Abstract
Perinatal testicular torsion is a relatively rare event that remains unrecognized in many patients or is suspected and treated accordingly only after an avoidable loss of time. The authors report their own experience with several patients, some of them quite atypical but instructive. Missed bilateral torsion is an issue, as are partial torsion, possible antenatal signs, and late presentation. These data are discussed together with the existing literature and may help shed new light on the natural course of testicular torsion and its treatment. The most important conclusion is that a much higher index of suspicion based on clinical findings is needed for timely detection of perinatal torsion. It is the authors’ opinion that immediate surgery is mandatory not only in suspected bilateral torsions but also in cases of possible unilateral torsions. There is no place for a more fatalistic “wait-and-see” approach. Whenever possible, even necrotic testes should not be removed during surgery because some endocrine function may be retained.
Keywords: Perinatal testicular torsion, Extravaginal torsion of testis, Bilateral spermatic cord torsion
Introduction
Perinatal testicular torsion (PTT), with its variable clinical presentation or its consequences when diagnosis is made only after several years have elapsed, is a rare but well-known entity in pediatric urology practice. PTT is—somewhat arbitrarily—defined by some authors to occur either prenatally or in the first 30 days of life [12, 19]. The mechanism of torsion, the clinical and surgical findings, and—unfortunately—the outcome are very different from the ones seen in testicular torsions at later ages. It is frustrating how few of these testes are actually salvaged according to literature [12, 14, 17–19, 26, 29]. Roughly 150 antenatal or immediately postnatal torsions have been reported so far with a significant percentage of bilateral cases. Some cautions are needed when interpreting data because literature pertaining to PTT mainly consists of (compilations of) case reports, with their inherent likelihood of bias. A testicular salvage rate of 5% or less was suggested in 1988 by Kaplan and Silber [27]. More recent papers are sometimes more optimistic, citing much higher success rates in small series, but not always clearly stratifying between prenatal and postnatal PTT [3, 35]. Clear prenatal PTT seems unsalvageable [28]. The controversy surrounding management of these patients was highlighted as recently as 2008 by a Canadian survey where 26 pediatric urologists were asked about their preferences [23]. A clear need for more controlled trials to better understand this clinical entity was the main conclusion.
Having seen many patients with sequelae of PTT and one particularly unfortunate case of bilateral torsion diagnosed only at the age of 4, the authors subsequently documented every aspect of history and clinical findings in an attempt to better understand this condition. This yielded some new and unexpected insights into the pathogenesis of PTT. These data, combined with the existing case reports and the evolving treatment recommendations in literature, may lead to a reappraisal of the concepts determining the management of these children.
Anatomical background
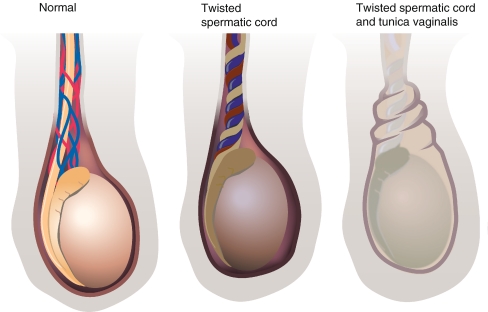
According to the traditional theories, there seem to exist two distinct mechanisms of testicular torsion: the extravaginal type which is typically found in newborns and the intravaginal type which is mostly seen in the older age groups. In the former type, the complete spermatic cord with all its contributing structures (vas, vessels, processus vaginalis, and investing fascias) is thought to undergo torsion (Figs. 1 and 2). A temporarily increased mobility of the involved tissue layers around the time of birth is generally accepted to lie at the root of this event, but the exact pathogenesis remains largely unknown [14]. It is generally assumed that about 70% of PTT occur during pregnancy, the rest at or shortly after birth [19].
Fig. 1.
Normal situation as opposed to intravaginal torsion of cord structures and extravaginal testicular torsion where tunica vaginalis (and investing outer layers) are also involved
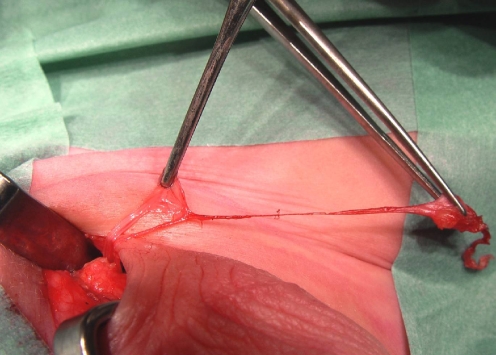
Fig. 2.
Clear demonstration of the point of torsion in the spermatic cord: Cremasteric tissues, internal spermatic fascia, and tunica vaginalis all seem involved. The testis is still covered by tunica vaginalis
In contrast, the intravaginal testicular torsion involves only the testis without its investing tunica vaginalis. This is due to a congenital variant of the tunica vaginalis where the normally present septum-like connection between the epididymis and the lamina parietalis of the fascia vaginalis is lacking, in effect creating a “bell clapper” situation with the testis only suspended at its top and thus relatively free to rotate axially (Fig. 1).
Data on testicular torsion in older children demonstrate that irreversible damage to spermatogenesis occurs after 4 to 6 h of ischemia, and (future) testosterone production is compromised by damage to Leydig cells after approximately 12 h of ischemia [2, 8, 46].
Patients
During a 6-year period between 2002 and 2008, 21 cases of PTT (or its sequelae) were diagnosed in our department. The majority were discovered during scrotal surgery or laparoscopic exploration for impalpable testes (Figs. 3 and 4). There were six acute testicular torsions (in four patients), four of these six torsions occurred bilaterally (in two of these four patients). Four were prenatal torsions; two occurred postnatally. The ones with the more salient features are reported in this text. A total of three boys had bilateral PTT (two acute cases and one delayed diagnosis), leading to anorchia.
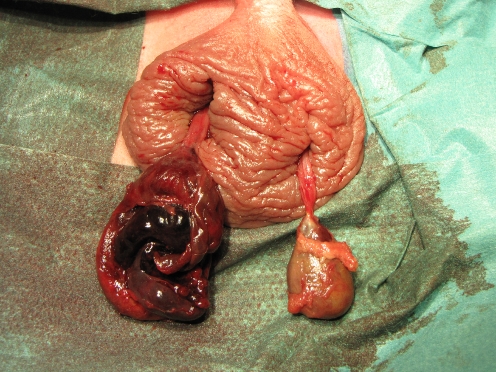
Fig. 3.
A testicular remnant found during scrotal exploration for cryptorchidism, when several years have elapsed after PTT. Note the highest point of torsion, proximal to which (atrophic) vas and vessels can again be identified
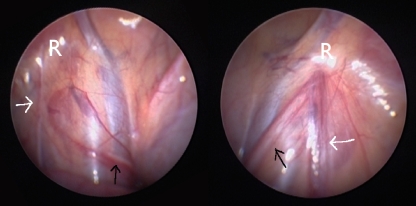
Fig. 4.
Typical laparoscopic image at the age of 5 of the internal inguinal rings in a patient with left-sided PTT. The black arrow indicates the vas crossing the iliac vessels and then ending blindly close to the closed internal inguinal ring (R), the level to which the torsion of the cord was transmitted. The white arrow shows the atrophic testicular vessels or their fibrotic remnants running toward the internal inguinal ring. Normal contralateral situation
Patient 1
On the fifth day of life, a baby was referred on suspicion of left-sided PTT. Physical examination showed a pronounced swelling of the left hemiscrotum which was inflamed and seemed tender. The swelling had been present for almost 48 h but gradually had become worse. Interestingly, the mother stated on admission that immediately after birth, she had actually had the distinct impression that the contralateral (right) side of the scrotum had been swollen and certainly not the left side. This right-sided swelling had gradually been replaced by a clinically more marked left-sided swelling.
Careful examination of the scrotum showed a right testicle of normal size but having an almost stone-like firmness. Immediate surgery was performed because bilateral nonsimultaneous PTT was anticipated based on the clinical findings. The hemorrhagically infarcted and completely necrotic left testis was removed for fear of infection. The right testis was twisted 720° and was avascular, but no signs of inflammation were noted. The torsion was corrected and the right testicle was left in place. Four months later, the size of the testis was pea like, suggesting resorption, but testosterone remained above detection limits. The significance of this finding for later life is unclear at present, but on follow-up 1 year later, the small testicle did remain palpable.
Patient 2
A 4-year-old boy was referred for acquired bilateral undescended and impalpable testes, also classified as ascended testes. Both the child’s parents and the medical records clearly stated that at least during the first months of life, two normal appearing testes had been noted in the scrotum. Trusting this information, laparoscopy was performed (omitting mandatory hormonal testing). This showed bilateral atrophic testicular vessels, ending blindly just proximal to the internal inguinal ring, and a vas entering the inguinal canal, the hallmark of PTT. Subsequent human chorionic gonadotropin (HCG) testing confirmed the diagnosis of anorchia. Testosterone remained below detection limits. Substitution therapy will be initiated when rising follicle-stimulating hormone and luteinizing hormone levels announce central puberty.
Patient 3
At the age of 10 weeks, a healthy baby boy was referred to the urology department with the diagnosis of PTT. Apparently, both testicles were considered normal at birth by the attending gynecologist. The typical red and swollen right hemiscrotum had been recognized on the third day after birth and diagnosis subsequently was confirmed sonographically. No action was taken by the neonatology department, however, and the child was not referred for immediate surgery, arguing that it was too late to save the testicle. The child was referred on a purely elective basis.
Further detailed history taking and reconstruction of the events were very instructive. Pregnancy had been uneventful until premature rupture of membranes with meconium-stained amniotic fluid in week 39. Fetal distress during delivery had led to a cesarean section. Moreover, ultrasound 2 weeks before birth had clearly shown normal appearing and perfectly symmetrical testes. Hence, it was assumed that the fetal distress leading to passage of meconium may have been caused by PTT.
After careful discussion by the pediatric urologist with the parents on the theoretical advantages and risks of preventive surgery on the contralateral testicle, it was decided to adopt a wait-and-see policy. The possible signs and symptoms of testicular torsion were clearly explained. Nothing untoward was reported on further follow-up 1 year later.
Patient 4
A 9-month-old boy who had been unwell since the morning and had started vomiting in the afternoon was found to have a painful red swelling of the left groin area. On ultrasound, an enlarged testicle was seen in the inguinal canal, and the child was referred to the pediatric urologist. Further history showed that the palpable left testicle had always been retractile or possibly undescended, but a clear hernia had never been suspected. Immediate surgery was performed through an inguinal incision; almost 4 h after the swelling had first been noted. Marked edema of both the subcutaneous tissues and the entire spermatic cord was found. The spermatic cord showed vascular congestion and a torsion of only 180° which was easily corrected once the correct planes of dissection had been identified. After opening the tunica vaginalis investing the testicle, pronounced adhesions between the testicle and tunica were found, precluding a more classical intravaginal torsion and highly suggestive of the longer-standing nature of this condition. After detorsion, the cord seemed to regain perfusion but the testicle remained blue. Orchidopexy was performed. On follow-up 18 months after the operation, the testicle had atrophied but was readily palpable in the scrotum with a size roughly that of a pea.
Patient 5
The diagnosis of bilateral PTT in a term baby was suspected within the first 2 h after birth in a referring general hospital. The pregnancy had been normal and labor had started at 40 weeks, but conversion to a cesarean section had become necessary due to failure to progress in labor. Physical examination showed a red and swollen right hemiscrotum with enlarged testis and a normal appearing left testis feeling very hard, however, on palpation (Fig. 5). Complete absence of flow in both testes on Doppler ultrasound confirmed the diagnosis, and the baby was urgently referred to the authors’ hospital where bilateral scrotal exploration was performed approximately 6 h after birth. A hemorrhagic and enlarged completely necrotic right testis was found (Fig. 6). The left testis of normal size was avascular, and there were pronounced adhesions between the testis and the lamina parietalis at the tunica vaginalis. Both spermatic cords seemed well vascularized. Interestingly, neither side showed a clear torsion. Every effort was made to remove any possibly constricting fibrotic tissue from the testes and the spermatic cords, and the testes were bathed for 15 min. The swelling of the right testicle decreased and the almost black discoloration diminished somewhat. Both testicles were replaced in the scrotum. Testosterone level at 4 weeks was 2.0 nmol/l and at 6 weeks 1.4 nmol/l. It was assumed that some Leydig cell function had remained. HCG testing with 1,500 IU, however, did not induce a rise of testosterone levels. At the age of 10 weeks, physical examination showed an almost complete resorption of the right testis but the left one could easily be palpated, again with the size of a large pea.
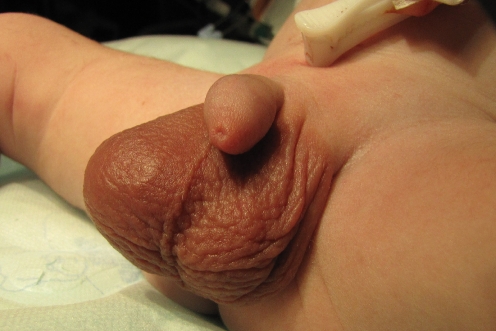
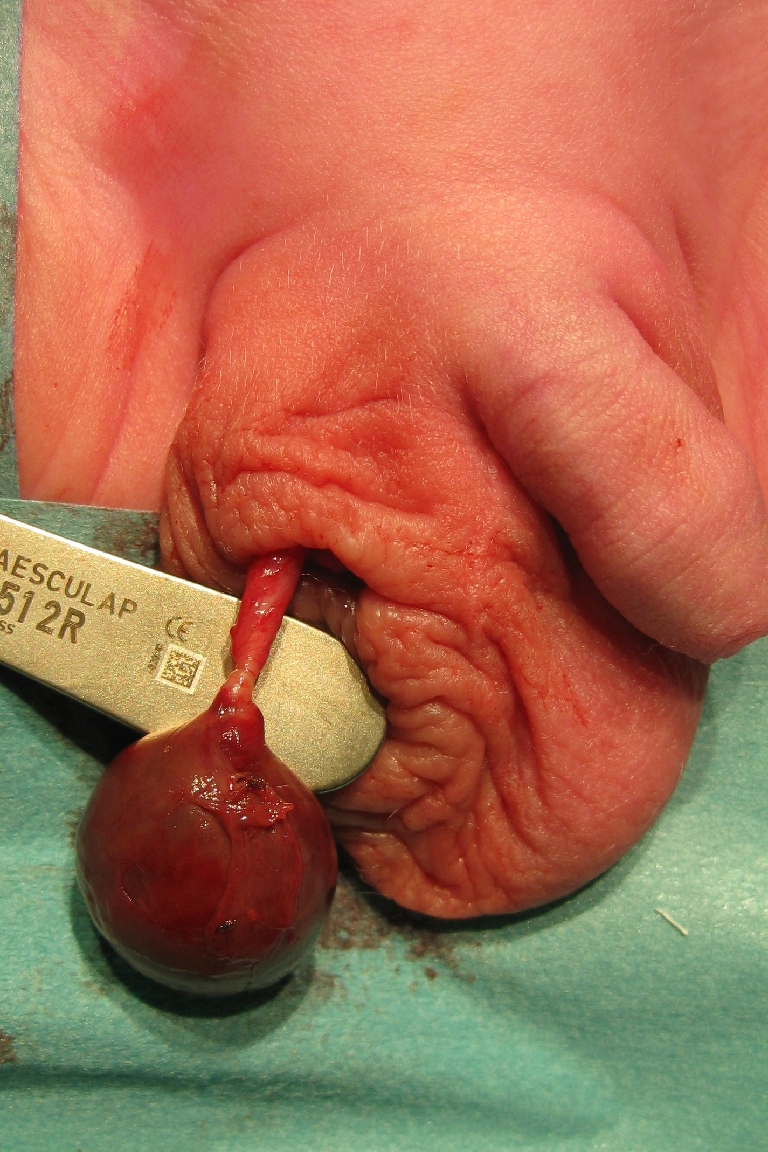
Fig. 5.
Scrotal appearance in asynchronous bilateral PTT as described in patient 5. Marked inflammation on the right side with the more recent torsion, only hardening of the left testis on palpation. Clinical underestimation of the severity is possible
Fig. 6.
Appearance of both testes in patient 5 immediately after opening the scrotum: The hemorrhagic and necrotic right testis after opening of the tunica vaginalis is demonstrated. Avascular left testis, tunica vaginalis still intact but strongly adherent to the testis. Normal vascularity and no clear torsion of both spermatic cords
Discussion
Most of the earlier publications on PTT reported only unilateral cases, almost exclusively of the prenatal type. Even when surgery was performed in an emergency setting, hardly any testes seemed salvable [12, 27, 28]. Hence, PTT was generally seen as an irreversible intrauterine event. This led to the recommendation to offer surgery in an (early) elective setting with contralateral fixation of the testis or even to omit surgery altogether when medical attention is sought after several weeks have elapsed, when the risk of contralateral torsion is considered unlikely [12, 14, 18]. Patient 3 was managed this way.
However, bilateral torsion was reported for the first time in 1967 by Papadatos and Moutsouris, but by 1995, 16 cases of bilateral PTT had been reported and more than 45 cases by 2007 [4, 5, 7, 9, 16, 28, 30, 32–35, 37, 42, 43, 45, 49, 50]. Based on compilations of these reports, it was estimated that approximately 10% to 22% of PTT are bilateral [19, 49]. Two thirds most likely occur synchronously
Time of torsion
PTT is generally considered to occur before birth or during the first 30 days of life, although this time frame is arbitrary. The relevant distinction to be made is the one between PTT occurring before or after birth. Prognosis in the former group is far worse for obvious reasons [43]. It is unclear how early in pregnancy testicular torsion can occur, but a case of bilateral torsion at 32 weeks of gestation has been reported [2, 40, 45]. As PTT almost always is a scrotal event, most torsions likely occur in the last trimester of pregnancy [10, 11, 25]. Extravaginal testicular torsion (the PTT type) in babies of more than a few months of age is rare but has been reported [9].
Interestingly, there are several reports on prenatally detected PTT on ultrasound, but the implications of this are unclear at present [2, 20, 24, 38, 45]. Urgent extraction of the fetus might be justified in the last weeks of pregnancy in suspected acute bilateral PTT, on the assumption that diagnosis was made shortly after torsion, but this intervention has not been reported so far. Intriguingly, a review in 2007 suggested that in the majority of asynchronous bilateral PTT cases, the first side to undergo torsion was the right [5]. Patient 1 in the present series adds one more to the list, but patient 5 suggests the inverse sequence. No logical explanation was provided.
Patient 4 seems to be the proverbial exception to the rule that PTT occurs before birth or in the first 30 days of life. The findings at surgery suggest a possible explanation. The adhesions clearly prove that the testis had been compromised much longer than the few hours’ duration of the acute symptoms (swelling, redness, vomiting). The partial extravaginal torsion, at first only impeding venous outflow, but gradually causing more edema, vascular congestion, reactive adhesions, and ultimately arterial obstruction, seems the only logical explanation. Intravaginal partial (or intermittent) torsion is unlikely because the adhesions would preclude rotation of the testis. The likely mechanism of partial torsion (180°) seems in a way reminiscent of the rare cases of idiopathic hemorrhagic testicular infarction without apparent torsion at birth where either spontaneous untwisting after infarction or a vascular incident are thought to lie at the root [36]. Patient 5 clearly is one of these exceptional cases.
These data clearly show that PTT consists of a broad spectrum both in respect to timing and severity of spermatic cord torsion.
Physical findings
Unlike the situation in testicular torsion in older children or adults, PTT often has an insidious presentation. There is a tremendous variation in clinical findings, most likely depending on the amount of time elapsed between the actual torsion and initial presentation [21]. This is highlighted by patient 1. Diagnosis of PTT is a clinical one and depends completely on the scrotal examination at birth by an obstetrician or pediatrician. A somewhat hardened testicle is pathognomonic of PTT. Attention to this fact was drawn by Baptist and Amin in 1996 [6]. In addition, in the acute phase, signs of inflammation will be more marked with some redness, swelling, and fixation of the overlying skin and sometimes clear tenderness. In children of darker races, diagnosis may be more difficult because redness of the scrotum is less conspicuous [42]. Hydroceles and edema may mask clinical presentation of PTT. Differential diagnosis at this point includes incarcerated scrotal hernia, hematoma, abscess, meconium peritonitis, epididymitis, or tumor (Table 1) [13, 47]. After a few days, the appearance of the testicle involved may normalize, but careful examination can probably still demonstrate differences between the two testicles. As time goes by, the testicle gradually decreases in size until it becomes (almost) undetectable. The sonographic appearance and evolution in time of PTT closely mirrors the clinical sequence [44].
Table 1.
Differential diagnosis of scrotal swelling in neonates
| PTT |
| Incarcerated scrotal hernia |
| Scrotal hematoma, hematocele |
| Scrotal abscess |
| Meconium peritonitis |
| Epididymitis |
| Tumor |
At first sight, it seems striking that many of the PTT cases discovered at a later age during surgical exploration for impalpable testes are reported to have had normal testes at birth, but when considering the sequence of events just mentioned, this should not surprise too much. Nevertheless, it is the authors’ impression that a detailed physical examination at birth with careful assessment of position, volume, and firmness of the testes can lead to earlier detection of PTT. In cases of (suspected) bilateral PTT, the stakes are high and hence management is straightforward: The only hope for salvage of testicular function is immediate surgery.
Risk factors
Vaginal delivery, prolonged labor, pre-eclampsia, gestational diabetes, twin pregnancy, and a higher birth weight all have been linked to a higher risk of PTT [2, 12, 28, 48]. This suggests that fetal stress and/or mechanical factors during pregnancy or delivery may play a role in the pathogenesis of PTT [28]. None of the babies in this series had any of the above risk factors. Patient 1 highlights the treacherous combination of prenatal (on the right) and postnatal (on the left) PTT, a clear case of asynchronous contralateral torsion. Several cases of contralateral torsions in babies waiting for elective scrotal exploration at a later age have been documented [9].
Expanding on fetal stress, the concept that fetal stress might actually be caused by PTT (and not the other way round) is a very tempting one. In the third trimester, a fetus probably can experience pain or show reaction to injury [31]. This may provide a hypothetical explanation for the sequence of events in patient 3. The authors later had an almost identical history in another patient (not reported on here). In both patients, it seems plausible that PTT was the cause of fetal stress leading to meconium discharge. A similar assumption can be made when reading the 2001 report by Ricci et al. During an emergency ultrasound study for reduced fetal activity at 37 weeks of gestation, they observed abnormalities of a testis that revealed itself after birth as a case of PTT [38]. There are two reports on siblings affected by PTT; the importance of possible hereditary factors remains unclear [15, 22].
Management
There remains some controversy surrounding management of unilateral torsions [41]. As outlined at the beginning of this paragraph, many authors advocate elective surgery (aimed mainly at contralateral orchidopexy) and some even prefer to remain conservative. This course of action was chosen in patient 3. In more recent publications, immediate emergency surgery is increasingly advocated for acute cases in the hope of saving the testis [1, 35]. This certainly applies to bilateral PTT. It is the authors’ opinion that the approach to suspected unilateral cases should be as strict as in bilateral cases because the risk of both a missed bilateral PTT or the occurrence of a subsequent asynchronous torsion of the contralateral testis should not be underestimated. As demonstrated by case report 4, even late presentations can occur, in effect rendering defining a safe age after which contralateral torsion can be considered highly unlikely a hazardous undertaking [9]. In the event, the consequences are disastrous. It could be very difficult to convince the parents of babies with unrecognized bilateral PTT that every effort was done to save testicular function when an elective surgical approach was chosen.
Patients 1 and 5 and histological data from literature suggest that there is a chance of some tissue surviving after torsion. Hence, it is a reasonable option to leave in place a testis, even if perfusion does not seem to resume after detorsion [2]. Especially the Leydig cells seem more resistant (however, it is difficult to interpret endocrine data in baby boys after bilateral PTT, for lack of validated reference values for this particular type of patient.)
When analyzing literature data, several small series reported some tissue surviving in approximately 5% of testes that were removed or underwent biopsy [5, 10, 43]. Later in life, the atrophied testicular remnants still contain viable germ cells in a small percentage of patients when exploration and orchiectomy are performed for cryptorchidism [39]. This in turn could be considered reason for resection because of the theoretical risk of tumor formation, but to the best of the authors’ knowledge, this has not been reported so far.
Conclusion
In conclusion:
A clear distinction needs to be made between “old” prenatal or missed cases of PTT, on the one hand, and the acute perinatal PTT cases. Only the latter group stands a chance of testicular salvage, provided that emergency surgical exploration is done.
The risk of bilateral, possibly asynchronous, PTT is real. Physical examination is too unreliable to guarantee noninvolvement of the contralateral testis. Hence, in each case of suspected unilateral PTT, every effort should be made to guarantee surgical exploration of both testicles on an emergency basis, even when it is unlikely to save the affected testis. Limiting the indication for surgery to cases of suspected acute (unilateral) PTT may increase the risk for the (until then) normal contralateral testis. Referral on an emergency basis is strictly indicated.
Mostly due to an important variation in clinical presentation, diagnosis of this condition in the acute phase requires a high degree of suspicion. A properly executed physical examination at birth is the mainstay of timely diagnosis. Special attention needs to be paid in the event of fetal distress without obvious hypoxemia as antenatal testicular torsion may have been the cause of distress. In general, increased awareness by both caregivers and parents seems mandatory.
Minor degrees of extravaginal torsion leading to delayed presentation can occur. The dictum that PTT only presents during the first weeks of life may be false.
Whenever possible and certainly in bilateral cases of PTT, every effort should be made to leave even necrotic testes in place, as some testicular function may still be possible.
Conflict of interest
None declared.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- PTT
Perinatal testicular torsion
References
- 1.Ahmed SJ, Kaplan GW, DeCambre ME. Perinatal testicular torsion: preoperative radiological findings and the argument for urgent surgical exploration. J Pediatr Surg. 2008;43(8):1563–1565. doi: 10.1016/j.jpedsurg.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 2.Arena F, Nicòtina PA, Romeo C, et al. Prenatal testicular torsion: ultrasonographic features, management and histopathological findings. Int J Urol. 2006;13(2):135–141. doi: 10.1111/j.1442-2042.2006.01247.x. [DOI] [PubMed] [Google Scholar]
- 3.Arnbjornsson E. Testicular survival after neonatal torsion. Z Kinderchir. 1986;41(5):293–294. doi: 10.1055/s-2008-1043362. [DOI] [PubMed] [Google Scholar]
- 4.Bachor R, Frohneberg D, Heymer B, Hautmann R. Bilateral intrauterine testicular torsion. Urologe A. 1987;26(4):216–219. [PubMed] [Google Scholar]
- 5.Baglaj M, Carachi R. Neonatal bilateral testicular torsion: a plea for emergency exploration. J Urol. 2007;177:2296–2299. doi: 10.1016/j.juro.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Baptist EC, Amin PV. Perinatal testicular torsion and the hard testicle. J Perinatol. 1996;16:67–68. [PubMed] [Google Scholar]
- 7.Barca PR, Dargallo T, Jardón JA, et al. Bilateral testicular torsion in the neonatal period. J Urol. 1997;158(5):1957–1959. doi: 10.1016/S0022-5347(01)64193-4. [DOI] [PubMed] [Google Scholar]
- 8.Bartsch G, Frank S, Marberger H, Mikuz G. Testicular torsion: late results with special regard to fertility and endocrine function. J Urol. 1980;124(3):375–378. doi: 10.1016/s0022-5347(17)55456-7. [DOI] [PubMed] [Google Scholar]
- 9.Beasley SW, McBride CA. The risk of metachronus (asynchronous) contralateral torsion following perinatal torsion. N Z Med J. 2005;118:1218. [PubMed] [Google Scholar]
- 10.Belman AB, Rushton HG. Is the vanished testis always a scrotal event? BJU Int. 2001;87:480–483. doi: 10.1046/j.1464-410X.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- 11.Belman AB, Rushton HG. Is an empty left hemiscrotum and hypertrophied right descended testis predictive of perinatal torsion? J Urol. 2003;170:1674–1675. doi: 10.1097/01.ju.0000083888.22807.b8. [DOI] [PubMed] [Google Scholar]
- 12.Brandt MT, Sheldon CA, Wacksman J, Matthews P. Prenatal testicular torsion: principles of management. J Urol. 1992;147:670–672. doi: 10.1016/s0022-5347(17)37342-1. [DOI] [PubMed] [Google Scholar]
- 13.Briggs C, Godbole P, MacKinnon AE, Vermeulen K. Neonatal paratesticular abscess mimicking perinatal torsion. J Pediatr Surg. 2005;40(7):1195–1196. doi: 10.1016/j.jpedsurg.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 14.Burge DM. Neonatal testicular torsion and infarction: aetiology and management. Br J Urol. 1987;59:70–73. doi: 10.1111/j.1464-410X.1987.tb04583.x. [DOI] [PubMed] [Google Scholar]
- 15.Castilla EE, Sod R, Anzorena O, Texido J. Neonatal testicular torsion in two brothers. J Med Genet. 1975;12(1):112–113. doi: 10.1136/jmg.12.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper CS, Snyder OB, Hawtrey CE. Bilateral neonatal testicular torsion. Clin Pediatr. 1997;36:653–656. doi: 10.1177/000992289703601107. [DOI] [PubMed] [Google Scholar]
- 17.Cuervo JL, Grillo A, Vecchiarelli C, et al. Perinatal testicular torsion: a unique strategy. J Pediatr Surg. 2007;42:699–703. doi: 10.1016/j.jpedsurg.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Cumming DC, Hyndman CW, Deacon JS. Intrauterine testicular torsion: not an emergency. Urology. 1979;14(6):603–604. doi: 10.1016/0090-4295(79)90534-X. [DOI] [PubMed] [Google Scholar]
- 19.Das S, Singer A. Controversies of perinatal torsion of the spermatic cord: a review, survey and recommendations. J Urol. 1990;143:231–233. doi: 10.1016/s0022-5347(17)39919-6. [DOI] [PubMed] [Google Scholar]
- 20.Devesa R, Muñoz A, Torrents M, et al. Prenatal diagnosis of testicular torsion. Ultrasound Obstet Gynecol. 1998;11:286–288. doi: 10.1046/j.1469-0705.1998.11040286.x. [DOI] [PubMed] [Google Scholar]
- 21.Giannakopoulos X, Chambilomatis P, Filiadis I, et al. Six cases of prenatal and neonatal torsion of the spermatic cord. Int J Urol. 1997;4:324–326. doi: 10.1111/j.1442-2042.1997.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 22.Gorbonos A, Cheng EY. Perinatal testicular torsion in siblings. J Pediatr Urol. 2007;3(6):514–515. doi: 10.1016/j.jpurol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Guerra LA, Wiesenthal J, Pike J, Leonard MP. Management of neonatal testicular torsion: which way to turn? Can Urol Assoc J. 2008;2(4):376–379. doi: 10.5489/cuaj.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernanz-Schulman M, Yenicesu F, Heller RM, Brock JW., 3rd Sonographic identification of perinatal testicular torsion. J Ultrasound Med. 1997;16:65–67. doi: 10.7863/jum.1997.16.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Huff DS, Wu HY, Snyder HM, 3rd, et al. Evidence in favor of the mechanical (intrauterine torsion) theory over the endocrinopathy (cryptorchidism) theory in the pathogenesis of testicular agenesis. J Urol. 1991;146:630–631. doi: 10.1016/s0022-5347(17)37876-x. [DOI] [PubMed] [Google Scholar]
- 26.John CM, Kooner G, Mathew DE, et al. Neonatal testicular torsion—a lost cause? Acta Paediatr. 2008;97(4):502–504. doi: 10.1111/j.1651-2227.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan GW, Silber I. Neonatal torsion—to pex or not? In: King LR, editor. Urologic surgery in neonates and young infants. Philadelphia: Saunders; 1988. pp. 386–395. [Google Scholar]
- 28.Kaye JD, Levitt SB, Friedman SC, et al. Neonatal torsion: a 14-year experience and proposed algorithm for management. J Urol. 2008;179(6):2377–2383. doi: 10.1016/j.juro.2008.01.148. [DOI] [PubMed] [Google Scholar]
- 29.LaQuaglia MP, Bauer SB, Eraklis A, et al. Bilateral neonatal torsion. J Urol. 1987;138:1051–1054. doi: 10.1016/s0022-5347(17)43499-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee SD, Cha CS. Asynchronous bilateral torsion of the spermatic cord in the newborn: a case report. J Korean Med Sci. 2002;17(5):712–714. doi: 10.3346/jkms.2002.17.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Ralston HJ, Drey EA, et al. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294(8):947–954. doi: 10.1001/jama.294.8.947. [DOI] [PubMed] [Google Scholar]
- 32.Mouritsen E, Boelskifte J, Rasmussen KL. Bilateral torsion of the testicles in a newborn. Arch Gynecol Obstet. 2002;266(2):118. doi: 10.1007/s004040100199. [DOI] [PubMed] [Google Scholar]
- 33.Olguner M, Akgür FM, Aktug T, Derebek E. Bilateral asynchronous perinatal testicular torsion: a case report. J Pediatr Surg. 2000;35:1348–1349. doi: 10.1053/jpsu.2000.9330. [DOI] [PubMed] [Google Scholar]
- 34.Papadatos C, Moutsouris C. Bilateral testicular torsion in the newborn. Arch Surg. 1967;71:249–251. doi: 10.1016/s0022-3476(67)80082-9. [DOI] [PubMed] [Google Scholar]
- 35.Pinto KJ, Noe HN, Jerkins GR. Management of neonatal testicular torsion. J Urol. 1997;158:1196–1197. doi: 10.1016/S0022-5347(01)64425-2. [DOI] [PubMed] [Google Scholar]
- 36.Pinto PS, Kiefer JN. Infarction of the testicle in the newborn infant. J Pediatr. 1957;51(1):80–84. doi: 10.1016/S0022-3476(57)80285-6. [DOI] [PubMed] [Google Scholar]
- 37.Reid M, Graham WJ. Bilateral testicular torsion in the newborn. Acta Paediatr Scand. 1976;65(5):647–648. doi: 10.1111/j.1651-2227.1976.tb04946.x. [DOI] [PubMed] [Google Scholar]
- 38.Ricci P, Cantisani V, Drudi FM, et al. Prenatal testicular torsion: sonographic appearance in the newborn infant. Eur Radiol. 2001;11(12):2589–2592. doi: 10.1007/s003300100868. [DOI] [PubMed] [Google Scholar]
- 39.Rozanski TA, Wojno KJ, Bloom DA. The remnant orchiectomy. J Urol. 1996;155(2):712–713. doi: 10.1016/S0022-5347(01)66507-8. [DOI] [PubMed] [Google Scholar]
- 40.Ryken TC, Turner JW, Haynes T. Bilateral testicular torsion in a pre-term neonate. J Urol. 1990;143:102–103. doi: 10.1016/s0022-5347(17)39879-8. [DOI] [PubMed] [Google Scholar]
- 41.Sakellaris G, Sifakis S, Hatzidaki E, et al. Early exploration in perinatal torsion of the spermatic cord: a case report. J Matern Fetal Neonatal Med. 2004;15:207–209. doi: 10.1080/14767050410001668338. [DOI] [PubMed] [Google Scholar]
- 42.Samnakay N, Tudehope D, Walker R. Spin on perinatal testicular torsion. J Paediatr Child Health. 2006;42(11):734–736. doi: 10.1111/j.1440-1754.2006.00961.x. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen MD, Galansky SH, Striegl AM, et al. Perinatal extravaginal torsion of the testis in the first month of life is a salvageable event. Urology. 2003;62:132–134. doi: 10.1016/S0090-4295(03)00402-3. [DOI] [PubMed] [Google Scholar]
- 44.Traubici J, Daneman A, Navarro O, et al. Original report. Testicular torsion in neonates and infants: sonographic features in 30 patients. AJR Am J Roentgenol. 2003;180:1143–1145. doi: 10.2214/ajr.180.4.1801143. [DOI] [PubMed] [Google Scholar]
- 45.Tripp BM, Homsy YL. Prenatal diagnosis of bilateral neonatal torsion: a case report. J Urol. 1995;153:1990–1991. doi: 10.1016/S0022-5347(01)67387-7. [DOI] [PubMed] [Google Scholar]
- 46.Tryfonas G, Violaki A, Tsikopoulos G, et al. Late postoperative results in males treated for testicular torsion during childhood. J Pediatr Surg. 1994;29(4):553–556. doi: 10.1016/0022-3468(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 47.van der Sluijs JW, den Hollander JC, Lequin MH, et al. Prenatal testicular torsion: diagnosis and natural course. An ultrasonographic study. Eur Radiol. 2004;14(2):250–255. doi: 10.1007/s00330-003-2019-0. [DOI] [PubMed] [Google Scholar]
- 48.Visani S, Gentile RL, Vijaya L. Perinatal torsion of spermatic cord. Urology. 1975;6:360–362. doi: 10.1016/0090-4295(75)90768-2. [DOI] [PubMed] [Google Scholar]
- 49.Yerkes EB, Robertson FM, Gitlin J, et al. Management of perinatal torsion: today, tomorrow or never? J Urol. 2005;174:1579–1582. doi: 10.1097/01.ju.0000179542.05953.11. [DOI] [PubMed] [Google Scholar]
- 50.Zafaranloo S, Gerard PS, Wise G. Bilateral neonatal testicular torsion: ultrasonographic evaluation. J Urol. 1986;135(3):589–590. doi: 10.1016/s0022-5347(17)45748-x. [DOI] [PubMed] [Google Scholar]