Abstract
Introduction
Placebo responses have been large across a number of clinical trials for treatment of women’s sexual dysfunction. Studying placebo responses may elucidate predictors of symptom reduction and responsiveness to intervention.
Aim
To determine the correlates of placebo response in participants enrolled in a clinical trial for female sexual dysfunction.
Methods
We analyzed data from 16 women with sexual arousal and orgasmic dysfunction who were randomized to receive 8 weeks of placebo treatment within a larger randomized controlled trial. Using nonparametric correlations, we tested whether age, length of relationship, psychological symptoms, and scores on self-report measures predicted change in sexual function with placebo treatment.
Main Outcome Measure
Female Sexual Function Index.
Results
Consistent with findings from other studies, we found a significant improvement in sexual function scores after 8 weeks of treatment with placebo. We also found that age and length of relationship predicted the magnitude of change in sexual function across treatment. Changes in relationship adjustment, but not relationship adjustment at baseline, predicted the magnitude of improvement in sexual function scores. We observed no relationship between psychological symptom severity and change in sexual function.
Conclusions
Participant age and length of relationship predicted subsequent magnitude of change in sexual function scores during treatment with placebo. In addition, relationship adjustment covaried with changes in sexual function. Our findings suggest that “placebo effects” may represent underlying factors that influence the way in which women respond to the process of treatment.
Keywords: Female Sexual Dysfunction, Placebo, Clinical Trial
Introduction
The incidence of sexual problems among women is high [1]. With the exception of specific techniques targeting primary female orgasmic disorder, there is little empirical support for specific psychosocial treatments for women’s sexual problems. In the late 1990s, the advent of sildenafil (Viagra) and similar agents to treat erectile disorder in males resulted in a surge of interest in pharmacological and other biomedical treatments for women’s sexual dysfunctions. Despite millions of dollars spent on nearly a decade of research to develop vasoactive agents to treat women’s sexual complaints, no such pharmacological treatments have been approved by the United States Food and Drug Administration, and several large-scale drug development programs in this area have been abandoned. This outcome is due in part to the fact that most studies have failed to find a clinically meaningful improvement in women’s sexual function beyond the effects of placebo. Interestingly, responses to placebo have been moderate to large in many such clinical trials; in some cases, the proportion of women showing improvement in sexual symptoms with placebo treatment has exceeded 40% or more [2,3].
Given the mixed success rates of biomedical treatments for women’s sexual dysfunctions, one might argue that ostensibly “active” treatment protocols are more similar (e.g., in setting, procedures, patient education, etc.) than dissimilar to placebo treatments, as the only difference between the two is the substance within the delivery vehicle. Theory and clinical wisdom may generate a wealth of speculations about the mechanism of a placebo response, but in fact, few empirical data are available to corroborate or negate these hypotheses in the case of sexual dysfunction treatment. It is therefore important to systematically examine placebo responses in the treatment of women’s sexual problems to better understand predictors and mechanisms of clinical change. We argue that simply aiming to minimize placebo responses (as in conventional clinical trial analysis) ignores a richer opportunity to understand participant-level factors that predict symptom reduction and responsiveness to treatment. In order to develop efficacious treatments for women’s sexual problems, whether biomedical or psychosocial, we believe it is worthwhile to investigate the phenomenon of clinical change in the absence of an “active” treatment.
Enrolling and participating in a clinical trial is not a uniform experience for all persons but rather an event shaped by experience, expectancies, motives for treatment, and interpersonal dynamics between the participant and the investigator [4,5]. Moreover, clinical trials focusing on sexual function are likely to affect not only the treatment-seeking person but also the sexual partner. For instance, Goldstein and colleagues [6] reported that pharmacologic treatment of male erectile dysfunction was associated with improved sexual desire, arousal, and satisfaction among the female partners of the clinical trial participants. The reactions of the partner and of the couple system to clinical trial procedures have been understudied in clinical research on individual treatments for sexual problems in women. Examining the influence of baseline predictors and treatment process variables in the absence of the active treatment itself can help provide a more complete picture of the true “ingredients” of an efficacious treatment.
Aims
In this pilot study, we isolated a group of treatment-seeking, sexually dysfunctional women who were randomized to receive treatment with placebo capsules as part of a larger controlled clinical trial of a vasoactive agent for sexual arousal and orgasm dysfunction. Our aim was to determine whether several variables predicted change on a validated measure of sexual function across the study among these women. We examined age and length of relationship as possible demographic predictors of treatment response. To determine whether the severity of sexual symptoms predicted a greater or lesser placebo response, we also assessed baseline sexual function as a predictor of subsequent change. Consistent with research indicating that substantial placebo responses are not limited to persons with neuroses or other psychological problems [7], we also sought to confirm that placebo responses were independent of psychological symptom severity. Finally, in light of our expectation that clinical trial participation was likely to affect not only the trial participants but also their partners, we examined relationship adjustment as a predictor of placebo response at baseline and across the trial period.
Methods
The data presented here are a subset from a larger placebo-controlled pharmacological trial that included 99 women seeking treatment for problems with sexual arousal or orgasm. Women between the ages of 18 and 65, who were currently involved with male partners, were eligible to participate. Exclusion criteria included amenorrhea; pregnancy, lactation, or less than 1 year postpartum; hypertension or other cardiovascular disease; diabetes; a history of major pelvic surgery such as hysterectomy; neurological impairments or diseases that could interfere with sexual response; a history of alcohol or other substance abuse within the past 6 months; and self-report of an untreated mental disorder. Women were also ineligible to participate if they were receiving concomitant biomedical or psychosocial treatment to address sexual concerns. We asked all participants to attempt at least two sexual encounters per week and to use a medically accepted form of birth control throughout the study. The study was approved by an institutional review board, and all participants provided written informed consent at the beginning of their participation in the trial.
Participants
Sixteen women assigned to placebo treatment completed the trial through the 4-week midtreatment assessment, and of these, 14 continued through the postassessment phase at 8 weeks. Participants ranged in age from 20 to 36 years (M = 25.75, standard deviation [SD] = 4.80) and were diagnosed either with female sexual arousal disorder (FSAD) (N = 13) or female orgasmic disorder (FOD) (N = 3) by a trained interviewer using criteria from the Fourth Edition, Text Revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) [8].Ten participants (62.5%) identified as white, and the remaining participants identified as African American (N = 1), Hispanic (N = 1), Asian (N = 2), or other (N = 2) ethnicity. Six participants were taking antidepressant medication at the time of enrollment in the study. All participants reported having completed some college education, with five having completed a bachelor’s degree or higher. Seven of the 16 participants were married, and the remaining nine women were single but in a steady sexual relationship with a male partner at the time of the study. The majority of participants (N = 10) had been involved with their partners between 1 and 5 years; five had been involved longer than 5 years; and one participant had been involved with her partner less than 1 year. We were able to obtain specific length of relationship data for 14 of the 16 participants and used this information in our statistical analyses.
Measures
In addition to a brief demographics questionnaire, we administered the following instruments to assess sexual function, relationship adjustment, and psychological symptom burden at baseline, midtreatment (4 weeks), and posttreatment (8 weeks).
Sexual Function
The Female Sexual Function Index (FSFI) [9] is a 19-item multidimensional self-report instrument used to assess women’s sexual function in six domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. The instrument yields scores for each of these six domains as well as a total score. The FSFI has demonstrated good test–retest reliability (α = 0.79–0.88) [9,10]. Wiegel and colleagues [10] developed a clinical cutoff score for the FSFI that was able to reliably distinguish women who did and did not meet DSM-IV [11] or DSM-IV-TR [8] criteria for female sexual dysfunctions. In the present study, we defined treatment responses as the difference in the FSFI total score from baseline to the 4-week assessment and from baseline to the 8-week assessments. The FSFI total score was chosen as the outcome endpoint because areas of difficulty were not limited to a single domain of functioning (for example, the mean FSFI desire domain score in this sample was 3.01, similar to means reported for women with both primary hypoactive sexual desire disorder and FSAD) [10], and we anticipated likewise that response to treatment would not be restricted to a single domain of functioning. The baseline FSFI total score in this sample was 19.10 (SD = 5.84), well below the clinical cutoff of 26.55 identified by Wiegel and colleagues [10], and only one participant had a baseline FSFI total score exceeding this cutoff.
Relationship Adjustment
The Dyadic Adjustment Scale (DAS) [12] is a 32-item self-report measure used to assess relationship adjustment. The DAS has four subscales (dyadic satisfaction, dyadic cohesion, dyadic consensus, and affectional expression) for which separate scores may be generated. The DAS also gives a total score, which we used in the present study as a measure of overall relationship adjustment. Spanier [12] reported a significant difference in DAS scores between couples who were married and couples who were divorced, suggesting good criterion-related validity, and the DAS correlated significantly with the Locke–Wallace Marital Adjustment Scale [13]. In our sample, the mean DAS score at baseline was 103.7 (SD = 22.5), somewhat lower than the mean of 114.8 (SD = 17.8) reported by Spanier [12] for a sample of 218 married persons. DAS data were missing from one participant who was excluded from analyses involving this measure.
Psychological Symptoms
The Brief Symptom Inventory (BSI) is a 53-item version of the Symptom Checklist-90-R [14] and a subsection of the Derogatis Sexual Functioning Inventory [15]. The BSI assesses the presence and severity of psychological symptoms in nine domains (e.g., depression, anxiety, hostility). The BSI can be scored on any of the nine domains but also yields a General Severity Index score, which we used in the present study as a measure of overall psychological symptom severity. In previous research, the General Severity Index score showed a test–retest reliability of 0.90 [16].
Procedure
Prior to treatment, participants (and when possible, their partners) attended a 30-minute orientation session to familiarize them with the rationale of the treatment study and to clarify the study procedures. After the orientation, participants attended two laboratory assessment sessions during which we measured their acute physiological reactions to both the placebo and the active treatment. Participants who agreed to continue to the chronic treatment phase received one of four possible 8-week treatments assigned at random: placebo only, active drug only, active drug plus psychotherapy, or psychotherapy alone.
The 16 women in the placebo group each received 28 placebo capsules prepared by a licensed pharmacist and designed to be identical in appearance to the active study drug. Participants received instructions to take a capsule once daily approximately 1 hour before the time that they would typically expect to engage in sexual activity. Throughout the study, we asked participants to record and rate their satisfaction with their sexual activities using diary forms supplied by the investigator. Using the measures described above, we assessed the participants’ psychological, sexual, and relationship function at baseline, midtreatment (4 weeks after initiating treatment), and immediately following 8 weeks of treatment.
Data Analysis
Though not the primary focus of the study, we examined the magnitude of change on the FSFI at midtreatment and posttreatment using descriptive statistics and t-tests. The purpose of our main analysis was to test whether age, length of relationship, baseline sexual function (FSFI total score), baseline self-reported psychological symptoms (BSI), and baseline relationship function (DAS) predicted subsequent change on the FSFI total score among women receiving placebo treatment. We also aimed to determine whether changes in psychological symptoms and relationship function covaried with changes in the FSFI total score at the midtreatment and posttreatment intervals. We entered and scored the data using SPSS for Windows version 14 (SPSS, Inc., Chicago, IL, USA). In all analyses we used the FSFI full scale change score as the dependent variable. Because of the small sample size and skewed distributions of several predictor variables, we used nonparametric correlations (Spearman’s rank order correlation, rho) to test the hypothesized relationships between our predictor variables of interest and FSFI change scores. Spearman’s rho transforms interval-scale data to ranks and is appropriate for data that are not normally distributed due to limited sample sizes or underlying distributions that do not meet the assumptions of parametric tests.
Results
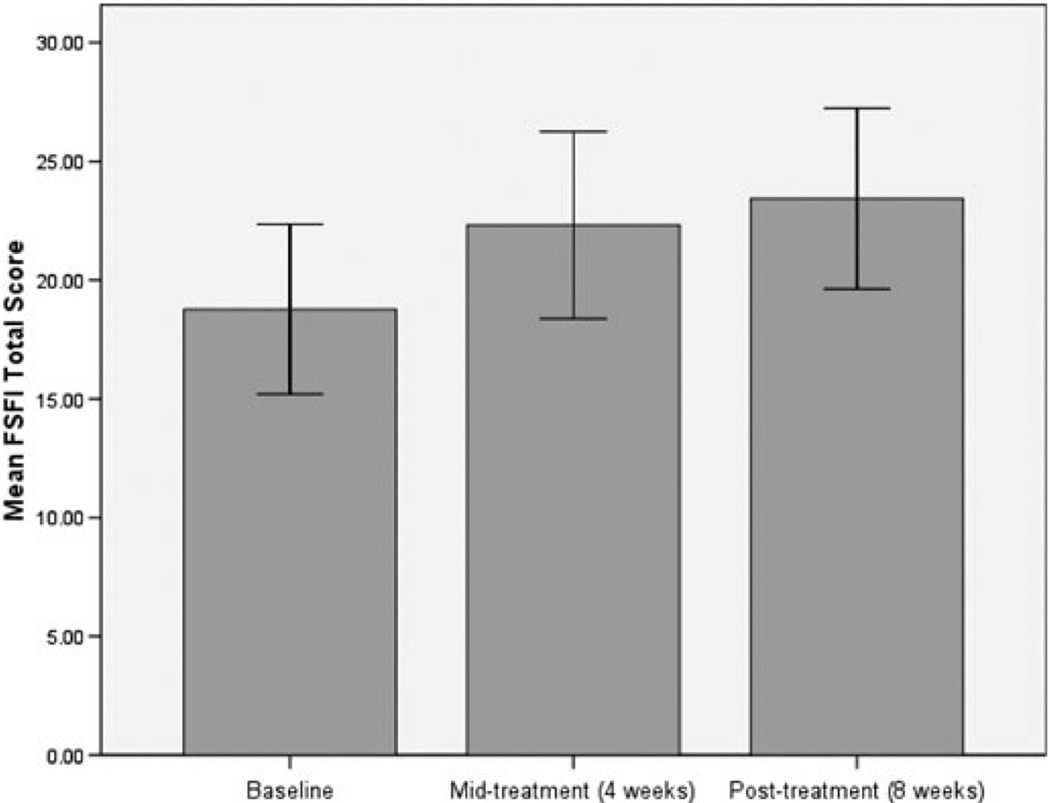
The average FSFI total score among the women who completed the study to midtreatment (N = 16) was 22.45, representing a mean within-person increase of 3.35 points from baseline. Seven women (43.8%) had unchanged or lower FSFI total scores at midtreatment than at baseline. By post-treatment, however, only 2 out of the 14 completers’ FSFI scores were lower than their baselines and the mean within-person change score at post-treatment was 4.66 points, representing a large effect size (Cohen’s d = 0.73) [17]. This effect size does not take into account the two drop-outs, both of whom had shown increased FSFI scores at midtreatment. Among the 14 completers, four (28.6%) scored above the clinical cutoff of 26.55 on the FSFI at post-treatment, not including one woman whose post-treatment score was just under the cutoff (a score of 26.50) and another woman who began with and maintained an FSFI score above the cutoff across the study. Overall, the change in FSFI scores among women receiving placebo was marginally significant at midtreatment, t(15) = 2.072, P = 0.056, and significant at post-treatment, t(13) = 3.246, P = 0.006. Figure 1 displays the means and confidence intervals of FSFI total scores at baseline, midtreatment, and post-treatment.
Figure 1.
Mean Female Sexual Function Index (FSFI) total scores at baseline, midtreatment, and post-treatment; error bars represent 95% confidence intervals.
Table 1 displays the nonparametric correlations between FSFI change scores (midtreatment and posttreatment) and our predictor variables of interest. Age and length of relationship at baseline were significantly correlated with the FSFI change score from baseline after 4 weeks and 8 weeks of treatment. However, age and length of relationship also appeared to be related (r = 0.507, P = 0.064), and thus, the interpretation of these two predictors was ambiguous. Baseline sexual function and relationship adjustment severity did not predict subsequent changes in sexual function at midtreatment or post-treatment. However, changes in sexual function at post-treatment were correlated with relationship adjustment change scores across the same 8-week period. Consistent with our hypothesis, psychological symptom severity did not predict response to placebo treatment.
Table 1.
Baseline predictors and covariates of Female Sexual Function Index (FSFI) change scores after 4 weeks and 8 weeks of placebo treatment
| Δ FSFI (midtreatment) N = 16 |
Δ FSFI (post-treatment) N = 14 |
|
|---|---|---|
| Demographics | ||
| Age | 0.583* | 0.689* |
| Relationship duration | 0.800* | 0.676* |
| Self-report variables at baseline | ||
| Female Sexual Function Index | −0.081 | −0.150 |
| Brief Symptoms Inventory | −0.188 | −0.244 |
| Dyadic Adjustment Scale | −0.265 | −0.312 |
| Change scores during clinical trial | ||
| Δ Brief Symptoms Inventory | 0.177 | −0.088 |
| Δ Dyadic Adjustment Scale | 0.303 | 0.588* |
All values are Spearman’s rho correlation coefficients.
Indicates that the correlation is statistically significant at P < 0.05 (two-tailed).
We conducted post hoc tests on several secondary variables to determine whether they might have also predicted outcomes in this sample. Specifically, a Mann–Whitney U-test revealed that married women (N = 8) experienced better outcomes than did nonmarried women (N = 6) at posttreatment (P = 0.043), although marital status was confounded with age and length of relationship. We also found that current antidepressant use, endorsed by 37.5% of the sample at baseline, was associated with a lower magnitude of change on the FSFI at both midtreatment (P = 0.042) and posttreatment (P = 0.043). We did not find a difference in outcomes, however, according to ethnic self-identification as white non-Hispanic (vs. other ethnic groups) or according to diagnosis (FSAD vs. FOD).
Discussion
Consistent with findings from other placebo-controlled studies for female sexual dysfunction, we found a substantial average increase in sexual function scores after 8 weeks of treatment with placebo. In addition, we found that age and relationship duration were positively correlated with changes in FSFI scores. Although the findings should be interpreted cautiously due to the small sample size, our study suggests that age and length of partner relationship may be important baseline variables to consider in clinical trials of treatments for women’s sexual dysfunctions. Unfortunately, our small sample size and the restricted range of some variables precluded examination of multiple predictor variables simultaneously, and therefore, it is unknown which variables are most strongly associated with placebo response when controlling for other variables. Therefore, a larger sample with greater age and demographic diversity is necessary to confirm our preliminary conclusions.
Interestingly, relationship adjustment at baseline did not predict the magnitude of improvement in sexual function symptoms; rather, it was the change in relationship adjustment during the study that appeared to be related to changes in sexual function symptoms. This is striking because, with the exception of the partner’s invitation to participate in the brief pretreatment orientation session, no part of our intervention targeted the trial participants’ partners. On the other hand, it is plausible that our intervention had some indirect effect on many participants’ relationships. Unfortunately, the nature of our data precludes any firm conclusions about the direction of the relationship between sexual function improvement and relationship adjustment. One possibility is that seeking treatment and attempting sexual activity during the course of the study generated emotional and other cues for sexual responses [18] by prompting greater communication and collaboration between partners. However, it is also possible that improved sexual function enhanced subsequent communication, affection, or intimacy between partners. The association of relationship functioning and sexual treatment outcome, though hardly surprising, is seldom discussed in the context of controlled clinical trials of pharmaceutical treatments. In future trials, measuring adjustment and satisfaction within the relationship, the partner’s reaction to treatment, and the partner’s own sexual function may yield useful data for understanding treatment outcomes.
The mean improvement of 4.66 points on the FSFI total score represents a statistically significant and relatively large effect, but the clinical importance of this change merits comment. In one of several studies validating the clinical utility of the FSFI, Wiegel and colleagues [10] reported mean FSFI total scores for a clinical population of women with FSAD (N = 152) and controls (N = 244). The means and SD for these groups were 20.05 (6.74) and 30.75 (4.80), respectively, a mean difference of 10.7 points. Therefore, a change of 4.66 points would represent nearly half the mean difference in scores between populations of women with FSAD and with no sexual dysfunctions. Unfortunately, we did not empirically assess participants’ subjective impressions of their outcomes, and therefore, the degree to which this magnitude of change is deemed an “improvement” is uncertain.
In neglecting to carefully analyze clinical responses among placebo recipients, conventional randomized, placebo-controlled clinical trial analysis ignores a potentially rich source of information about how women with sexual problems respond to the process of treatment, which is necessarily imbedded in the “active” treatment itself. Multiple studies have indicated that the treatment process can show efficaciousness in its own right, and it is therefore more likely than not that some degree of “placebo” response is present in any treatment outcome. We can only speculate about the components of the clinical trial itself that might have had an influence on placebo group outcomes in this study, but several possibilities are worthy of further exploration, including those already discussed above as well as the participant–researcher alliance, the participants’ conceptualization of their problems and their expectancies for treatment, the nature of the information given to participants, and the process of self-evaluation through the use of daily diaries. In future trials, we plan to closely investigate the relationship of participant-level and study design factors to placebo-group outcomes.
Conclusions
The findings from this pilot study suggest that demographic and relationship-related factors may predict the magnitude of improvement in sexual function in female clinical trial participants assigned to receive placebo treatment for sexual problems. These preliminary results are subject to further testing with larger sample sizes. However, the findings may have important implications for both future clinical trial design and understanding predictors of change in the treatment of sexual dysfunction in women.
Acknowledgments
This publication was made possible by Grant Number 5 RO1 AT00224-02 from the National Center for Complementary and Alternative Medicine to Cindy Meston. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine.
Footnotes
Conflict of Interest: None declared.
-
Category 1
-
Conception and DesignAndrea Bradford; Cindy Meston
-
Acquisition of DataAndrea Bradford
-
Analysis and Interpretation of DataAndrea Bradford
-
-
Category 2
-
Drafting the ArticleAndrea Bradford
-
Revising It for Intellectual ContentAndrea Bradford; Cindy Meston
-
-
Category 3
-
Final Approval of the Completed ArticleAndrea Bradford; Cindy Meston
-
References
- 1.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 2.Basson R, McInnes R, Smith MD, Hodgson G, Koppiker N. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J Womens Health Gend Based Med. 2002;11:367–377. doi: 10.1089/152460902317586001. [DOI] [PubMed] [Google Scholar]
- 3.Berman JR, Berman LA, Toler SM, Gill J, Haughie S. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: A double-blind, placebo controlled study. J Urol. 2003;170:2333–2338. doi: 10.1097/01.ju.0000090966.74607.34. [DOI] [PubMed] [Google Scholar]
- 4.Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: A meta-analytic review. J Consult Clin Psychol. 2000;68:438–450. [PubMed] [Google Scholar]
- 5.Stewart-Williams S. The placebo puzzle: Putting together the pieces. Health Psychol. 2004;23:198–206. doi: 10.1037/0278-6133.23.2.198. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein I, Fisher WA, Sand M, Rosen RC, Mollen M, Brock G, Karlin G, Pommerville P, Bangerter K, Bandel TJ, Derogatis LR. Women’s sexual function improves when partners are administered vardenafil for erectile dysfunction: A prospective, randomized, double-blind, placebo-controlled trial. J Sex Med. 2005;2:819–832. doi: 10.1111/j.1743-6109.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 7.Bootzin RR, Caspi O. Explanatory mechanisms for placebo effects: Cognition, personality and social learning. In: Guess HA, Kleinman A, Kusek JW, Engel LW, editors. The science of placebo: Toward an interdisciplinary agenda. London: BMJ Books; 2002. pp. 108–132. [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: Author; 2000. text revision. [Google Scholar]
- 9.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D’Agostino R., Jr The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 10.Wiegel M, Meston C, Rosen R. The Female Sexual Function Index: Cross-validation and development of clinical cut-off scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: Author; 1994. [Google Scholar]
- 12.Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976;38:15–28. [Google Scholar]
- 13.Locke HJ, Wallace KM. Short marital-adjustment and prediction tests: Their reliability and validity. Marriage Fam Living. 1959;21:251–255. [Google Scholar]
- 14.Derogatis LR. Manual II. Towsen, MD: Clinical Psychometric Research; 1983. SCL-90-R: Administration, scoring and procedures. [Google Scholar]
- 15.Derogatis LR, Melisaratos N. The DSFI: A multidimensional measure of sexual functioning. J Sex Marital Ther. 1979;5:244–281. doi: 10.1080/00926237908403732. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 17.Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups of repeated measures designs. Psychol Methods. 1996;1:170–177. [Google Scholar]
- 18.McCall K, Meston CM. Cues resulting in desire for sexual activity in women. J Sex Med. 2006;3:838–852. doi: 10.1111/j.1743-6109.2006.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]