Abstract
Eosinophilic inflammation is a characteristic feature of asthma. Integrins are highly versatile cellular receptors that regulate extravasation of eosinophils from the postcapillary segment of the bronchial circulation to the airway wall and airspace. Such movement into the asthmatic lung is described as a sequential, multistep paradigm, whereby integrins on circulating eosinophils become activated, eosinophils tether in flow and roll on bronchial endothelial cells, integrins on rolling eosinophils become further activated as a result of exposure to cytokines, eosinophils arrest firmly to adhesive ligands on activated endothelium, and eosinophils transmigrate to the airway in response to chemoattractants. Eosinophils express seven integrin heterodimeric adhesion molecules: alpha4beta1 (CD49d/29), alpha6beta1 (CD49f/29), alphaMbeta2 (CD11b/18), alphaLbeta2 (CD11a/18), alphaXbeta2 (CD11c/18), alphaDbeta2 (CD11d/18), and alpha4beta7 (CD49d/beta7). The role of these integrins in eosinophil recruitment has been elucidated by major advances in the understanding of integrin structure, integrin function, and modulators of integrins. Such findings have been facilitated by cellular experiments of eosinophils in vitro, studies of allergic asthma in humans and animal models in vivo, and crystal structures of integrins. Here, we elaborate on how integrins cooperate to mediate eosinophil movement to the asthmatic airway. Antagonists that target integrins or the effectors that regulate integrins of eosinophils represent potentially promising therapies in the treatment of asthma.
Keywords: asthma, cytokine, extravasation, podosome, recruitment, VCAM-1
INTRODUCTION
The eosinophil is a highly motile white blood cell containing distinctive cytoplasmic granules [1, 2]. First described in 1879 by Paul Ehrlich, eosinophils have been studied extensively as potent defenders against parasitic helminths. The cuticles of helminths are destroyed by granule proteins and superoxide radicals released or generated by eosinophils [3, 4]. The view of eosinophils as benign immune sentinels and effectors has become complicated over the past few decades by recognition of the possible deleterious role of eosinophils in immune hypersensitivity syndromes, including asthma, dermatitis, and rhinitis [2, 5–9].
The prevalence of asthma worldwide is increasing for reasons that are unclear [10–12]. Recruitment of eosinophils to the airway is believed to exacerbate asthma and contribute to the chronic character of asthma [13, 14]. Thus, the study of how eosinophils traffic from blood to the airway is of considerable importance. Integrins, which are among the most versatile of known cellular receptors [15, 16], have generated particular interest as likely determinants of how eosinophils arrest in the postcapillary venules of the bronchi and mediate extravasation of eosinophils from blood to the airway wall and airspace [17]. Specifically, integrins have been studied in relationship to rolling and arrest of eosinophils on endothelium, migration through endothelium and the underlying basement membrane, and traversing of bronchial epithelium into the airway lumen [18, 19]. It is important to note that this multi-step paradigm of eosinophil and granulocyte diapedesis applies to postcapillary vessels of the bronchial circulation; that movement to the parenchymal tissue of the lung is little understood [20].
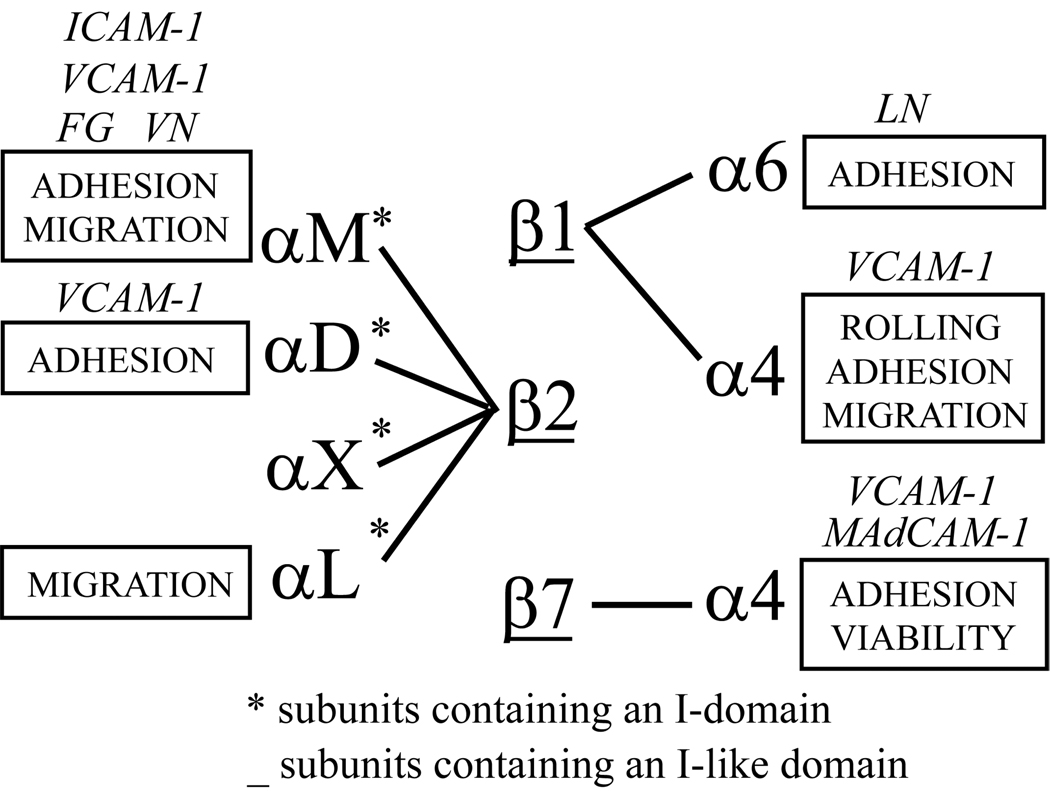
As depicted in Figure 1, purified human blood eosinophils express seven integrin heterodimers: α4β1 (CD49d/29), α6β1 (CD49f/29), αMβ2 (CD11b/18), αLβ2 (CD11a/18), αXβ2 (CD11c/18), αDβ2 (CD11d/18), and α4β7 (CD49d/β7) [21–23]. Each type of heterodimer interacts with its own set of ligands, which may be deposited in extracellular matrix or a counter-receptor on other cells. Understanding functions of the integrin complement on a given cell type is complicated by the fact that each integrin may be present in several conformational states, have varying levels of expression, and cluster in the plasma membrane [24, 25]. The movement of eosinophils into the asthmatic lung, therefore, can be expected to involve a complex interplay of integrin receptors in different states of activation interacting with a diverse set of ligands on bronchial endothelium and cells within tissue.
Figure 1. Integrins of eosinophils.
Schematic of the seven heterodimeric integrin adhesion receptors expressed by eosinophils. Functions and ligands assigned to integrins have been deduced in various assays employing eosinophils. Asterisks represent subunits that contain the I (Insert)-domain. Subunits that are underlined contain the I-like domain. ICAM, intercellular adhesion molecule; MAdCAM-1, mucosal addressin cell adhesion molecule-1; FG, fibrinogen; LN, laminin; VCAM, vascular cell adhesion molecule; VN, vitronectin.
Although less is known about integrins of the eosinophil in comparison to integrins of lymphocytes, neutrophils, or platelets, considerable information is available about the surface phenotype of the eosinophil, how integrins and integrin effectors dynamically regulate interactions of eosinophils with ligands, and how these interactions lead to properties of eosinophil adhesion, migration, survival, and recruitment in asthma. Here, we summarize recent advances in the comprehension of integrin structure, integrin function, and integrin modulation of eosinophils. Therapeutic implications of such advances are discussed.
INTEGRINS OF EOSINOPHILS
α4β1 (CD49d/29, VLA-4)
Roles of α4β1 of eosinophils
α4β1 has been studied more extensively than other eosinophil integrins because of its role in mediation of rolling at physiological shear rates [26, 27] and adhesion [21, 28–31] of purified blood or airway eosinophils on vascular cell adhesion molecule 1 (VCAM-1, CD106). VCAM-1 is an integrin counter-receptor upregulated in the asthmatic lung [32]. Figure 2 depicts the arrangement of immunoglobulin (Ig)-like modules of VCAM-1 and the loop in Ig-like module 1 that contains the Ile-Asp-Ser-Pro-Leu (IDSPL) recognition sequence that interacts with α4β1. Expression of endothelial VCAM-1 is induced by T helper cell type 2 (Th2) mediators, strongly implicating VCAM-1 in α4β1-mediated eosinophil recruitment from blood to the airway of human asthmatics [33, 34]. Endothelial VCAM-1 may also be expressed in response to activators of NF-κB or of activator protein (AP)-1, including thrombin, homocysteine, angiotensin II, reactive oxygen species, or migration inhibitory factor [35–39]. Eosinophils may even upregulate local amounts of VCAM-1 on endothelial cells through production of hypothiocyanous acid (HOSCN), a membrane permeable product that is generated in response to oxidation of thiocyanate (SCN−) by eosinophil peroxidase [40]. There is little [41, 42] or no [34, 43] expression of α4β1 on purified human neutrophils, suggesting that eosinophil recognition of VCAM-1 mediated by α4β1 is an important mechanism by which selective infiltration of eosinophils over neutrophils is achieved in asthma [44, 45]. Although it has been reported that α9β1 on neutrophils can mediate migration on VCAM-1 [42], in our hands neutrophils do not adhere to immobilized recombinant soluble VCAM-1 (our unpublished results) under conditions in which eosinophils adhere readily [30, 31].
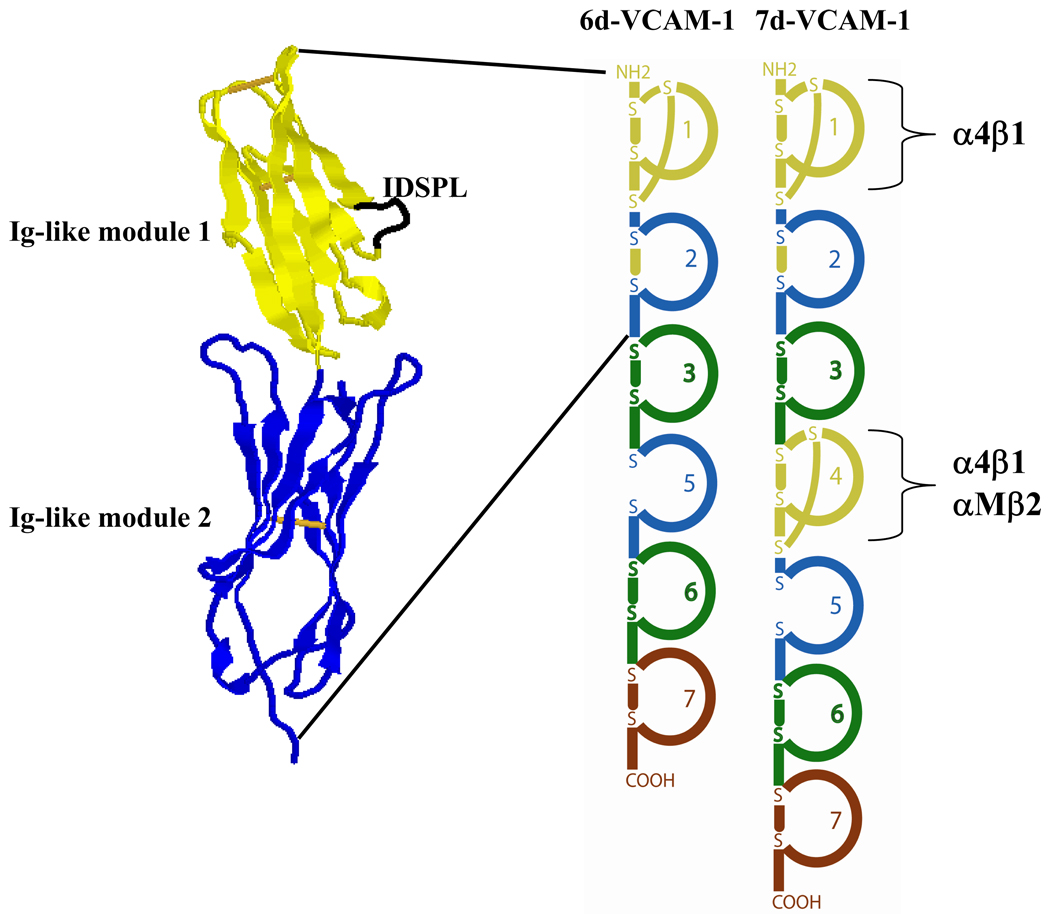
Figure 2. Structure and schematic of VCAM-1 splice forms.
Crystal structure of the first two N-terminal immunoglobulin (Ig)-like modules of 6d- and 7d-VCAM-1 [75]. The crystal structure is color coded to match the schematic of VCAM-1 modules depicted to the right. In the crystal structure, module 1 contains an IDSPL loop (highlighted in black) that is recognized by α4β1 of eosinophils. A second IDSPL motif recognized by α4β1 of eosinophils is present in module 4 of 7d-VCAM-1, which is also recognized by αMβ2. Module 4 is absent from 6d-VCAM-1, the likely result of exon skipping during mRNA splicing [78]. Two intra-domain disulfide bonds are present in modules 1 and 4 while modules 2, 3, 6, and 7 are predicted to contain only one such disulfide. The disulfide is missing from module 5. Individual modules have been color coded based on amino acid sequence identity: modules 1 and 4 = 73%, modules 2 and 5 = 60%, modules 3 and 6 = 60%. The internal homology and similarity of intron sizes between modules 1–3 and 4–6 suggests that modules 4–6 may have arisen in evolution by gene duplication of ancestral modules 1–3 [163]. The figure was created with RasMol.
In addition to mediating rolling and firm adhesion, α4β1 supports eosinophil migration on VCAM-1. Thus, blocking antibodies recognizing the α4 subunit inhibit eosinophil migration through Transwell pores coated with recombinant VCAM-1 in response to Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) and across VCAM-1-expressing endothelial cells induced by supernatants of peripheral blood mononuclear cells obtained from antigen-sensitized atopic asthmatics [46, 47]. Migration of eosinophils across endothelial cell monolayers in response to eotaxin is inhibited by anti-α4 [48]. Ligation of α4β1 by VCAM-1 has been shown to modulate functions beyond eosinophil rolling, adhesion, and migration. That is, adhesion of eosinophils to VCAM-1 potentiates superoxide anion generation [49–51]. The respiratory burst is inhibited following preincubation with anti-α4 antibody or the WAY103 small molecule α4 antagonist [50]. In contrast, anti-α4 or WAY103 enhances eosinophil-derived neurotoxin release from granules of eosinophils adherent to VCAM-1 [50]. Thus, ligation of α4β1 by these two antagonists has different activities – stimulation of granule release and inhibition of respiratory burst.
The β1 subunit localizes to podosomes of blood eosinophils adherent to VCAM-1 [52] or of airway eosinophils adherent to VCAM-1, intercellular adhesion molecule (ICAM)-1 (CD54), fibrinogen, vitronectin, or albumin [31]. The podosome is a transient structure that mediates interaction with and proteolysis of adhesive ligands in extracellular matrix [53]. Podosomes are enhanced in both size and quantity of airway eosinophils adherent to VCAM-1 in comparison to blood eosinophils [31], and the increase can be replicated by treatment of blood eosinophils with interleukin (IL)-5 or tumor necrosis factor (TNF)-α [52]. It is noteworthy that eosinophils do not form the more stable focal adhesions found in fibroblasts adherent to VCAM-1 [52]. Airway eosinophils are more migratory than blood eosinophils [54]. The increased size and/or number of podosomes of airway eosinophils may, therefore, contribute to the increased mobility.
As described above, the conformational states of integrins determine whether an integrin is functional for cell adhesion and migration. Activation of integrins is accomplished by “inside-out” signaling whereby chemokines and cytokines interact with cell-surface receptors and initiate signaling pathways that target the cytoplasmic domain of integrins. A comparison of α4β1 activity on eosinophils and the Jurkat T lymphocytic cell line suggests that α4β1 on eosinophils is tightly controlled. That is, the 15/7, HUTS-21, or 9EG7 activation-sensitive antibodies do not react with β1 of purified blood eosinophils using identical protocols in which the antibodies react robustly with β1 of Jurkat cells [28, 30], indicating that β1 of Jurkat cells is in a higher activation state in comparison to eosinophils. Thus, purified blood eosinophils do not adhere or migrate on the α4β1 ligand, fibronectin [28, 55], whereas Jurkat cells constitutively adhere to fibronectin [28] and migrate on surfaces coated with fibronectin in response to serum (our unpublished results). Coating of Transwell membranes with fibronectin inhibits formyl-Met-Leu-Phe (fMLP)-stimulated migration of eosinophils in comparison to uncoated membranes, indicating that fibronectin may even be inhibitory to eosinophil migration [55]. Adhesion and migration on fibronectin, therefore, requires a highly active form of α4β1 that is not present on purified blood eosinophils. “Forcing” β1 into a high activation state by incubation of blood eosinophils with the 8A2 activating antibody to β1 results in adherent eosinophils that roll less well on VCAM-1 [27], stimulates eosinophil adhesion to fibronectin [28], and decreases migration of eosinophils across monolayers of human umbilical vein endothelial cells [56]. Incubation of purified eosinophils from blood of allergic subjects with Mn2+ exposes the activation-sensitive epitope in the β1 hybrid domain recognized by mAb 15/7 [57] and enhances adhesion to VCAM-1 [58]. RANTES, monocyte chemotactic protein (MCP)-3, and eotaxin transiently increase eosinophil interaction with VCAM-1 and of a Leu-Asp-Val (LDV)-containing peptide [59, 60]. Thus, steps in eosinophil recruitment mediated by α4β1 are dynamically regulated by the allosteric structure of α4β1 present on the eosinophil surface.
Conformations of β1 of eosinophils
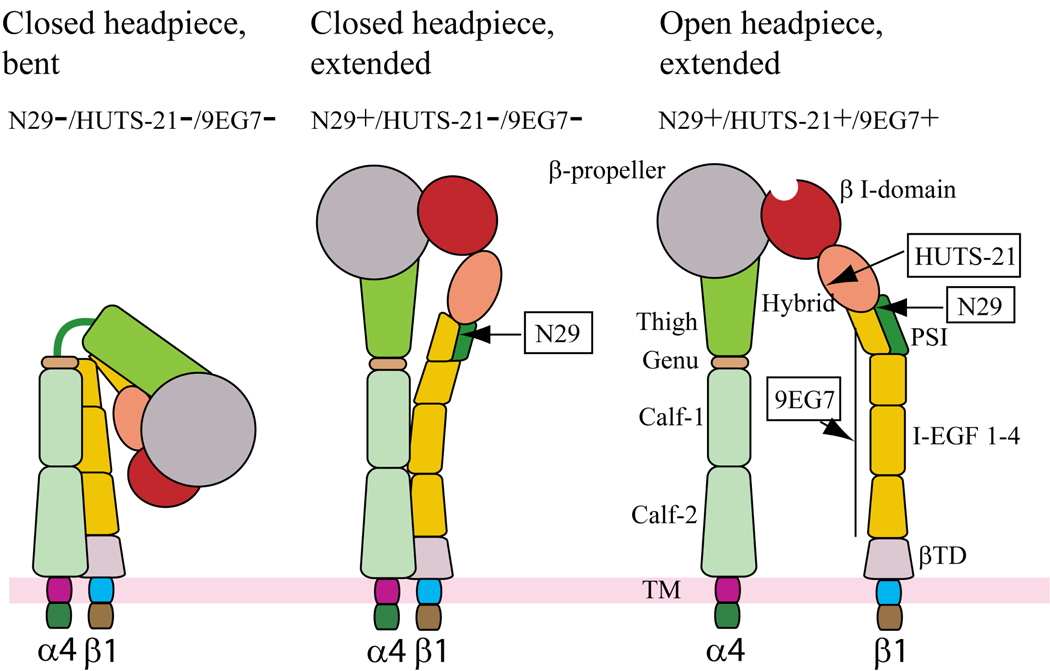
We have employed conformation-sensitive antibodies to probe β1 conformation on purified or nonpurified blood or airway eosinophils in segmental antigen challenge or steroid withdrawal clinical models of allergic human asthma. Figure 3 depicts a model in which several states of β1 activation are queried with three conformation-sensitive antibodies: N29, HUTS-21, and 9EG7. N29 recognizes an activation-induced epitope in the N-terminal region of the plexin, semaphorin, and integrin (PSI) domain [61], HUTS-21 recognizes an activation-induced epitope in the hybrid domain [62] that is also recognized by mAb 15/7 [57, 63], and 9EG7 recognizes an activation-induced epitope in the epidermal growth factor (EGF) domains of the “leg” [64, 65]. The locations of these epitopes in various structures adopted by β1, based on structures of αVβ3 and αIIbβ3 deduced from X-ray crystallographic and electron microscopic studies [24, 66], suggest that the antibodies recognize increasingly activated forms in the order N29<HUTS-21<9EG7. We have found that N29 but not HUTS-21 or 9EG7 recognize purified blood and airway eosinophils [31]. Such eosinophils adhere to immobilized VCAM-1 and not fibronectin [31], indicating that the N29+/HUTS-21−/9EG7− putative form of α4β1 depicted to the middle in Figure 3 recognizes VCAM-1 but not fibronectin. Jurkat cells, which express the N29+/HUTS-21+/9EG7+ putative form of α4β1 depicted to the right in Figure 3, react highly with all three antibodies [30], adhere to fibronectin [67], and adhere even better to VCAM-1 compared to eosinophils [30]. Thus, both N29+/HUTS-21−/9EG7− and N29+/HUTS-21+/9EG7+ forms of α4β1 prefer VCAM-1 over fibronectin. Eosinophils that are purified from blood are more reactive to N29 compared to nonpurified eosinophils in whole blood from the same donor, indicating that β1 is susceptible to activation during the purification procedure [68]. In asthmatics with a dual response phenotype, N29 reactivity of unfractionated blood and bronchoalveolar lavage (BAL) eosinophils 48 hours after segmental antigen challenge is increased compared to blood eosinophils before challenge (our unpublished results). Moreover, N29 reactivity of BAL eosinophils correlates with the percentage of eosinophils in BAL fluid. These results indicate that the N29 immunoreactive form of β1 is likely important in movement of primed blood eosinophils out of the circulation and into the airway in response to segmental antigen challenge. Indeed, we speculate that VCAM-1 may not be recognized by the N29−/HUTS-21−/9EG7− putative form of α4β1 illustrated to the left in Figure 3.
Figure 3. Antibody probes of α4β1 conformation and affinity.
Schematic of three putative conformations of α4β1 that might be expressed by eosinophils as modeled on the crystal structures of αVβ3 and αIIbβ3 and adapted from Luo BH et al. [164]. The bent, closed form of α4β1 is presumably not recognized by any of the activation-sensitive antibodies (left). Extension of α4β1 reveals the epitope in the β1 PSI domain recognized by N29 - this conformation is presumed to most closely depict the conformation of α4β1 of blood or airway eosinophils (middle). Further activation results in swing-out of the hybrid domain and exposure of the epitope recognized by HUTS-21, along with separation of the integrin legs and exposure of the epitope recognized by 9EG7 (right). The latter form of α4β1 is presumably present on Jurkat, EoL-3, or Mn2+- or PMA-activated AML14.3D10 eosinophilic cells [30].
Based on the utility of N29 as a measure for activation of α4β1 in the antigen-challenge model, N29 positivity of blood eosinophils was assayed in the inhaled corticosteroid (ICS) withdrawal model of controlled elicitation of asthma. In the ICS model, mild asthmatics undergo true or sham ICS withdrawal in a randomized, placebo-controlled, two-period crossover study. ICS withdrawal results in a decrease in the forced expiratory volume in one second (FEV1) [69], increase in circulating and sputum eosinophils [69], and a higher percentage of VCAM-1-positive vessels in comparison to subjects before withdrawal [32]. We found that N29 epitope expression, which was quantified by flow cytometry on eosinophils in whole blood, correlated inversely with FEV1 both after ICS withdrawal and throughout the ICS study [68]. In response to ICS withdrawal, N29 epitope expression correlated with FENO (fraction of exhaled nitric oxide) [68], an established asthma marker and indicator of airway inflammation [70, 71]. Indeed, receiver-operator characteristic curve analysis [72] indicates that N29 reactivity is a better predictor of FEV1 < 95% of baseline than FENO or sputum eosinophils [68]. The ICS study may be the first demonstrated example in which clinical measurements of a disease are predicted by and/or correlate with the activation state of a particular integrin subunit expressed on a circulating cell type.
α4β1 recognition of VCAM-1 modules
The allosteric structural forms assumed by α4β1 of eosinophils in antigen challenge and ICS withdrawal models of asthma are important in the recognition of VCAM-1 modules and alternatively spliced VCAM-1 forms. Shown in Figure 2, the loop between β strands C and D in the first immunoglobulin (Ig)-like module of VCAM-1 is accessible on the protein surface and contains the core integrin-recognition sequence IDSPL [73–77]. A second IDSPL site is present in the CD loop in module 4 [74, 76]. Alternative splicing of mRNA encoding VCAM-1 generates two protein forms in humans: a variant consisting of seven Ig-like modules, 7d-VCAM-1, containing both putative integrin-binding sites in modules 1 and 4, and a variant containing six Ig-like modules, 6d-VCAM-1, that is missing the putative integrin-binding site in module 4 and contains only the site in module 1 [78]. Endothelial cells appear to express more 7d-VCAM-1 compared to 6d-VCAM-1 based on quantitation of mRNA from HUVEC cells treated with TNF-α [78]. The distance of module 1 from the endothelial surface may be expected to facilitate α4β1-mediated eosinophil capture from the circulation. That is to say, module 1 of 7d-VCAM-1 may extend 3.7 nanometers further from the endothelial surface than module 1 of 6d-VCAM-1 or 11.1 nanometers further than module 4 based on the length of individual Ig-like modules calculated from rotary shadowing electron microscopic images of recombinant soluble 7d-VCAM-1 adsorbed to mica [77]. The first two N-terminal modules of 7d-VCAM-1 can mediate both rolling and firm adhesion of blood eosinophils via α4β1 [27], indicating that this extension allows ready interaction with eosinophil α4β1. By studying a set of recombinant forms of VCAM-1, we have shown that α4β1 mediates robust adhesion of purified blood eosinophils to constructs containing module 1, including 6d-VCAM-1, 7d-VCAM-1, and the construct containing only modules 1–3, 1–3VCAM-1, whereas there is less adhesion mediated by α4β1 to the construct containing only modules 4–7, 4–7VCAM-1 [30]. Thus, purified blood eosinophils more readily recognize module 1 in comparison to module 4 of VCAM-1. While module 1 may promote the initial capture of eosinophils, the close proximity of module 4 to the endothelial wall may help strengthen adhesion and facilitate movement of activated blood eosinophils out of the circulation. Static adhesion to module 4 of eosinophils or eosinophilic cell lines can be enhanced following incubation with IL-5, Mn2+, or PMA [30]. That 7d-VCAM-1 is divalent may be of additional consequence for α4β1-mediated eosinophil diapedesis. In other words, it may be that module 1 and 4 of a single VCAM-1 molecule can be ligated simultaneously by two different α4β1 integrin molecules co-expressed on an individual eosinophil cell. The head of α4β1, if modeled after the crystal structure of αVβ3, has dimensions between 4.5–9.0 nanometers [66], smaller than the distance that bridges modules 1 and 4 [77]. Structural variations between modules 2 and 5 may differentially orient modules 1 and 4, respectively, for recognition by α4β1 of eosinophils. That is, while module 2 of VCAM-1 is disulfide-bonded in the crystal structure shown in Figure 2 [75], module 5 is unusual for Ig-like modules in lacking such a bond based on proteolysis studies with endoproteinase Glu-C [79]. Of note, EoL-3 and AML14.3D10 eosinophilic leukemic cell lines do not recognize module 4 of VCAM-1 even though these cells express more surface α4β1 and display a more conformationally active form of β1 that is better recognized by N29, HUTS-21, and 9EG7 [30]. Recognition of modules 1 and 4 by eosinophils is, therefore, controlled by more than α4β1 expression level and activation state alone. The β1 subunit of eosinophils but not of eosinophilic cell lines is in podosomes [52], which may partly explain the differences between the cell lines and eosinophils. Experiments described below indicate a role for αMβ2 as well in eosinophil recognition of VCAM-1 module 4.
αMβ2 (CD11b/18, Mac-1)
αMβ2 is present on purified blood eosinophils in a conformational state that is constitutively less active than α4β1. αMβ2 of unstimulated purified blood eosinophils mediates low levels of static adhesion to module 4 of 7d-VCAM-1 [30]. Baseline interaction is regulated by phosphoinositide-3 kinase (PI3K), in as much as adhesion to module 4 and not module 1 of 7d-VCAM-1 is blocked completely by wortmannin or LY294002 [30], both known to block αMβ2 integrin-mediated adhesion of eosinophils to ICAM-1 or albumin in response to IL-5 [80, 81]. That inhibitors specific for αMβ2 completely block adhesion to module 4 even though the recognition is partly α4β1-dependent [30], raises the possibility of integrin crosstalk and/or cooperativity between αMβ2 and α4β1. Further evidence that recognition of module 4 by αMβ2 is significant comes from the AML14.3D10 and EoL-3 eosinophilic cell lines, which do not express αMβ2 and do not adhere to module 4 [30].
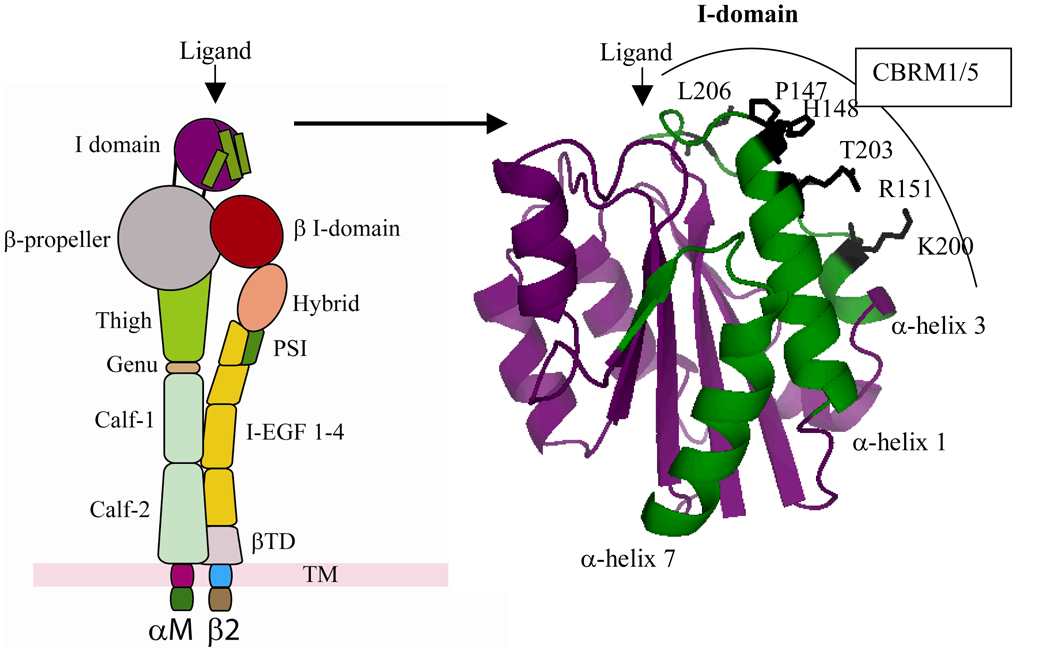
Whereas α4β1 of blood eosinophils constitutively ligates VCAM-1 even in the absence of stimulation by cytokines and irrespective of “inside-out” signaling, αMβ2 recognition is influenced greatly by activation. Incubation with IL-5 enhances αMβ2-mediated static adhesion of blood eosinophils to ICAM-1 or modules 1 or 4 of VCAM-1 [30, 80, 81]. The αMβ2-mediated adhesion of IL-5-stimulated eosinophils to modules 1 or 4 of VCAM-1 is mostly refractory to inhibition by wortmannin or LY294002 [30]. Adhesion of IL-5-stimulated eosinophils to ICAM-1 or albumin is, however, blocked by these inhibitors [80, 81]. Thus, recognition of diverse ligands by αMβ2 of eosinophils is differentially regulated. Indeed, under assay conditions that mimic blood flow, IL-5 incubation has the paradoxical effect of decreasing, rather than increasing, adhesion of blood eosinophils to 7d-VCAM-1 in the parallel plate flow chamber within minutes [30]. The decrease in adhesion is dependent on αMβ2 since it can be reversed by anti-αM antibody [30]. Such a finding is consistent with the observation that IL-5 promotes the release in vivo of eosinophils from bone marrow via a mechanism that is dependent on β2 integrins and PI3K and that can be inhibited by wortmannin [82]. The release of eosinophils may be facilitated further by L-selectin shedding from the surface of eosinophils in response to IL-5 stimulation [83]. These results suggest that the recognition of VCAM-1 by αMβ2 may be tightly controlled by IL-5. Namely, the IL-5-induced conformation of αMβ2 may transiently enhance and then inhibit eosinophil adhesion in a process that may depend on αMβ2 activation and be associated with L-selectin shedding. The consequence of such release would be to promote the subsequent movement of eosinophils through the vascular wall into the airway lumen. In support of this conjecture, we have shown that airway eosinophils purified following segmental antigen challenge express a conformationally active form of αMβ2 recognized by the CBRM1/5 anti-αM conformation-sensitive antibody [31]. Figure 4 depicts the epitope in the I (insert)-domain of the αM subunit recognized by CBRM1/5 [84]. Two different structures believed to represent the inactive or active form of the αM I-domain have been crystallized in buffers containing manganese or magnesium, respectively [85, 86]. Even though the CBRM1/5 epitope is exposed in the presumed inactive crystal form, the epitope is not well recognized by CBRM1/5 [84]. Thus, the I-domain of αM likely undergoes a change in shape or conformation upon activation that facilitates better recognition of the I-domain by CBRM1/5 [84, 87]. As such, the enhanced reactivity of CBRM1/5 of airway compared to blood eosinophils indicates that the αM I-domain of airway eosinophils is structurally different. Such airway eosinophils recognized by CBRM1/5 adhere to diverse ligands, including VCAM-1, albumin, ICAM-1, fibrinogen, and vitronectin via αMβ2 [31]. That is to say, a prominent characteristic of αMβ2 is its ability to recognize a plethora of structurally diverse ligands and extracellular matrix proteins through the Lys245-Arg261 segment of the αM I-domain [88]. Blood eosinophils purified before or after antigen challenge are not well recognized by CBRM1/5 and exhibit little or no adhesion to such ligands [31]. Intranasal administration of IL-5, a regulator of αMβ2, causes eosinophil migration into the airway lumen of mice [89]. This movement is blocked by intraperitoneal administration of the p85a dominant negative form of the PI3K regulatory subunit fused to human immunodeficiency virus-transactivator of transcription (HIV-TAT) [89]. αMβ2 expression is upregulated on human or mouse airway eosinophils following antigen challenge in comparison to blood eosinophils [90–93]. Elevation of αMβ2 has also been observed on migratory human blood eosinophils [48]. The upregulation of αMβ2 on eosinophils can be achieved following incubation of blood eosinophils with IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF), fMLP, or platelet activating factor (PAF) [91, 94]. Expression of αM on blood eosinophils is increased following incubation with calcium ionophore or after only minutes of phorbol 12-myristate 13-acetate (PMA) stimulation, indicating that blood eosinophils have preformed stores of αMβ2 that can be rapidly mobilized to the cell surface [21].
Figure 4. I-domain of αMβ2.
Schematic (left) of the I-domain of αMβ2 color coded to match the crystal structure (right) [86]. α-helices-1, 3, and -7 of the I-domain (shown in green) undergo conformational changes in response to αMβ2 activation [84]. Residues recognized by CBRM1/5 are labeled and highlighted in black. The figure was created with PyMol.
Transendothelial migration of eosinophils triggered by eotaxin is inhibited by anti-αM [48]. The chemokines RANTES, MCP-3, and complement (C)5a expose the CBRM1/5 activation epitope of αMβ2 [59, 95] and promote migration of blood eosinophils [59, 95–98]. Treatment with IL-5 exposes the CBRM1/5 epitope of blood eosinophils and mimics the phenotype of airway eosinophils by inducing αMβ2-mediated adhesion of blood eosinophils to ICAM-1, albumin, vitronectin, and fibrinogen [31, 99]. IL-5 is upregulated in the blood and airway of human asthmatics [100–103], and the IL-5 receptor is down-regulated on airway eosinophils compared to blood eosinophils, an indication that the IL-5 receptor on airway eosinophils has been occupied [104]. Degranulation of eosinophils stimulated by GM-CSF and PAF is mediated by αMβ2 [105]. Therefore, upregulation and activation of αMβ2 appears to represent a major and long-lasting consequence of exposure of eosinophils to mediators of eosinophilic inflammation.
αDβ2 (CD11d/18)
The αD and αM I-domains share 60% amino acid sequence identity [106]. As such, αDβ2 on eosinophils may be expected to resemble αMβ2 in the promiscuous recognition of several ligands. In αDβ2-transfected HEK293 human embryonic kidney or IC-21 macrophage cells, αDβ2 mediates adhesion to fibrinogen, vitronectin, VCAM-1, or fibronectin and migration on vitronectin [106]. In unstimulated or IL-5-treated purified blood eosinophils, αDβ2 has been reported to contribute to adhesion on VCAM-1 in static or flow conditions [21, 107]. Like αM, airway eosinophils express higher levels of αD in comparison to blood eosinophils [21, 31], and expression of αD on blood eosinophils is increased to a level similar to the expression on airway eosinophils following minutes of treatment with PMA, calcium ionophore, or three days of culture in IL-5 [21, 107]. Eosinophils that are purified from blood express elevated surface levels of αD compared to eosinophils in whole blood from the same donor indicating that upregulation of αDβ2, like activation of β1 integrins [68], is a consequence of eosinophil activation during purification [68]. Thus, as with αMβ2, eosinophils appear to have preformed stores of αDβ2 that can be rapidly mobilized. We have found that αD stains diffusely in unstimulated blood eosinophils adherent to VCAM-1 but is present in structures in the substrate plane in airway eosinophils or PMA-activated blood eosinophils adherent to VCAM-1 [52]. These αD-positive structures do not co-localize with podosomes, raising the possibility that αDβ2 recognizes VCAM-1 independent of recognition by α4β1. We have not observed αD-dependent adhesion of unstimulated or IL-5-stimulated purified blood eosinophils to VCAM-1 using anti-αD antibodies but do not exclude the possibility that αD, like αM, contributes to eosinophil recognition of VCAM-1 [30].
α4β7 (CD49d/β7)
α4β7 of blood eosinophils supports static adhesion on mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and mediates rolling on MAdCAM-1 and VCAM-1 under flow [27]. Incubation with PAF induces elevated static adhesion of eosinophils to MAdCAM-1 via α4β7 [29]. Crosslinking of β7 on blood eosinophils by soluble VCAM-1 or Fib 30 antibody increases GM-CSF mRNA expression and survival of blood eosinophils, indicating that α4β7 is a regulator of eosinophil survival [108]. The increased survival is blocked by anti-GM-CSF, but not by antibodies to IL-3 or IL-5, pointing to GM-CSF synthesis as the likely target by which ligation of α4β7 increases viability [108].
α6β1 (CD49f/29)
α6β1 is a well-known receptor for laminins. α6β1 supports adhesion of eosinophils purified from allergic donors to human laminin obtained from pepsin digestion of placenta, while eosinophils purified from nonallergic donors adhere less well [23]. We did not find specific adhesion of blood eosinophils purified from normal, allergic, or asthmatic subjects or of airway eosinophils to mouse laminin-1, raising the question of if, when, and/or how eosinophils are able to recognize laminin [31]. Eosinophil migration through Matrigel, a basement membrane mix that contains laminin-1, is reduced in response to anti-β1 [109].
αLβ2 (CD11a) and αXβ2 (CD11c/18)
αLβ2 is mostly selective for ICAMs and has a narrower ligand-binding specificity than αMβ2 or αDβ2 [110]. In vitro transendothelial chemotaxis of eosinophils to eotaxin is partly inhibited by anti-αL [48]. αXβ2 shares ligands with αMβ2 [111]. There is little, if any, known about the role of αXβ2 on eosinophils, making αXβ2 the most enigmatic of integrin heterodimers expressed by eosinophils. We have found no inhibition of blood or airway eosinophil adhesion to ICAM-1, albumin, or fibrinogen by antibodies to αLβ2 or αXβ2, indicating that these two integrins are inactive on eosinophils for these particular ligands compared to αMβ2 [31].
SUMMARY OF EOSINOPHIL INTEGRINS
The unique set of integrin heterodimers expressed by eosinophils mediates diverse functions of eosinophil rolling, firm adhesion, migration, respiratory burst, degranulation, and viability. In asthma, these functions are important in orchestrating interactions of eosinophils with ligands expressed by airway cells. α4β1 and αMβ2 are likely the two most important integrins that mediate eosinophil adhesion and movement. These two integrin heterodimers differ in several respects. α4β1 of unstimulated blood eosinophils mediates rolling along VCAM-1-expressing airway endothelium and constitutively ligates modules 1 and 4 of VCAM-1, whereas αMβ2 is constitutively less active and primarily recognizes only module 4 of VCAM-1. α4β1 in the blood and αMβ2 en route to or within the airway can undergo activation. We propose that α4β1 and αMβ2 may cooperate to mediate adhesion and release from luminal ligands like VCAM-1, followed by adhesion to and movement on diverse pericellular endothelial ligands mediated by activated αMβ2. Specifically, α4β1 expressed on circulating eosinophils may undergo activation, i.e., in response to allergen, allowing α4β1 to better recognize module 1 of 7d-VCAM-1 or 6d-VCAM-1. Following eosinophil capture on endothelium, both α4β1 and αMβ2 co-expressed on the eosinophil may compete for binding of module 4 of the aforementioned 7d-VCAM-1 molecule to facilitate firm adhesion. Subsequent activation of αMβ2 by IL-5 or chemokines may cause αMβ2 to assume a dominant role in recognition of module 4 and a significant role in recognition of module 1. Such recognition may involve a transient increase in adhesion to VCAM-1 mediated by αMβ2, followed by release from VCAM-1 of αMβ2 within minutes under flow in the presence of IL-5 and release of α4β1 due to possible proteolysis in podosomes [52]. The consequence of such release may be to promote αMβ2-mediated movement of eosinophils on diverse endothelial ligands in transit to the airway. In essence, movement through airway endothelium may, therefore, involve a “hand-off” whereby αMβ2 replaces α4β1 as the principal adhesive and migratory integrin. The “hand-off” may be facilitated by IL-5 and/or chemokines including members of the eotaxin family, which can shift integrin usage away from α4β1 to β2 integrins [112]. In fact, IL-5 can even inhibit binding of the 15/7 anti-β1 conformation-sensitive antibody to eosinophils from allergic donors [58] and, as noted, induce L-selectin shedding and release from endothelial selectins. 6d-VCAM-1 with one integrin recognition module, and 7d-VCAM-1 with two, may regulate differentially such recruitment by virtue of valency of the splice form, distance of the integrin binding site from the endothelial surface, and/or role of module 4. Activation of αMβ2 and diapedesis to the pulmonary vasculature may be facilitated further by degradation of matrix proteins by metalloproteinases in podosomes, transient adhesive structures of IL-5- and TNFα-activated blood eosinophils or airway eosinophils.
The remaining five integrins of eosinophils – αDβ2, α4β7, α6β1, αLβ2, and αXβ2 – likely play supportive roles to α4β1 and αMβ2 in eosinophil recruitment. αDβ2 and αXβ2 of activated eosinophils potentially recognize VCAM-1 and additional ligands similar to those recognized by αMβ2. The enhanced survival of eosinophils in the airway lumen may involve ligation of α4β7 to soluble adhesive ligands such as fibronectin. α6β1 of migrating eosinophils may interact with laminin in transit through vascular basement membrane, while αLβ2 potentially ligates ICAMs. The functions of αDβ2, α4β7, α6β1, αLβ2, and αXβ2 of eosinophils, however, warrant further exploration.
TARGETING EOSINOPHIL INTEGRINS IN VIVO
Several competitive inhibitors that target integrin heterodimers have been devised to interfere with the recruitment or number of eosinophils in the asthmatic airway, and thus, diminish airway eosinophilic inflammation and particular aspects of asthma, to which eosinophils are thought to contribute [113]. These therapies have been evaluated in clinical human trials and diverse animal models including primate, guinea pig, sheep, rabbit, mouse, and rat. Enigmatically, as elaborated below, the studies in humans and in animal models have reached different conclusions.
Most therapeutic strategies involving integrins of eosinophils have targeted α4, the common subunit of α4β1, the most important counter-receptor of eosinophils recognizing VCAM-1, and α4β7, potentially important in recognition of MAdCAM-1. The utility of α4 subunit antagonists as therapeutic agents in clinical disease was first realized with the development of Antegren (Tysabri, Natalizumab) a humanized anti-α4 antibody that has shown effectiveness in clinical trials for multiple sclerosis and Crohn’s disease [114]. In parallel, a number of small molecule inhibitors including BIO-1211 (compound 28) have been developed. BIO-1211 has a 200-fold greater selectivity for the active compared to inactive form of α4β1 and is based on the LDV sequence from the alternatively spliced connecting segment-1 (CS-1) peptide of cellular fibronectin [115, 116]. Despite promising results in animal models of asthma, development of BIO-1211 was discontinued due to lack of efficacy in phase II clinical trials of asthma conducted by Merck and Biogen [117]. In preliminary studies, a second LDV mimetic modeled after BIO-1211, IVL745, caused only a modest reduction in sputum eosinophils in human patients with mild-to-moderate atopic asthma following inhalation and had no effect on the early or late asthmatic response to inhaled allergen or markers of airway inflammation [118]. The futures of IVL745 and another α4β1 antagonist, compound HMR 1031, under investigation by Sanofi-Aventis in phase II trials of asthma, are unclear [117, 119]. HMR 1031 is a potent α4β1 small molecule antagonist that selectively blocks binding of α4β1 to VCAM-1 and fibronectin [120]. When administered by aerosol for 8 days to patients with mild-to-moderate persistent asthma, HMR 1031 failed to relieve house dust mite- or methacholine- induced airway eosinophilia, exhaled nitric oxide production, or soluble markers of airway hyperresponsiveness [121]. Two orally active dual α4β1/α4β7 antagonists, TR14035 and R411, were examined in clinical trials by Tanabe/GlaxoSmithKline and Roche, respectively [117]. TR14035 is no longer listed in GlaxoSmithKline’s therapeutic pipeline [117], R411 was discontinued as noted on Roche’s 2006 annual investor report [122], and several other α4β1 antagonists, including GW-559090 from GlaxoSmithKline [123, 124] or RBx-7796 (Clafrinast) from Ranbaxy [125], are no longer listed in either company’s pipeline.
The relative failure of α4 antagonists in humans is puzzling given the effectiveness of these compounds in animal models of asthma. Thus, while described above, BIO-1211, HMR 1031, IVL745, TR14035, or GW559090X α4β1 antagonists have negligible benefit in human asthma clinical trials, these compounds have had significant effects in sheep [118, 121, 126], mouse [121, 127], rat [118, 123, 128], or guinea pig [123] models of asthma. The HP1/2, PS2/3, PS/2, TA-2, or Max-68P anti-α4 blocking antibodies, CS-1 peptide ligand anti-α4β1 mimic, or α4β1 small molecule inhibitors reduce either numbers of eosinophils in the airway or ameliorate inflammatory histopathology or airway allergic responses in the guinea pig [129–133], sheep [134–137], mouse [138–140], rat [141, 142], or rabbit [143]. A complication of such studies is the failure to differentiate between effects resulting from eosinophils, roles of integrins of eosinophils, or roles of other cell types [144–147]. Of additional consequence, there are reports of cellular movement independent of β1 or β2-integrins in a model of T cell migration within a 3D-collagen gel [148] or by granulocytes null for β2 in response to fMLP [149], suggesting that movement of eosinophils or leukocytes in disease may involve processes other than, or in addition to, integrins. In any case, more informative models of inflammation that are better able to define the specific role of integrins of eosinophils in asthma and disease may result in improved therapeutic outcome.
Approaches that target integrins in eosinophil-associated diseases including asthma likely can be significantly improved. One problem in the development of such inhibitors may be the functional overlap and redundancy of integrins. In other words, α4β1, αLβ2, and αMβ2 may be able to compensate for one another to some extent in vivo [150–152]. Thus, simultaneous targeting of several integrins may be a potentially more advantageous strategy than antagonizing only one integrin molecule. Indeed, the success of corticosteroids or β2-adrenergic receptor agonists (bronchodilators) in dampening airway inflammation could be said to result from such broad activity on several inflammatory targets at once [119]. Nonetheless, morbidity and mortality continue to increase in eosinophil-related diseases including asthma despite the availability of corticosteroids formulated for delivery to the lung [153]. Clearly, there is a need for additional therapies that inhibit eosinophilic inflammation. Therapies that broadly target integrins would offer the possibility of potently suppressing eosinophil-related pathologies with greater specificity and with lesser side effects compared to current treatments. Such therapy could target recruitment mechanisms involving both α4 and β2 integrins. This strategy may prove efficacious against neutrophils as well as eosinophils and be of use in subjects with severe or persistent asthma with predominantly neutrophilic inflammation [154–156]. As noted previously, β2 integrins of human eosinophils recognize VCAM-1 and numerous matrix proteins of the pulmonary vasculature, mediate adhesion and movement of eosinophils, and are regulated by PI3K, a target of therapeutic value in the murine asthma model [89]. Movement of human eosinophils is antagonized by anti-β2 [54, 157–160]. In fact, such migration is inhibited even more effectively with a combination of anti-α4 and anti-β2 than either alone [160]. Several models of allergic inflammation in animals indicate that β2 integrins may be promising therapeutic targets in future treatment regimens of asthma [142, 143]. A major worry is immunocompetency arising from antagonism of β2 integrins. An example is efalizumab (Raptiva®, Genentech), a humanized anti-αL monoclonal antibody that has been approved for the treatment of psoriasis [161]. Efalizumab has shown moderate efficacy in reducing accumulation of eosinophils in the airway and in attenuating the late asthmatic response of human subjects with atopic asthma [162]. Efalizumab is immunosuppressive and may, therefore, be contra-indicated in patients with infection, who are already immunocompromised, or who are pregnant [161]. One strategy to improve integrin antagonists like efalizumab may be to develop inhibitors that bind only certain integrin activation states. Targeting specific structural conformers of integrins, in turn, may block only certain integrin-ligand interactions and minimize side effects.
REFERENCES
- 1.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- 2.Adamko DJ, Odemuyiwa SO, Vethanayagam D, Moqbel R. The rise of the phoenix: the expanding role of the eosinophil in health and disease. Allergy. 2005;60:13–22. doi: 10.1111/j.1398-9995.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth AE, Vadas MA, Wassom DL, Dessein A, Hogan M, Sherry B, Gleich GJ, David JR. Interactions between human eosinophils and schistosomula of Schistosoma mansoni. II. The mechanism of irreversible eosinophil adherence. J Exp Med. 1979;150:1456–1471. doi: 10.1084/jem.150.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterworth AE, David JR. Eosinophil function. N Engl J Med. 1981;304:154–156. doi: 10.1056/NEJM198101153040305. [DOI] [PubMed] [Google Scholar]
- 5.Filley WV, Holley KE, Kephart GM, Gleich GJ. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet. 1982;2:11–16. doi: 10.1016/s0140-6736(82)91152-7. [DOI] [PubMed] [Google Scholar]
- 6.Frigas E, Gleich GJ. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986;77:527–537. doi: 10.1016/0091-6749(86)90341-6. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw AJ, Moqbel R, Kay AB. Eosinophils: biology and role in disease. Adv Immunol. 1995;60:151–266. doi: 10.1016/s0065-2776(08)60586-6. [DOI] [PubMed] [Google Scholar]
- 8.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 9.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 10.Busse W, Banks-Schlegel S, Noel P, Ortega H, Taggart V, Elias J. Future research directions in asthma: an NHLBI Working Group report. Am J Respir Crit Care Med. 2004;170:683–690. doi: 10.1164/rccm.200311-1539WS. [DOI] [PubMed] [Google Scholar]
- 11.Woolcock AJ, Peat JK. Evidence for the increase in asthma worldwide. Ciba Found Symp. 1997;206:122–134. doi: 10.1002/9780470515334.ch8. discussion 134-9, 157-9. [DOI] [PubMed] [Google Scholar]
- 12.Meza C, Gershwin ME. Why is asthma becoming more of a problem? Curr Opin Pulm Med. 1997;3:6–9. doi: 10.1097/00063198-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Picado C. Early and late-phase asthmatic reactions: a hypothesis. Allergy. 1992;47:331–333. doi: 10.1111/j.1398-9995.1992.tb02064.x. [DOI] [PubMed] [Google Scholar]
- 14.Pawankar R, Yamagishi S, Takizawa R, Yagi T. Mast cell-IgE-and mast cell-structural cell interactions in allergic airway disease. Curr Drug Targets Inflamm Allergy. 2003;2:303–312. doi: 10.2174/1568010033484016. [DOI] [PubMed] [Google Scholar]
- 15.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 16.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–641. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 17.Bochner BS. Adhesion molecules as therapeutic targets. Immunol Allergy Clin North Am. 2004;24:615–630. doi: 10.1016/j.iac.2004.06.003. vi. [DOI] [PubMed] [Google Scholar]
- 18.Wardlaw AJ. Eosinophil trafficking in asthma. Clin Med. 2001;1:214–218. doi: 10.7861/clinmedicine.1-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol Rev. 2001;179:163–172. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 20.Doerschuk CM. Leukocyte trafficking in alveoli and airway passages. Respir Res. 2000;1:136–140. doi: 10.1186/rr24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grayson MH, Van der Vieren M, Sterbinsky SA, Michael Gallatin W, Hoffman PA, Staunton DE, Bochner BS. alphadbeta2 integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1) J Exp Med. 1998;188:2187–2191. doi: 10.1084/jem.188.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tachimoto H, Bochner BS. The surface phenotype of human eosinophils. Chem Immunol. 2000;76:45–62. doi: 10.1159/000058780. [DOI] [PubMed] [Google Scholar]
- 23.Georas SN, McIntyre BW, Ebisawa M, Bednarczyk JL, Sterbinsky SA, Schleimer RP, Bochner BS. Expression of a functional laminin receptor (alpha 6 beta 1, very late activation antigen-6) on human eosinophils. Blood. 1993;82:2872–2879. [PubMed] [Google Scholar]
- 24.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humphries MJ. Monoclonal antibodies as probes of integrin priming and activation. Biochem Soc Trans. 2004;32:407–411. doi: 10.1042/BST0320407. [DOI] [PubMed] [Google Scholar]
- 26.Sriramarao P, von Andrian UH, Butcher EC, Bourdon MA, Broide DH. L-selectin and very late antigen-4 integrin promote eosinophil rolling at physiological shear rates in vivo. J Immunol. 1994;153:4238–4246. [PubMed] [Google Scholar]
- 27.Sriramarao P, DiScipio RG, Cobb RR, Cybulsky M, Stachnick G, Castaneda D, Elices M, Broide DH. VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling under conditions of shear flow. Blood. 2000;95:592–601. [PubMed] [Google Scholar]
- 28.Matsumoto K, Sterbinsky SA, Bickel CA, Zhou DF, Kovach NL, Bochner BS. Regulation of alpha 4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1. J Allergy Clin Immunol. 1997;99:648–656. doi: 10.1016/s0091-6749(97)70027-7. [DOI] [PubMed] [Google Scholar]
- 29.Walsh GM, Symon FA, Lazarovils AL, Wardlaw AJ. Integrin alpha 4 beta 7 mediates human eosinophil interaction with MAdCAM-1, VCAM-1 and fibronectin. Immunology. 1996;89:112–119. doi: 10.1046/j.1365-2567.1996.d01-713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barthel SR, Annis DS, Mosher DF, Johansson MW. Differential engagement of modules 1 and 4 of vascular cell adhesion molecule-1 (CD106) by integrins alpha4beta1 (CD49d/29) and alphaMbeta2 (CD11b/18) of eosinophils. J Biol Chem. 2006;281:32175–32187. doi: 10.1074/jbc.M600943200. [DOI] [PubMed] [Google Scholar]
- 31.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol. 2006;35:378–386. doi: 10.1165/rcmb.2006-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ten Hacken NH, Postma DS, Bosma F, Drok G, Rutgers B, Kraan J, Timens W. Vascular adhesion molecules in nocturnal asthma: a possible role for VCAM-1 in ongoing airway wall inflammation. Clin Exp Allergy. 1998;28:1518–1525. doi: 10.1046/j.1365-2222.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- 33.Masinovsky B, Urdal D, Gallatin WM. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990;145:2886–2895. [PubMed] [Google Scholar]
- 34.Weller PF, Rand TH, Goelz SE, Chi-Rosso G, Lobb RR. Human eosinophil adherence to vascular endothelium mediated by binding to vascular cell adhesion molecule 1 and endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88:7430–7433. doi: 10.1073/pnas.88.16.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minami T, Abid MR, Zhang J, King G, Kodama T, Aird WC. Thrombin stimulation of vascular adhesion molecule-1 in endothelial cells is mediated by protein kinase C (PKC)-delta-NF-kappa B and PKC-zeta-GATA signaling pathways. J Biol Chem. 2003;278:6976–6984. doi: 10.1074/jbc.M208974200. [DOI] [PubMed] [Google Scholar]
- 36.Carluccio MA, Ancora MA, Massaro M, Carluccio M, Scoditti E, Distante A, Storelli C, De Caterina R. Homocysteine induces VCAM-1 gene expression through NF-{kappa}B and NAD(P)H oxidase activation - protective role of Mediterranean diet polyphenolic antioxidants. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00432.2007. [DOI] [PubMed] [Google Scholar]
- 37.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 38.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25:1401–1407. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 39.Amin MA, Haas CS, Zhu K, Mansfield PJ, Kim MJ, Lackowski NP, Koch AE. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood. 2006;107:2252–2261. doi: 10.1182/blood-2005-05-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JG, Mahmud SA, Nguyen J, Slungaard A. Thiocyanate-dependent induction of endothelial cell adhesion molecule expression by phagocyte peroxidases: a novel HOSCN-specific oxidant mechanism to amplify inflammation. J Immunol. 2006;177:8714–8722. doi: 10.4049/jimmunol.177.12.8714. [DOI] [PubMed] [Google Scholar]
- 41.Bochner BS, Luscinskas FW, Gimbrone MA, Jr, Newman W, Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirveskari J, Bono P, Granfors K, Leirisalo-Repo M, Jalkanen S, Salmi M. Expression of alpha4-integrins on human neutrophils. J Leukoc Biol. 2000;68:243–250. [PubMed] [Google Scholar]
- 44.Ohkawara Y, Yamauchi K, Maruyama N, Hoshi H, Ohno I, Honma M, Tanno Y, Tamura G, Shirato K, Ohtani H. In situ expression of the cell adhesion molecules in bronchial tissues from asthmatics with air flow limitation: in vivo evidence of VCAM-1/VLA-4 interaction in selective eosinophil infiltration. Am J Respir Cell Mol Biol. 1995;12:4–12. doi: 10.1165/ajrcmb.12.1.7529029. [DOI] [PubMed] [Google Scholar]
- 45.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto H, Nagata M, Sakamoto Y. CC chemokines and transmigration of eosinophils in the presence of vascular cell adhesion molecule 1. Ann Allergy Asthma Immunol. 2005;94:292–300. doi: 10.1016/S1081-1206(10)61311-7. [DOI] [PubMed] [Google Scholar]
- 47.Nagata M, Yamamoto H, Tabe K, Sakamoto Y. Eosinophil transmigration across VCAM-1-expressing endothelial cells is upregulated by antigen-stimulated mononuclear cells. Int Arch Allergy Immunol. 2001;125 Suppl 1:7–11. doi: 10.1159/000053844. [DOI] [PubMed] [Google Scholar]
- 48.Jia GQ, Gonzalo JA, Hidalgo A, Wagner D, Cybulsky M, Gutierrez-Ramos JC. Selective eosinophil transendothelial migration triggered by eotaxin via modulation of Mac-1/ICAM-1 and VLA-4/VCAM-1 interactions. Int Immunol. 1999;11:1–10. doi: 10.1093/intimm/11.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Nagata M, Sedgwick JB, Kita H, Busse WW. Granulocyte macrophage colony-stimulating factor augments ICAM-1 and VCAM-1 activation of eosinophil function. Am J Respir Cell Mol Biol. 1998;19:158–166. doi: 10.1165/ajrcmb.19.1.3001. [DOI] [PubMed] [Google Scholar]
- 50.Sedgwick JB, Jansen KJ, Kennedy JD, Kita H, Busse WW. Effects of the very late adhesion molecule 4 antagonist WAY103 on human peripheral blood eosinophil vascular cell adhesion molecule 1-dependent functions. J Allergy Clin Immunol. 2005;116:812–819. doi: 10.1016/j.jaci.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Nagata M, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J Immunol. 1995;155:2194–2202. [PubMed] [Google Scholar]
- 52.Johansson MW, Lye MH, Barthel SR, Duffy AK, Annis DS, Mosher DF. Eosinophils adhere to vascular cell adhesion molecule-1 via podosomes. Am J Respir Cell Mol Biol. 2004;31:413–422. doi: 10.1165/rcmb.2004-0099OC. [DOI] [PubMed] [Google Scholar]
- 53.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 54.Ebisawa M, Liu MC, Yamada T, Kato M, Lichtenstein LM, Bochner BS, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. II. Potentiation of eosinophil transendothelial migration by eosinophil-active cytokines. J Immunol. 1994;152:4590–4596. [PubMed] [Google Scholar]
- 55.Holub A, Byrnes J, Anderson S, Dzaidzio L, Hogg N, Huttenlocher A. Ligand density modulates eosinophil signaling and migration. J Leukoc Biol. 2003;73:657–664. doi: 10.1189/jlb.0502264. [DOI] [PubMed] [Google Scholar]
- 56.Kuijpers TW, Mul EP, Blom M, Kovach NL, Gaeta FC, Tollefson V, Elices MJ, Harlan JM. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J Exp Med. 1993;178:279–284. doi: 10.1084/jem.178.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puzon-McLaughlin W, Yednock TA, Takada Y. Regulation of conformation and ligand binding function of integrin alpha5beta1 by the beta1 cytoplasmic domain. J Biol Chem. 1996;271:16580–16585. doi: 10.1074/jbc.271.28.16580. [DOI] [PubMed] [Google Scholar]
- 58.Werfel SJ, Yednock TA, Matsumoto K, Sterbinsky SA, Schleimer RP, Bochner BS. Functional regulation of beta 1 integrins on human eosinophils by divalent cations and cytokines. Am J Respir Cell Mol Biol. 1996;14:44–52. doi: 10.1165/ajrcmb.14.1.8534485. [DOI] [PubMed] [Google Scholar]
- 59.Weber C, Kitayama J, Springer TA. Differential regulation of beta 1 and beta 2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci U S A. 1996;93:10939–10944. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chigaev A, Blenc AM, Braaten JV, Kumaraswamy N, Kepley CL, Andrews RP, Oliver JM, Edwards BS, Prossnitz ER, Larson RS, Sklar LA. Real time analysis of the affinity regulation of alpha 4-integrin. The physiologically activated receptor is intermediate in affinity between resting and Mn(2+) or antibody activation. J Biol Chem. 2001;276:48670–48678. doi: 10.1074/jbc.M103194200. [DOI] [PubMed] [Google Scholar]
- 61.Mould AP, Travis MA, Barton SJ, Hamilton JA, Askari JA, Craig SE, Macdonald PR, Kammerer RA, Buckley PA, Humphries MJ. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J Biol Chem. 2005;280:4238–4246. doi: 10.1074/jbc.M412240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 63.Ni H, Li A, Simonsen N, Wilkins JA. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain. J Biol Chem. 1998;273:7981–7987. doi: 10.1074/jbc.273.14.7981. [DOI] [PubMed] [Google Scholar]
- 64.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci U S A. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 66.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilkins JA, Stupack D, Stewart S, Caixia S. Beta 1 integrin-mediated lymphocyte adherence to extracellular matrix is enhanced by phorbol ester treatment. Eur J Immunol. 1991;21:517–522. doi: 10.1002/eji.1830210239. [DOI] [PubMed] [Google Scholar]
- 68.Johansson MW, Barthel SR, Swenson CA, Evans MD, Jarjour NN, Mosher DF, Busse WW. Eosinophil beta 1 integrin activation state correlates with asthma activity in a blind study of inhaled corticosteroid withdrawal. J Allergy Clin Immunol. 2006;117:1502–1504. doi: 10.1016/j.jaci.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 69.Gibson PG, Wong BJ, Hepperle MJ, Kline PA, Girgis-Gabardo A, Guyatt G, Dolovich J, Denburg JA, Ramsdale EH, Hargreave FE. A research method to induce and examine a mild exacerbation of asthma by withdrawal of inhaled corticosteroid. Clin Exp Allergy. 1992;22:525–532. doi: 10.1111/j.1365-2222.1992.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 70.Wenzel SE, Covar R. Update in asthma 2005. Am J Respir Crit Care Med. 2006;173:698–706. doi: 10.1164/rccm.2601007. [DOI] [PubMed] [Google Scholar]
- 71.Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005;115:953–959. doi: 10.1016/j.jaci.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 72.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 73.Chiu HH, Crowe DT, Renz ME, Presta LG, Jones S, Weissman IL, Fong S. Similar but nonidentical amino acid residues on vascular cell adhesion molecule-1 are involved in the interaction with alpha 4 beta 1 and alpha 4 beta 7 under different activity states. J Immunol. 1995;155:5257–5267. [PubMed] [Google Scholar]
- 74.Vonderheide RH, Tedder TF, Springer TA, Staunton DE. Residues within a conserved amino acid motif of domains 1 and 4 of VCAM-1 are required for binding to VLA-4. J Cell Biol. 1994;125:215–222. doi: 10.1083/jcb.125.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor P, Bilsland M, Walkinshaw MD. A new conformation of the integrin-binding fragment of human VCAM-1 crystallizes in a highly hydrated packing arrangement. Acta Crystallogr D Biol Crystallogr. 2001;57:1579–1583. doi: 10.1107/s0907444901011209. [DOI] [PubMed] [Google Scholar]
- 76.Clements JM, Newham P, Shepherd M, Gilbert R, Dudgeon TJ, Needham LA, Edwards RM, Berry L, Brass A, Humphries MJ. Identification of a key integrin-binding sequence in VCAM-1 homologous to the LDV active site in fibronectin. J Cell Sci. 1994;107(Pt 8):2127–2135. doi: 10.1242/jcs.107.8.2127. [DOI] [PubMed] [Google Scholar]
- 77.Osborn L, Vassallo C, Browning BG, Tizard R, Haskard DO, Benjamin CD, Dougas I, Kirchhausen T. Arrangement of domains, and amino acid residues required for binding of vascular cell adhesion molecule-1 to its counter-receptor VLA-4 (alpha 4 beta 1) J Cell Biol. 1994;124:601–608. doi: 10.1083/jcb.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hession C, Tizard R, Vassallo C, Schiffer SB, Goff D, Moy P, Chi-Rosso G, Luhowskyj S, Lobb R, Osborn L. Cloning of an alternate form of vascular cell adhesion molecule-1 (VCAM1) J Biol Chem. 1991;266:6682–6685. [PubMed] [Google Scholar]
- 79.Pepinsky B, Hession C, Chen LL, Moy P, Burkly L, Jakubowski A, Chow EP, Benjamin C, Chi-Rosso G, Luhowskyj S, et al. Structure/function studies on vascular cell adhesion molecule-1. J Biol Chem. 1992;267:17820–17826. [PubMed] [Google Scholar]
- 80.Zhu X, Subbaraman R, Sano H, Jacobs B, Sano A, Boetticher E, Munoz NM, Leff AR. A surrogate method for assessment of beta(2)-integrin-dependent adhesion of human eosinophils to ICAM-1. J Immunol Methods. 2000;240:157–164. doi: 10.1016/s0022-1759(00)00192-7. [DOI] [PubMed] [Google Scholar]
- 81.Sano M, Leff AR, Myou S, Boetticher E, Meliton AY, Learoyd J, Lambertino AT, Munoz NM, Zhu X. Regulation of interleukin-5-induced beta2-integrin adhesion of human eosinophils by phosphoinositide 3-kinase. Am J Respir Cell Mol Biol. 2005;33:65–70. doi: 10.1165/rcmb.2005-0076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM. Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin 5: the role of specific adhesion molecules and phosphatidylinositol 3-kinase. J Exp Med. 1998;188:1621–1632. doi: 10.1084/jem.188.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neeley SP, Hamann KJ, White SR, Baranowski SL, Burch RA, Leff AR. Selective regulation of expression of surface adhesion molecules Mac-1, L-selectin, and VLA-4 on human eosinophils and neutrophils. Am J Respir Cell Mol Biol. 1993;8:633–639. doi: 10.1165/ajrcmb/8.6.633. [DOI] [PubMed] [Google Scholar]
- 84.Oxvig C, Lu C, Springer TA. Conformational changes in tertiary structure near the ligand binding site of an integrin I domain. Proc Natl Acad Sci U S A. 1999;96:2215–2220. doi: 10.1073/pnas.96.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee JO, Bankston LA, Arnaout MA, Liddington RC. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995;3:1333–1340. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 86.Lee JO, Rieu P, Arnaout MA, Liddington R. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- 87.Liddington R, Bankston L. The integrin I domain: crystals, metals and related artefacts. Structure. 1998;6:937–938. doi: 10.1016/s0969-2126(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 88.Yakubenko VP, Lishko VK, Lam SC, Ugarova TP. A molecular basis for integrin alphaMbeta 2 ligand binding promiscuity. J Biol Chem. 2002;277:48635–48642. doi: 10.1074/jbc.M208877200. [DOI] [PubMed] [Google Scholar]
- 89.Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, Liu J, Munoz NM, Zhu X. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med. 2003;198:1573–1582. doi: 10.1084/jem.20030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- 91.Kroegel C, Liu MC, Hubbard WC, Lichtenstein LM, Bochner BS. Blood and bronchoalveolar eosinophils in allergic subjects after segmental antigen challenge: surface phenotype, density heterogeneity, and prostanoid production. J Allergy Clin Immunol. 1994;93:725–734. doi: 10.1016/0091-6749(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 92.Georas SN, Liu MC, Newman W, Beall LD, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial cell activation accompany the recruitment of human granulocytes to the lung after segmental antigen challenge. Am J Respir Cell Mol Biol. 1992;7:261–269. doi: 10.1165/ajrcmb/7.3.261. [DOI] [PubMed] [Google Scholar]
- 93.Hisada T, Hellewell PG, Teixeira MM, Malm MG, Salmon M, Huang TJ, Chung KF. alpha4 integrin-dependent eotaxin induction of bronchial hyperresponsiveness and eosinophil migration in interleukin-5 transgenic mice. Am J Respir Cell Mol Biol. 1999;20:992–1000. doi: 10.1165/ajrcmb.20.5.3473. [DOI] [PubMed] [Google Scholar]
- 94.Sedgwick JB, Quan SF, Calhoun WJ, Busse WW. Effect of interleukin-5 and granulocyte-macrophage colony stimulating factor on in vitro eosinophil function: comparison with airway eosinophils. J Allergy Clin Immunol. 1995;96:375–385. doi: 10.1016/s0091-6749(95)70057-9. [DOI] [PubMed] [Google Scholar]
- 95.Ulfman LH, Alblas J, van Aalst CW, Zwaginga JJ, Koenderman L. Differences in Potency of CXC Chemokine Ligand 8-, CC Chemokine Ligand 11-, and C5a-Induced Modulation of Integrin Function on Human Eosinophils. J Immunol. 2005;175:6092–6099. doi: 10.4049/jimmunol.175.9.6092. [DOI] [PubMed] [Google Scholar]
- 96.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, Minty A, Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med. 1994;179:751–756. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127–133. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 99.Zhu X, Munoz NM, Kim KP, Sano H, Cho W, Leff AR. Cytosolic phospholipase A2 activation is essential for beta 1 and beta 2 integrin-dependent adhesion of human eosinophils. J Immunol. 1999;163:3423–3429. [PubMed] [Google Scholar]
- 100.Lalani T, Simmons RK, Ahmed AR. Biology of IL-5 in health and disease. Ann Allergy Asthma Immunol. 1999;82:317–332. doi: 10.1016/S1081-1206(10)63281-4. quiz 332-3. [DOI] [PubMed] [Google Scholar]
- 101.Alexander AG, Barkans J, Moqbel R, Barnes NC, Kay AB, Corrigan CJ. Serum interleukin 5 concentrations in atopic and non-atopic patients with glucocorticoid-dependent chronic severe asthma. Thorax. 1994;49:1231–1233. doi: 10.1136/thx.49.12.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corrigan CJ, Haczku A, Gemou-Engesaeth V, Doi S, Kikuchi Y, Takatsu K, Durham SR, Kay AB. CD4 T-lymphocyte activation in asthma is accompanied by increased serum concentrations of interleukin-5. Effect of glucocorticoid therapy. Am Rev Respir Dis. 1993;147:540–547. doi: 10.1164/ajrccm/147.3.540. [DOI] [PubMed] [Google Scholar]
- 103.Kotsimbos AT, Hamid Q. IL-5 and IL-5 receptor in asthma. Mem Inst Oswaldo Cruz. 1997;92 Suppl 2:75–91. doi: 10.1590/s0074-02761997000800012. [DOI] [PubMed] [Google Scholar]
- 104.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 105.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]
- 106.Yakubenko VP, Yadav SP, Ugarova TP. Integrin alphaDbeta2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties. Blood. 2006;107:1643–1650. doi: 10.1182/blood-2005-06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kikuchi M, Tachimoto H, Nutku E, Hudson SA, Bochner BS. Phorbol esters alter alpha4 and alphad integrin usage during eosinophil adhesion to VCAM-1. Cell Commun Adhes. 2003;10:119–128. [PubMed] [Google Scholar]
- 108.Meerschaert J, Vrtis RF, Shikama Y, Sedgwick JB, Busse WW, Mosher DF. Engagement of alpha4beta7 integrins by monoclonal antibodies or ligands enhances survival of human eosinophils in vitro. J Immunol. 1999;163:6217–6227. [PubMed] [Google Scholar]
- 109.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Transmigration of eosinophils through basement membrane components in vitro: synergistic effects of platelet-activating factor and eosinophil-active cytokines. Am J Respir Cell Mol Biol. 1997;16:455–463. doi: 10.1165/ajrcmb.16.4.9115757. [DOI] [PubMed] [Google Scholar]
- 110.Hayflick JS, Kilgannon P, Gallatin WM. The intercellular adhesion molecule (ICAM) family of proteins. New members and novel functions. Immunol Res. 1998;17:313–327. doi: 10.1007/BF02786454. [DOI] [PubMed] [Google Scholar]
- 111.Davis GE. The Mac-1 and p150,95 beta 2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Exp Cell Res. 1992;200:242–252. doi: 10.1016/0014-4827(92)90170-d. [DOI] [PubMed] [Google Scholar]
- 112.Tachimoto H, Kikuchi M, Hudson SA, Bickel CA, Hamilton RG, Bochner BS. Eotaxin-2 alters eosinophil integrin function via mitogen-activated protein kinases. Am J Respir Cell Mol Biol. 2002;26:645–649. doi: 10.1165/ajrcmb.26.6.4741. [DOI] [PubMed] [Google Scholar]
- 113.Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005;11:148–152. doi: 10.1016/j.molmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 114.Baker DE. Natalizumab: Overview of Its Pharmacology and Safety. Rev Gastroenterol Disord. 2007;7:38–46. [PubMed] [Google Scholar]
- 115.Lin K, Ateeq HS, Hsiung SH, Chong LT, Zimmerman CN, Castro A, Lee WC, Hammond CE, Kalkunte S, Chen LL, Pepinsky RB, Leone DR, Sprague AG, Abraham WM, Gill A, Lobb RR, Adams SP. Selective, tight-binding inhibitors of integrin alpha4beta1 that inhibit allergic airway responses. J Med Chem. 1999;42:920–934. doi: 10.1021/jm980673g. [DOI] [PubMed] [Google Scholar]
- 116.Singh J, Adams S, Carter MB, Cuervo H, Lee WC, Lobb RR, Pepinsky RB, Petter R, Scott D. Rational design of potent and selective VLA-4 inhibitors and their utility in the treatment of asthma. Curr Top Med Chem. 2004;4:1497–1507. doi: 10.2174/1568026043387520. [DOI] [PubMed] [Google Scholar]
- 117.Vanderslice P, Biediger RJ, Woodside DG, Berens KL, Holland GW, Dixon RA. Development of cell adhesion molecule antagonists as therapeutics for asthma and COPD. Pulm Pharmacol Ther. 2004;17:1–10. doi: 10.1016/j.pupt.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 118.Norris V, Choong L, Tran D, Corden Z, Boyce M, Arshad H, Holgate S, O’Connor B, Millet S, Miller B, Rohatagi S, Kirkesseli S. Effect of IVL745, a VLA-4 antagonist, on allergen-induced bronchoconstriction in patients with asthma. J Allergy Clin Immunol. 2005;116:761–767. doi: 10.1016/j.jaci.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 119.Walsh GM. Novel therapies for asthma--advances and problems. Curr Pharm Des. 2005;11:3027–3038. doi: 10.2174/1381612054864984. [DOI] [PubMed] [Google Scholar]
- 120.Shah B, Jensen BK, Zhang J, Hunt T, Rohatagi S. Effect of food on pharmacokinetics of an inhaled drug: a case study with a VLA-4 antagonist, HMR1031. J Clin Pharmacol. 2003;43:1341–1349. doi: 10.1177/0091270003258172. [DOI] [PubMed] [Google Scholar]
- 121.Diamant Z, Kuperus J, Baan R, Nietzmann K, Millet S, Mendes P, Miller B, Amin D, Rohatagi S, Sterk PJ, Hoogsteden HC, Prins JB. Effect of a very late antigen-4 receptor antagonist on allergen-induced airway responses and inflammation in asthma. Clin Exp Allergy. 2005;35:1080–1087. doi: 10.1111/j.1365-2222.2005.02296.x. [DOI] [PubMed] [Google Scholar]
- 122.http://www.roche.com/home/investors/inv_news_upd/inv_news_upd_2006/inv-update-2006-10-17.htm
- 123.Ravensberg AJ, Luijk B, Westers P, Hiemstra PS, Sterk PJ, Lammers JW, Rabe KF. The effect of a single inhaled dose of a VLA-4 antagonist on allergen-induced airway responses and airway inflammation in patients with asthma. Allergy. 2006;61:1097–1103. doi: 10.1111/j.1398-9995.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- 124.http://www.gsk.com/investors/reps06/annual-report-2006.pdf
- 125.http://www.ranbaxy.com/history_ranbaxy.htm
- 126.Abraham WM, Gill A, Ahmed A, Sielczak MW, Lauredo IT, Botinnikova Y, Lin KC, Pepinsky B, Leone DR, Lobb RR, Adams SP. A small-molecule, tight-binding inhibitor of the integrin alpha(4)beta(1) blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am J Respir Crit Care Med. 2000;162:603–611. doi: 10.1164/ajrccm.162.2.9911061. [DOI] [PubMed] [Google Scholar]
- 127.Koo GC, Shah K, Ding GJ, Xiao J, Wnek R, Doherty G, Tong XC, Pepinsky RB, Lin KC, Hagmann WK, Kawka D, Singer II. A small molecule very late antigen-4 antagonist can inhibit ovalbumin-induced lung inflammation. Am J Respir Crit Care Med. 2003;167:1400–1409. doi: 10.1164/rccm.200207-696OC. [DOI] [PubMed] [Google Scholar]
- 128.Cortijo J, Sanz MJ, Iranzo A, Montesinos JL, Nabah YN, Alfon J, Gomez LA, Merlos M, Morcillo EJ. A small molecule, orally active, alpha4beta1/alpha4beta7 dual antagonist reduces leukocyte infiltration and airway hyper-responsiveness in an experimental model of allergic asthma in Brown Norway rats. Br J Pharmacol. 2006;147:661–670. doi: 10.1038/sj.bjp.0706658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pretolani M, Ruffie C, Lapa e Silva JR, Joseph D, Lobb RR, Vargaftig BB. Antibody to very late activation antigen 4 prevents antigen-induced bronchial hyperreactivity and cellular infiltration in the guinea pig airways. J Exp Med. 1994;180:795–805. doi: 10.1084/jem.180.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Milne AA, Piper PJ. Role of the VLA-4 integrin in leucocyte recruitment and bronchial hyperresponsiveness in the guinea-pig. Eur J Pharmacol. 1995;282:243–249. doi: 10.1016/0014-2999(95)00340-q. [DOI] [PubMed] [Google Scholar]
- 131.Sagara H, Matsuda H, Wada N, Yagita H, Fukuda T, Okumura K, Makino S, Ra C. A monoclonal antibody against very late activation antigen-4 inhibits eosinophil accumulation and late asthmatic response in a guinea pig model of asthma. Int Arch Allergy Immunol. 1997;112:287–294. doi: 10.1159/000237468. [DOI] [PubMed] [Google Scholar]
- 132.Kraneveld AD, van Ark I, Van Der Linde HJ, Fattah D, Nijkamp FP, Van Oosterhout AJ. Antibody to very late activation antigen 4 prevents interleukin-5-induced airway hyperresponsiveness and eosinophil infiltration in the airways of guinea pigs. J Allergy Clin Immunol. 1997;100:242–250. doi: 10.1016/s0091-6749(97)70231-8. [DOI] [PubMed] [Google Scholar]
- 133.Weg VB, Williams TJ, Lobb RR, Nourshargh S. A monoclonal antibody recognizing very late activation antigen-4 inhibits eosinophil accumulation in vivo. J Exp Med. 1993;177:561–566. doi: 10.1084/jem.177.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abraham WM, Sielczak MW, Ahmed A, Cortes A, Lauredo IT, Kim J, Pepinsky B, Benjamin CD, Leone DR, Lobb RR, et al. Alpha 4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J Clin Invest. 1994;93:776–787. doi: 10.1172/JCI117032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abraham WM, Ahmed A, Sielczak MW, Narita M, Arrhenius T, Elices MJ. Blockade of late-phase airway responses and airway hyperresponsiveness in allergic sheep with a small-molecule peptide inhibitor of VLA-4. Am J Respir Crit Care Med. 1997;156:696–703. doi: 10.1164/ajrccm.156.3.9609039. [DOI] [PubMed] [Google Scholar]
- 136.Huryn DM, Konradi AW, Ashwell S, Freedman SB, Lombardo LJ, Pleiss MA, Thorsett ED, Yednock T, Kennedy JD. The identification and optimization of orally efficacious, small molecule VLA-4 antagonists. Curr Top Med Chem. 2004;4:1473–1484. doi: 10.2174/1568026043387467. [DOI] [PubMed] [Google Scholar]
- 137.Abraham WM, Delehunt JC, Yerger L, Marchette B. Characterization of a late phase pulmonary response after antigen challenge in allergic sheep. Am Rev Respir Dis. 1983;128:839–844. doi: 10.1164/arrd.1983.128.5.839. [DOI] [PubMed] [Google Scholar]
- 138.Kanehiro A, Takeda K, Joetham A, Tomkinson A, Ikemura T, Irvin CG, Gelfand EW. Timing of administration of anti-VLA-4 differentiates airway hyperresponsiveness in the central and peripheral airways in mice. Am J Respir Crit Care Med. 2000;162:1132–1139. doi: 10.1164/ajrccm.162.3.9910100. [DOI] [PubMed] [Google Scholar]
- 139.Borchers MT, Crosby J, Farmer S, Sypek J, Ansay T, Lee NA, Lee JJ. Blockade of CD49d inhibits allergic airway pathologies independent of effects on leukocyte recruitment. Am J Physiol Lung Cell Mol Physiol. 2001;280:L813–L821. doi: 10.1152/ajplung.2001.280.4.L813. [DOI] [PubMed] [Google Scholar]
- 140.Kudlacz E, Whitney C, Andresen C, Duplantier A, Beckius G, Chupak L, Klein A, Kraus K, Milici A. Pulmonary eosinophilia in a murine model of allergic inflammation is attenuated by small molecule alpha4beta1 antagonists. J Pharmacol Exp Ther. 2002;301:747–752. doi: 10.1124/jpet.301.2.747. [DOI] [PubMed] [Google Scholar]
- 141.Richards IM, Kolbasa KP, Hatfield CA, Winterrowd GE, Vonderfecht SL, Fidler SF, Griffin RL, Brashler JR, Krzesicki RF, Sly LM, Ready KA, Staite ND, Chin JE. Role of very late activation antigen-4 in the antigen-induced accumulation of eosinophils and lymphocytes in the lungs and airway lumen of sensitized brown Norway rats. Am J Respir Cell Mol Biol. 1996;15:172–183. doi: 10.1165/ajrcmb.15.2.8703473. [DOI] [PubMed] [Google Scholar]
- 142.Rabb HA, Olivenstein R, Issekutz TB, Renzi PM, Martin JG. The role of the leukocyte adhesion molecules VLA-4, LFA-1, and Mac-1 in allergic airway responses in the rat. Am J Respir Crit Care Med. 1994;149:1186–1191. doi: 10.1164/ajrccm.149.5.8173758. [DOI] [PubMed] [Google Scholar]
- 143.Gascoigne MH, Holland K, Page CP, Shock A, Robinson M, Foulkes R, Gozzard N. The effect of anti-integrin monoclonal antibodies on antigen-induced pulmonary inflammation in allergic rabbits. Pulm Pharmacol Ther. 2003;16:279–285. doi: 10.1016/S1094-5539(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 144.Henderson WR, Jr, Chi EY, Albert RK, Chu SJ, Lamm WJ, Rochon Y, Jonas M, Christie PE, Harlan JM. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 1997;100:3083–3092. doi: 10.1172/JCI119863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998;161:1501–1509. [PubMed] [Google Scholar]
- 146.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Justice JP, Borchers MT, Crosby JR, Hines EM, Shen HH, Ochkur SI, McGarry MP, Lee NA, Lee JJ. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol. 2003;284:L169–L178. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]
- 148.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 149.Sixt M, Hallmann R, Wendler O, Scharffetter-Kochanek K, Sorokin LM. Cell adhesion and migration properties of beta 2-integrin negative polymorphonuclear granulocytes on defined extracellular matrix molecules. Relevance for leukocyte extravasation. J Biol Chem. 2001;276:18878–18887. doi: 10.1074/jbc.M010898200. [DOI] [PubMed] [Google Scholar]
- 150.Issekutz TB. Dual inhibition of VLA-4 and LFA-1 maximally inhibits cutaneous delayed-type hypersensitivity-induced inflammation. Am J Pathol. 1993;143:1286–1293. [PMC free article] [PubMed] [Google Scholar]
- 151.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Issekutz AC, Issekutz TB. The contribution of LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18) to the in vivo migration of polymorphonuclear leucocytes to inflammatory reactions in the rat. Immunology. 1992;76:655–661. [PMC free article] [PubMed] [Google Scholar]
- 153.Amon A, Pahl A, Szelenyi I. Can corticosteroids be beaten in future asthma therapy? Pharmazie. 2006;61:122–124. [PubMed] [Google Scholar]
- 154.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 155.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 156.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kimani G, Tonnesen MG, Henson PM. Stimulation of eosinophil adherence to human vascular endothelial cells in vitro by platelet-activating factor. J Immunol. 1988;140:3161–3166. [PubMed] [Google Scholar]
- 158.Lamas AM, Mulroney CM, Schleimer RP. Studies on the adhesive interaction between purified human eosinophils and cultured vascular endothelial cells. J Immunol. 1988;140:1500–1505. [PubMed] [Google Scholar]
- 159.Walsh GM, Hartnell A, Wardlaw AJ, Kurihara K, Sanderson CJ, Kay AB. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leucocyte integrin (CD11/18)-dependent manner. Immunology. 1990;71:258–265. [PMC free article] [PubMed] [Google Scholar]
- 160.Ebisawa M, Yamada T, Bickel C, Klunk D, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. III. Effect of the chemokine RANTES. J Immunol. 1994;153:2153–2160. [PubMed] [Google Scholar]
- 161.Scheinfeld N. Efalizumab: a review of events reported during clinical trials and side effects. Expert Opin Drug Saf. 2006;5:197–209. doi: 10.1517/14740338.5.2.197. [DOI] [PubMed] [Google Scholar]
- 162.Gauvreau GM, Becker AB, Boulet LP, Chakir J, Fick RB, Greene WL, Killian KJ, O’Byrne P M, Reid JK, Cockcroft DW. The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2003;112:331–338. doi: 10.1067/mai.2003.1689. [DOI] [PubMed] [Google Scholar]
- 163.Cybulsky MI, Fries JW, Williams AJ, Sultan P, Eddy R, Byers M, Shows T, Gimbrone MA, Jr, Collins T. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci U S A. 1991;88:7859–7863. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18:579–686. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]