Abstract
Expression of developmental and unconventional myosin heavy chain (MHC) isoforms in some adult head and neck muscles is thought to reflect specific contractile demands of muscle fibers active during kinematically complex movements. Mammalian tongue muscles are active during oromotor behaviors that encompass a wide range of tongue movement speeds and tongue shape changes (e.g. respiration, oral transport, swallowing, rejection), but the extent to which tongue muscles express developmental and unconventional MHC is not known. Quantitative PCR was used to determine the mRNA content of conventional MHC-beta, MHC-2a, MHC-2b and MHC-2x, the developmental isoforms embryonic MHC and neonatal MHC and the unconventional isoforms atrial/cardiac-α MHC (MHC-alpha), extraocular MHC, masseter MHC and slow tonic MHC in tongue body muscles of the rat, macaque and human. In all species, conventional MHC isoforms predominate. MHC-2b and MHC-2x account for 98% of total MHC mRNA in the rat. MHC-2a, MHC-2x and MHC-beta account for 94% of total MHC mRNA in humans and 96% of total MHC mRNA in macaque. With the exception of MHC-alpha in humans (5%), developmental and unconventional MHC mRNA represents less than 0.3% of total MHC mRNA. We conclude that in these species, there is limited expression of developmental and unconventional MHC and that diversity of tongue body muscle fiber contractile properties is achieved primarily by MHC-beta, MHC-2a, MHC-2x and MHC-2b. Whether expression of MHC-alpha mRNA in tongue is unique to humans or present in other hominoids awaits further investigation.
Key Words: Myosin, Tongue, PCR
Introduction
Sarcomeric myosin heavy chain (MHC) is a primary determinant of muscle contractile properties [Reiser et al., 1985; Galler et al., 1997; Bottinelli, 2001]. Contractile diversity may be modified by the expression of MHC isoforms with different properties (e.g. MHC-beta, MHC-2a) and by simultaneous expression of multiple MHC isoforms in single muscle fibers, thereby creating fibers with intermediate properties [Reiser et al., 1985]. Adult mammalian appendicular muscle achieves contractile diversity by expression of conventional MHC-beta (slow), MHC-2a, MHC-2x and MHC-2b (fast) and limited hybridization [Caiozzo et al., 2003; Kohn et al., 2007].
MHC isoform expression is also highly plastic and changes in response to hormonal, neural, mechanical and energetic stimuli [Pette and Staron, 2000]. Chronic electrical stimulation increases the expression of ‘slower’ isoforms at the expense of ‘faster’ isoforms [Termin et al., 1989; Jaschinski et al., 1998]. Mechanical stretch promotes the expression of MHC-beta and represses MHC-2b [Goldspink et al., 1991], and both circulating and locally produced growth factors influence the MHC profile [Russell et al., 1988; Yang et al., 1996]. The majority of myosin genes are located in clusters on 2 chromosomes: a slow cluster of atrial/cardiac-α MHC (MHC-alpha) and MHC-beta on human chromosome 14q2 and a fast cluster of embryonic MHC (MHC-emb), neonatal MHC (MHC-neo), MHC-2a, MHC-2x, MHC-2b and extraocular MHC (MHC-eo) on human chromosome 17p13 [Leinwand et al., 1983]. Selection of isoforms within these clusters appears to result from a mixture of promoter-specific mechanisms and intergenic transcription [Allen et al., 2001; Rinaldi et al., 2008]. Expression in most adult appendicular muscles is limited to MHC-beta, MHC-2a, MHC-2x and MHC-2b, with limited expression of MHC-neo and MHC-emb. Within the spectrum of conventional myosins, expression of slower isoforms increases with body size [Hamalainen and Pette, 1995].
Exceptions to these rules are found in some head and neck muscles which routinely express developmental MHCs, including MHC-emb and MHC-neo, and unconventional MHCs, including MHC-alpha, MHC-eo, masseter MHC (MHC-ma) and slow tonic MHC (MHC-st) [Bormioli et al., 1979; Wieczorek et al., 1985; Hoh, 2002; Kjellgren et al., 2003]. These muscles may contain individual fibers containing 3 or more MHC isoforms [Kranjc et al., 2000; Yu et al., 2002]. The comparatively complex MHC expression patterns in head and neck muscles are thought to reflect more complex neural signals or requirements for greater contractile diversity in muscles active during kinematically diverse movements [Hoh, 2005; Toniolo et al., 2008]. For example, extraocular muscles, which must accomplish both rapid reorientation of the eye and maintenance of an extremely steady position, display specialized fiber morphology and expression of MHC-alpha, MHC-emb, MHC-eo, MHC-neo and MHC-st [Porter, 2002; Kjellgren et al., 2003; Lim et al., 2006]. Many of the head and neck muscles derive from branchial arches of somitomeres, in contrast to limb and trunk muscles, which derive from somites. Branchial arch muscles, including the laryngeal muscles and muscles of mastication, appear to be dependent on Tbx1 for myogenic commitment, rather than pax3/7, seen in somite-derived muscles of the limb and tongue [Buckingham et al., 2003; Kelly et al., 2004; Noden and Francis-West, 2006], and this difference in lineage may contribute to MHC expression.
Of particular interest is the expression of MHC-ma and MHC-st, which lie outside of the conventional myosin clusters. MHC-ma, or MYH16, is found on chromosome 7, while MHC-st, tentatively identified as MYH15, is found on chromosome 20 [Desjardins et al., 2002; Stedman et al., 2004]. MHC-ma is expressed in jaw-closing muscles of some carnivores, primates and bats [Hoh, 2002], but its presence has not been widely tested in other head and neck muscles. The human MHC-ma gene produces a truncated and nonfunctional protein but is still transcribed in masseter [Stedman et al., 2004]. MHC-st is demonstrated by immunohisotochemistry (IHC) in extraocular muscles, but its presence in other head and neck muscles is in dispute [Han et al., 1999; Brandon et al., 2003; Sokoloff et al., 2007a; Sokoloff et al., 2009]. To our knowledge, MHC-st has not been studied by PCR, which enables identification of low levels of mRNA expression. The unique expression of these developmental and unconventional isoforms in head and neck muscles raises the question of whether it reflects the unique mechanical demands of specific muscles, the unique developmental origin of some muscles or a general phenomenon of the head and neck environment.
Mammalian tongue muscles are active during oromotor behaviors that encompass a wide range of tongue behaviors, including tonic, phasic and ballistic tongue activation [van Willigen and Weijs-Boot, 1984; Saboisky et al., 2006], and might be expected to exhibit complex patterns of MHC expression. Immunohistochemical studies of extrinsic tongue muscles (i.e. muscles with their origin outside of the tongue body) and intrinsic tongue muscles (i.e. muscles with their origin and insertion within the tongue body) have identified MHC-beta, MHC-2a and MHC-2x in the adult macaque and human, and MHC-2b in macaque [Stal et al., 2003; Smith et al., 2006; Sokoloff et al., 2007b; Sokoloff et al., 2009], but have found no or limited developmental and unconventional MHC [Sokoloff et al., 2007a; Sokoloff et al., 2009]. However, these studies are limited by discordance in reports of antibody specificities [Liu et al., 2002; Mu et al., 2004; Sokoloff et al., 2007b], the limited availability of antibodies specific for MHC-alpha and, with the exception of one test for MHC-st [Sokoloff et al., 2007a], no study of unconventional MHC in intrinsic tongue muscles. Electrophoresis studies of MHC in tongue muscles of the adult mouse and rat have identified MHC-2a, MHC-2b and MHC-2x and no or limited MHC-beta, MHC-emb and MHC-neo [Brozanski et al., 1993; Hartmann et al., 2001; Agbulut et al., 2003], but may not allow confident determination of MHC with similar migration mobilities, such as MHC-neo and MHC-emb [Brozanski et al., 1993; Lloyd et al., 1996]. The dynamic range of silver-stained gels is approximately 1 log, while the dynamic range of Coomassie-stained gels is approximately 2 logs, so the expression of unconventional MHC isoforms in a few fibers could be easily overlooked. The dynamic range of real-time, quantitative PCR (qPCR) is approximately 7 logs, making detection of very low-level expression possible.
To our knowledge, the MHC composition of adult mammal tongue body muscles has not been characterized by sensitive real-time PCR of MHC mRNA. To address this gap in our understanding of tongue muscle biology, we determined the presence and quantity of mRNA of conventional MHC, developmental MHC and unconventional MHC-alpha, MHC-eo, MHC-ma and MHC-st in tongue body muscles in the rat, macaque and human.
Methods
Specimens and Tissue Preparation
Tongue body tissue for mRNA study was harvested from the left or right side of the tongue from 6 rats, 11 macaques (Macaca rhesus) and 6 humans (see table 1 for ages and sex). To maximize the similarity of tongue body muscles studied across these species, we sought to primarily sample the intrinsic tongue muscles inferior longitudinalis, superior longitudinalis, transversus and verticalis of the anterior tongue body and to minimize involvement of the extrinsic tongue muscles genioglossus, hyoglossus and styloglossus. To this aim, we harvested tissue approximately 5–10 mm from the tongue tip in the rats, approximately 10–15 mm from the tongue tip in the monkeys and 0.8–2.0 mm from the tongue tip in the humans, excluding midline tissue in the macaques and humans. Although intrinsic muscles predominate in these tongue regions, it is likely that we sampled some fibers of extrinsic tongue muscles, particularly human genioglossus and styloglossus, which may have anterior tongue body insertions [Gaige et al., 2007]. Control muscle tissue was sampled from adult human biceps brachii and extraocular medial rectus, fetal human tongue, adult macaque ventricle, adult mouse heart, adult mouse gastrocnemius and fetal mouse hindlimb muscle.
Table 1.
Sex and age of monkey and human subjects
| Sex | Age |
||
|---|---|---|---|
| years | months | ||
| Monkey | F | 3 | 11 |
| Monkey | F | 22 | 7 |
| Monkey | F | 23 | 4 |
| Monkey | F | 23 | 9 |
| Monkey | F | 24 | 7 |
| Monkey | F | 31 | |
| Monkey | M | 5 | 8 |
| Monkey | M | 6 | |
| Monkey | M | 6 | 6 |
| Monkey | M | 7 | 5 |
| Monkey | M | 23 | 7 |
| Human | F | 63 | |
| Human | F | 66 | |
| Human | F | 86 | |
| Human | M | 56 | |
| Human | M | 63 | |
| Human | M | 80 | |
Rat tissue was harvested within 15 min postmortem, and macaque (California National Regional Primate Center, Yerkes National Primate Research Center) and human tissue (Emory University School of Medicine Body Donor Program, National Disease Research Interchange) within 12 h postmortem. Tissue was immediately frozen in isopentane cooled by liquid nitrogen and stored until use at −80°C. Animal tissue was collected from animals sacrificed during the course of studies approved by the Institutional Animal Care and Use Committee and not involving the orofacial musculature. Human tissue used in this study is exempt from Institutional Review Board approval.
Sample Preparation
A sample of 50–100 mg was homogenized using a Tissuemiser (Fisher Scientific) in 1 ml of Trizol reagent (Invitrogen) according to the manufacturer's protocol. Total RNA yield was determined by UV spectroscopy. Reverse transcription was performed with 1 μg of total RNA using the MultiScribe RT kit (Invitrogen) according to the manufacturer's protocol using random primers.
qPCR Primer Design
To achieve species independence, human and mouse mRNA sequences of each isoform were aligned using the Biology Workbench 3.2 web tool [Subramaniam, 1998]. Primers were designed to identical regions of the aligned sequences using the Primaclade web tool [Gadberry et al., 2005], and specificity was confirmed by Nucleotide Blast search (National Center for Biotechnology Information). Species independence for 2 targets required degenerate bases, and all possible base combinations were tested to verify target specificity.
Candidate primer sets were further validated by conventional PCR and by melting curve analysis. For conventional PCR validation, cDNA was amplified from specifically identified positive control samples, i.e. human biceps and mouse gastrocnemius for MHC-beta, MHC-2a, MHC-2x, MHC-2b and MHC-st, macaque ventricle and mouse heart for MHC-alpha, human and mouse fetal muscle for MHC-emb and MHC-neo, human medial rectus muscle for MHC-eo and cat masseter for MHC-ma. The selection of limb muscle as control for MHC-st was based on the presence of muscle spindles. Products were separated through 2% agarose-Tris-borate-EDTA buffer gels, and only primer sets that produced unitary products of the expected molecular weight were accepted. Further validation by melting curve analysis required that primer sets produce a single melting temperature within 2°C of temperatures predicted using a nearest neighbor model [Sugimoto et al., 1996].
PCR Standards
To quantitatively compare isoforms, expression was quantified using target-specific standard curves. Standards for absolute quantification were prepared by band purification of PCR products. Products were separated by electrophoresis through a 7% Tris-borate-EDTA-buffered acrylamide gel and visualized by ethidium bromide staining. Specific bands were excised from the gel and minced using a razor blade, and DNA was extracted overnight at 4°C in 100 mM Tris, pH 7.5, 150 mM NaCl and 5 mM EDTA (TNE). DNA was recovered by repeated ethanol precipitation, and the final product was diluted in water and quantified by Hoechst 33258 (0.1 μg/ml in TNE) fluorescence.
Quantitative PCR
Amplification reactions contained 250 nM forward and reverse primers in Platinum SYBR Green qPCR Supermix (Invitrogen). The thermal protocol for all targets was 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 62°C for 1 min and a terminal melting curve. All samples were run in triplicate along with standards. Reverse transcription products were diluted by a factor specific to the PCR target, determined by pilot experiments with the goal of producing threshold cycles around 20 (table 2). Each PCR assay was validated by standard curve analysis, requiring efficiency within the range of 100 ± 20% (table 3). Individual reactions were validated by melting curve analysis, and samples that did not pass the melting curve validation were discarded. Samples falling below the gene-specific threshold quantity are reported as not detectable; however, numerical values associated with these samples were carried through calculations of total myosin content. These values are generally 1,000 times smaller than the total myosin content and do not substantially affect the results.
Table 2.
Real-time PCR parameters
| Target | Forward primer (5′-3′) | Reverse primer (5′-3′) | Amplicon size, bp | Dilution factor | Predicted melting peak, °C |
|---|---|---|---|---|---|
| β-Actin | TTCAACACCCCAGCCATGT | GTAGATGGGCACAGTGTGGGT | 120 | 1:50 | H: 83.8 |
| Mu: 83.5 | |||||
| MHC-2x (MYH1) | GTACCACAGGGAAACTGGCTT | CTTGGTCATCAATGCTGGG | 222 | 1:250 | H: 82.5 |
| Mu: 83.2 | |||||
| MHC-2a (MYH2) | GTCTGCCAACTTCCAGAAGC | CAGCTTGTTCAAATTCTCTCTGAA | 307 | 1:25 | H: 85.9 |
| Mu: 86.0 | |||||
| MHC-emb (MYH3) | CTTGAGCACTGAGCTCTTCAAA | CAGCCTTYTCCAGCTCMATC | 180 | 1:25 | H: 82.4 |
| Mu: 83.0 | |||||
| MHC-2b (MYH4) | AAAGGTGGCCATTTACAAGCT | CAGCAGAGTTCAGACTTGTCAG | 146 | 1:25 | H: 84.2 |
| Mu: 84.8 | |||||
| MHC-alpha (MYH6) | AAGTCCTCCCTCAAGCTCATGGC | ATTTTCCCGGTGGAGAGC | 135 | 1:250 | H: 83.9 |
| Mu: 83.1 | |||||
| MHC-beta (MYH7) | GCTGTTATTGCAGCCATTG | TTCCTGTTGCCCCAAAATG | 184 | 1:250 | H: 85.8 |
| Mu: 84.3 | |||||
| MHC-neo (MYH8) | CCTCACTTCGTACGGTGTATCA | CCTCTGGAATAGCACTTGCAT | 199 | 1:25 | H: 82.0 |
| Mu: 84.8 | |||||
| MHC-eo (MYH13) | TGAGAGAYGAGAAGCTGGTGAC | TGAAGAACAGGTTCATCCAGGG | 177 | 1:25 | H: 85.1 |
| Mu: 84.1 | |||||
| MHC-st (MYH15) | GGCAAATGCTGAGAAACTCTG | GCCGAGTGAAGTTGCTCTTT | 206/187 | 1:25 | H: 83.7 |
| Mu: 82.0 | |||||
| MHC-ma (MYH16) | GGGGAGATCCAGTCTGAACA | GTAGATGGGCAGCCACTTGT | 258 | 1:25 | H: 85.0 |
Species-independent primer sets for MYH isoforms, amplicon size, dilution factor used in the assay and predicted melting temperatures for human (H) and mouse/rat (Mu) samples.
Table 3.
PCR target validation results
| Target | Average efficiency % | Detection threshold molecules | Detection limit pg/μg RNA | Observed melting temperature °C |
|---|---|---|---|---|
| β-Actin | 97 ± 2 | 600 | 0.06 | H: 86.6 |
| M: 87.8 | ||||
| R: 85.6 | ||||
| MHC-2x (MYH1) | 90 ± 5 | 6,000 | 9.2 | H: 80.5 |
| M: 80.7 | ||||
| R: 81.6 | ||||
| MHC-2a (MYH2) | 82 ± 3 | 600 | 0.09 | H: 85.6 |
| M: 85.1 | ||||
| R: 85.5 | ||||
| MHC-emb (MYH3) | 86 ± 1 | 60 | 0.01 | H: 79.8 |
| M: 79.8 | ||||
| R: 80.7 | ||||
| MHC-2b (MYH4) | 91 ± 3 | 6,000 | 0.9 | H: 83.0 |
| M: 83.5 | ||||
| R: 84.4 | ||||
| MHC-alpha (MYH6) | 90 84 | 60 | 0.09 | H: 84.1 |
| M: 82.9 | ||||
| Mu: 82.3 | ||||
| MHC-beta (MYH7) | 90 ± 1 | 600 | 0.9 | H: 87.8 |
| M: 87.1 | ||||
| Mu: 84.9 | ||||
| MHC-neo (MYH8) | 90 ± 0.4 | 600 | 0.09 | H: 82.2 |
| M: 82.9 | ||||
| R: 85.6 | ||||
| MHC-eo (MYH13) | 87 ± 2 | 6,000 | 0.9 | H: 84.7 |
| M: 84.6 | ||||
| R: 84.7 | ||||
| MHC-st (MYH15) | 84.7 ± 3 | 3,010 | 0.6 | H: 83.2 |
| M: 83.5 | ||||
| Mu: 81.6 | ||||
| MHC-ma (MYH16) | 83.8 ± 2 | 301 | 0.05 | H: 85.0 |
| M: 85.3 | ||||
| cat: 85.0 | ||||
H = Human; M = monkey; R = rat; Mu = mouse/rat.
Determination of Sensitivity
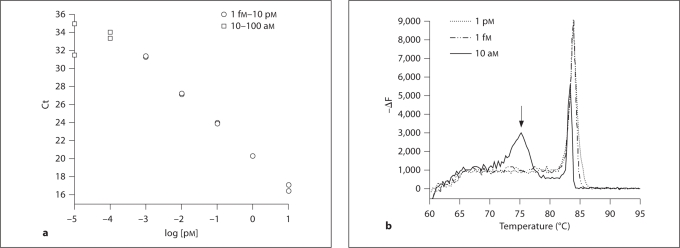
The detection threshold was determined by amplification of serial dilutions of each standard. Such standard curves often deviate from the expected amplification curve at low concentrations (fig. 1), reflecting the appearance of primer dimer or other amplification anomalies. The detection threshold was defined as the number of molecules in the lowest standard observed before this deviation. The detection limit was defined by dividing the target-specific quantity of cDNA input to the PCR reaction.
Fig. 1.
Determination of detection threshold. Detection threshold was defined as the number of molecules in the lowest standard before observation of primer dimer formation. a The standard curve for MHC-2b shows that low concentrations (□) deviate from the line defined by higher concentrations (○). Ct = Threshold cycle. b Melting peak analysis reveals that these peaks contain primer dimer reaction, indicated by the peak at a lower temperature (arrow). In this case, the detection threshold would be 1 fM × 1 μl of sample volume, or 600 molecules. ΔF = Change in fluorescence per change in temperature.
Statistics
Data are expressed as means ± SD except where noted otherwise. Analysis by 2-way ANOVA (species × isoform) was used to determine main effects with a significance threshold of p < 0.05. Post hoc comparisons to determine gene-specific differences between species used the Bonferroni/Dunn correction for multiple comparisons, which gives a significance threshold of p < 0.0167.
Results
Validation of Species-Independent MHC Primers for qPCR
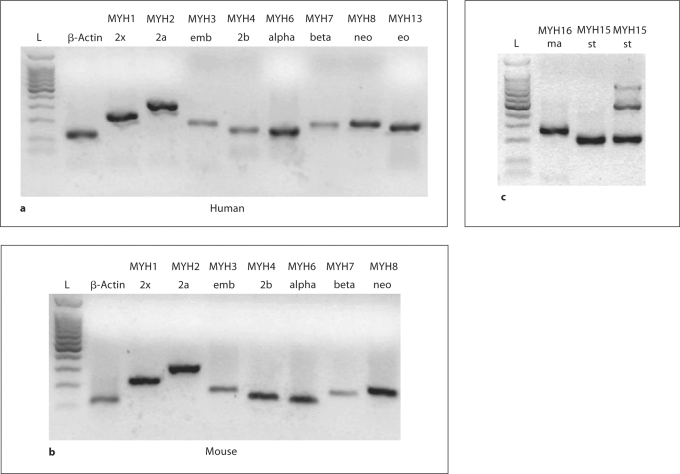
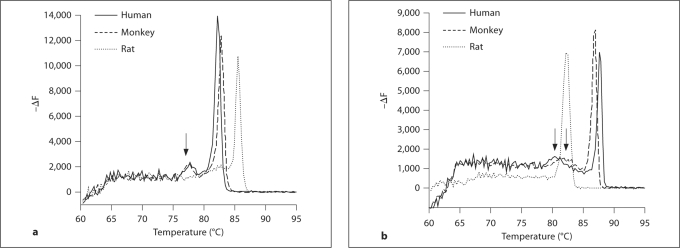
Primers were designed for 10 MHC isoforms, and conventional PCR reveals a single band at the correct weight for each isoform (fig. 2). A single melting peak for 8 of 10 isoforms (data not shown), near the expected temperature, confirmed the validity of the selected primers (table 2). Melting curves for MHC-beta and MHC-neo (MYH7 and MYH8, respectively) consistently produced a small, secondary melting peak (fig. 3) that was unaffected by changes in primer concentration, template concentration or annealing temperature. The magnitude of this peak was a constant ratio of the primary melting peak, and the temperature was greater than expected for a primer dimer. Agarose gel analysis of PCR products consistently revealed only a single product weight, and we concluded that the secondary peak represents secondary structure within the product.
Fig. 2.
a, b Amplifications of positive control samples from humans (a) and mice (b). No mouse or rat extraocular muscle was available. c MHC-ma primers amplify a strong band from cat masseter, and MHC-st primers amplify a strong band from human extraocular muscle. MHC-st primers also amplify the expected 187 bp product from a mouse soleus (lane 4), with an additional 446 bp band from genomic DNA. All products are intron-spanning, and no study samples contained genomic DNA products.
Fig. 3.
Multiple peaks in melting curves. A small secondary peak appeared in melting curves for MHC-neo (a) and MHC-beta (b) isoforms after qPCR of human, monkey and rat samples (arrows). The ratio of this secondary peak to the primary peak is independent of primer or template concentration or annealing temperature. Separation on a 2% agarose gel yields 1 product at the appropriate weight (see fig. 2), suggesting that the smaller peak likely represents a secondary structure within the sequence. ΔF = Change in fluorescence per change in temperature.
The efficiency of standard curves ranged from 82 to 97%. The principal limitation to detection was the formation of primer dimers, indicated by a plateau of the threshold cycle, generally at fewer than 6,000 copies, depending on the isoform (table 3; fig. 1). Establishing the detection threshold provided a guideline to determine sample dilutions and detection limits (table 3).
Absolute Quantification of Myosin Isoform Expression in the Tongue
Total MHC content averaged 1,700 ± 2,300 pg/μg RNA (table 4). Intersample variability was quite high, with total content ranging from 17 to 10,000 pg/μg RNA. β-Actin variability was also high, ranging from 1 to 23 pg/μg RNA, suggesting that some of this variability reflects variability of RNA quality or reverse transcription efficiency. Average β-actin content was 9.5 ± 6.4 pg/μg total RNA, which is about a third of that reported in other studies [Schmittgen and Zakrajsek, 2000; Ambion, 2009].
Table 4.
Species-specific expression of MHC isoforms
| Human | Monkey | Rat | |
|---|---|---|---|
| β-Actin | 6.2 ± 3.7 | 3.7 ± 2.5 | 5.3 ± 3.7 |
| MHC-2x (MYH1) | 87 ± 67 | 350 ± 370 | 590 ± 560 |
| MHC-2a (MYH2) | 670 ± 1,200 | 1,700 ± 2,400 | 42 ± 53 |
| MHC-emb (MYH3) | 1.8 ± 2.5 | 0.75 ± 0.55 | 0.14 ± 0.15 |
| MHC-2b (MYH4) | 0.43 ± 0.64 | 5.2 ± 3.0 | 290 ± 240 |
| MHC-alpha (MYH6) | 6.7 ± 15 | 0.11 ± 0.10 | ND (<0.09) |
| MHC-beta (MYH7) | 400 ± 800 | 260 ± 350 | 0.12 ± 0.048 |
| MHC-neo (MYH8) | 0.41 ± 0.38 | 6.7 ± 8.8 | 0.38 ± 0.45 |
| MHC-eo (MYH13) | ND (<0.9) | 4.1 ± 4.3 | ND |
| MHC-st (MYH15) | 0.82 ± 0.80 | 1.1 ± 0.87 | ND (<0.5) |
| MHC-ma (MYH16) | ND (<0.05) | ND | ND |
Values are shown as means ± SD (pg/μg). Isoforms for which all samples within a species fell below the detection limit are indicated as not detectable (ND).
Conventional MHC Content
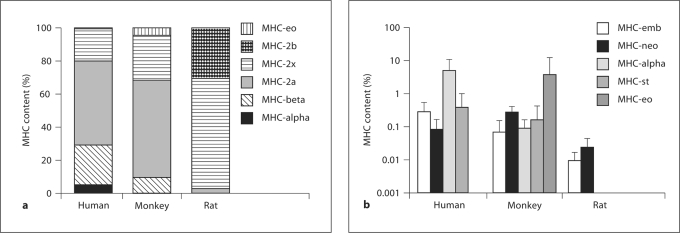
MHC isoform expression was summed across isoforms, and the fractional expression of each isoform was calculated (fig. 4). Although intersample variability in absolute units was high, isoform profiles were relatively consistent within each species. Conventional, appendicular MHCs predominated in all species. Human and monkey profiles were similar, consisting primarily of MHC-2x (20 ± 14% in human, 27 ± 21% in monkey), MHC-2a (50 ± 21%, 59 ± 22%) and MHC-beta (24 ± 13%, 10 ± 5%). In monkeys, MHC-2b contributed less than 0.25% of total MHC and was undetectable in humans. Statistical resolution was limited, but humans do express a significantly higher fraction of MHC-beta than monkeys (p = 0.0026). Rats primarily express MHC-2x (68 ± 10%), with substantial MHC-2b (31 ± 10%) but no detectable MHC-beta. Expression of MHC-2b, MHC-2x and MHC-2a in rats was significantly different from both primates (p < 0.003).
Fig. 4.
MHC isoform profiles for each species. a Conventional and prominently expressed isoforms. b Developmental and unconventional isoforms.
Developmental and Unconventional MHC Content
Combined expression of MHC-neo and MHC-emb isoforms contributed less than 0.8% of MHC in all individuals of all 3 species. The detection limits were 0.009 pg/μg RNA for MHC-emb and 0.09 pg/μg RNA for MHC-neo. MHC-emb was detectable in all human and monkey samples but in only 4 of 6 rats. MHC-neo was detectable in 2 of 6 humans, 10 of 11 monkey samples and 3 of 6 rats. In those samples in which developmental isoforms were above the threshold, their sum was 0.36 ± 0.21% of total MHC (fig. 4), with the highest value being 0.72% of total MHC. MHC-emb expression is significantly higher in humans compared to other species (p = 0.015), while MHC-neo expression is greatest in monkeys (p = 0.007).
Unconventional myosins were also expressed at much lower levels than conventional myosins and were differentially expressed in human and monkey tongues. MHC-alpha was observed in all human tongue samples and constitutes 5 ± 6% of total MHC. In monkey samples, MHC-alpha constitutes <0.1% of total myosin, averaging 1.1 ± 1.0 pg/μg RNA, and was under the detection limit of 0.09 pg/μg RNA in 1 sample. MHC-alpha was not detected in any rat sample. MHC-eo was detectable in 8 of 11 monkey samples, but was extremely variable. In 6 samples, MHC-eo was 0.17 ± 0.16% of total MHC, but 2 samples contained 12 and 27%, bringing the population mean to 3.7 ± 8.5%. The absolute content of MHC-eo in these samples was 8 pg/mg, similar to the other samples, but the absolute content of conventional myosins (MHC-beta, MHC-2a and MHC-2x) was less than 58 pg/μg, compared with 2,300 pg/μg for the whole population, which suggests that the higher percentages of MHC-eo were not representative. MHC-eo was not detectable in any human or rat sample. MHC-st was not detected in any rat and was less than 0.05% of total MHC in both humans and monkeys. MHC-ma was not detectable in any sample.
Discussion
The principal finding of this study is that conventional MHC isoforms predominate in rat, macaque and human muscles of the anterior tongue body. Also notable is the greater expression of MHC-alpha in humans (5% of total MHC) versus macaques (<0.2%) and the unique expression of MHC-eo in most macaques. In all species, developmental MHC is expressed at very low levels (MHC-neo and MHC-emb together 0.35% of total MHC).
MHC content was extremely variable between samples. Of particular note are the 2 monkey samples that contained roughly 100 times less conventional MHC than the rest of the population. Variability in MHC content probably reflects a combination of variable RNA quality, shown also by the high variability of β-actin abundance, and variability in the amount of muscle contained in the small samples used for homogenization. Variability in the anatomical origin of samples may also contribute to the variability in isoform profiles. Our goal was to test the same muscles in each species, but due to the complexity of tongue muscle fiber architecture, our samples likely varied with respect to the relative amount of different intrinsic tongue muscles, the contribution of extrinsic tongue muscles and the contribution of nonmuscular tongue tissue.
Comparison to Other Tongue Studies
Previous studies of adult mammal tongue muscles have documented isoforms typical of appendicular muscles (i.e. MHC-beta, MHC-2a, MHC-2x, MHC-2b) but little or no unconventional or developmental MHC. This is consistent with the results of the present study, in which expression of unconventional and developmental myosins is substantially below the detection threshold expected of protein gels.
Rats. Using protein electrophoresis, rat tongue body has been reported to contain 8% MHC-2a, 64–72% MHC-2x, 20–24% MHC-2b and no MHC-beta [Termin and Pette, 1991; Jee et al., 2009], which is similar to our findings. D’Albis et al. [1989] could not detect either MHC-neo or MHC-emb after postnatal day 30, and the combined expression of MHC-neo and MHC-emb of <0.2% that we report would be expected to be below the detection threshold of protein electrophoresis. Electrophoresis studies of the adult rat extrinsic tongue muscles report similar profiles, with a predominance of MHC-2x (35–60%) and the balance consisting of MHC-2a (19–24.2%) and MHC-2b (20–45%), with no MHC-beta or developmental MHC [Brozanski et al., 1993; Vincent et al., 2002; Moore et al., 2007; Volz et al., 2007].
Macaques. MHC composition of the macaque tongue body is consistent with the general predominance of MHC-2a in the primate tongue. By examining ATPase activity of intrinsic muscles from tongue regions similar to those of the current study, DePaul and Abbs [1996] reported approximately 80% type IIA fibers, 20% type I fibers and minimal type IIB and type IIC fibers [DePaul and Abbs, 1996, fig. 4, tongue regions 2 and 3]. In contrast, by examining mRNA content, we found 59% MHC-2a, 27% MHC-2x and 10% MHC-beta in macaque tongue body muscles. Differences in tongue muscle composition reported in these studies may be related to the poor correspondence between fiber type and MHC content, especially for hybrid fibers [Serrano et al., 2001; Smerdu and Erzen, 2001], differences in the muscles sampled and the difference in species (M. fascicularis and M. rhesus). Studies of macaque extrinsic tongue muscles also report a predominance of MHC-2a, with 64.4–69.6% MHC-2a, 19.8–29.6% MHC-beta, 0–6.6% MHC-2x and 3.4–6.0% MHC-2b in genioglossus, hyoglossus and styloglossus by electrophoresis [Smith et al., 2006]. Using IHC, we previously identified MHC-2a and MHC-beta in 71.3 and 33.5%, respectively, of macaque styloglossus muscle fibers, including some hybrid fibers [Sokoloff et al., 2007b].
Humans. The MHC expression in human tongue is very similar to other primates. By examining ATPase activity, Stål et al. [2003] reported 59% type IIA, 21% type I, 11% type IIAB, 8% type IM/IIC and 1% type IIB fibers in human anterior intrinsic tongue muscles (57% type IIA, 38% type I and 5% type IM/IIC fibers in the middle-anterior tongue body). Our findings are similar with respect to MHC-2a (50% of total MHC) and MHC-beta (24% of total MHC). We also noted a 20% prevalence of MHC-2x, greater than the 11% type IIAB + 1% type IIB fibers reported by Stål et al. [2003]. In the human styloglossus and hyoglossus, we previously reported 13–19% MHC-beta-MHC-2x fibers but few MHC-2x fibers (we could not distinguish MHC-2a-MHC-2x fibers [Sokoloff et al., 2007b; Sokoloff et al., 2009]), suggesting that this isoform may be primarily expressed in hybrid fibers. In previous IHC studies of adult human extrinsic tongue muscles, we found limited or no MHC-emb, MHC-eo, MHC-neo and MHC-st [Sokoloff et al., 2007a, b; Sokoloff et al., 2009]. Here, we extend these findings to anterior tongue body muscles.
Developmental and Unconventional MHC Expression
Developmental Isoforms. Expression of developmental MHC isoforms was approximately 0.3% in humans and macaques and 0.05% in rats. Similar limited expression of developmental MHC has been demonstrated in many head and neck muscles by PCR and IHC [Jung et al., 1999; Sundman et al., 2004; Tellis et al., 2004]. Detection of developmental MHC in these muscles is expected to be below the detection threshold of silver- or Coomassie-stained protein gels, consistent with the general observation that these isoforms are not identified by electrophoresis in adult muscle. Developmental myosins are expressed with greater prevalence in a few specific head and neck muscles, i.e. masseter (hard diet) 12%, thyroarytenoid 4.4%, lateral cricoarytenoid, 3.3%, tensor tympani 7.5% and extraocular muscles 11–14% [Jung et al., 1998; Jung et al., 1999; Saito et al., 2002; Jung et al., 2004]. Developmental myosins are also expressed in muscle regenerating after traumatic or contractile damage. The role of the tongue in mastication places it under compressive stresses not associated with appendicular muscle and places it at risk for accidental biting, and the low level of developmental MHC mRNA in the tongue body suggests that fiber regeneration is not routine. MHC-neo has been reported to be expressed in the tapered ends of fibers, particularly in birds [Rosser et al., 1995], and the low level of MHC-neo we found in tongue homogenates is consistent with expression restricted to very few fibers or fiber subdomains, such as tapered ends.
Unconventional Isoforms. MHC-st expression is teleologically consistent with tonic activation of head and neck muscles during respiration and with the activation of muscles for structural support of a muscular hydrostat [Saboisky et al., 2006; Gilbert et al., 2007]. MHC-st was detected in humans and monkeys at less than 0.5%, consistent with our previous observation of limited MHC-st in human tongue muscles by IHC [Sokoloff et al., 2007a]. Expression of MHC-st in the head and neck appears to be limited to extraocular muscles [Kranjc et al., 2000; Kjellgren et al., 2003], middle ear muscles [Mascarello et al., 1983] and other branchial arch musculature [Han et al., 1999; Mu et al., 2004]. MHC-st is also expressed in muscle spindles [Liu et al., 2002], but it seems that a special developmental program may be required to permit expression of either MHC-ma or MHC-st.
MHC-eo has been reported in extraocular and laryngeal muscles in many mammal species [Jung et al., 1999; Hoh, 2005; Toniolo et al., 2008]. Although the MHC-eo gene is found in the conventional fast MHC cluster, we found only a trace of this isoform and only in macaques. Since we could detect every other MHC from the fast cluster in all 3 species, this suggests that MHC-eo is under greater repression than other MHC isoforms.
Expression of MHC-alpha has been reported in jaw-closing muscles from several species [Bredman et al., 1991; Hoh et al., 2000] and to be the dominant slow isoform in rabbit temporalis [Korfage et al., 2006]. Peuker and Pette [1995] report MHC-alpha mRNA content of 18 pg/μg RNA in rabbit masseter, with a ratio of MHC-alpha to MHC-beta of 18:1. In humans, we found that MHC-beta is the dominant slow isoform in tongue. Although we found significant expression of MHC-alpha (67 pg/μg; 5% of total), the ratio of MHC-alpha to MHC-beta was 1:6. This is similar to jaw-closing and laryngeal muscles of humans [Korfage et al., 2000; Horton et al., 2008], so it does seem that both somitic and branchial arch muscles can express MHC-alpha from the slow myosin cluster. IHC suggests that MHC-alpha is typically coexpressed with 1 or more other isoforms [Bredman et al., 1991; Korfage et al., 2000; Kjellgren et al., 2003]. The contractile properties of MHC-alpha are intermediate between those of MHC-beta and MHC-2a, though closer to MHC-beta [Andruchov et al., 2004]. MHC-alpha expression in the human tongue might thus enable an increased gradation of muscle fiber contractile properties. However, a similar gradation of fiber contractile properties might also be achieved by hybridization of slow and fast MHC, as demonstrated by ATPase activity and MHC co-expression in intrinsic and extrinsic human tongue muscles [Stal et al., 2003; Sokoloff et al., 2007a] and in the macaque styloglossus [Sokoloff et al., 2007b].
Scaling
We observed a progressive increase in slow MHC isoforms from small rats to large humans. Myosin isoform expression in the limbs also varies systematically with organism size, with larger animals expressing a greater proportion of slow isoforms [Hamalainen and Pette, 1995]. This isoform shift partially resolves the scaling of muscle-shortening velocity with the inverse cube root of mass [Hill, 1950]. Both bite size and food intake rate scale with the 2/3 power of mass, while the time required to crop a bite is uncorrelated with mass [Shipley et al., 1994], which suggests no special speed requirement for jaw and tongue musculature. Nonetheless, we observed the same trend in tongue MHC profile as is seen in limb musculature, with a progressive decrease in MHC-2x expression from rat to macaque to human and a progressive increase in MHC-beta.
The purpose of this project was to determine whether tongue muscle expresses unconventional and developmental MHC isoforms, as has been reported for other head and neck muscles. We found that the intrinsic musculature of the tongue is more similar to limb musculature than to other, nonsomitic head and neck muscles, almost exclusively expressing the conventional MHCs, i.e. MHC-beta, MHC-2a, MHC-2x and MHC-2b, in rats, monkeys and humans. This supports a regulatory model in which developmental origin takes precedence over mechanical signals in the expression of unconventional myosins.
Acknowledgement
This project was supported by National Institutes of Health grant DC005017 to A.J.S. The National Institutes of Health had no role in the design, performance or interpretation of the study. The authors thank the Emory University School of Medicine Body Donor Program and the National Disease Research Interchange for human tissue and Ms. Sona Santos and the California National Primate Research Center (Research Center Base Grant RR00169, National Center for Research Resources, NIH) and the Yerkes Primate Research Center (Research Center Base Grant RR000165, National Center for Research Resources, NIH) for non-human primate tissue.
Abbreviations used in this paper
- IHC
immunohistochemistry
- MHC
myosin heavy chain
- MHC-alpha
atrial/cardiac-α myosin heavy chain
- MHC-emb
embryonic myosin heavy chain
- MHC-eo
extraocular myosin heavy chain
- MHC-ma
masseter myosin heavy chain
- MHC-neo
neonatal myosin heavy chain
- MHC-st
slow tonic myosin heavy chain
References
- Agbulut O., Noirez P., Beaumont F., Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell. 2003;95:399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Allen D.L., Sartorius C.A., Sycuro L.K., Leinwand L.A. Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem. 2001;276:43524–43533. doi: 10.1074/jbc.M108017200. [DOI] [PubMed] [Google Scholar]
- Ambion (2009) Use of internal and external standards or reference RNAs for accurate quantitation of RNA levels. Ambion Technical Bulletins. Technical Bulletin 151. Austin, Ambion.
- Andruchov O., Wang Y., Andruchova O., Galler S. Functional properties of skinned rabbit skeletal and cardiac muscle preparations containing alpha-cardiac myosin heavy chain. Pflugers Arch. 2004;448:44–53. doi: 10.1007/s00424-003-1229-2. [DOI] [PubMed] [Google Scholar]
- Bormioli S.P., Torresan P., Sartore S., Moschini G.B., Schiaffino S. Immunohistochemical identification of slow-tonic fibers in human extrinsic eye muscles. Invest Ophthalmol Vis Sci. 1979;18:303–306. [PubMed] [Google Scholar]
- Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflugers Arch. 2001;443:6–17. doi: 10.1007/s004240100700. [DOI] [PubMed] [Google Scholar]
- Brandon C.A., Rosen C., Georgelis G., Horton M.J., Mooney M.P., Sciote J.J. Staining of human thyroarytenoid muscle with myosin antibodies reveals some unique extrafusal fibers, but no muscle spindles. J Voice. 2003;17:245–254. doi: 10.1016/s0892-1997(03)00013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredman J.J., Wessels A., Weijs W.A., Korfage J.A., Soffers C.A., Moorman A.F. Demonstration of ‘cardiac-specific’ myosin heavy chain in masticatory muscles of human and rabbit. Histochem J. 1991;23:160–170. doi: 10.1007/BF01046587. [DOI] [PubMed] [Google Scholar]
- Brozanski B.S., Daood M.J., Watchko J.F., LaFramboise W.A., Guthrie R.D. Postnatal expression of myosin isoforms in the genioglossus and diaphragm muscles. Pediatr Pulmonol. 1993;15:212–219. doi: 10.1002/ppul.1950150406. [DOI] [PubMed] [Google Scholar]
- Buckingham M., Bajard L., Chang T., Daubas P., Hadchouel J., Meilhac S., Montarras D., Rocancourt D., Relaix F. The formation of skeletal muscle: from somite to limb. J Anat. 2003;202:59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiozzo V.J., Baker M.J., Huang K., Chou H., Wu Y.Z., Baldwin K.M. Single-fiber myosin heavy chain polymorphism: how many patterns and what proportions? Am J Physiol Regul Integr Comp Physiol. 2003;285:R570–R580. doi: 10.1152/ajpregu.00646.2002. [DOI] [PubMed] [Google Scholar]
- d'Albis A., Couteaux R., Janmot C., Roulet A. Specific programs of myosin expression in the postnatal development of rat muscles. Eur J Biochem. 1989;183:583–590. doi: 10.1111/j.1432-1033.1989.tb21087.x. [DOI] [PubMed] [Google Scholar]
- DePaul R., Abbs J.H. Quantitative morphology and histochemistry of intrinsic lingual muscle fibers in Macaca fascicularis. Acta Anat (Basel) 1996;155:29–40. doi: 10.1159/000147787. [DOI] [PubMed] [Google Scholar]
- Desjardins P.R., Burkman J.M., Shrager J.B., Allmond L.A., Stedman H.H. Evolutionary implications of three novel members of the human sarcomeric myosin heavy chain gene family. Mol Biol Evol. 2002;19:375–93. doi: 10.1093/oxfordjournals.molbev.a004093. [DOI] [PubMed] [Google Scholar]
- Gadberry M.D., Malcomber S.T., Doust A.N., Kellogg E.A. Primaclade – a flexible tool to find conserved PCR primers across multiple species. Bioinformatics. 2005;21:1263–1264. doi: 10.1093/bioinformatics/bti134. [DOI] [PubMed] [Google Scholar]
- Gaige T.A., Benner T., Wang R., Wedeen V.J., Gilbert R.J. Three dimensional myoarchitecture of the human tongue determined in vivo by diffusion tensor imaging with tractography. J Magn Reson Imaging. 2007;26:654–61. doi: 10.1002/jmri.21022. [DOI] [PubMed] [Google Scholar]
- Galler S., Hilber K., Gohlsch B., Pette D. Two functionally distinct myosin heavy chain isoforms in slow skeletal muscle fibres. FEBS Lett. 1997;410:150–152. doi: 10.1016/s0014-5793(97)00556-5. [DOI] [PubMed] [Google Scholar]
- Gilbert R.J., Napadow V.J., Gaige T.A., Wedeen V.J. Anatomical basis of lingual hydrostatic deformation. J Exp Biol. 2007;210:4069–4082. doi: 10.1242/jeb.007096. [DOI] [PubMed] [Google Scholar]
- Goldspink G., Scutt A., Martindale J., Jaenicke T., Turay L., Gerlach G.F. Stretch and force generation induce rapid hypertrophy and myosin isoform gene switching in adult skeletal muscle. Biochem Soc Trans. 1991;19:368–373. doi: 10.1042/bst0190368. [DOI] [PubMed] [Google Scholar]
- Hamalainen N., Pette D. Patterns of myosin isoforms in mammalian skeletal muscle fibres. Microsc Res Tech. 1995;30:381–389. doi: 10.1002/jemt.1070300505. [DOI] [PubMed] [Google Scholar]
- Han Y., Wang J., Fischman D.A., Biller H.F., Sanders I. Slow tonic muscle fibers in the thyroarytenoid muscles of human vocal folds; a possible specialization for speech. Anat Rec. 1999;256:146–157. doi: 10.1002/(SICI)1097-0185(19991001)256:2<146::AID-AR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hartmann N., Martrette J.M., Westphal A. Influence of the Lurcher mutation on myosin heavy chain expression in skeletal and cardiac muscles. J Cell Biochem Suppl. 2001;suppl 36:222–231. doi: 10.1002/jcb.1109. [DOI] [PubMed] [Google Scholar]
- Hill A.V. The dimensions of animals and their muscular dynamics. Sci Prog. 1950;38:209–230. [Google Scholar]
- Hoh J.F. ‘Superfast’ or masticatory myosin and the evolution of jaw-closing muscles of vertebrates. J Exp Biol. 2002;205:2203–2210. doi: 10.1242/jeb.205.15.2203. [DOI] [PubMed] [Google Scholar]
- Hoh J.F. Laryngeal muscle fibre types. Acta Physiol Scand. 2005;183:133–149. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- Hoh J.F., Kim Y., Sieber L.G., Zhong W.W., Lucas C.A. Jaw-closing muscles of kangaroos express alpha-cardiac myosin heavy chain. J Muscle Res Cell Motil. 2000;21:673–680. doi: 10.1023/a:1005676106940. [DOI] [PubMed] [Google Scholar]
- Horton M.J., Rosen C., Close J.M., Sciote J.J. Quantification of myosin heavy chain RNA in human laryngeal muscles: differential expression in the vertical and horizontal posterior cricoarytenoid and thyroarytenoid. Laryngoscope. 2008;118:472–477. doi: 10.1097/MLG.0b013e31815c1a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaschinski F., Schuler M., Peuker H., Pette D. Changes in myosin heavy chain mRNA and protein isoforms of rat muscle during forced contractile activity. Am J Physiol. 1998;274:C365–C370. doi: 10.1152/ajpcell.1998.274.2.C365. [DOI] [PubMed] [Google Scholar]
- Jee H., Sakurai T., Kawada S., Ishii N., Atomi Y. Significant roles of microtubules in mature striated muscle deduced from the correlation between tubulin and its molecular chaperone alphaB-crystallin in rat muscles. J Physiol Sci. 2009;59:149–155. doi: 10.1007/s12576-008-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.H., Han S.H., Choi J.O. Expression of myosin heavy chain mRNA in rat laryngeal muscles. Acta Otolaryngol. 1999;119:396–402. doi: 10.1080/00016489950181459. [DOI] [PubMed] [Google Scholar]
- Jung H.H., Lieber R.L., Ryan A.F. Quantification of myosin heavy chain mRNA in somatic and branchial arch muscles using competitive PCR. Am J Physiol. 1998;275:C68–C74. doi: 10.1152/ajpcell.1998.275.1.C68. [DOI] [PubMed] [Google Scholar]
- Jung H.H., Han S.H., Nam S.Y., Kim Y.H., Kim J.L. Myosin heavy chain composition of rat middle ear muscles. Acta Otolaryngol. 2004;124:569–573. doi: 10.1080/00016480310002249. [DOI] [PubMed] [Google Scholar]
- Kelly R.G., Jerome-Majewska L.A., Papaioannou V.E. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Mol Genet. 2004;13:2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- Kjellgren D., Thornell L.E., Andersen J., Pedrosa-Domellöf F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci. 2003;44:1419–1425. doi: 10.1167/iovs.02-0638. [DOI] [PubMed] [Google Scholar]
- Kohn T.A., Hoffman L.C., Myburgh K.H. Identification of myosin heavy chain isoforms in skeletal muscle of four Southern African wild ruminants. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:399–407. doi: 10.1016/j.cbpa.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Korfage J.A., Brugman P., Van Eijden T.M. Intermuscular and intramuscular differences in myosin heavy chain composition of the human masticatory muscles. J Neurol Sci. 2000;178:95–106. doi: 10.1016/s0022-510x(00)00372-5. [DOI] [PubMed] [Google Scholar]
- Korfage J.A., Van Wessel T., Langenbach G.E., Van Eijden T.M. Heterogeneous postnatal transitions in myosin heavy chain isoforms within the rabbit temporalis muscle. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1095–104. doi: 10.1002/ar.a.20375. [DOI] [PubMed] [Google Scholar]
- Kranjc B.S., Sketelj J., Albis A.D., Ambroz M., Erzen I. Fibre types and myosin heavy chain expression in the ocular medial rectus muscle of the adult rat. J Muscle Res Cell Motil. 2000;21:753–761. doi: 10.1023/a:1010362926221. [DOI] [PubMed] [Google Scholar]
- Leinwand L.A., Fournier R.E., Nadal-Ginard B., Shows T.B. Multigene family for sarcomeric myosin heavy chain in mouse and human DNA: localization on a single chromosome. Science. 1983;221:766–769. doi: 10.1126/science.6879174. [DOI] [PubMed] [Google Scholar]
- Lim S.J., Jung H.H., Cho Y.A. Postnatal development of myosin heavy chain isoforms in rat extraocular muscles. Mol Vis. 2006;12:243–50. [PubMed] [Google Scholar]
- Liu J.X., Eriksson P.O., Thornell L.E., Pedrosa-Domellöf F. Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem. 2002;50:171–183. doi: 10.1177/002215540205000205. [DOI] [PubMed] [Google Scholar]
- Lloyd J.S., Brozanski B.S., Daood M., Watchko J.F. Developmental transitions in the myosin heavy chain phenotype of human respiratory muscle. Biol Neonate. 1996;69:67–75. doi: 10.1159/000244280. [DOI] [PubMed] [Google Scholar]
- Mascarello F., Veggetti A., Cerpenè E., Rowlerson A. An immunohistochemical study of the middle ear muscles of some carnivores and primates, with special reference to the IIM and slow-tonic fibre types. J Anat. 1983;137:95–108. [PMC free article] [PubMed] [Google Scholar]
- Moore W.A., Goldberg S.J., Shall M.S. Effects of artificial rearing on contractile properties of genioglossus muscle in Sprague-Dawley rat. Arch Oral Biol. 2007;52:133–141. doi: 10.1016/j.archoralbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mu L., Su H., Wang J., Han Y., SandersI I. Adult human mylohyoid muscle fibers express slow-tonic, alpha-cardiac, and developmental myosin heavy-chain isoforms. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:749–760. doi: 10.1002/ar.a.20065. [DOI] [PubMed] [Google Scholar]
- Noden D.M., Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Pette D., Staron R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Peuker H., Pette D. Reverse transcriptase-polymerase chain reaction detects induction of cardiac-like alpha myosin heavy chain mRNA in low frequency stimulated rabbit fast-twitch muscle. FEBS Lett. 1995;367:132–136. doi: 10.1016/0014-5793(95)00545-k. [DOI] [PubMed] [Google Scholar]
- Porter J.D. Extraocular muscle: cellular adaptations for a diverse functional repertoire. Ann NY Acad Sci. 2002;956:7–16. doi: 10.1111/j.1749-6632.2002.tb02804.x. [DOI] [PubMed] [Google Scholar]
- Reiser P.J., Moss R.L., Giulian G.G., Greaser M.L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985;260:9077–9080. [PubMed] [Google Scholar]
- Rinaldi C., Haddad F., Bodell P.W., Qin A.X., Jiang W., Baldwin K.M. Intergenic bidirectional promoter and cooperative regulation of the IIx and IIb MHC genes in fast skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;295:R208–R218. doi: 10.1152/ajpregu.00134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser B.W., Waldbillig D.M., Lovo S.D., Armstrong J.D., Bandman E. Myosin heavy chain expression within the tapered ends of skeletal muscle fibers. Anat Rec. 1995;242:462–470. doi: 10.1002/ar.1092420404. [DOI] [PubMed] [Google Scholar]
- Russell S.D., Cambon N., Nadal-Ginard B., Whalen R.G. Thyroid hormone induces a nerve-independent precocious expression of fast myosin heavy chain mRNA in rat hindlimb skeletal muscle. J Biol Chem. 1988;263:6370–6374. [PubMed] [Google Scholar]
- Saboisky J.P., Butler J.E., Fogel R.B., Taylor J.L., Trinder J.A., White D.P., Gandevia S.C. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- Saito T., Ohnuki Y., Yamane A., Saeki Y. Effects of diet consistency on the myosin heavy chain mRNAs of rat masseter muscle during postnatal development. Arch Oral Biol. 2002;47:109–115. doi: 10.1016/s0003-9969(01)00094-2. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Zakrajsek B.A. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/s0165-022x(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Serrano A.L., Perez M., Lucía A., Chicharro J.L., Quiroz-Rothe E., Rivero J.L. Immunolabelling, histochemistry and in situ hybridisation in human skeletal muscle fibres to detect myosin heavy chain expression at the protein and mRNA level. J Anat. 2001;199:329–337. doi: 10.1046/j.1469-7580.2001.19930329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley L.A., Gross J.E., Spalinger D.E., Hobbs N.T., Wunder B.A. The scaling of intake rate in mammalian herbivores. Am Nat. 1994;143:1055–1082. [Google Scholar]
- Smerdu V., Erzen I. Dynamic nature of fibre-type specific expression of myosin heavy chain transcripts in 14 different human skeletal muscles. J Muscle Res Cell Motil. 2001;22:647–655. doi: 10.1023/a:1016337806308. [DOI] [PubMed] [Google Scholar]
- Smith J.C., Goldberg S.J., Shall M.S. Myosin heavy chain and fibre diameter of extrinsic tongue muscles in rhesus monkey. Arch Oral Biol. 2006;51:520–525. doi: 10.1016/j.archoralbio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Sokoloff A.J., Li H., Burkholder T.J. Limited expression of slow tonic myosin heavy chain in human cranial muscles. Muscle Nerve. 2007a;36:183–189. doi: 10.1002/mus.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff A.J., Yang B., Li H., Burkholder T.J. Immunohistochemical characterization of slow and fast myosin heavy chain composition of muscle fibres in the styloglossus muscle of the human and macaque (Macaca rhesus) Arch Oral Biol. 2007b;52:533–543. doi: 10.1016/j.archoralbio.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff, A.J., M. Daugherty, H. Li (2009) Myosin heavy-chain composition of the human hyoglossus muscle. Dysphagia, E-pub ahead of print. [DOI] [PMC free article] [PubMed]
- Stål P., Marklund S., Thornell L.E., De Paul, Eriksson P.O. Fibre composition of human intrinsic tongue muscles. Cells Tissues Organs. 2003;173:147–161. doi: 10.1159/000069470. [DOI] [PubMed] [Google Scholar]
- Stedman H.H., Kozyak B.W., Nelson A., Thesier D.M., Su L.T., Low D.W., Bridges C.R., Shrager J.B., Minugh-Purvis N., Mitchell M.A. Myosin gene mutation correlates with anatomical changes in the human lineage. Nature. 2004;428:415–418. doi: 10.1038/nature02358. [DOI] [PubMed] [Google Scholar]
- Subramaniam S. The Biology Workbench – a seamless database and analysis environment for the biologist. Proteins. 1998;32:1–2. [PubMed] [Google Scholar]
- Sugimoto N., Nakano S., Yoneyama M., Honda K. Improved thermodynamic parameters and helix initiation factor to predict stability of DNA duplexes. Nucleic Acids Res. 1996;24:4501–4505. doi: 10.1093/nar/24.22.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundman E., Ansved T., Margolin G., Kuylenstierna R., Eriksson L.I. Fiber-type composition and fiber size of the human cricopharyngeal muscle and the pharyngeal constrictor muscle. Acta Anaesthesiol Scand. 2004;48:423–429. doi: 10.1111/j.1399-6576.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- Tellis C.M., Rosen C., Thekdi A., Sciote J.J. Anatomy and fiber type composition of human interarytenoid muscle. Ann Otol Rhinol Laryngol. 2004;113:97–107. doi: 10.1177/000348940411300203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termin A., Staron R. S., Pette D. Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. A single-fiber study. Eur J Biochem. 1989;186:749–754. doi: 10.1111/j.1432-1033.1989.tb15269.x. [DOI] [PubMed] [Google Scholar]
- Termin A., Pette D. Myosin heavy-chain-based isomyosins in developing, adult fast-twitch and slow-twitch muscles. Eur J Biochem. 1991;195:577–584. doi: 10.1111/j.1432-1033.1991.tb15740.x. [DOI] [PubMed] [Google Scholar]
- Toniolo L., Cancellara P., Maccatrozzo L., Patruno M., Mascarello F., Reggiani C. Masticatory myosin unveiled: first determination of contractile parameters of muscle fibers from carnivore jaw muscles. Am J Physiol Cell Physiol. 2008;295:C1535–C1542. doi: 10.1152/ajpcell.00093.2008. [DOI] [PubMed] [Google Scholar]
- van Willigen J.D., Weijs-Boot J. Phasic and rhythmic responses of the oral musculature to mechanical stimulation of the rat palate. Arch Oral Biol. 1984;29:7–11. doi: 10.1016/0003-9969(84)90035-9. [DOI] [PubMed] [Google Scholar]
- Vincent H.K., Shanely R.A., Stewart D.J., Demirel H.A., Hamilton K.L., Ray A.D., Michlin C., Farkas G.A., Powers S.K. Adaptation of upper airway muscles to chronic endurance exercise. Am J Respir Crit Care Med. 2002;166:287–293. doi: 10.1164/rccm.2104120. [DOI] [PubMed] [Google Scholar]
- Volz L.M., Mann L.B., Russell J.A., Jackson M.A., Leverson G.E., Connor N.P. Biochemistry of anterior, medial, and posterior genioglossus muscle in the rat. Dysphagia. 2007;22:210–214. doi: 10.1007/s00455-006-9075-y. [DOI] [PubMed] [Google Scholar]
- Wieczorek D.F., Periasamy M., Butler-Browne G.S., Whalen R.G., Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Alnaqeeb M., Simpson H., Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil. 1996;17:487–495. doi: 10.1007/BF00123364. [DOI] [PubMed] [Google Scholar]
- Yu F., Stål P., Thornell L.E., Larsson L. Human single masseter muscle fibers contain unique combinations of myosin and myosin binding protein C isoforms. J Muscle Res Cell Motil. 2002;23:317–326. doi: 10.1023/a:1022061706126. [DOI] [PubMed] [Google Scholar]