 17 adults who stutter. Of these 17, results of the SSI were lost for one individual during a laboratory move; the remaining 16 scored in the very mild or mild range.
Standardized Nonword Repetition Task
Prior to the kinematic recording session, all participants completed the Nonword Repetition Task designed by Dollaghan and Campbell (1998). This test includes 16 nonwords of 1 to 4 syllables in length and is scored as percent phonemes correct for the nonwords of each length. Participants heard each nonword produced by a female experimenter and then repeated the word. The session was videotaped for later scoring. This test was included to carefully assess any differences in errors in nonword repetition at the behavioral level in adults who stutter.
Kinematic Recording Apparatus
Participants were seated in front of an Optotrak 3020 motion tracking system (Northern Digital). This system tracks the 3-d positions of small (5 mm diameter) infrared light emitting diodes (IREDs) attached to the surface of the skin with adhesive collars. Upper and lower lip motions were recorded with IREDs attached to the center of the vermillion border of each lip. Data from an IRED attached to the jaw were recorded but not analyzed in the present experiment. Head motion was recorded with 5 IREDs attached to the forehead and to specially modified sports goggles. The motion of these 5 IREDS was used to create a rigid body, and Optotrak software was used to compute a 3-dimensional head coordinate system for each participant. Superior-inferior upper lip and lower lip (plus jaw) movements were then calculated relative to the head coordinate system, a process which corrects for head motion artifact (see Smith & Goffman, 1998, Appendix A, and Smith, Johnson, McGillem, & Goffman, 2000). Motion of each IRED was sampled at 250 Hz.
Experimental Protocol
After positioning the IREDs, the experimenter explained the protocol, informing the participant that the task required saying “new words.” These novel nonwords were adapted from those used by Dollaghan and Campbell (1998), so that they ranged in length from one to four syllables and contained primarily labial sounds (e.g., m, p, b), which highly constrain lip motion to reach the consonant targets. In an earlier experiment, we employed an identical protocol in a study of 20 normally fluent 9-10-year olds and 20 normally fluent young adults (Walsh, Smith, & Weber-Fox, 2006). As described in Walsh et al. (2006), the nonwords increased in length and phonological complexity: “mab” (/mæb/), “mabshibe” (/mæbʃaIb/), “mabfieshabe” (/mæbfaIʃeIb/), and “mabshaytiedoib” (/mæbʃeitaIdɔIb/). A fifth nonword, “mabteebeebee” (/mæbtibibi/), was included as a length control (four simple syllables). In order to select consistent starting and ending points to segment the articulatory movement trajectories for analysis, all of the nonwords had bilabials at the beginning and end, so that the peak velocity of the opening oral motion following bilabial closure could be used as the kinematic landmark for segmenting the data. Finally, with regard to the nonword set we used, we note that there are many approaches to manipulating phonological complexity. Some of these include more rigorous control of variables such as syllable frequency and intra- and inter-syllable complexity (e.g., Riecker et al. 2008). Given our requirements to target bilabial consonants (so that the nonwords primarily involve movements we can track with the optical system) and to use nonwords that begin and end with the same consonants (for the purpose of segmenting the kinematic data for analysis), we had to compromise on more precise control of complexity in the design of the nonword stimulus set.
On the basis of our earlier study (Walsh et al., 2006), we expected that adults who stutter would be able to fluently and accurately produce most, if not all, of the nonwords designed for the kinematic experiment, because 9 and 10-year-old children could accurately produce these words. This strategy was adopted, because the purpose of the experiment was to examine the dynamics of fluent speech production in the face of increased phonological and motor processing demands. Also, on the basis of this earlier study, we expected that if practice effects were to be observed over the course of the present experiment, 10 trials would be an adequate number. To ensure that participants could correctly produce the novel nonwords before movement recording was started, each participant heard a recorded model of each nonword presented via loudspeaker. They then repeated it. Emphatic stress was consistently placed on the initial syllable (mab) of each model. The experimenter determined whether the participant's nonword production was accurate. If not, the model of that nonword was repeated. All participants were required to produce each nonword correctly twice consecutively. After all five nonwords were trained to criterion, participants were then instructed that when they heard each nonword over the speaker, they should produce it in the carrier phrase, “Bob says ____ again” using their preferred rate and loudness.
Movement data collection was then started. During the movement data collection trials, participants heard the recorded model for each nonword, then produced it embedded in the carrier phrase. The five nonwords were pseudo-randomized within 11 blocks with each nonword occurring once in each block. There was a pause of approximately 2 sec between the presentation of each nonword within a block. There was a short break (approximately 1 minute) after completion of six blocks. The experimenters kept track online of the numbers of fluent productions obtained, and data collection continued until at least ten fluent exemplars of each utterance had been obtained. A fluent production of the entire phrase was judged to be free from errors (i.e. substitutions, omissions, additions, distortions, aberrant prosody, or inappropriate pauses) by one experimenter online and later during data analysis by a second experimenter. If differences in the judgments of fluency arose between the online and later judgment, the later, offline judgment was used, because this individual could repeatedly play the utterance and view it on videotape. Offline judgments of accuracy and fluency were completed by one of the authors who is a certified speech-language pathologist with extensive experience in the area of fluency. This individual also scored the Nonword Repetition Task (Dollaghan & Campbell, 1998) for each subject.
Data Analysis
The first correct production for each nonword in the kinematic recording trials was not included in the data analysis. This strategy was adopted, because it is conservative, biasing the results against finding significant differences between early and later trials. One could argue, for example, the first trial produced after the cameras were activated and data collection began is more likely to be an outlier, despite the fact that subjects produced two correct practice trials before the cameras were turned on. Kinematic analysis, then, was completed on the next five (e.g., in most cases trials 2-6) and last five (e.g., in most cases 7-11) accurate and fluent productions of each nonword for each participant.
After head motion correction, the upper lip and lower lip (including the jaw component) movements associated with the nonword productions were imported into MATLAB (Mathworks, 2005) signal processing software for analysis. Movement signals were low-pass filtered (cut-off 10 Hz) in the forward and reverse directions, An interactive program that offered a simultaneous display of the superior-inferior displacement and velocity records from the lower lip for each trial was used to extract the kinematic data associated with each nonword from the longer records produced for the carrier phrase. As described in Walsh et al. (2006), the kinematic record for each nonword was segmented by selecting consistent kinematic landmarks from the velocity records. Starting points were chosen as the peak velocity of the opening movement for the /m/ in “mab”, while end points were selected as the peak lower lip opening velocity for the final opening movement of each nonword. The lower lip start and end points were then used as reference points to segment the data from the upper lip signal and also were used to compute the duration of the total movement trajectory for each nonword. The speech acoustic signal was digitized at 7.5 kHz with an A/D unit synchronized to the Optotrak kinematic data collection time base. The experimenter used this audio signal during data analysis to ensure that the kinematic data segmented for each nonword corresponded to the appropriate target utterance.
To examine whether practice effects occurred during the course of the experimental session, we designed an analysis to compare the coordinative consistency of the earlier compared to the later productions of each nonword. We used the lip aperture signal, which is simply the point by point subtraction of the lower lip from the upper lip superior-inferior displacement (Smith & Zelaznik, 2004).2 The use of the lip aperture signal in the present study is a departure from the method of our earlier study of the effects of syntactic complexity on speech kinematics in adults who stutter (Kleinow & Smith, 2000) in which only the motion of the lower lip marker (plus jaw) was analyzed. We elected to use lip aperture on the basis of the results of a large-scale, cross-sectional study of 180 children and adults (Smith & Zelaznik, 2004). We compared the coordinative consistency of the lip aperture trajectory (upper lip IRED minus lower lip IRED; which, because jaw movement was not subtracted from the lower lip IRED signal, represents the pattern of coordination of the upper lip, lower lip and jaw) with the consistency of coordination of the lower lip/jaw synergy (lower lip IRED minus jaw IRED). In all age groups, the lip aperture signal was less variable on repeated utterances compared to the lower lip/jaw trajectory. This result was notable, because if each articulator's trajectory variability is computed separately, the upper lip and lower lip signals show much higher trajectory variability as reflected in indices of spatial and temporal variability (STIs) computed separately for each articulator (Walsh & Smith, 2002). These results thus support the idea that the lip aperture signal represents the functioning of a higher level coordinative synergy to achieve dynamically targeted lip aperture distances during the production of speech (Smith & Zelaznik, 2004). As such, it seemed a good strategy to examine the coordinative consistency of this higher order synergy in relation to the linguistic goals of the speaker, rather than to restrict the analysis to a single point trajectory.
In order to examine potential differences in inter-articulatory coordination between groups and to uncover any potential changes that might have occurred with practice, lip aperture variability indices were computed using the five early (e.g., usually trials 2-6) and five later (e.g., usually trials 7-11) productions of each nonword for each subject. We refer to these as the “early” and “later” trials. These indices reflect the degree of spatial and temporal variability in coordinative patterns among the upper lip, lower lip, and jaw for early and later sets of lip aperture trajectories for each nonword production. As in earlier studies from our laboratory, the variability indices were calculated by time- and amplitude-normalizing the lip aperture movement trajectories (Smith et al., 2000; Smith & Zelaznik, 2004). Figure 1 illustrates this procedure for data from one participant, an adult who stutters, producing the nonword “mabfieshabe.” For time-normalization, a cubic spline procedure was used to project each lip aperture record onto a constant axis length of 1000 points. Each record was amplitude normalized by subtracting the mean of the displacement signal and dividing by its standard deviation. Finally, as shown in the bottom panel of Figure 1, the standard deviations of the five early and five later normalized lip aperture trajectories were calculated at fixed 2% intervals in relative time. These 50 standard deviations were summed resulting in the lip aperture variability index. Lower values of the coordination indices reflect convergence of the coordinative patterns over the 5 trials.
Figure 1.
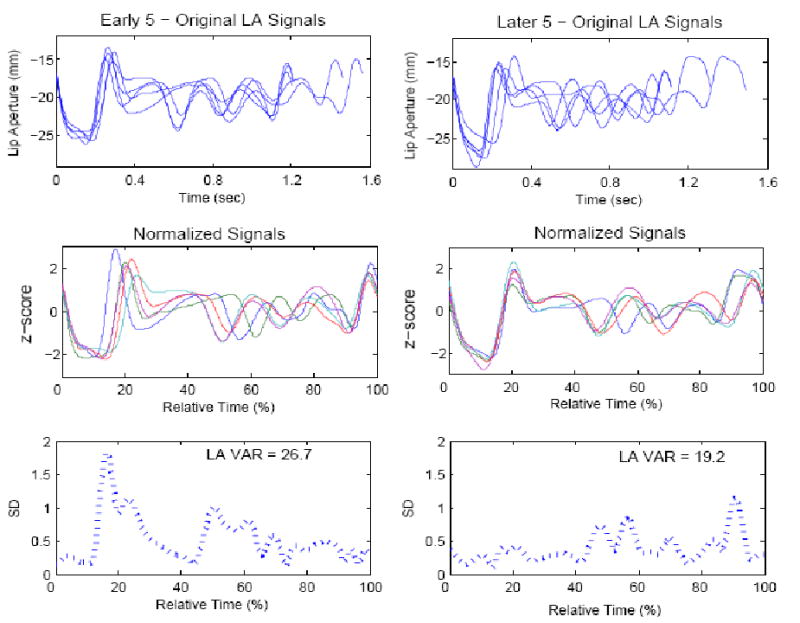
Example of one nonword, “mabfieshabe,” produced by an adult who stutters. The plots in the left column are the set of 5 productions from the “early” trials; right column plots are the set of five “later” trials. Top panel: original difference signals before normalization. Note different duration of the various productions of the nonword. Middle panel: plots after time and amplitude normalization. Bottom panel: Standard deviations computed at 2% intervals in relative time. Inset in bottom plots are the lip aperture variability indices for the early and later trials. Note that the LA variability index is reduced for the later set of trials.
A repeated-measures ANOVA was computed to detect between-subjects effect ‘group’ (stuttering vs. fluent adults) and within-subject effects of ‘nonword’ (5 nonwords) and ‘trial’ (early versus later productions). Separate ANOVAs were computed for the lip aperture variability index and for nonword duration. A t-test was used for a planned comparison of the lip aperture variability indices on the two nonwords of the same length, 4 syllables, but higher and lower phonological complexity. Behavioral data also were compiled to characterize the performance of the participants in each group in producing the novel nonwords for both the kinematic data collection trials and on the Nonword Repetition Test (Dollaghan & Campbell, 1998).
Results
Behavioral Results Nonword Repetition Test
Due to a faulty tape, the Dollaghan and Campbell (1998) Nonword Repetition Test (NRT) for two participants, one normally fluent adult and one adult who stutters, were lost. For the remaining 16 participants in each group, percent phoneme correct scores were computed for one, two, three, and four-syllable nonwords. Minimum, maximum, and median scores are shown in Table 1. Participants in both groups performed at ceiling or near-ceiling in the one, two, and three-syllable nonword repetition. For the four-syllable nonword repetition, median scores were 89 % and 85% for normally fluent and stuttering adults respectively, with no differences in the best and worst performance. Thus performance on this standard test was not different for the two groups.
Table 1.
Performance of both groups on the Dollaghan & Campbell Nonword Repetition Task (1998). Scores are percent phonemes correct.
| 1 Syl | 2 Syl | 3 Syl | 4 Syl | Total | ||
|---|---|---|---|---|---|---|
| Min | 92 | 90 | 89 | 61 | 81 | |
| NORM | Max | 100 | 100 | 100 | 94 | 98 |
| Mdn | 100 | 100 | 100 | 89 | 96 | |
| Min | 100 | 100 | 93 | 64 | 84 | |
| STUT | Max | 100 | 100 | 100 | 94 | 97 |
| Mdn | 100 | 100 | 98 | 85 | 94 |
Behavioral Results “Mab” Nonword Set
For the nonword repetition task designed for kinematic data collection, the “mab” nonword set, performance of the two groups (minimum, maximum, and median percent scores) is summarized in Table 2. The column labeled total productions indicates the total number of utterances produced by a participant to obtain the required 10 accurate and fluent productions of each nonword and the surrounding carrier phrase. The percent errors column indicates the number of nonwords produced incorrectly and/or disfluently. We counted the target word as a whole and did not score on a phoneme correct basis as in the Dollaghan and Campbell NRT above. The remaining two columns provide a breakdown of the nonword production as either fluent or disfluent. Again, the performance of the two groups is remarkably similar, except that the normally fluent group had a median score of zero disfluencies, while the stuttering group had median score of 1.2 disfluencies on the target words. The last column indicates the number of disfluent productions of the carrier phrase, “Bob says _____ again.” The adults who stutter had a median of one disfluency on the carrier phrase, while the normally fluent group had a median of zero.
Table 2.
Behavioral results for the “mab” word set. See text for more detailed explanation of column headers.
| Total # Nonword Prod. | % Error in Nonword | % Fluent Nonword Errors | % Disfluent Nonword Errors | Number Disfl. In Carrier Phrase | ||
|---|---|---|---|---|---|---|
| Min | 72 | 1.4 | 0 | 0 | 0 | |
| NORM | Max | 100 | 25.0 | 22.7 | 4.9 | 4 |
| Mdn | 83 | 7.0 | 6.0 | 0 | 0 | |
| Min | 72 | 1.2 | 0 | 0 | 0 | |
| STUT | Max | 94 | 19.0 | 13.8 | 4.4 | 4 |
| Mdn | 88 | 9.0 | 5.6 | 1.2 | 1 |
Overall the behavioral analysis of both the standard NRT test of Dollaghan and Campbell (1998) and performance on the nonword set designed for kinematic analysis in the present experiment reveals that these adults who have a mild stuttering problem perform much like their normally fluent peers. The only difference is that the adults who stutter were, as one would expect, more disfluent.
Kinematic Analyses of Coordination Consistency
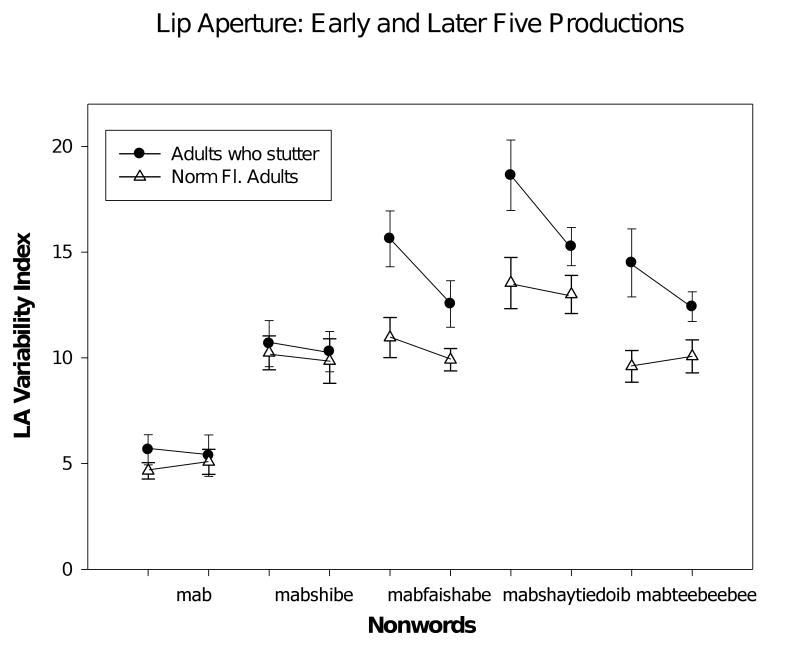
In Figure 2 mean lip aperture variability indices are plotted for each group of participants, adults who stutter and normally fluent adults. Recall that this index reflects the degree of convergence of sets of lip aperture trajectories for 5 early and later productions of each nonword. A higher variability index indicates less trial-to-trial consistency in the sets of lip aperture trajectories, which we interpret as less trial-to-trial consistency in the coordination of the upper lip, lower lip, and jaw. A reduction in the lip aperture variability index from early to later sets of trials is evidence of a practice effect; that is, the pattern of upper lip, lower lip, and jaw coordination is becoming more consistent with the within-session practice.
Figure 2.
Plot of lip aperture variability indices (mean and std. error) for early and later trials for the two groups of speakers. For each nonword and each speaker group, the symbols for the early and later sets of trials are connected by a line.
It is clear from the graph in Figure 2 that the group of 17 adults who stutter had higher lip aperture variability indices on the longer, more complex nonwords. Also apparent is the fact that the adults who stutter show remarkable reductions in their lip aperture variability indices in the later compared to the earlier trials. This effect is especially apparent for the more complex, longer nonwords. In contrast the normally fluent participants show an effect of the complexity and length of the nonword, but little apparent effect of practice (i.e., the lip aperture variability indices are about the same for the early and later five productions of each nonword).
These observations were supported by statistical analysis. A repeated measures ANOVA on the lip aperture variability indices revealed that the adults who stutter had, on average, higher variability indices (F (1,32) = 8.8, p = .006). As expected, there was an effect of nonword (F (4,128) = 60.2, p < .0001); there was also a nonword × group interaction (F (4,128) = 3.3, p < .01). There was also a significant effect of trials, such that, on average, later trials were characterized by lower lip aperture variability scores (F (1,32) = 8.1, p < .01); there was also a significant trial × group interaction (F (1,32) = 5.1, p = .03). Given this interaction, separate, follow-up ANOVAs were completed to test the effect of trial, early vs. late, on each group. For the group of adults who stutter, there was a significant effect of trial (F (1,32) = 31.3, p < .001); while no effect of trial was seen in the normally fluent control group (F < 1). Thus, this analysis reveals that a practice effect, evidenced by significantly lower lip aperture variability indices on the sets of later nonword productions, was characteristic of the stuttering participants only.
We planned t-tests to highlight the comparison of the lip aperture variability indices for the two nonwords that had equal lengths in syllables but high and low levels of phonological complexity (“mabshaytiedoib” vs. “mabteebeebee”). Given the significant differences between groups and the nonword by group interaction in the ANOVA reported above, separate paired t-tests were computed for each group to compare coordination consistency for the most complex 4-syllable word and the length control. For the normally fluent group and for the stuttering group the lip aperture variability indices for “mabshaytiedoib” (computed from all 10 tokens) were higher than those for the length control, “mabteebeebee,” (normally fluent group, means, 15.7 and 11.4, t (16) = 3.13, p < .01); stuttering group, means, 18. 2 and 14.5, t (16) = 2.45, p < .03).
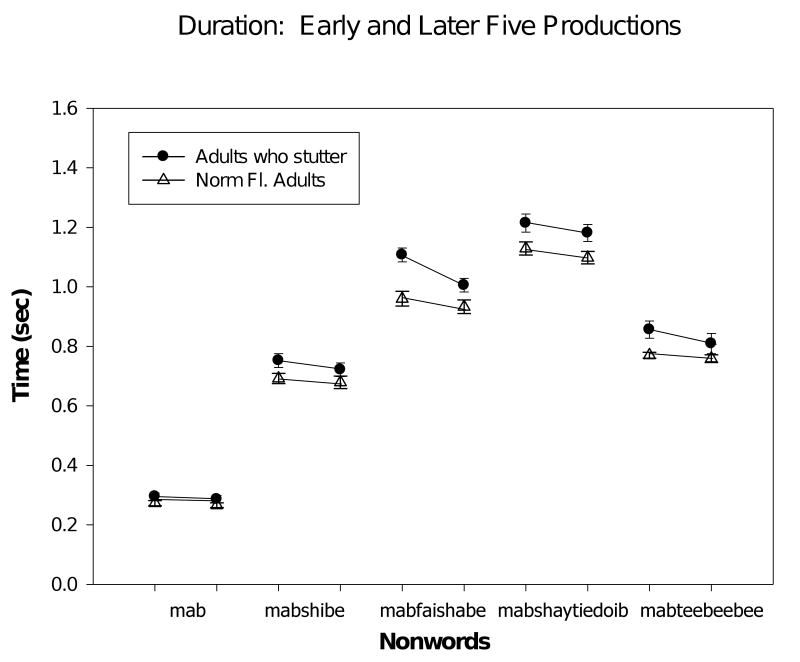
Nonword Duration
The duration of the lip aperture trajectory for each nonword was also measured. In Figure 3, mean durations are plotted for each group of participants, adults who stutter (AWS) and normally fluent controls. Duration of the five nonwords ranged from about 0.2 sec for the shortest to approximately 1.2 sec for the longest nonword. Adults who stutter had longer nonword durations than the normally fluent controls (F (1,32) = 12.9, p < .001). There was the expected word effect (F (4, 128) = 444.6, p < .0001). The word × group interaction was also significant (F (4, 128) = 10.8, p < .001). There was an effect of trial, such that the last five trials tended to be shorter than the first five (F (1,32) = 5.2, p < .03). Interestingly and in contrast to the results for the lip aperture variability index, there was no group × trial interaction for nonword duration (F (1,32) = 1.2, p = .28). Thus, both groups of speakers had, on average, shorter durations of the nonwords with repeated productions.
Figure 3.
Plot of duration of the nonwords (mean and std error) for the early and later trials for the two groups of speakers. For each nonword and each speaker group, the symbols for the early and later sets of trials are connected by a line.
Relationship Between Lip Aperture Coordination Variability and Duration
To determine if the faster speakers in each group tended to be the most consistent in their coordination patterns over the 10 productions, we computed correlation coefficients between the mean duration and lip aperture variability index for each nonword separately for each subject group. For adults who stutter, the five correlations were not significant, ranging in value from -.25 (for “mab”) to .22 (for “mabteebeebee”). Similarly, none of the five correlations between lip aperture variability indices and nonword duration were significant for the nonstuttering group; the range was from -.31 (for “mabshaytiedoib”) to .37 (for “mabfieshabe”).
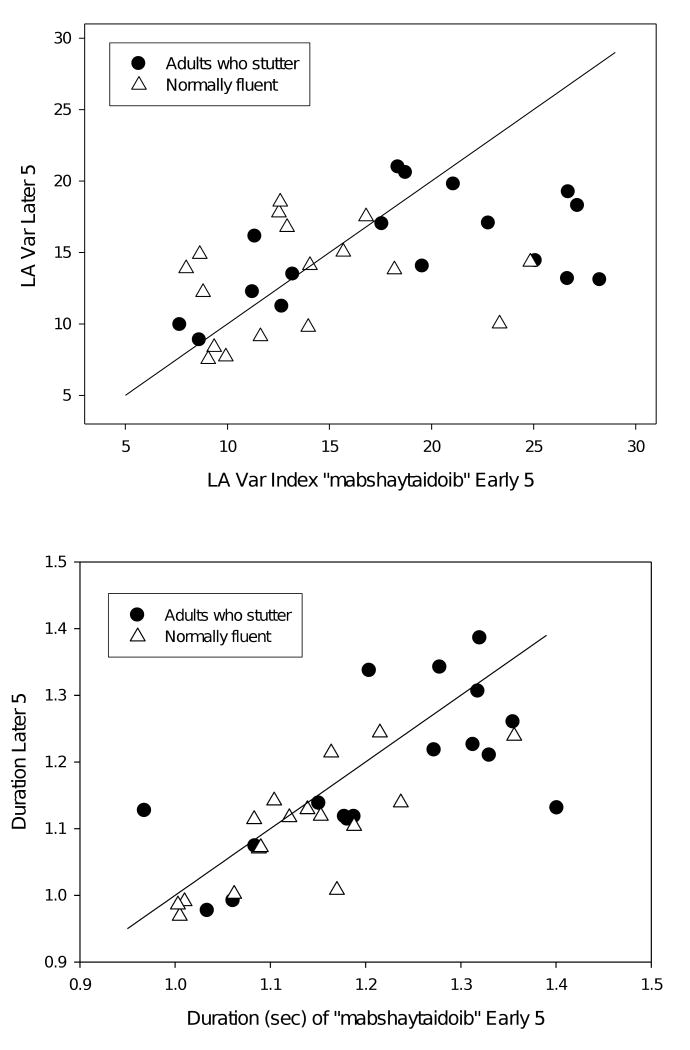
Individual Differences
Throughout this section, we highlight the differences between groups, adults who stutter and their normally fluent peers. Individuals who stutter are a highly heterogeneous group, as expected in a disorder likely to have many underlying contributing factors that operate with different weights in affected individuals (Smith & Kelly, 1997). Thus it is important to recognize that the behavior of many of the adults who stutter overlapped that of their normally fluent peers. To illustrate this point, the early and later lip aperture variability indices and nonword duration for the longest, most complex nonword (“mabshaytaidoib”) are plotted for each individual in Figure 4. Recall that from the group statistics, we concluded that adults who stutter have higher variability indices compared to the normally fluent group and show a practice effect such that the coordinative variability for later trials is lower than that of the earlier trials, while the normal adults showed no practice effect. In the upper plot of lip aperture variability, it is clear that approximately six of the adults who stutter have lip aperture variability indices within the normal range. Further these six individuals do not show a practice effect (their early and late indices fall approximately along the diagonal). Another subgroup, 4 adults who stutter, has relatively high variability indices but do not show a practice effect. Also apparent is a group of 7 adults who stutter whose lip aperture variability indices clearly do not fall in the normal performance range, and these individuals show practice effects, in some cases very large practice effects (e.g., falling by approximately 50% from a lip aperture variability index >25 in the early trials to <15 in the later trials).
Figure 4.
Plot of lip aperture variability indices (top) and durations (bottom) for each subject's early 5 and later 5 productions of the nonword “mabshaytaidoib.”
In the plot of the durations of the early vs. the later trials for each individual in Figure 4, we can also ascertain that subgroups of adults who stutter are present. One subgroup of 8 individuals has nonword durations in the normal range, while 9 adults who stutter are clearly slower in producing the nonwords. Most of the points on the graph, for both groups, fall slightly below the diagonal, suggesting a slight speeding up for most speakers on the later trials, evidence of a modest practice effect for both groups, as the statistical analyses suggested.
Discussion
Repeating a novel phonetic sequence, the nonword repetition task, engages multiple widely distributed neural networks, from those involved in auditory decoding and phonological encoding to those involved in motor planning and execution. We exploited this task in the present experiment as a probe into the speech production systems of adults who stutter when they are speaking fluently. Clearly this task reveals properties of the stuttering adults' speech production system that are unlike that of their normally fluent peers. First, interarticulator coordination is less consistent in the adults who stutter. As predicted, this effect is enhanced by increased length and phonological complexity of the nonwords. Also, unexpectedly, a significant number of the adults who stutter showed a dramatic within-session practice effect for coordination consistency. Coordination consistency increased on the later trials only for the stuttering group. The normally fluent adults, as in our earlier experiment with this stimulus set, apparently were at ceiling in the early trials and showed no improvement in coordinative consistency (Walsh et al., 2006). Both groups showed a practice effect as reflected in slightly increased speaking rate in the later trials. Adults who stutter were heterogeneous, with some performing well within the normal range for both coordination consistency and speed of production. In general, these results support our multifactorial model of stuttering and demonstrate that increasing linguistic complexity of the utterance, both syntactic (Kleinow & Smith, 2000) and phonological (present results), increases variability in the output of speech motor systems of a significant number of adults who stutter. Nonword Length vs. Complexity?
Ideally, the effects of phonological complexity could be disambiguated experimentally from the effects of utterance length, but as we argued in the Introduction, this is very difficult if not impossible. Phonological complexity can be defined at many different levels (Kenstowicz, 1994). Word length also can be indexed in different ways. For example, our two 4-syllable nonwords were of equal length in syllables, but it takes much less time to produce the less complex “mabteebeebee” compared to “mabshaytaidoib.” Also, the more complex nonword is phonologically more complex, but it is also motorically more complex as indexed by the nature of the component gestures required to produce it. Therefore, we do not attempt to make an argument that linguistic complexity, in this case at the phonological and/or phonetic levels, is operating independently of the demands of planning and producing a longer utterance. On the other hand, we would argue that any differences in lip aperture coordination variability are not an epiphenomenon of differences in nonword length. The results were not the same for the coordination consistency and duration measures, that is, both groups tended to speed up speech rates on later trials, so that a practice effect was evident for both groups. In contrast, the adults who stutter also increased in coordinative consistency on later trials, while the normally fluent group did not. Another line of evidence relevant to this argument is that there was no association between speed of production and coordinative consistency across individuals. The slowest speakers in each group did not tend to have the higher lip aperture variability indices as revealed by correlations computed for each group. These dissociations between the results for utterance length and lip aperture variability point to the fact that the two measures reflect the operation of different underlying processes.
Despite the disclaimers above, we do think our findings shed some light on the role of utterance length and complexity in challenging the speech motor systems of adults who stutter. First, it is clear that utterance length plays a role in decreasing coordinative consistency in these speakers. The adults who stutter are most like the normally fluent group when the utterance is shortest (“bob says mab again”). The greatest differences between groups emerge for the longer nonwords spoken in the carrier phrase. In addition, utterance length is strongly implicated as a significant, destabilizing factor for adults who stutter when we see the large differences between groups on the very simple, 4-syllable word, “mabteebeebee.” The syllables in this nonword are phonetically simple, yet adults who stutter, as a group, show higher coordinative variability and a clear practice effect for this simple nonword. For the single syllable nonword, “mab” no such patterns emerge. Therefore, this result strongly reinforces the idea that when adults who stutter plan and execute utterances of greater length, the speech motor system is operating with a greater instability compared to when shorter utterances are planned and produced.
With regard to phonological complexity, the present results also point to the suggestion that increasing linguistic processing demands has destabilizing influences on the speech motor system of adults who stutter. Two results support this assertion. First the 4-syllable, most complex nonword resulted in higher lip aperture variability indices compared to the 4-syllable, simple nonword. Second, the lip aperture variability indices of the adults who stutter (see Figure 2) for the 3-syllable, but more complex nonword, “mabfaishabe,” are approximately equal to those of the 4-syllable simple nonword, “mabteebeebee”.
In summary, we argue that the results of the present study of nonword production in adults who stutter implicate utterance length and complexity as destabilizing factors for the speech motor systems of adults who stutter. Also it is apparent that, even with practice during the experimental session (see Figures 2 and 3), the adults who stutter, on average do not reach the levels of performance consistency and speed characteristic of the control participants. Another factor that may contribute to the higher inconsistency and lower speech rate of adults who stutter in this task is the novel nature of the nonwords. This suggestion is supported by the finding that adults who stutter as a group did not differ from controls in producing highly familiar short sentences (e.g., “buy Bobby a puppy”) on the lower lip trajectory variability index computed for the entire sentence (Smith & Kleinow, 2000). A Phonological Encoding Deficit in Stuttering?
A number of theories of the cause of stuttering have employed psycholinguistic models of language production and have hypothesized that the core problem leading to disfluency lies in phonological encoding. These accounts include the Covert Repair Hypothesis (Postma & Kolk, 1993), the EXPLAN theory (Howell, 2007), and the neuropsycholinguistic model (Perkins, Kent, & Curlee, 1991). While these accounts differ in their details, they have in common that the core problem in stuttering is proposed to be delays and/or errors in generating the phonetic string, which must be serially ordered, so that the appropriate articulatory commands can be issued. Thus, hypothetically, a temporal misalignment occurs, and there are intermittent errors and/or lags that lead to disfluencies. As we have detailed in earlier papers (Smith & Kelly, 1997), our theoretical approach to stuttering is multifactorial and therefore not consistent with models, such as these, which target a single, primary cause of stuttering. However, within either type of framework, one can ask whether stuttering is characterized by a deficit in phonological processing abilities.
Do the present results support the idea that adults who stutter differ in their phonological processing abilities compared to normally fluent controls? At the behavioral level, the data do not support the notion of a phonological deficit. The adults who stutter performed just as well as their normally fluent peers in both nonword repetition tasks we assessed. Ludlow et al., we note, did find differences in novel nonword production accuracy for adults who stutter, but the novel nonwords used in their experiment were much more difficult than those employed here. Therefore, our behavioral results may indicate a ceiling effect was operating for both groups in the standard nonword repetition task (Dollaghan & Campbell, 1998) and in the nonword set we designed for the kinematic experiment.
All three psycholinguistic theories cited above implicate slowed, inefficient phonological encoding resulting in asynchronies between a phonological processing level and the motor output level. The present results demonstrated that adults who stutter, on average, produced the nonwords at a slower speaking rate. This could be the result of slowed phonological encoding, slowed motor planning, and slowed execution processes. We cannot pinpoint any one of these processes as more likely to be an explanatory factor in the slowed output. In fact given evidence that adults who stutter are slower in silent reading (Bosshardt, 1990), often slower in speech response times (van Lieshout et al., 1996b), and often slower in speech production rates, it is likely that all of these levels of processing are slower and less efficient in adults who stutter.
In contrast to the adults who stutter assessed in the present experiment, children who stutter, including 3-5 year-olds (Anderson et al., 2006) and 4-8-year-olds (Hakim & Ratner, 2004), do produce more errors than their normally fluent peers on nonword repetition tasks. A significant portion of young children who stutter show delays in phonology (Arndt & Healey,2001; Nippold 2001). Thus, the characteristics of stuttering in childhood and in the mature speaker may be quite different with regard to a number of the underlying factors, including a phonological factor. This suggestion is also supported by results of ERP experiments examining the neural correlates of a rhyme judgment task. Adults who stutter were similar to control participants in their rhyme judgment accuracy and latency (except in the most complex rhyming condition), and their ERPs were not different from those of the normally fluent controls (Weber-Fox, Spencer, Spruill, & Smith, 2004). Interestingly, a follow-up study of a group of 9-12 year-old children who stutter revealed dramatic differences in behavioral measures of the stuttering children's rhyme judgments, reflected in reduced accuracy and speed of their responses (Weber-Fox, Spruill, Spencer, & Smith, 2008). Further, the children who stutter had atypical, immature ERP responses to the target words and CNVs preceding the target words. These findings reinforce the idea that stuttering is a dynamic disorder, one that changes on many time scales, from minutes, to days, to decades (Smith, 1999).
Developmental Patterns
In our earlier study of the effects of syntactic complexity on speech movement patterning (Kleinow & Smith, 2000) and in the present kinematic results, the performance of adults who stutter resembles that of typically developing children rather than that of normally fluent age-matched adults. Normally fluent adults have highly stable speech motor systems that remain consistent in the face of demands that destabilize the system in adults who stutter. The dependent variable we used in the present study to assess speech motor system performance, the lip aperture variability index, reflects interarticulator (upper lip, lower lip, and jaw) coordination. We (Smith & Zelaznik, 2004) have studied the time course of development of this measure of interarticulator coordination in 180 children and adults from age 4-years to young adult (20-22 years). We used this method to explore the development of functional synergies (or coordinative structures) for speech:
“Consistent inter-effector relationships is one type of evidence for the operation of functional synergies, defined as ‘classes of movement patterns involving collections of muscle or joint variables that act as basic units’ (Sporns & Edelman, 1993, p. 963; see also Turvey, 1977). For example, there are many combinations of motions of the upper lip, lower lip, and jaw that could be used to achieve a specific oral opening-closing pattern, but during development, it is hypothesized that speech motor control systems converge on preferred regions of the movement space (Sporns & Edelman, 1993; Thelen & Smith, 1994), such that on repeated productions of a motor task, the variability in inter-effector relationships is very low (Smith & Zelaznik, 2004; p. 24).
In our earlier study we found a protracted course of development, such that even at age 14-years, adult levels of interarticulatory consistency were not achieved. Given all of the changes to be encountered by the speech production system during adolescence, we suggested the protracted course of development is adaptive, reflecting the adaptive neural plasticity of developing systems.
Within this framework the present results and those of Kleinow & Smith (2000) provide evidence that adults who stutter have not developed normally mature, stable functional synergies for speech and/or that the linking and sequencing of these functional synergies necessary to produce novel phonetic sequences shows signs of an immature system. The finding that the later five trials of adults who stutter were significantly more consistent than the early five trials provides clear evidence that these speakers were adjusting their articulatory coordinative patterns as a function of practice. Recall that the five nonwords were randomized within blocks, so this finding does not reflect a simple adaptation effect.
It is sometimes noted that variability is inconsistently interpreted. Higher variability in children's movement output is interpreted as adaptive, while the present findings of higher variability and the learning effect in adults who stutter are interpreted as a sign of an immature system. As we have argued before (Smith, in press), any measure of variability must be interpreted within the context of the experiment and the populations being studied. Here we demonstrate that the normal, mature motor pattern in this task produced by adults who do not stutter is highly stable performance that shows no improvement in coordinative consistency, albeit they do increase rate of production in the later trials. The performance of the matched group of adults who stutter on this task is remarkably different, one which we interpret as reflecting the operation of a speech motor system that has failed to reach mature levels of stability in formation of functional synergies for speech and in the processes involved in sequencing and linking these synergies to produce speech. This is a system that is therefore more susceptible to disruption especially when task demands, including linguistic, emotional, and cognitive aspects of speaking, are increased (Smith, 1999).
Of course, it is also important to note the individual differences among the adults who stutter in the present experiment. Clearly, there are subgroups. Some adults who stutter do not show increased coordination instability in the face of increased utterance length and phonological processing demands, while the performance of other individuals was remarkably affected and well outside the normal performance range. This result is also consistent with an account of stuttering that emphasizes an “esoteric formula” (Van Riper, 1982), a complex weighting of many, interactive factors that contribute to the probability of stuttering (Smith, 1999; Smith & Kelly, 1997).
Conclusion and Final Caveats
The present findings clearly support a model of stuttering in which speech motor system performance is affected by the linguistic goals of the speaker and the length of the utterance to be produced. The nonword repetition task proved to be a useful window through which to observe differences in underlying motor system dynamics during fluent speech production in adults who stutter. Furthermore, the dependent measures selected, which reflect inter-articulatory dynamics over the entire movement sequence for each nonword, revealed clear differences in motor system operation for adults who stutter compared to normally fluent adults speaking fluently. Given that the performance of the adults who stutter and their response to practice trials resembled that of normally fluent school-age children, rather than age-matched peers, we have provided further evidence that adults who stutter have speech motor systems that have not followed a normal developmental course.
One aspect of our study is a limitation, that is, only individuals with a mild stuttering problem were assessed; two potential participants with more severe stuttering could not do the experimental task. This was a necessary limitation of the study, as we wanted to examine the fluent speech of adults who stutter. One could argue that the strategy of including only mildly affected adults created a bias against finding significant differences between the two groups, but in fact, remarkable differences in coordinative stability and in the effects of practice were observed. We would suggest that in adults with moderate or severe stuttering, the same processes contribute to the probability of fluency failure. That is, increased task demands increase the probability of overt speech motor system failure, and we would suggest that these more profoundly affected individuals operate in a different region of the instability continuum, with a generally greater probability of disfluency due to the interaction of motor, language, emotional and other factors.
In future experiments with extended practice trials, perhaps adults with moderate and severe stuttering could be tested on this protocol. In addition, we did not examine potential motor learning effects that might be observed in a re-test session, for example on the next day. We have completed a follow-up study to Walsh et al. (2006) of normally fluent 9 and 10-year-olds and young adults in which we added more difficult nonwords to the “mab” word set (e.g., “mabspokweeflaib”), and we re-tested participants a day later (Sasikekaran, Smith, & Weber-Fox, in press). This study revealed that normally fluent young adults do show practice effects in later trials (practice effects included both a reduction in coordinative variability and increased speech rate) on the more difficult nonwords. Further, for both children and adults, the practice effects were retained, such that on Day 2 testing the normally fluent adults and children performed with the same coordinative consistency and speed achieved after practice on Day 1.
In summary, the present experiment clearly lends more support to the hypothesis that phonological factors play an important role in affecting speech motor output in stuttering. We also have provided evidence that behaviorally there is a change in the performance of the person who stutters from childhood to adulthood, with children who stutter lagging their normally developing peers on percent correct performance on a nonword repetition task (Hakim & Ratner; Anderson et al., 2006), while the adults we assessed performed as well as their normally fluent peers. While the behavioral impact of phonological complexity may be reduced in the mature person who stutters, we see that the underlying interactions of linguistic and motor processes continue to destabilize the speech motor systems of adults who stutter. Whether this destabilization occurs to a lesser degree in adults compared to young children who are stuttering will be determined in an ongoing study from our laboratory.
Continuing Education
QUESTIONS (bolded letter is the correct answer)
-
A central tenet of the authors' multifactorial, dynamic theory of stuttering is that
a. Phonological processing deficits cause stuttering.
b. Motor timing problems are the primary cause of the development of stuttering.
c. Many factors interact with the operation of the speech motor system to increase or decrease the probability of disfluency.
d. Genetic factors produced language processing deficits in all individuals who stutter.
e. Stuttering behaviors are essentially constant throughout the lifespan of the individual who stutters, because the underlying cause of stuttering does not change over the lifespan.
-
The authors hypothesize that increasing the linguistic complexity of an utterance
a. Increases instabilities in the operation of speech motor systems of adults who stutter.
b. Decreases the instability in the operation of speech motor systems of adults who stutter.
c. Has no effect on the operation of speech motor systems of adults, whether they stutter or not.
d. Improves fluency, because more attention is demanded in the speaking task.
e. Has no effect on motor processing in speech, because linguistic encoding occurs at much higher levels of the nervous system.
-
According to general motor learning theory
a. Practice effects cannot be observed within a single experimental session; rather a return to the laboratory later for retesting is necessary to reveal the effects of practice.
b. Motor learning occurs immediately within a single session in certain individuals.
c. Because speech is so over learned in adults and there is little plasticity left in their speech production systems, adults will not show practice or motor learning effects on speaking tasks.
d. Improvements in speed and accuracy of performance are the hallmark of motor learning.
e. Adults who stutter should not show practice effects, because their speech motor systems are extremely stable.
-
A nonword repetition task was used in the present study
a. Because it probes syntactic and semantic processing skills.
b. Because the participant must generate a new motor program for speech based on the phonological encoding of novel phonetic strings.
c. Because it has been demonstrated that adults who stutter cannot generate novel phonetic sequences.
d. To test whether the core deficit in stuttering is motor timing.
e. Because adults who stutter have atypical language processing skills as determined by standardized language tests.
-
Adults who stutter
a. Performed the nonword repetition task with much lower accuracy compared to the normally fluent control participants.
b. Showed no changes in their speech motor processes as a function of practice.
c. Increased the consistency of their oral coordinative patterns with practice during a single experimental session.
d. Performed precisely the same as the normally fluent control subjects, because even in adults who stutter, speeches a highly over learned in while practice motor behavior.
e. Showed high levels of coordination stability, because disfluencies did not occur during the experiment.
-
The results of the present study can be interpreted to suggest that:
a. After years of stuttering, the speech motor systems of adults who stutter have adapted such that their oral motor coordination is normal.
b. Adults who stutter have highly consistent patterns of articulation which do not change even in the face of novel or highly complex speaking demands.
c. Only children have enough plasticity in their speech motor system to show changes in oral motor coordination as a result of practice.
d. The nonword repetition task revealed differences in the underlying dynamics of language/motor interactions and adults who stutter compared to their normally fluent peers.
e. The multifactorial dynamic theory of stuttering is incorrect, because speech motor processes were not affected by increased linguistic demands.
Acknowledgments
This work was supported by Grant DC00559 from the NIH's National Institute on Deafness and Other Communication Disorders. We thank Janna Berlin for her help in all stages of data collection and analysis.
Biographies
Anne Smith is a Distinguished Professor at Purdue University. She has investigated the physiological bases of stuttering for over two decades. Her current work focuses on language and motor interactions in the development of stuttering. She co-directs The Produce Stuttering Project, a longitudinal investigation of young children who stutter.
Neeraja Sadagopan received her doctoral degree at Purdue University with an emphasis on speech motor control. She is currently an assistant professor at the University of Colorado, where she is developing a research program on speech motor control processes and aging.
Bridget Walsh received her doctoral degree at Purdue University with an emphasis on speech motor control and motor disorders. She is currently working at Purdue on speech motor control in patients with Parkinson's disease.
Christine Weber-Fox is an associate professor at Purdue University. She co-directs The Purdue Stuttering Project. Her major research interests are in the neural bases of language processing, and she studies children who stutter in children with specific language impairment.
Footnotes
In the motor behavior literature (e.g., Schmidt & Wriesberg, 2004) practice effects are generally defined as short-term, within-session improvements in performance, observed as increased accuracy and speed of responses; while motor learning is established when participants retain these improvements upon retest on the task in a later experimental session.
The lip aperture signal we computed as the distance between the two lip markers as a function of time is a rough, but reasonable estimate of lip aperture (Westbury & Hashi, 1997). A better estimate of this complex variable would require tracking multiple points on the upper and lower lips, but “point parameterization” of motions of the complex structures involved in speech has a long history in speech production research (Westbury & Hashi, 1997). In any case, we follow the tradition in the speech production literature of referring to the result of the subtraction of the two mid-line upper and lower lip markers in the vertical dimension as the “lip aperture” signal.
Educational Objectives: (1)To help the reader understand potential language/motor interactions in stuttering and (2)To test and discuss the hypothesis that phonologically complex utterances have a destabilizing effect on the speech motor system in individuals who stutter.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anne Smith, Department of Speech, Language, Hearing Sciences, Purdue University
Neeraja Sadagopan, Department of Speech, Language, Hearing Sciences, University of Colorado, Boulder
Bridget Walsh, Department of Speech, Language, Hearing Sciences, Purdue University.
Christine Weber-Fox, Department of Speech, Language, Hearing Sciences, Purdue University.
References
- Anderson JD, Wagovich SA, Hall NE. Nonword repetition skills in young children who do and do not stutter. Journal of Fluency Disorders. 2006;31:177–199. doi: 10.1016/j.jfludis.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt J, Healey EC. Concomitant disorders in school-aged children who stutter. Language, Speech, and Hearing Services in Schools. 2001;32:68–78. doi: 10.1044/0161-1461(2001/006). [DOI] [PubMed] [Google Scholar]
- Bosshardt HG. Subvocalization and reading rate differences between stuttering and nonstuttering children and adults. Journal of Speech and Hearing Research. 1990;33:776–785. doi: 10.1044/jshr.3304.776. [DOI] [PubMed] [Google Scholar]
- DeNil LF. The influence of phonetic context on temporal sequencing of upper lip, lower lip, and jaw peak velocity and movement onset during bilabial consonants in stuttering and nonstuttering adults. Journal of Fluency Disorders. 1995;20:127–144. [Google Scholar]
- Dollaghan CA, Campbell TF. Nonword repetition and child language impairment. Journal of Speech, Language, and Hearing Research. 1998;41:1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Graf Estes K, Evans JL, Else-Quest NM. Differences in the nonword repetition performance of children with and without specific language impairment: A meta-analysis. Journal of Speech, Language, and hearing Research. 2007;50:177–195. doi: 10.1044/1092-4388(2007/015). [DOI] [PubMed] [Google Scholar]
- Hammill DD, Brown VL, Larsen SC, Wiederholt JL. Test of Adolescent and Adult Language. 3rd. Austin, TX: Pro-Ed, Inc.; 1994. [Google Scholar]
- Hakim HB, Ratner NB. Nonword repetition abilities of children who stutter: An exploratory study. Journal of Fluency Disorders. 2004;29:179–199. doi: 10.1016/j.jfludis.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Howell P. The effects of gated speech on the fluency of speakers who stutter. Folia Phoniatrica et Logopaedica. 2007;59:250–255. doi: 10.1159/000104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenstowicz M. Phonology in generative grammar. Oxford: Blackwells; 1994. [Google Scholar]
- Kleinow J, Smith A. Influences of length and syntactic complexity on the speech motor stability of the fluent speech of adults who stutter. Journal of Speech, Language, and Hearing Research. 2000;43:548–559. doi: 10.1044/jslhr.4302.548. [DOI] [PubMed] [Google Scholar]
- Levelt W. Speaking: From intention to articulation. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Levelt W, Roelofs A, Meyer A. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22:1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Ludlow C, Siren K, Zikira M. Speech production learning in adults with chronic developmental stuttering. In: Hulstijn W, Peters H, Van Lieshout P, editors. Speech production: Motor control, brain research and fluency disorders. NY: Elsevier; 1997. pp. 221–230. [Google Scholar]
- Maner KJ, Smith A, Grayson L. Influences of utterance length and complexity on speech motor performance in children and adults. Journal of Speech, Language, and Hearing Research. 2000;43:560–573. doi: 10.1044/jslhr.4302.560. [DOI] [PubMed] [Google Scholar]
- Mathworks, Inc. Matlab: High performance numeric computation and visualization software. Natick, MA: Author; 2005. computer program. [Google Scholar]
- McClean MD, Kroll RM, Loftus NS. Kinematic analysis of lip closure in stutterers' fluent speech. Journal of Speech and Hearing Research. 1990;33:755–760. doi: 10.1044/jshr.3304.755. [DOI] [PubMed] [Google Scholar]
- McClean MD, Levandowski DR, Cord MT. Intersyllabic movement timing in the fluent speech of stutterers with different disfluency levels. Journal of Speech and Hearing Research. 1994;37:1060–1066. doi: 10.1044/jshr.3705.1060. [DOI] [PubMed] [Google Scholar]
- Namasivayam AK, Van Lieshout P. Investigating speech motor practice and learning in people who stutter. Journal of Fluency Disorders. 2008;33:32–51. doi: 10.1016/j.jfludis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Nippold MA. Phonological disorders in stuttering in children: What is the frequency of co-occurrence? Clinical Linguistics and Phonetics. 2001;15:219–228. [Google Scholar]
- Perkins WH, Kent R, Curlee R. A theory of neuropsycholinguistic functions in stuttering. Journal of Speech and Hearing Research. 1991;34:734–752. doi: 10.1044/jshr.3404.734. [DOI] [PubMed] [Google Scholar]
- Postma A, Kolk H. The covert repair hypothesis: Prearticulatory repair processes in normal and stuttered disfluencies. Journal of Speech and Hearing Research. 1993;36:472–487. [PubMed] [Google Scholar]
- Riecker A, Brendel B, Ziegler W, Erb M, Ackermann H. The influence of syllable onset complexity and syllable frequency on speech motor control. Brain and Language. 2008;107:102–113. doi: 10.1016/j.bandl.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Sasikekaran J, Smith A, Weber-Fox C. Nonword repetition in children and adults: Effects on movement coordination. Developmental Science. doi: 10.1111/j.1467-7687.2009.00911.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, Wrisberg CA. Motor performance and learning. 3rd. Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- Smith A. Stuttering: A unified approach to a multifactorial, dynamic disorder. In: Ratner N, Healey C, editors. Research and treatment of fluency disorders: Bridging the gap. Mahwah, NJ: Erlbaum; 1999. pp. 27–44. [Google Scholar]
- Smith A. Development of neural control of orofacial movements for speech. In: Hardcastle W, Gibbon F, Laver J, editors. Handbook of phonetic sciences. Oxford: Blackwell; In Press. [Google Scholar]
- Smith A, Goffman L. Interaction of language and motor factors in speech production. In: Maasen B, Kent RD, Peters HFM, Peters H, van Lieshout P, Hulstijn W, editors. Speech motor control in normal and disordered speech. Oxford University Press; 2004. pp. 225–252. [Google Scholar]
- Smith A, Johnson M, McGillem C, Goffman L. On the assessment of stability and patterning of speech movements. Journal of Speech, Language, and Hearing Research. 2000;43:277–286. doi: 10.1044/jslhr.4301.277. [DOI] [PubMed] [Google Scholar]
- Smith A, Kelly E. Stuttering: A dynamic, multifactorial model. In: Curlee RF, Siegel GM, editors. Nature and treatment of stuttering: New directions. 2nd. Needham Heights, MA: Allyn & Bacon; 1997. pp. 204–217. [Google Scholar]
- Smith A, Kleinow J. Kinematic correlates of speaking rate changes in stuttering and normally fluent adults. Journal of Speech, Language, and Hearing Research. 2000;43:521–536. doi: 10.1044/jslhr.4302.521. [DOI] [PubMed] [Google Scholar]
- Smith A, Zelaznik H. The development of functional synergies for speech motor coordination in childhood and adolescence. Developmental Psychobiology. 2004;45:22–33. doi: 10.1002/dev.20009. [DOI] [PubMed] [Google Scholar]
- Smits-Bandstra S, De Nil LF, Saint-Cyr JA. Speech and nonspeech sequence skill learning in adults who stutter. Journal of Fluency Disorders. 2006;31:116–136. doi: 10.1016/j.jfludis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- St. Louis KO, Ruscello DM. Oral Speech Mechanism Screening Exam—Revised. Austin, TX: Pro-Ed.; 1987. [Google Scholar]
- Sporns O, Edelman GM. Solving Berstein's problem: A proposal for the development of coordinated movement by selection. Child Development. 1993;64:960–981. [PubMed] [Google Scholar]
- Thelen E, Smith L. A dynamic systems approach to the development of cognition and action. Cambridge, MA: The MIT Press; 1994. [Google Scholar]
- Van Lieshout PHHM, Hulstijn W, Peters HFM. From planning to articulation in speech production: What differentiates a person who stutters from a person who does not stutter? Journal of Speech and Hearing Research. 1996a;39:546–564. doi: 10.1044/jshr.3903.546. [DOI] [PubMed] [Google Scholar]
- Van Lieshout PHHM, Hulstijn W, Peters HFM. Speech production in people who stutter: Testing the motor plan assembly hypothesis. Journal of Speech and Hearing Research. 1996b;39:76–92. doi: 10.1044/jshr.3901.76. [DOI] [PubMed] [Google Scholar]
- Van Lieshout PHHM, Peters HFM, Starkweather CW, Hulstijn W. Physiological differences between stutterers and nonstutterers in perceptually fluent speech. Journal of Speech and Hearing Research. 1993;36:55–63. doi: 10.1044/jshr.3601.55. [DOI] [PubMed] [Google Scholar]
- Van Riper C. The nature of stuttering. 2nd. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Walsh B, Smith A. Articulatory movements in adolescents: Evidence for protracted development of speech motor control processes. Journal of Speech, Language, and Hearing Research. 2002;45:1119–1133. doi: 10.1044/1092-4388(2002/090). [DOI] [PubMed] [Google Scholar]
- Walsh B, Smith A, Weber-Fox C. Short-term plasticity in children's speech motor systems. Developmental Psychobiology. 2006;48:660–674. doi: 10.1002/dev.20185. [DOI] [PubMed] [Google Scholar]
- Westbury J, Hashi M. Lip pellet positions during vowels and labial consonants. Journal of Phonetics. 1997;25:405–419. [Google Scholar]
- Weber-Fox C, Spencer R, Spruill JE, III, Smith A. Phonological processing in adults who stutter: Electrophysiological and behavioral evidence. Journal of Speech, Language, and Hearing Research. 2004;47:1244–1258. doi: 10.1044/1092-4388(2004/094). [DOI] [PubMed] [Google Scholar]
- Weber-Fox C, Spruill JE, III, Spencer R, Smith A. Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science. 2008;11:321–337. doi: 10.1111/j.1467-7687.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G. Stuttering: A disorder of movement. Journal of Speech and Hearing Research. 1980;23:122–136. [PubMed] [Google Scholar]