Abstract
Four hundred and thirty-seven solid tumor cancer patients undergoing chemotherapy were enrolled, interviewed, and randomized to receive either a six-contact, eight-week, nurse-directed intervention or an automated telephone symptom management intervention. Patients were assessed at 10 and 16 weeks. Patients were queried at intake, 10 and 16 weeks to determine severity of their symptoms and if they had been hospitalized, how many times and location of the hospital. The fixed and variable costs associated with production of each arm were identified. Both total fixed and variable costs were greater for the nurse arm; total costs per patient were $69 and $167 for the automated and nurse arm respectively. Overall symptom severity declined significantly over baseline and equally between groups at 10 and 16 weeks. The relations between reductions in symptom severity and the numbers of hospitalizations and days in the hospital were investigated using zero inflated Poisson regression model. The cost of a hospitalization was estimated at $1800 per day in 2004. At 16 weeks, those with 50% or greater reductions in severity had adjusted mean of 1.1 days in the hospital, while those with increased symptom severity had the mean of 2.23. Reductions in hospitalizations related to lower severity suggest that the telephone arm could produce a net savings over cost of its development and implementation. While promising, the links between reductions in severity of symptoms and fewer hospitalizations remain difficult to isolate.
Keywords: Symptom management, trials, offset costs
Introduction
A remarkable number of trials have been conducted to determine the impact of novel strategies for delivering self-care intervention on reducing the number and severity of symptoms among cancer patients (1,2). Most compare experimental interventions against conventional care alone and some contrast more elaborate cognitive behavioral strategies with information and education interventions to manage symptoms. When compared, the less resource intensive educational/information interventions appear to produce similar reductions in symptom burden as resource intensive interventions (3–6). To date, few reports compare the costs of delivering these interventions with their corresponding reductions in symptom severity or how improved symptom management might offset other costs, such as emergency department visits or hospitalizations, and thereby offset expenditures for enhanced strategies to manage symptoms. Symptom management interventions that engage patients in self-care strategies and demonstrate improved processes of care, while not imposing added demands on outpatient oncology personnel, should be considered for inclusion as part of routine care and covered by insurers.
Cost-effectiveness analysis is an essential component in determining if novel interventions compare favorably with established care. The importance of these analyses is reflected in a series of articles that begin to demonstrate how to compare costs with their effectiveness (7–15). However, these analyses rely on units of impact such as quality-adjusted life-years. In the case of symptom management trials, establishing a correspondence between symptom reduction and subsequent quality-adjusted life-years cannot be established (16–18).
In order to compare the impact of interventions on multiple symptoms against the costs of producing those effects, it is necessary to reduce outcome observation to a single severity score. A summation of severity of multiple symptoms into an index produces such score and provides a measure of the total symptom severity burden. Although a summation of the severity across all symptoms is not without limitations (6, 19–21), percent reductions of 50% or more in symptom burden generally are considered to be clinically and statistically significant (22–24).
This research compared fixed and variable costs associated with the corresponding reductions in severity produced by each arm of a two-arm symptom management trial and links these reductions with the rates of hospitalization reported by cancer patients in each trial arm during and immediately following their treatment. The average total cost per trial arm was compared with savings in hospitalization costs as one estimate of the possible effectiveness of the symptom management interventions.
Methods
Sample
Following approval by the institutional review boards of the sponsoring university and the collaborating cancer centers, cancer patients meeting the following criteria were accrued: 1) 21 years of age or older, 2) having a diagnosis of a solid tumor cancer or non-Hodgkins lymphoma, 3) undergoing a course of chemotherapy, 4) being able to speak and read English, and 5) having a touchtone telephone. Participating patients signed an informed consent form and had all sociodemographic information entered into a Web-based tracking system. Next, all patients were screened for symptom severity using an automated voice response (AVR) version of the M. D. Anderson Symptom Inventory (25). Patients scoring 2 or higher on severity of at least one symptom (range 0–10) entered the trial. Those not reaching this threshold after twice weekly calls covering six weeks were sent a letter thanking them for participation but were not entered into the trial.
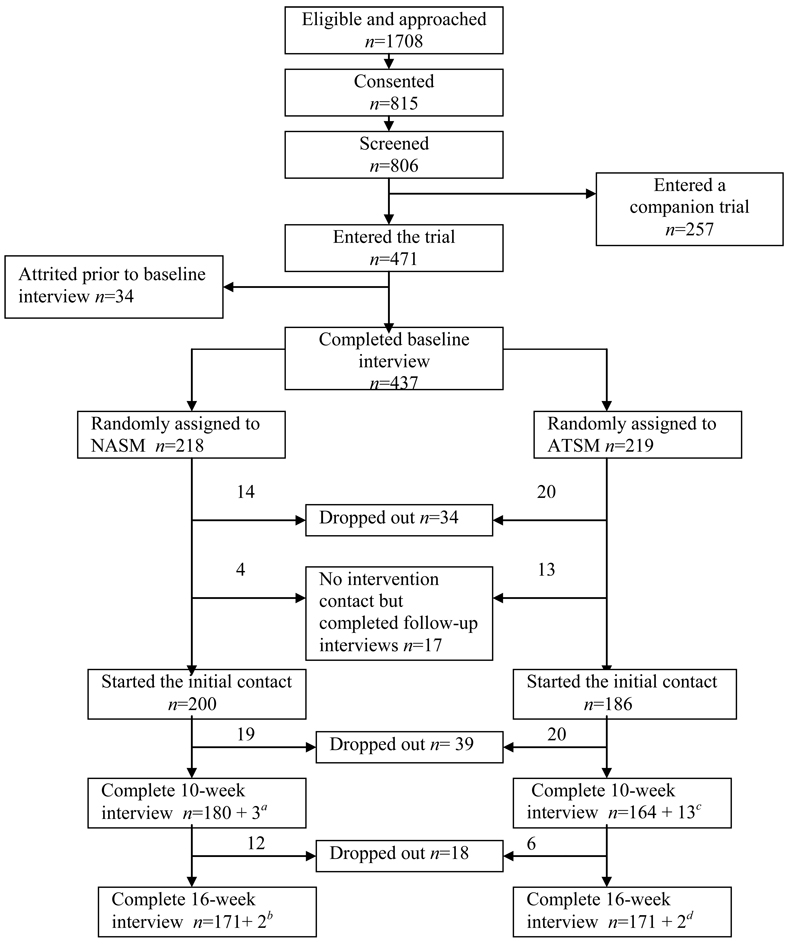
Patients who scored 2 or higher on severity received an intake interview and a copy of the Symptom Management Guide (SMG), and were randomized into either a Nurse Administered Symptom Management (NASM) arm or to an Automated Telephone Symptom Management (ATSM) arm using a computer minimization program (26) that balanced patients by arm with respect to recruitment location and site of cancer. Both arms of the trial received one call each for the first four weeks, skipped week 5, were called week 6, skipped week 7, and received a final call on week 8. At 10 weeks, outcome data were obtained through a second interview. Figure 1 summarizes the number of enrolled and attrited patients at each step, and number analyzed.
Figure 1.
Flow chart of the trial.
a NASM arm: Three patients did not enter intervention but complete the 10-week interview.
b NASM arm: One patient skipped 10-week interview and one skipped intervention and 10-week interview.
c ATSM arm: Thirteen patients did not enter intervention but complete the 10-week interview.
d ATSM arm: Two patients skipped 10-week interview.
Procedure
This trial compared an NASM intervention plus referral to an SMG manual with an information education intervention delivered by an ATSM system with referral to the SMG. Each arm assessed the severity and offered intervention strategies for any of 17 symptoms: fatigue, pain, dyspnea, insomnia, distress, nausea, fever, difficulty remembering, lack of appetite, dry mouth, vomiting, numbness and tingling, diarrhea, cough, constipation, weakness, and alopecia rated by patients at a 4 or higher at any of the six contacts. In the NASM, nurses delivered up to four strategies for each symptom supplemented with references to the SMG.
In the ATSM arm, a pre-recorded pleasant female voice queried patients regarding their severity for the 17 symptoms. To rate severity, patients pressed the appropriate numbers on their telephone keypads. For symptoms rated at 4 or higher, patients were directed to the section of the SMG that informed them about strategies to manage each symptom. The trial is described more fully in Sikorskii et al. (6).
Measures
Information on the sociodemographic characteristics of patients were obtained at enrollment and confirmed at the baseline interview. Site and stage of cancer were obtained from patients’ records following enrollment. Comorbid conditions were obtained from patients using a standardized instrument (27).
Severity of 17 symptoms was assessed at intake, each intervention contact, and at the 10-and 16-week interviews. Severity was assessed on an 11-point scale (0 not present, 1 very mild to 10 worst imaginable). The list of symptoms evaluated in this study was developed in multiple prior studies and the literature confirms that these symptoms are the most prevalent in the population of interest that includes multiple sites of cancer (17). Severity was assessed on an 11-point scale (0 not present, 1 very mild to 10 worst imaginable). To measure effectiveness, a summed severity index was computed with scores potentially ranging from 0–170. The internal consistency reliability ranged from 0.79 to 0.82 at all observations. This measure is an index of total symptom burden; no assumptions are made as to the scalar properties of the index. For example, two patients could achieve an identical score of 20, one through reporting five symptoms at a severity of 4 and a second patient reporting two different symptoms at a severity of 10 (17). Thus, unlike traditional scales where each item taps an underlying construct as an indicator (28), the severity index is based on experience with common cancer and treatment-related symptoms. The strengths, weaknesses, and alternatives to this approach are discussed in detail in other research reported by this team (6, 19, 20).
During the development and implementation of the trial fixed and variable costs were identified. Total costs (fixed plus variable) were divided by the number of patients in each arm to obtain unit costs per patient per arm. Three components of total cost were included: personnel time, fixed costs to create the software that drove the interventions, and variable or operating costs for the interventions. We did not include opportunity costs borne by patients such as time to respond to interviews or to each intervention contact, or opportunity costs associated with these activities. We chose not to include these costs since patients who perceived the costs of participation to be greater than the benefits could withdraw at any time. Finally, all cost calculations were based on patients who completed all 6 intervention contacts.
The nurse-administered cognitive behavioral arm involved direct telephone interactions with patients as well as time to enter data into the electronic protocol. On average, nurses spent 40 minutes per call with an additional 16 minutes per call for recording information into the nursing protocol. Overall, nurses spent slightly over 55 minutes per contact for the six contact interventions. These data were obtained from nurses in real time. A responsibility of the quality assurance coordinator was to make certain nurses documented time for each of their activities for each intervention contact completed during the preceding week. No time was allocated for nurses for the time they devoted to unanswered calls to patients.
The average number of minutes per contact for the AVR arm of the trial was 18.9 minutes. In the ATSM arm, the calls were automated and differences among patients were due to the number of symptoms above threshold where the system first reminded patients of the symptoms reported over threshold of 4, and the prompt to read the appropriate sections of the SMG. Finally, mean time to complete a call in each arm was stable across the six contacts. Given the stability of contact time across intervention contacts, it is possible to adjust variable costs by fewer numbers of contacts. In other analyses we estimated the upper bounds of each arm to be around 1,500 patients per year. This assumes six nurses working full time and full capacity for the ATSM arm.
Hospitalization data were obtained from patient reports at the intake, 10-week (end of trial) and at the 16-week follow-up period. We chose to rely on patient reports of hospitalization rather than information from the medical record. When compared, we found that patients, particularly those who were out of area from where they received treatment, reported more hospital admissions than were found in their medical records. Thus, we concluded that, given the significance of a hospital admission, and the relatively short recall period that patient report may better reflect hospitalizations than information from the medical record. Specific questions included: the number of times the patient was hospitalized (a three month recall from baseline observation and then at 10 and 16 weeks), the numbers of nights they spent in the hospital, the city in which they were hospitalized. Finally, when the facility where patients were treated was the same as the city in which they resided, rates of hospitalization were concordant with declines occurring as distance between residence and treatment facility increased.
Data Analysis
Both arms produced significant and virtually identical levels of reductions in severity between baseline and 10 weeks, and baseline and 16 weeks. To examine the relations between reductions in symptom severity produced (equally) by both arms, numbers of hospitalizations, and the numbers of days in the hospital, the distributions of the hospitalization variables were evaluated. To model substantial proportion of zeros, zero-inflated Poisson (ZIP), negative binomial, and zero-inflated negative binomial models were considered. The choice of ZIP model was made based on comparison of observed and predicted counts for each equation. In the first, the outcome was the number of hospitalizations at 16 weeks, and in the second the outcome of the number of days in the hospital at 16 weeks. The covariates included the number of hospitalizations or the number of days spent in the hospital as reported by patients at intake, baseline symptom severity, trial arm, and the percent reduction in symptom severity between baseline and 16 weeks: >50% improvement, 0–49% improvement, or deterioration. Creation of these categories was informed by the guideline of clinically significant change of 50% or more in symptom severity (22–24). To estimate the impact of the covariates on the numbers of hospitalizations and the number of days in the hospital, the ZIP models first test the significance of each covariate in distinguishing between zeros and non-zeros. Second, the effects of covariates on non-zero frequencies were examined. The ZIP models were implemented using SAS (version 9.1) experimental procedure PROC COUNTREG.
Results
Table 1 describes the age, sex, sites and stages of cancer, comorbidity and symptom severity at baseline and 10 weeks. The groups were comparable at baseline and there were no differences in attrition by group by baseline severity scores. Unadjusted means of symptom severity at baseline and 16 weeks are in Table 1. After adjusting for age, cancer site, comorbidity and symptom severity at intake, the least square mean symptom severity at 16 weeks was 21 with an standard error of 1.3 for the ATSM and 19 (SE of 1.3) for the NASM arm. Between baseline and 16 weeks, 83% of the patients remained the same or improved in the ATSM arm and 75% remained the same or improved in the NASM arm. Each arm produced a statistically significant reduction in severity over baseline but no differences in symptom severity were observed between arms at 16-weeks.
Table 1.
Characteristics of the Participants by Trial Arm
| ATSM | NASM | ||||||
|---|---|---|---|---|---|---|---|
| n | % | N | % | ||||
| Gender | |||||||
| Male | 53 | 24.20 | 57 | 26.15 | |||
| Female | 166 | 75.80 | 161 | 73.85 | |||
| Site of Cancer | |||||||
| Lung | 37 | 17.29 | 39 | 18.14 | |||
| Breast | 86 | 40.19 | 88 | 40.93 | |||
| Colon | 33 | 15.42 | 30 | 13.95 | |||
| Other | 58 | 27.10 | 58 | 26.98 | |||
| Stage of Cancer | |||||||
| Early | 66 | 30.84 | 53 | 24.65 | |||
| Late | 148 | 69.16 | 162 | 75.35 | |||
| Reduction in severity | |||||||
| >50% | 90 | 52.02 | 79 | 45.66 | |||
| 0–49% | 55 | 31.79 | 51 | 29.48 | |||
| Deterioration | 28 | 16.18 | 43 | 24.86 | |||
| n | Mean | SD | n | Mean | SD | ||
| Age | 218 | 57.12 | 12.00 | 218 | 57.28 | 11.84 | |
| Comorbidity | 219 | 2.01 | 1.63 | 218 | 2.10 | 1.61 | |
| Symptom severity at baseline |
219 | 36.13 | 22.84 | 218 | 32.53 | 20.90 | |
| Symptom severity at 16 weeks |
173 | 18.33 | 18.74 | 173 | 19.33 | 18.62 | |
SD = standard deviation.
Given the comparability of the symptom severities at the 16-week endpoint, we then determined if one arm produced these outcomes at a lower cost than the other. Table 2 presents the costs per trial arm in 2003 dollars. Each arm required space on a server, hosting site, and security, which were split evenly between the two arms. This separation was made for the purpose of our comparison and is arbitrary; space and cost for server rental will vary by the intervention used. Support for computer maintenance was based on annual contact costs; two computers were used for the ATSM and one computer each for the nurses. Similarly, costs for production and printing of the SMG were divided equally since each patient received a guide at the time of enrollment. The differences in software programs and leases are due to the costs of producing the customized computer assisted protocol software that nurses used to record assessments of patients’ symptoms, manipulate the plans of care that linked symptoms with selected interventions from a standard protocol list stored in the roster of interventions for each symptom. The AVR software was purchased and then modified and extended to fit the requirements of this study. While costs of producing the nurse protocol software were higher, the costs for hardware, software maintenance, and management were greater for the ATSM and are reflected by allocating 80% of those costs to the AVR and only 20% to NASM arm. In sum, fixed costs were $60,577 and $102,185 for the ATSM and the NASM, respectively.
Table 2.
Fixed, Variable, and Total Costs for Each Intervention Arm
| ATSM | NASM | |
|---|---|---|
| Fixed Costs | ||
| Server | 3,430 | 3,430 |
| Hosting | 600 | 600 |
| Security | 1,004 | 1,004 |
| Publish Symptom Guide | 2,912 | 2,912 |
| Information Technology | 890 | 3,116 |
| Software Programs and Lease |
5,635 | 67,677 |
| Hardware/Software Maintenance and Management |
42,120 | 10,000 |
| Nurse Interviewer Training |
9,360a | |
| Total Fixed Costs | $60,577 | $102,185 |
| Variable Costs | ||
| Telephone Costs at $.36/min |
1.89 hrs/ × $.36/min $41.00 |
5.5 hrs × $30/hr Nurse Salary × 1.3 Fringe = $214.50+$.36 = $188.80 = $333.30 |
| Personnel Coordination Time |
1.89 hrs × .5 = .95 × $14 × 1.3 $17.29 |
5.5 hrs × .15 = .82 × $14/hr × 1.3 = $14.92 |
| Quality Assurance Time | 1.89 hrs × .1 = .19 × $25 × 1.3 $6.14 |
5.5 hrs × .3 = 1.65 × $30/hr × 1.3 $64.35 |
|
Variable Costs per Patient |
$64.43 | $412.57 |
| Patients | 173 | 173 |
| Total Variable Costs | $11146 | $71374 |
| Total Costs | $71723 | $173,559 |
| Total Cost per patient | $414.59 | $1003.23 |
6 nurses × 40/hrs × 30 hrs 30% fringe benefits
Variable costs are based on a completed set of six contacts. Telephone calls were assessed based on charges from telephone carriers during the course of the intervention and were allocated at $.36 per minute for local and long distance calls and multiplied by the mean number of minutes per contact and six contacts for each arm. For the ATSM arm, this amounted to $41 for local or long distance calls, 1.6 hours per six contacts for a graduate assistant to monitor the AVR system for missed calls, patients who forgot PIN numbers, or other complications, and quality assurance. In both arms a fringe rate of 1.3 of direct labor costs was used. Variable costs for the NASM arm were labor (calculated in hours) at $30/hour, telephone ($86), and 15% of a graduate student’s time and 33% of quality assurance nurse to listen to tapes of the nurse interveners and to review their documentation in the electronic protocol.
The cost per patient is $414.59 for six ATSM contacts and $1,060.66 for six NASM contacts. The costs for patients who completed all six contacts of the ATSM and NASM interventions were $69 per patient and $167 per patient for the ATSM and the NASM respectively.
Since neither arm differed with respect to symptom reduction at 16 weeks, we wanted to learn if cases that improved over the course of the trial were less likely to use other costly services such as hospitalizations. Table 3 summarizes the numbers of patients with none, one or two or more hospitalizations and the numbers of nights hospitalized as reported at baseline, 10 and 16 weeks. No differences by trial arm were found for these variables. Finally, Table 4 contains analyses of the numbers of hospitalizations and the numbers of nights hospitalized in relation to the reductions in symptom severity. After adjusting for hospitalizations in the three months prior to entry into the trial as reported at baseline, symptom severity at baseline, and trial arm, for patients who were hospitalized at least once during the 16 weeks of the trial, patients whose severity declined equal to or greater than 50% were significantly less likely to be hospitalized (P<0.01, Table 4) when contrasted with patients whose symptom severity increased between baseline and 16 weeks. Similarly, symptom reduction was related to significantly fewer numbers of nights of hospitalization. A significant reduction in the number of nights in the hospital was also observed for those patients whose symptom severity remained the same or declined up to 49% compared to those whose symptoms worsened (P<0.01). The effects found in ZIP models are further quantified in Table 5. Patients whose symptom status remained the same or improved reported a mean number of hospitalizations of 0.33 and 0.34 versus 0.52 for those whose severity worsened during the course of the trial. Similarly, once hospitalized, patients whose severity remained the same or declined up to 49% reported 1.08 mean number nights of hospitalization; patients with 50% or more symptom improvement spent 1.28 nights, while those with worsening severity had a mean of 2.23 nights (data not in tables).
Table 3.
Number of Admissions and Nights of Admission at the Baseline, 10- and 16-Week Observation Points
| ATSM n (%) |
NASM n (%) |
|
|---|---|---|
| Number of hospitalizations reported at baseline 0 1 2+ |
105 (60.69) 47 (27.17) 21 (12.14) |
101 (58.38) 47 (27.17) 25 (14.45) |
| Number of hospitalizations reported at 10 weeks 0 1 2+ |
138 (80.70) 27 (15.79) 6 (3.51) |
143 (83.63) 23 (13.45) 5 (2.92) |
| Number of hospitalizations reported at 16 weeks 0 1 2+ |
155 (89.60) 14 (8.09) 4 (2.31) |
147 (84.97) 23 (13.29) 3 (1.73) |
| Number of nights in the hospital reported at baseline 0 1 2+ |
114 (65.90) 14 (8.09) 45 (26.01) |
108 (62.43) 12 (6.94) 53 (30.64) |
| Number of nights in the hospital reported at 10 weeks 0 1 2+ |
140 (81.87) 8 (4.68) 23 (13.45) |
145 (84.80) 7 (4.09) 19 (11.11) |
| Number of nights in the hospital reported at 16 weeks 0 1 2+ |
157 (90.75) 5 (2.89) 11 (6.36) |
147 (84.97) 8 (4.62) 18 (10.40) |
Table 4.
The Effects of the Covariates in the Zero-Inflated Model Predicting Number of Hospitalizations at 16 Weeks and Number of Days in the Hospital
| Outcome: Number of Hospitalizations at 16 Weeks | ||||
| Covariate | Estimated Coefficient |
Standard Error | t | P-value |
| Number of hospitalization before baseline |
0.38 | 0.16 | 2.40 | 0.0165 |
| Overall severity reduced (≥50%) | −0.87 | 0.31 | −2.79 | 0.0053 |
| Overall severity reduced (0–49%) | −0.66 | 0.38 | −1.75 | 0.0802 |
| Baseline symptom severity | 0.02 | 0.01 | 2.10 | 0.0357 |
| Trail Arm (ATSM vs. NASM) | −0.25 | 0.30 | −0.82 | 0.4112 |
| Outcome: Number of Nights in the Hospital | ||||
| Covariate | Estimated Coefficient |
Standard Error | t | P-value |
| Number of Nights Stayed in Hospital Before Wave 1 |
0.03 | 0.01 | 2.46 | 0.0139 |
| Overall severity reduced (≥50%) | −0.53 | 0.11 | −4.74 | <0.0001 |
| Overall severity reduced (0–49%) | −0.60 | 0.13 | −4.67 | <0.0001 |
| Baseline symptom severity | 0.01 | 0.002 | 5.12 | <0.0001 |
| Trail Arm (ATSM vs. NASM) | 0.02 | 0.09 | 0.25 | 0.8024 |
Table 5.
Mean Number of Hospitalizations and Number of Nights Spent in the Hospital at 16 Weeks by Overall Reduction in Severity and Trial Arm
| Reduction in Symptom Severity |
Number of Hospitalizations Mean (SD) |
Number of Nights in the Hospital Mean (SD) |
Estimated Cost Per Patient |
|---|---|---|---|
| ≥50% (n=169) | 0.34 (0.73) | 1.28 (3.67) | $783.36 |
| 0–49% (n=106) | 0.33 (0.74) | 1.08 (2.97) | $641.52 |
| Deteriorated (n=71) | 0.52 (0.97) | 2.23 (4.59) | $2088.00 |
Finally, the median cost per day of hospitalization was $1,800 in 2004 (29). We estimate the costs per symptom severity group as follows: for patients with a 50% or greater reduction in severity and where the rate of hospitalization (0.34) is multiplied by the number of nights in the hospital (1.28) and the sum is multiplied by $1800, the total is $783.36. For patients who remained the same or achieved at least a 49% reduction the costs were $641.52 (0.33 × 1.08 × $1800) and for patients whose severity worsened during the trial, the cost was $2088 per patient (0.52 × 2.23 × $1800). The expected cost overall was approximately $1015 per patient. The savings to payers of symptom management protocols (assuming that they also pay for other medical care costs) ranges from $640 to $2088, depending on the level of symptom change, or an average expected savings of $1015 per patient hospitalized. If we assume an average of 0.33 hospitalizations (a very low number) and a cost per hospitalization of $1800 per day (again a very conservative estimate) it appears that adding a program of symptom management, especially one that relies on the use of an automated system, could contribute to a cost savings for cancer patients during and immediately following chemotherapy.
Discussion
Despite the large number of educational and psychobehavioral trials of symptom management interventions none have been adopted by purchasers of care or by third party payers. No doubt, this is related to the fact that the moderate effect sizes observed from these studies have not been translated into reductions in the costs of subsequent care. This study is among the first to compare costs associated with the delivery of two trial arms against reductions in symptom severity and to assess the downstream savings associated with fewer hospital admissions and shorter lengths of stay. The evidence presented here clearly points toward the merits of a relatively low cost automated approach that engages patients in undertaking self-care approaches to manage their cancer related symptoms.
Alternatively, given that this was a two-arm trial, one could argue that symptom severity would have declined without interventions. In an earlier trial where a five-contact, nurse-directed intervention delivered by telephone was compared with conventional care alone, we found that symptom severity measured using a summed score across 15 symptoms was reported at intake to be 28.6 and 26.4 for the experimental and conventional arms respectively. At 10 weeks, the mean severities were 22 for experimental and 28 for control arm, and this difference was sustained at 16 weeks (means of 22 and 29, respectively) (4). These differences were significant and the experimental arm produced a greater effect on patients entering with higher severity scores. In this two arm trial, patients entered with severity scores of 35 and 32 for a 17-symptom index respectively for the nurse and voice response arms. By 16 weeks, these scores were 19 and 18 for the two arms respectively. The fact that both arms in the current trial produced greater responses than the experimental arm alone in the prior trial using the same measures, same inclusion criteria and with only one additional contact strongly supports an effect for each arm and reduces the likelihood that such reductions in severity would have occurred without intervention over this period of time.
Since a significant association was present between maintaining or lowering symptoms and fewer hospitalizations or shorter stays among those patients who are hospitalized, then a case can be made for comparing the costs related to producing the intervention against the savings attributed to fewer hospitalizations and shorter lengths of stay among those patients who were hospitalized. In a meta-analysis that focused on determining the offset costs (reductions in hospital admissions and lengths of stay) produced by behavioral interventions, 31% produced saving after subtracting costs of the intervention. These findings are consistent with this research where the ATSM model which required lower overall costs produced greater savings given that the reductions in severity were essentially the same. However, as the authors of the meta-analysis report, cost offsets, particularly those that occur in outpatient settings, are difficult to attribute solely to novel interventions (30). This reservation pertains to this research as well. Isolating the sequential or even causal events linking reductions in symptoms to lower hospitalizations is difficult. Issues related to timing, when the severity of a symptom is lowered, or when severity increases, and the occurrence or prevention of an admission to a hospital is confounded by a host of other potential determinants including; biological, psychological and behavioral factors of patients, clinical decisions made by physicians, as well as access to care and hospitals (30).
While hospitalizations were greater among those whose symptom severity increased during the trial, the overall number of cases hospitalized was relatively small. Thus, we can only suggest that the rates of savings in hospitalizations from symptom management might lower overall costs of care during treatment. Further, changes in severity were combined across trial arms, each arm produced similar reductions in symptom severity. We argue that the costs to produce the ATSM could be justified based on numbers of patients with the observed levels of hospitalization. Had each arm served the maximum numbers of patients, then, depending upon rates of hospitalization, the ATSM or even the NASM may have been found to be cost-effective. Considering the increased cost per day of hospitalization, and the reductions in costs of technology, it is possible that the ATSM could prove even more effective. This research took only hospitalization into account; no corresponding improvements in quality of life were considered such as ability to work, reductions in treatment delays or interruptions. Finally, the arguments posed here might prove more convincing had the ATSM arm been compared with conventional care alone where differences in severity as well as hospitalization rates might have demonstrated greater differences. However, what remains unknown is the overall savings that might occur if the ATSM (lower cost arm) was compared with a no treatment or conventional care control group.
In addition to the limitations noted in the preceding paragraphs, the index measuring severity does not capture differences according to symptoms. It is possible that hospitalizations could be an artifact of certain highly severe symptoms. Finally, and most important, a true control group is needed for this work. Such a comparison would allow us to implement traditional cost effectiveness analysis.
Future work in this area needs to consider the cumulative effects of multiple outcomes such as additional unscheduled visits to oncology practices and primary care settings by patients seeking relief from their symptoms, and the roles of symptoms in treatment delays, reductions, and stoppages, and thus compromised therapeutic beneficiaries. In addition, the use of prescription and over the counter medications should also be included in future analyses of cost. Finally, a study that incorporates productivity lost as reflected by days lost from work by both patients and caregivers is relevant when examining societal cost and benefits of symptom control interventions.
In conclusion, this model of linking reductions in symptom severity with savings in high cost items such as hospitalizations represents an important approach to justifying the value added by symptom interventions that are relatively low cost, highly scalable to larger populations, and place little or no burden on the performance of the oncology system of care. These data suggest that a savings of one to two hospitalizations could go a long way toward paying for an ATSM that manages patient symptoms through self-care strategies that patients can implement at home and that do not rely on already over burdened oncology practices.
Acknowledgments
This work was supported by National Cancer Institute Grant #RO1 CA30724 (Automated Telephone Monitoring for Symptom Management, C. Given, PI, B. Given, Co-PI) and The Walther Cancer Foundation, Indianapolis, Indiana.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive behavioral therapy: a review of meta-analysis. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: overview and recommendations for future research. J Natl Cancer Inst. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen P, Meade C, Stein K, et al. Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. J Clin Oncol. 2002;20:2851–2862. doi: 10.1200/JCO.2002.08.301. [DOI] [PubMed] [Google Scholar]
- 4.Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22:507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 5.Yates P, Aranda S, Hargraves M, et al. Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy. J Clin Oncol. 2005;23:6027–6036. doi: 10.1200/JCO.2005.01.271. [DOI] [PubMed] [Google Scholar]
- 6.Sikorskii A, Given CW, Given B, et al. Symptom management for cancer patients: a trial comparing two multimodal interventions. J Pain Symptom Manage. 2007;34:253–264. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977;296:716–721. doi: 10.1056/NEJM197703312961304. [DOI] [PubMed] [Google Scholar]
- 8.Eddy DM. Investigational treatments: how strict should we be? JAMA. 1997;278:179–185. doi: 10.1001/jama.278.3.179. [DOI] [PubMed] [Google Scholar]
- 9.Eddy DM. Clinical decision making: from theory to practice. Benefit language: criteria that will improve quality while reducing costs. JAMA. 1996;275:650–657. doi: 10.1001/jama.275.8.650. [DOI] [PubMed] [Google Scholar]
- 10.Eddy DM. Clinical decision making: from theory to practice. Rationing resources while improving quality. How to get more for less. JAMA. 1994;272:817–824. doi: 10.1001/jama.272.10.817. [DOI] [PubMed] [Google Scholar]
- 11.Eddy DM. Clinical decision making: from theory to practice. Applying cost-effectiveness analysis. The inside story. JAMA. 1992;268:2575–2582. doi: 10.1001/jama.268.18.2575. [DOI] [PubMed] [Google Scholar]
- 12.Eddy DM. Clinical decision making: from theory to practice. Cost effectiveness analysis. Is it up to the task? JAMA. 1992;267:3342–3348. doi: 10.1001/jama.267.24.3342. [DOI] [PubMed] [Google Scholar]
- 13.Eddy DM. Clinical decision making: from theory to practice. Cost-effectiveness analysis. A conversation with my father. JAMA. 1992;267:1669–1675. doi: 10.1001/jama.267.12.1669. [DOI] [PubMed] [Google Scholar]
- 14.Drummond MF, Richardson WS, O’Brien BJ, Levin M, Heyland D. Users’ guides to the medical literature. XIII. How to use an article on economic analysis of clinical practice. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1997;277:1552–1557. doi: 10.1001/jama.277.19.1552. [DOI] [PubMed] [Google Scholar]
- 15.Chiles JA, Lambert MJ, Hatch AL. The impact of psychological interventions on medical cost offset: a meta-analytic review. Clin Psychol Sci Pract. 2006;6:204–220. [Google Scholar]
- 16.Sloan JA, Berk L, Roscoe J, et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-Sponsored Clinical Trials Networks. J Clin Oncol. 2007;25:5070–5077. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 18.Mandelblatt JS, Silliman R. Hanging in the balance: making decisions about benefits and harms of breast cancer screening among the oldest old without a safety net of scientific evidence. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.19.4928. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Given B, Given C, Sikorskii A, et al. Establishing mild, moderate and severe scores for cancer related symptoms: how consistent and clinically meaningful are interference based severity cut points? J Pain Symptom Manage. 2008;35:126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon S, Given CW, Sikorskii A, Given B. Do interference-based cut-points differentiate mild, moderate, and severe levels of 16 cancer-related symptoms over time? J Pain Symptom Manage. 2008 doi: 10.1016/j.jpainsymman.2008.01.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikorskii A, Given B, Given C, Jeon S. Differential symptom reporting by mode of administration of the assessment: automated voice response system versus a live telephone interview. Med Care. 2009 doi: 10.1097/MLR.0b013e3181a31d00. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 23.Miaskowski C, Dodd M, West C, et al. The use of a responder analysis to identify differences in patient outcomes following a self-care intervention to improve cancer pain management. Pain. 2007;129:55–63. doi: 10.1016/j.pain.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul SM, Zelman DC, Smith M, Miaskowski C. Categorizing the severity of cancer pain; further exploration of the establishment of cutpoints. Pain. 2005;113:37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 27.Katz JN, Chang LC, Sangha O, Fossel A, Bates D. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Fayers PM, Hand DJ. Factor analysis, causal indicators and quality of life. Qual Life Res. 1997;6:139–150. doi: 10.1023/a:1026490117121. [DOI] [PubMed] [Google Scholar]
- 29.Love TP, Reilly TW, editors. Health care financing review. Washington, DC: Centers for Medicare & Medicaid Services; 2008. [Google Scholar]
- 30.Hunsley J. The cost-effectiveness of psychological interventions. Ottawa: Canadian Psychological Association; 2002. [Google Scholar]