Abstract
Background
Alzheimer's disease (AD) is the most prevalent form of dementia predominantly affecting the elderly. It is believed that soluble amyloid-β (Aβ) oligomers are involved in the pathogenesis of AD, yet the underlying mechanisms remain elusive.
Objectives
Emerging evidence suggests that mitochondrial dysfunction likely plays a critical role in Aβ-induced neuronal degeneration. Previously, we demonstrated that Aβ-derived diffusible ligands (ADDLs) induce reduced mitochondrial density in neurites, and we suspect that an impaired mitochondrial trafficking might be involved, which is tested in this study.
Methods
Using live cell imaging, anterograde and retrograde transport of mitochondria in primary hippocampal neurons treated with sub-lethal doses of ADDLs was measured.
Results
We found that ADDLs induced significant impairment in both anterograde and retrograde transport of mitochondria along axons.
Conclusion
These results suggest that an impaired mitochondrial transport likely contributes to ADDL-induced abnormal mitochondrial distribution and dysfunction and also reinforce the idea that axonal transport is likely involved in AD pathogenesis.
Key Words: Aβ-derived diffusible ligands, Mitochondria, Axonal transport, Alzheimer's disease
Introduction
Alzheimer's disease (AD) is the most common neurodegenerative disorder in the aged population and is characterized by two pathological hallmarks, i.e., senile plaques and neurofibrillary tangles, along with extensive neuronal death in selected brain regions [1]. Despite extensive research efforts, the mechanisms underlying neurodegeneration in AD remain elusive. Largely due to the fact that AD-causing mutations found in amyloid-β (Aβ) precursor protein (APP) and presenilin 1 and 2 have a primary effect on Aβ processing and plaque formation, a myriad of studies have focused on the central role of Aβ in the pathogenesis and progression of AD, and it is generally believed that the soluble oligomers of Aβ are the major toxic species. Emerging evidence suggest that mitochondria are a major intracellular target of soluble Aβ oligomers [2]. Interestingly, we found abnormal mitochondrial dynamics in AD fibroblasts [3] and demonstrated that APP overexpression, likely through Aβ production, induced abnormal mitochondrial dynamics in neuronal cells [4]. Most recently, we confirmed abnormal mitochondrial dynamics in AD neurons and demonstrated that Aβ-derived diffusible ligand (ADDLs) induced abnormal mitochondrial dynamics in neurons [5]. One of the common features in all these models demonstrating abnormal mitochondrial dynamics is an abnormal mitochondrial distribution, i.e., perinuclear accumulation of mitochondria in AD fibroblasts or M17 cells overexpressing mutant APP or reduced mitochondrial density in neurites of primary hippocampal neurons. Since fast axonal transport (FAT) of mitochondria underlies the uniform distribution of mitochondria along the axon [6], these findings prompted us to question whether an abnormal mitochondrial transport is involved. In this study, by using live cell imaging, we investigated axonal transport in ADDL-treated primary hippocampal neurons.
Materials and Methods
Cell Culture and Transfection
Primary neurons from E18 rat hippocampus (BrainBits) were seeded at 30,000–40,000 cells per well on 35 × 10 mm culture dishes coated with poly-D-lysine/laminin in neurobasal medium supplemented with 2% B27 (Invitrogen)/0.5 mM glutamine/25 mM glutamate. Half the culture medium was changed every 3 days with neurobasal medium supplemented with 2% B27 (Invitrogen) and 0.5 mM glutamine. All cultures were kept at 37°C in a humidified 5% CO2-containing atmosphere. More than 90% of cells were neurons after they were cultured for 12 days in vitro (DIV), verified by positive staining for neuronal specific markers microtubule-associated protein-2 (MAP2, dendritic marker) and Tau-1 (axonal marker). At DIV 9, neurons were transfected with Mito-DsRed2 construct (Clontech) using Neurofect (Genlantis) according to the manufacturer's protocol.
Time-Lapse Imaging and Image Analysis
ADDLs were prepared as previously described [5]. Two days after transfection with Mito-DsRed2, neurons were treated with ADDLs and then put in a well-equipped environmental chamber with controlled CO2 content, humidity and temperature. Time-lapse images were captured with an inverted Leica DMI6000 fluorescence microscope (Leica) with a fully automated scanning stage and Leica EL6000 alignment-free metal halide bulb. The microscope was driven by MetaMorph software (Version 7.04, Molecular Devices). Images were acquired using a ×40 1.24 NA objective and a Retiga-EXi CCD digital camera (QImaging). Positive transfected neurons were identified by their expression of red fluorescence, and three different neurons were selected for time-lapse imaging at the same time. Images were acquired every 10 s for a total of 121 images (20 min) without apparent phototoxicity or photobleaching. Kymographs were generated using MetaMorph software (Molecular Devices). Image analysis was performed with WCIF ImageJ (developed by W. Rasband) and MetaMorph software (Molecular Devices).
Results
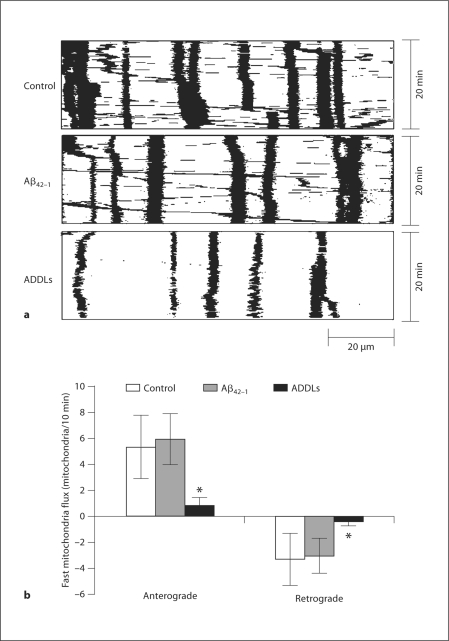
To investigate FAT of mitochondria, rat E18 hippocampal neurons were plated on poly-D-lysine/laminin-coated glass dishes and transfected with Mito-DsRed2 to label mitochondria at DIV 9. 48 h after transfection, neurons were treated with 800 nM ADDLs or 10 μM Aβ42–1. 24 h after treatment, mitochondria movement in neurons was imaged for up to 20 min under fluorescent microscope for time-lapse recordings. No cell death was observed under these conditions as determined by LDH assay (not shown). Because of high mitochondria density in the proximal segment of axon, we just measured FAT of mitochondria in the relative distal segment of axon (around 100 μm in length beginning 300 μm from the cell body of neurons; fig. 1a). Consistent with our prior report [5], ADDL treatment led to less mitochondria in axons. FAT of mitochondria included anterograde and retrograde movements, and mitochondria with velocity less than 0.1 μm/s were classified as stationary. The mitochondria flux that passed a single point in the center of kymographs was counted as described before [7]. Notably, in ADDL-treated neurons, both anterograde (0.80 ± 0.67) and retrograde FAT (0.44 ± 0.21) of mitochondria were significantly reduced compared to nontreated control neurons (5.34 ± 2.47 for anterograde and 3.31 ± 2.02 for retrograde transport of mitochondria; fig. 1b). Neurons treated with Aβ42–1 demonstrated similar FAT of mitochondria to control neurons.
Fig. 1.
Effect of ADDLs on FAT of mitochondria. Rat E18 hippocampal neurons (DIV 9) were transfected with Mito-DsRed2. 24 h after incubation with or without 800 n M ADDLs at DIV 11, neurons were imaged in time-lapse (10 s interval, 20 min). a Representative kymographs showing transport of mitochondria in the segment of axon around 100 μm in length beginning 300 μm from the cell body of control neurons or neurons treated with Aβ42–1 and ADDLs. b Quantification of mitochondria flux (number mitochondria/10 min) revealed that both anterograde and retrograde FAT of mitochondria were greatly impaired by ADDLs rather than negative control or Aβ42–1. At least 20 neurons were analyzed in three independent experiments (∗ p ≤ 0.05, Student's t test).
Discussion
Previously, it was demonstrated that acute treatment of Aβ monomers and fibrils induces significant reduction in motile mitochondria [8]. In this study, we performed a more detailed study on axonal transport of mitochondria and found that soluble Aβ oligomers induced significantly reduced mitochondrial axonal transport, suggesting that impaired axonal transport of mitochondria likely contributes to ADDL-induced abnormal mitochondrial distribution in neurites. This is consistent with a recent study demonstrating an overall disruption of FAT induced by soluble Aβ oligomers in isolated squid axoplasms [9]. In fact, deficits in axonal transport may represent an early step in AD pathogenesis since axonal swelling and reduced axonal transport were observed before apparent AD hallmarks [10]. Overall, our studies, along with others, suggest that Aβ is likely one of the major mediators causing deficits in axonal transport.
Acknowledgements
This study is supported by the NIH (AG 031852), Alzheimer's Association (IIRG-07-60196, Zen-07-59500) and a pilot grant from Memory and Cognition Center at Case Western Reserve University.
References
- 1.Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 2.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 8.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci USA. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]