Abstract
Malaria is a major public health problem in the developing world owing to its high rates of morbidity and mortality. Of all the malarial parasites that infect humans, Plasmodium falciparum is most commonly associated with neurological complications, which manifest as agitation, psychosis, seizures, impaired consciousness and coma (cerebral malaria). Cerebral malaria is the most severe neurological complication; the condition is associated with mortality of 15–20%, and a substantial proportion of individuals with this condition develop neurocognitive sequelae. In this Review, we describe the various neurological complications encountered in malaria, discuss the underlying pathogenesis, and outline current management strategies for these complications. Furthermore, we discuss the role of adjunctive therapies in improving outcome.
Introduction
Malaria is not only common in malaria-endemic areas; the disease is seen increasingly in Western countries as a result of people emigrating or traveling from such areas. The Plasmodium falciparum parasite is responsible for almost all the neurological complications associated with malaria, although P. vivax causes seizures in children, and is also associated with coma in both children and adults.
In 2002, an estimated 2.2 billion individuals were exposed to P. falciparum in malaria-endemic areas, with 515 million clinical episodes and over 1 million deaths.1 Over 70% of these infections occurred in children living in sub-Saharan Africa, although P. falciparum can infect humans at any age. The neurological manifestations of malaria include seizures, psychosis, agitation, impaired consciousness and coma; the latter two features are the hallmarks of cerebral malaria.2-4 In malaria-endemic areas, neurological features are found in nearly half of children admitted to hospital with falciparum malaria.5 In areas where individuals develop severe disease, the proportions of patients who develop cerebral form are similar between children and adults.6 Cerebral malaria occurs in 2.4% of travelers with falciparum malaria,7 and has been well described; however, the other neurological complications of falciparum malaria have received relatively little attention.
In this Review, we describe the neurological manifestations of falciparum malaria in adults and children, and the pathogenesis and management of these complications, with a particular focus on the results obtained to date with adjunctive therapies. In addition, we highlight areas of future research with the aim of improving outcomes.
Diagnosis
The diagnosis of falciparum malaria should be considered in any patient who has a febrile illness with neuro logical symptoms and has passed through a malaria endemic area in the past 3 months. Occasional cases of malaria are seen in people living near airports, or are contracted from blood transfusions. The usual diagnostic test is the microscopic examination of stained blood smears, but rapid tests such as the immunochromatographic test for P. falciparum histidine-rich protein 2 and lactate dehydrogenase are also available.8 Polymerase chain reaction testing for parasite messenger RNA or DNA is more sensitive than microscopy, but is expensive and does not provide an estimate of the parasite load.
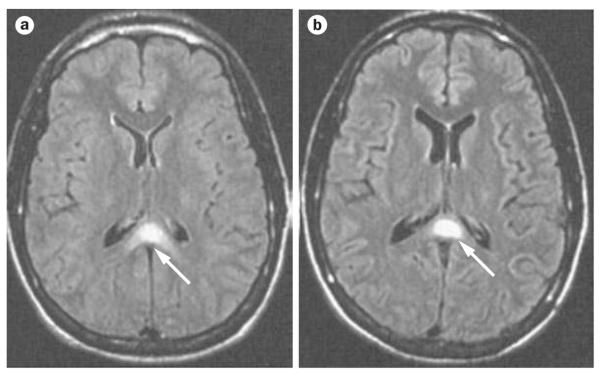
In malaria-endemic areas, falciparum malaria is a diagnosis of exclusion, in which three negative blood smears at 8–12 h intervals are required before the diagnosis can be ruled out. Many infective and metabolic processes cause the types of neurological symptoms and signs associated with malaria, and the malaria parasitemia might be incidental in some cases. In a recent study from Malawi, 24% of the children who fulfilled the criteria for cerebral malaria before death had evidence at post mortem of an alternative cause for the coma.9 The presence of malarial retinopathy (Figure 1)10 was the only clinical feature that distinguished patients with typical histopathological features of cerebral malaria from those with alternative pathologies. A lumbar puncture must be performed to exclude other causes for the encephalopathy, although there can be a mild pleocytosis11 and an increase in protein in malaria, possibly as a result of seizures.12 Neuroimaging in patients with cerebral malaria might demonstrate brain swelling, cortical infarcts, and hyperintense areas in the white matter (Figure 2);13-15 however, none of these features are diagnostic of cerebral malaria.
Figure 1.
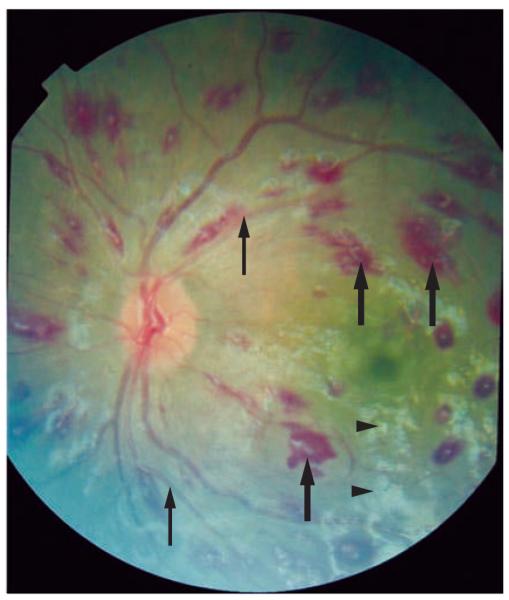
Malarial retinopathy. Photograph of the retina in patient with malaria, which shows exudates (arrowheads), hemorrhages (thick arrows) and changes in the color of the blood vessels (thin arrows). Permission obtained from Nick Beare, Royal Liverpool University Hospital, Liverpool, UK and Terri Taylor, Blantyre Malaria Project, Queen Elizabeth Central Hospital, Blantyre, Malawi.
Figure 2.
FLAIR images of the brain of a patient with cerebral malaria. a | Loss of sulci and narrowing of the ventricles (brain swelling), with hyperintensity in the semiovale centrum along with abnormal signal in the splenium of the corpus callosum (arrow). b | One week after the onset of illness, the image shows widening of the sulci and ventricles, with resolution of hyperintensities, except for the lesion in the splenium of the corpus callosum (arrow). Abbreviation: FLAIR, fluid-attentuated inversion recovery. Permission obtained from the American Society of Neuroradiology © Cordoliani Y. S. et al. AJNR Am. J. Neuroradiol. 19, 871–874 (1998).
Pathogenesis
The unique characteristic of malaria caused by P. falciparum compared with the other species of malarial parasite is the sequestration of infected erythrocytes in the venules of various organs, particularly the brain. This feature is thought to cause many of the complications of malaria, especially the neurological features.
After the sporozoites are injected into the human bloodstream by the female Anopheles mosquito, the parasite goes through an asymptomatic hepatic stage. The infected hepatocytes burst, and release the merozoites, which enter the erythrocytes. It is the 48 h lifecycle of merozoites within the erythrocytes that produces the classic symptoms of malaria. In the late stages, infected erythrocytes adhere to the endothelial cells of the venules, to promote growth of the parasite in a relatively hypoxic environment and evade destruction in the spleen. This cytoadherence is mediated by proteins encoded by the var genes of the parasite. These proteins are exported to the surface of infected erythrocytes, where they act as points of attachment to ligands upregulated in the venule endothelium. In these late stages, the parasites are metabolically active, and consume glucose and produce lactate via anaerobic glycolysis. Infected erythro cytes are sequestered in many organs of the body, with the brain being preferentially targeted, followed by the heart, liver and skin.16 The degree of sequestration can be increased by binding of adherent infected erythrocytes to other infected erythrocytes (autoagglutination) or noninfected erythrocytes (rosetting), or by platelet-mediated clumping.
The sequestration of infected erythrocytes reduces microvascular flow, a process that is aggravated by reduced deformability of infected as well as uninfected erythrocytes. Tissue necrosis is unlikely to occur, as little evidence of ischemic damage exists and the clinical signs reverse rapidly. However, a critical reduction might occur in the supply of metabolic substrate to the brain, aggravated by anemia or hypoglycemia in the presence of increased basal metabolism with seizures and fever.
P. falciparum increases levels of both proinflammatory and anti-inflammatory cytokines, but the roles of these molecules in disease pathogenesis are unclear. Levels of (proinflammatory) tumor necrosis factor (TNF), interleukin (IL)-6 and anti-inflammatory IL–10 are increased in patients with cerebral malaria compared with those without malaria. Several polymorphisms in the TNF gene promoter are associated with an increased risk of cerebral malaria, neurological sequelae and death.17 In a sample of Vietnamese adults, levels of IL-6, IL-10 and TNF were raised in patients with multiorgan severe disease, but were less elevated (but still not normal) in patients with cerebral malaria alone, which suggests that these cytokines are involved in the pathogenesis of severe malaria, but not coma per se.18
Recent studies reported an association between elevated serum levels of IL-1 receptor antagonist and severe malaria in children.19 Another study suggested that plasma levels of inducible protein 10 (IP-10), the soluble TNF receptor sTNF-R2 and soluble Fas proteins might be potential biomarkers of cerebral malaria severity and mortality.20 By contrast, high levels of vascular endothelial growth factor were protective against death in cerebral malaria. Post-mortem analysis of brains from patients with cerebral malaria has suggested increased local production of TNF, IL-1 and transforming growth factor in brain tissue.21 However, production of or staining for these cytokines did not correlate with parasite sequestration.
Nitric oxide (NO) has been suggested as a key effector for TNF in the pathogenesis of malaria. Cytokines upregulate inducible NO synthase in brain endothelial cells and increase the production of NO, which diffuses into the brain tissue.22 Increased NO activity might change blood flow and reduce glutamate uptake, leading to excitotoxicity. NO is likely to reduce the level of consciousness rapidly and reversibly, because this short-lived molecule can easily diffuse across the blood–brain barrier to interfere with neuronal function.
Given that parasites are largely confined to the intravascular space, one major question regarding the pathogenesis of cerebral malaria is: how do these parasites cause neuronal dysfunction?23 The blood–brain barrier seems to be impaired in patients with cerebral malaria,17 and in those with other neurological manifestations of malaria.24 Postmortem analysis of the brains of adults with cerebral malaria has shown widespread vascular endothelial cell activation with disruption of cell junctional proteins (zonula occludens 1, occludin and vinculin), particularly in vessels that contain infected erythrocytes.21 This process might be sufficient to allow metabolites to impair consciousness or precipitate seizures.
Brain swelling is commonly seen on neuroimaging in patients with cerebral malaria (Figure 2).13-15 This swelling is not associated with vasogenic edema, although cytotoxic edema is seen in some patients.14,15 The brain swelling is probably attributable to increased blood volume that occurs as a result of sequestration of the infected erythrocytes and/or an increase in cerebral blood flow, particularly in response to anemia, fever and seizures. Raised intracranial pressure occurs in all children with cerebral malaria, and some children develop severe intracranial hypertension, which might be associated with death or neurological sequelae.25 Intracranial hypertension is less common in adults.
Post-mortem examination in patients with cerebral malaria demonstrates sequestration of infected erythrocytes in all parts of the brain. The brain is often swollen—evidence for frank herniation is rare in adults, but more common in children. Extraerythrocytic hemazoin (a product of hemoglobin metabolism by malaria parasites) is found inside cerebral vessels,26 which suggests that rupture of sequestered, infected erythrocytes might lead to an inflammatory process within and around brain capillaries. These findings are not seen consistently in adults,27 and might reflect a difference between adults and children. The combination of vascular clogging, mononuclear cell margination and enhanced vascular permeability has been seen in Asian adults.28 In addition, pathogenic roles for heme,29 activation of the blood coagulation cascade30 and platelet-induced clumping of P. falciparum-infected erythrocytes31 have been proposed. Apoptosis of endothelial cells caused by P. falciparum-infected erythrocytes from children has been reported in an ex vivo model, but no statistical association with neurological complications or sequelae was formed.32 Apoptosis has been seen in the brainstems of adults dying with cerebral malaria, but the frequency of staining for active caspase 3, a marker of apoptosis, was not significantly higher than it was in control individuals without malaria.33
Neurological complications of malaria
The neurological complications of falciparum malaria can manifest as acute headaches, irritability, agitation, seizures, psychosis and impaired consciousness (from confusion to deep coma). Many of these features can occur in the same patient at different times during the course of the illness.
Cerebral malaria
Cerebral malaria has features of a diffuse encephalopathy similar to that seen in metabolic conditions, occasionally with focal signs.
Definition
The WHO defines cerebral malaria as unarousable coma (unable to localize a painful stimulus) in a patient in whom the presence of P. falciparum asexual parasitemia has been demonstrated, after other causes of encephalopathy have been excluded. This definition was developed for research purposes, and many patients present with less severe impairment of consciousness. These patients should still be treated with parenteral antimalarial therapy as a medical emergency, despite not meeting the WHO criteria.4
In most hospitals, hypoglycemia and acute bacterial meningitis can be easily excluded, but the exclusion of other causes, such as viral encephalitis and metabolic causes, can be problematic, particularly in malaria-endemic areas where facilities are limited. Seizures can impair the level of consciousness, either in the form of subclinical seizures or a transient postictal state.
Clinical presentation
Almost all patients with cerebral malaria present with fever, rigors and/or chills. Some patients might have headache or vomiting. Altered sensorium might be present from the outset, or might develop slowly over a period of several days. Signs of irritability, restlessness or psychotic behavior can be the initial manifestations of cerebral involvement.3,4 A history of travel and blood transfusions should be sought in patients who present with these symptoms.
In children with cerebral malaria, coma usually develops rapidly, and often follows seizures. In adults, coma develops gradually and is only occasionally associated with seizures.2 Around 15–20% of adults with cerebral malaria have seizures, compared with 80% of children. Most seizures seem to be generalized on clinical observation, but EEG identifies a focal origin in many patients. Electrical discharges without clinical manifestations or with subtle clinical manifestations are common.34-36
Neck stiffness is occasionally encountered,37 but other signs of meningism (for example, Kernig sign) are uncommon in our experience. Photophobia is also rare. Cranial nerve involvement is sometimes observed in adults, but is rare in children. Convergent spasms that imply an upper-brainstem lesion, transient ocular bobbing, horizontal and vertical nystagmus, and sixth nerve palsies are also occasionally observed.3 Disorders of conjugate gaze are typically present, but pupillary abnormalities are seen more commonly in children than in adults. Abnormal oculocephalic and oculovestibular reflexes occur in children with deep coma.38
Neurological examination of the limbs usually suggests symmetrical upper motor neuron dysfunction,3 although muscle tone and tendon jerks, which are usually increased in this condition, can be normal or even depressed. Ankle or patellar clonus can be present in some cases. Bilateral extensor plantar reflexes are seen in comatose patients. Focal deficits such as hemiparesis and isolated cranial nerve lesions can also occur.3
Decorticate or decerebrate posturing is more common in children than adults with cerebral malaria. This posturing can occur in association with hypoglycemia, but often indicates raised intracranial pressure. Generalized extensor spasms associated with phasic upward deviations of the eyes, pouting, and periods of stertorous breathing that resemble dyskinesia have been observed. Extrapyramidal signs can be observed, often during convales cence. A pout reflex, bruxism or a brisk jaw jerk might also be present. The gag reflex is usually preserved, except in deep coma.
Patients with cerebral malaria often exhibit a change in diurnal rhythm, with difficulty in sleeping or excessive sleepiness. Patients might have a dazed or vacant look, or experience somnambulism. Talkativeness and yawning are common during recovery from coma.
Seizures
In African children admitted to hospital with seizures, malaria is a common cause.6 The seizures can be febrile—although over half occur when the child is afebrile—and in many cases they are repetitive, focal or prolonged.39 Clinical seizures are associated with a rise in intracranial pressure. Initial EEG recordings of very slow frequency, with background asymmetry, burst suppression, or interictal discharges, are associated with an adverse outcome.34,35 In adults, seizures occur in patients with cerebral malaria or as part of a postmalarial neurological syndrome (see below). Status epilepticus is unusual in adults, though multiple convulsions are common. By contrast, status epilepticus is common in children, and is usually seen in the context of cerebral malaria. Epilepsy has been documented after recovery from cerebral malaria in African children.40 However, additional research will be required to determine the clinical and electrographic features, pathogenesis and outcomes of this sequela.
Other neurological features
Psychiatric manifestations, such as hallucinations, psychoses, fugue, delusions and illusions, can occur as presenting symptoms or during recovery in patients with malaria. These symptoms can be caused by antimalarial drugs; in particular, chloroquine, quinine and mefloquine.
Neurocognitive sequelae
Neurological sequelae of cerebral malaria are more severe in children than in adults. The prevalence of neurological deficits varies between 6% and 29% at the time of hospital discharge.41 In children, neurological abnormalities might be transient (for example, psychosis or ataxia), might improve rapidly over months (for example, cortical blindness or aphasia), or might persist for a prolonged period (for example, hemiparesis or extrapyramidal syndrome).42,43
In adults, sequelae are less common, and range from 3–10% in prevalence.44-46 In india, 10% of adults had neurological sequelae on discharge, including psychosis (4%), cerebellar ataxia (3%), and extrapyramidal rigidity or hemiplegia.45 The depth and duration of coma and multiple convulsions were independent risk factors for neurological sequelae.
Subtle deficits (for example, cognitive difficulties, language and behavior problems) are increasingly recognized, and have been documented in 24% of children after recovery from cerebral malaria.47,48 The prevalence of these impairments in adults or after other forms of severe malaria is not known, because of lack of long-term follow-up studies with neuropsychological examinations.
In a study of 187 ugandan children, 26% of children with cerebral malaria and 13% with uncomplicated malaria had cognitive deficits in one or more areas at a 2-year follow-up examination, as compared with 8% of healthy children. The deficits in children with cerebral malaria were primarily in the area of attention (18% cerebral malaria vs 3% controls).49 In children with cerebral malaria, high levels of TNF production in the brain are associated with subsequent neurological and cognitive morbidity.50
Postmalarial syndromes
Two neurological syndromes have been observed during the recovery from severe malaria, when the parasitemia has cleared. Cerebellar ataxia has been reported in patients recovering from cerebral malaria, with spontaneous resolution of the ataxia over a period of several months.51,52 A self-limiting ‘postmalaria neurological syndrome’ that consists of acute confusional state, acute psychosis, generalized convulsions and/or tremor is seen in about 0.1% of patients after severe malaria.53 This condition is associated with the use of mefloquine.
Management of cerebral malaria
Patients with suspected cerebral malaria should be transferred to the best available health care facility.4 In addition to parenteral administration of antimalarial drugs, management should be directed towards early recognition and appropriate management of common complications such as hypoglycemia, fluid and electrolyte imbalance, convulsions, anemia, acidosis, and renal and respiratory impairment.
Antimalarial drugs
Antimalarial drugs are the only interventions that unequivocally reduce mortality in patients with malaria. The cinchoids (quinine and quinidine) and artemisinin compounds are most commonly used, and artemisinins are becoming the drugs of choice (see below). A loading dose is recommended to enable parasiticidal levels to be achieved rapidly.54 Both the cinchoids and artemisinins are usually combined with other antimalarial drugs to shorten the duration of therapy (in the case of quinine) and prevent resistance (regimens outlined in Box 1).
Box 1. Antimalarial drugs used in the treatment of cerebral malaria.
General principles
Administer antimalarial drug parenterally for at least 48 h; thereafter administer orally as soon as patient can swallow
Administer a loading dose
Total duration of therapy depends on sensitivity of parasite, but usually 7 days
Artemisinin derivatives
Artesunate: two 2.4 mg/kg doses given intravenously 12 h apart on day 1, then 2.4 mg/kg daily for 6 days, given orally if patient can swallow. Administer intramuscularly or rectally if no intravenous access
Artemether: 3.2 mg/kg intramuscular loading dose, then 1.6 mg/kg per day for 6 days, given orally if patient can swallow. Absorption might be erratic in patients in shock
Arteether: 150 mg once a day by deep intramuscular injection for 3 consecutive days (adults)
Short-duration artemisinin (3 days): followed with mefloquine or sulfadoxine–pyremethamine compounds to prevent recrudescence
Quinine
Dilute quinine dihydrochloride with normal or dextrose saline and infused over 4 h (preferably via syringe or infusion pump)
Loading dose: 20 mg/kg body weight (salt) infused over 4 h
Maintenance dose: 10 mg/kg body weight infused every 8 h until oral administration possible
Quinidine gluconate
Loading dose: 6.25 mg/kg base (=10 mg/kg salt) infused intravenously over 1–2 h, then continuous infusion of 0.0125 mg/kg/min base (=0.02 mg/kg/min salt). If continuous infusion is impractical, use 15 mg/kg base (=24 mg/kg salt) loading dose infused intravenously over 4 h, then 7.5 mg/kg base (=12 mg/kg salt) infused over 4 h every 8 h, starting 8 h after loading dose
Maintain blood quinidine levels in range of 3–8 mg/l. At least 24 h of quinidine gluconate infusion are recommended (or 3 intermittent doses); once parasite density is <1% and the patient can take oral medication, patient can complete treatment course with 10 mg/kg (salt) given orally every 8 h
Initial (including loading) doses of parenteral quinine or quinidine need not be reduced in individuals with renal failure. If renal failure persists or the patient does not improve, reduce maintenance dosage by 25–33% on third treatment day
Obtain history of recent use of drugs that might prolong QT interval (e.g. quinine or mefloquine) before deciding loading dose
Quinine has a narrow therapeutic window because it only kills parasites in the late stages of the erythrocytic cycle. It can induce hypoglycemia by promoting insulin secretion. Other complications such as dizziness and cinchonism become evident as the patient regains consciousness. Quinine causes hypotension if given rapidly via the intravenous route, and also slows ventricular repolarization (as indicated by prolongation of the QT interval).4 Quinidine is used in preference to quinine in the US.55
The artemisinins—artemether, arteether and artesunate—are the derivatives of Chinese drug qinghaosu. These drugs are the most rapidly acting and potent anti-malarial compounds available; they kill all the stages of the parasite within the erythrocyte, and also kill the gametocytes. In a large, open-label, randomized trial of Asian adults with severe malaria, artesunate significantly reduced mortality by 34.7% (P = 0.0002, 95% CI 18.5–47.6%) compared with quinine,56 and a similar study in African children is currently underway.57 The WHO now recommends artesunate as the drug of choice in the treatment of severe malaria.54 The artemisinin compounds are not licensed in many countries, but in the US intravenous artesunate can be used as an investigational new drug for severe malaria.55 Artemisinin derivatives can be administered via the intramuscular route, and have few local or systemic adverse effects.
Management of other complications
The neurological complications of falciparum malaria are often associated with dysfunction of other organs and systems, with effects such as severe anemia, hypoglycemia, acute renal failure, hepatic impairment, and coagulation disorders. The presence of these other complications influences the patient’s overall outcome.58 Bacterial infections are common in children who present with features of severe malaria,59 and appropriate antimicrobial therapy should be administered.
Hypoglycemia occurs in 3% of adults with severe falciparum malaria,56 and is particularly common in children, pregnant women, and patients on quinine therapy. Hypoglycemia can be associated with deteriorating consciousness, generalized seizures, extensor posturing, shock and coma, but often no clinical signs are evident, and blood glucose levels should, therefore, be monitored at regular intervals. Hypoglycemia responds well to standard therapy,60,61 although hyperinsulinemic hypoglycemia in association with quinine therapy also responds well to long-acting somatostatin analogs.62
Acute seizures are often terminated by benzodiazepines,63-65 although these drugs might be less effective in malaria than in other conditions, as malaria seems to downregulate γ-aminobutyric acid receptors.66 Prolonged seizures can usually be terminated with phenobarbital67 or phenytoin.67,68
The fluid balance is critical in severe malaria. Children with this condition are often hypovolemic and require fluids, but no consensus exists in relation to the optimal type and amount of fluid-replacement therapy. Adults can develop renal impairment and/or pulmonary edema, and fluids might, therefore, need to be restricted. In patients with cerebral malaria who have raised intracranial pressure the cerebral perfusion pressure needs to be optimized, although a role for fluid administration in this process has not been clearly established. Perturbations of potassium levels and hyponatremia occur frequently in malaria, but are rarely associated with clinical features or poor outcomes.
Blood transfusions are used to correct severe anemia; the amount of blood transfused and the rapidity of administration differ between children and adults. In African children, fresh whole blood (10–20 ml/kg) is administered to enable rapid correction of the underlying hypovolemia. In adults, a fairly small quantity of blood is often adminis tered over a 4 h period because of concerns about fluid overload. The role of exchange transfusion in malaria is controversial. The potential benefits of this intervention include rapid removal of circulating parasite69 and other compounds that might have deleterious effects in severe malaria (for example, lactate), as well as the capability to correct severe anemia while reducing the risk of circulatory overload. The WHO recommends that an exchange transfusion should be considered if the patient has severe malaria (deep coma, hypoglycemia, acidosis or severe anemia) with a parasitemia of >20%. However, no clinical trial data are available that demonstrate improved outcomes with exchange transfusions.
Adjunctive therapy
In view of the fact that antimalarial drugs often take at least 12–18 h to kill the parasites, adjunctive therapies administered in the meantime might reduce the risk of mortality and neurocognitive sequelae, particularly in patients with cerebral malaria. A number of agents have been or are being tested, but none has shown unequivocal evidence of improvement in clinical trials (Tables 1 and 2). Consequently, none of these agents can be recommended as part of the standard management strategy at present. Many of the trials were poorly conducted,70 and quinine was used as the standard antimalarial drug in most of them. The outcomes of adjunctive therapy might differ if it is administered in conjunction with artemisinins.
Table 1.
Randomized trials of anti-inflammatory and antioxidant therapies in severe malaria with neurological complications
| Reference | Design and regimena | Patients | Outcomes |
|---|---|---|---|
| Corticosteroids | |||
| Warrell et al. (1982)44 |
RDPCT of dexamethasone (1.5–2.0 mg/kg over 48 h) |
100 Thai children and adults with cerebral malaria |
Dexamethasone prolonged DoC (63 h vs 47 h in placebo group); complications in 26% of dexamethasone group versus 11% of placebo group |
| Hoffman et al. (1988)71 |
RDPCT of dexamethasone (11.4 mg/kg per 48 h) |
38 Indonesian children and adults with impaired consciousness |
No significant difference in FCT, PCT and DoC |
| Rampengan (1991)72 |
Randomized trial of dexamethasone (4.5 mg/kg per 72 h) versus heparin (300 U/kg intramuscularly daily for 3 days) versus neither |
62 Indonesian children and adults with impaired consciousness |
No significant difference between groups in mortality or neurological sequelae |
| Immunomodulatory therapy | |||
| Taylor et al. (2002)81 |
RDPCT of HIG purified from plasma of local semi-immune blood donors (400 mg/kg by intravenous infusion over 3 h) versus placebo (albumin and sucrose infusion) |
31 Malawian children with cerebral malaria |
No difference in mortality: 5 of 16 patients given HIG died compared with 1 of 15 receiving placebo; no differences in DoC, FCT or PCT |
| van Hensbroek et al. (1996)80 |
RDPCT of monoclonal antibody (B-C7) against TNF |
610 Gambian children with cerebral malaria |
No difference in mortality; neurological sequelae in 15 (6.8%) of survivors in B-C7 group compared with 5 (2.2%) survivors in the placebo group (adjusted odds ratio 3.35, 95% CI 1.08–10.4) |
| Iron-chelation therapy | |||
| Gordeuk et al. (1992)73 |
RDPCT of deferoxamine (100 mg/kg per day infused for a total of 72 h) |
83 Zambian children (<6 years old) with cerebral malaria |
Shorter DoC in deferoxamine group (20 h) versus in placebo group (43 h); rapid PCT in deferoxamine group |
| Thuma et al. (1998)74 |
RDPCT of deferoxamine (100 mg/kg per day infused for a total of 72 h) |
352 Zambian children (<6 years old) with cerebral |
No significant effect on mortality or DoC |
| Mohanty et al. (2002)99 |
RDPCT of oral deferiprone (75 mg/kg) | 45 indian patients | Deferiprone improved FCT, PCT and DoC |
| Pentoxifylline | |||
| Di Perri et al. (1995)78 |
RDPCT of pentoxifylline (10 mg/kg continuous infusion) |
56 Burundian children with cerebral malaria |
Shorter median DoC in pentoxifylline group (6 vs 46 h); trend towards reduced mortality in pentoxifylline group |
| Looareesuwan et al. (1998)100 |
Randomized trial of low-dose pentoxifylline (0.83 [mg/kg]/h) versus high-dose pentoxifylline (1.67 [mg/kg]/h) versus placebo |
45 Thai adults, 18 of whom had cerebral malaria |
No difference in DoC or mortality |
| Das et al. (2003)79 |
RDPCT of pentoxifylline (10 mg/kg intravenously) for 3 days |
52 Indian adults with cerebral malaria |
Shorter DoC in pentoxifylline group (22 h) versus placebo group (64 h); decrease in TNF levels by day 3 and trend towards reduced mortality in pentoxifylline group |
All patients were given the antimalarial drug quinine, except for some patients in the van Hensbroek study, who were given artemether. Abbreviations; DoC, duration of coma after treatment; FCT, fever clearance time; HIG, hyperimmune immunoglobin; PCT, parasite clearance time; RDPCT, randomized, double-blind, placebo-controlled trial; TNF, tumor necrosis factor.
Table 2.
Randomized trials of osmotic diuretics, fluids and phenobarbital in severe malaria with neurological complications
| Reference | Design and regimena | Patients | Outcomes |
|---|---|---|---|
| Osmotic diuretic therapy | |||
| Namutangula et al. (2007)85 |
RCT of mannitol (1 g/kg) as a single dose on admission |
156 Ugandan children with cerebral malaria |
No benefit with respect to DoC, FCT or mortality |
| Fluids | |||
| Maitland et al. (2005)86 |
Randomized trial of 4% albumin (20 ml/kg) versus normal saline (20 ml/kg) versus maintenance (patients with moderate acidosis only) |
150 Kenyan children with acidosis, 123 of whom had impaired consciousness |
Lower mortality with albumin than with saline (4% vs 18%) in patients with moderate acidosis; in patients with coma, 5% of those given albumin died compared with 46% of those given saline |
| Seizure prophylaxis | |||
| White NJ et al. (1988)90 |
RDPCT of phenobarbital (3.5 mg/kg single intramuscular dose) versus placebo |
48 Thai children (>6 years old) and Thai adults with cerebral malaria |
Seizures occurred after admission in 54% of placebo vs 12.5% of phenobarbital group |
| Kochar DL et al. (1997)91 |
Randomized trial of phenobarbital (10 mg/ kg single Intramuscular dose) |
184 Indian adults with cerebral malaria |
Seizures occurred after admission In 3% of phenobarbital group vs 23% of placebo group |
| Crawley JC et al. (2000)92 |
RDPCT of phenobarbital (20 mg/kg single Intramuscular dose) versus placebo |
340 Kenyan children with cerebral malaria |
Seizures occurred after admission in 11% of phenobarbital group vs 27% of placebo group; mortality was 18% in phenobarbital group versus 8% of placebo group |
All patients were given the antimalarial drug quinine. Abbreviations: DoC, duration of coma after treatment; FCT, fever clearance time; RCT, randomized, controlled trial; RDPCT, randomized, double-blind, placebo-controlled trial.
Corticosteroids are anti-inflammatory agents that might improve the integrity of the blood–brain barrier, and reduce both intracranial pressure and the inflammatory response. These compounds were the first adjunctive agents to be tested in randomized, controlled trials in severe malaria. However, two randomized trials of different doses of dexamethasone in South East Asian adults44,71 and one small trial in Indonesian children with cerebral malaria72 did not show any benefit. In fact, in one of the trials, dexamethasone was associated with increased complications (gastrointestinal bleeding, sepsis and prolonged coma recovery time).44 These compounds have not been tested in African children, who often exhibit intracranial hypertension and seem to have a more marked inflammatory response than Asian adults.
Desferrioxamine chelates iron and acts as an anti-oxidant. Preliminary findings in Zambian children with cerebral malaria indicated that treatment with this drug was associated with a rapid resolution of fever, a reduction in seizures and a reduced duration of coma,73 but a larger, randomized trial showed no effect on mortality.74 N-acetyl cysteine is another antioxidant, which improves erythrocyte deformability and inhibits TNF release. This drug normalizes serum lactate levels in adults with severe malaria75 and is currently being tested in adults with cerebral malaria.57
Pentoxifylline reduces secretion of cytokines such as TNF, prevents rosetting, increases erythrocyte deformability, and might also decrease cytoadherence.76 An initial trial of this drug in German travelers with falciparum malaria did not show any clinical benefit or a decrease in TNF levels,77 but this study did not address CNS complications. In an open-label, randomized, controlled, therapeutic trial 56 Burundian children with cerebral malaria, pentoxifylline reduced both the duration of coma and TNF levels.78 In 52 Indian adults with cerebral malaria, pentoxifylline significantly reduced the duration of coma, with a trend towards a reduction in mortality.79
In view of the proposed central role of TNF in the pathophysiology of cerebral malaria, a trial of B-C7, a monoclonal antibody against TNF, was performed in Gambian children with cerebral malaria. This agent did not reduce mortality, and was, furthermore, associated with an increase in neurological sequelae, possibly because the antibody retains TNF within the circulation, which prolongs its effects on the vascular endothelium.80
Hyperimmune globulin inhibits and reverses the cytoadherence of infected erythrocytes in vitro. A trial of hyperimmune globulin, purified from the plasma of local semi-immune blood donors in 31 Malawian children with cerebral malaria, was stopped because the immunoglobulin was demonstrated not to be superior to placebo.81 Other compounds that interfere with cytoadherence, such as heparin,72 curdlan sulfate82 and levamisole,83 have been tried in patients with malaria, including cerebral malaria. Levamisole is currently being tested in indian and Bangledeshi adults with severe malaria.57
Osmotic diuretics such as mannitol lower intracranial pressure, and might improve microcirculatory flow and reduce oxygen free-radical damage.84 Mannitol was found to reduce intracranial pressure in Kenyan children with cerebral malaria, but did not prevent a poor outcome.25 A single dose of mannitol did not reduce mortality or rates of neurological sequelae in Ugandan children with cerebral malaria,85 and multiple doses do not seem to improve outcomes in adults with cerebral malaria (Mishra SK, unpublished data).
Albumin might improve microcirculatory flow and treat hypovolemia, and thereby reduce lactic acidosis. In children with malaria and acidosis, phase II clinical trials have suggested that 4% albumin improves mortality compared with saline, particularly in children with coma.86 A large trial is being conducted to address the use of 4% albumin in children with sepsis and malaria.57 Other colloid solutions such as gelofusin, dextran 70 and hetastarch are also being examined.57 Other compounds that improve microcirculatory flow, such as low-dose heparin, did not improve outcome in indonesian children with cerebral malaria. Dichloroacetate stimulates pyruvate dehydrogenase, which leads to a decrease in lactate levels. In Ghanaian children with severe malaria and hyperlactemia, dichloroacetate did not improve outcome in those with neurological features.87
Evidence is growing that erythropoietin has a neuro-protective role in cerebral malaria.88 Animal studies have shown that it increases survival in a dose-dependent manner, and reduces both the inflammatory response in the brain and neuronal apoptosis. Recently, children with cerebral malaria and high levels of erythropoietin were demonstrated to have a significantly better chance of surviving without neurological sequelae than children with cerebral malaria and low erythropoietin levels.89 A trial of this compound in children with cerebral malaria is underway.57
Prophylactic anticonvulsants might improve outcomes in patients with cerebral malaria, particularly with regard to neurocognitive sequelae. A single intramuscular injection of phenobarbital reduced the frequency of seizures in Thai adults,90 Indian adults91 and Kenyan children with cerebral malaria.92 However, this treatment was associated with increased mortality in the children, probably because it depressed the hyperpnea that usually compensates for acidosis in these unventilated patients. A trial of fosphenytoin, which has fewer sedative properties than phenobarbital, is currently being conducted.57
Several other compounds such as prostacylin have been tried as adjunctive therapies in patients with cerebral malaria, but clinical trials have not been conducted. At the present time, phase II clinical trials of oral activated charcoal to reduce endotoxemia, and l-arginine to improve endothelial function, lactate clearance time and tissue oxygen delivery,93 are being conducted.57 Other compounds, such as agents that prevent platelet aggregation,94 statins,95 immunomodulators,96 and antiapoptotic agents,97 have been suggested as adjunctive therapies, but have not yet been tested in humans.
Future research
The pathogenesis of the neurological complications of falciparum malaria requires further elucidation. Studies using MRI, particularly with novel contrast media,98 might be helpful in this regard. EEG will determine the role of seizures as a cause of poor outcome, and can be used to monitor the effect of antiepileptic drugs. In the future, efforts to establish a highly specific definition of cerebral malaria will also be impotant, and noninvasive monitoring of intracranial pressure and cerebral blood flow might be informative in this regard. The further investigation of potential adjunctive therapies, such as antiapoptotic agents, is warranted, and neurocognitive impairment or markers of brain damage should be used as outcomes in these studies.
Conclusions
The neurological complications of falciparum malaria are common and encompass a wide spectrum of clinical presentation. These complications can manifest during acute illness, or can present during convalescence. Treatment of the neurological complications is warranted, but in trials conducted to date, adjunctive interventions have not been shown to improve the overall outcome of severe falciparum malaria.
Key points.
Neurological complications are common in falciparum malaria; cerebral malaria is the most severe and is associated with almost all neurocognitive sequelae and deaths from neurological complications
The pathogenesis of the neurological complications is multifactorial, with sequestration of infected erythrocytes in the brain the probable cause of most neurological complications
Subtle deficits such as cognitive impairments are often undetected, and few data are available on the incidence of and therapeutic options to prevent brain damage
The mainstay of malaria therapy is parenteral quinidine, quinine or artesunate; artesunate is the treatment of choice where available
Adjunctive therapies might help to improve outcomes, but as yet none has unequivocally been shown to be beneficial
Review criteria.
PubMed was searched using Entrez for articles published before 1 November 2008, including electronic early release publications. Search terms included “Plasmodium falciparum” or “falciparum malaria” or “cerebral malaria” and “brain” or “central nervous system” or “neurological complications” and “therapy” or “adju* therapy”. We chose only papers written in English and French, the languages read by the authors. In addition, we searched the clinical trials registries http://clinicaltrials.gov/ and http://www.controlled-trials.com/mrct/ with the term “severe malaria”.
Acknowledgments
S. K. Mishra expresses his sincere thanks to the Director of Intensive Care (Dr O. P. Agrawal), Director (Dr P. K. Rath) and the staff of the Malaria Unit of Ispat General Hospital, India.
C. R. J. C. Newton is funded by The Wellcome Trust, UK, and wishes to thank his colleagues in Kenya and the UK who have contributed to the ideas outlined in this Review, and Karren Visser for help in the preparation of this manuscript.
Footnotes
Competing interests The authors declared no competing interests.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra SK, Mohanty S, Satpathy SK, Mohapatra DN. Cerebral malaria in adults—a description of 526 cases admitted to Ispat General Hospital in Rourkela, India. Ann. Trop. Med. Parasitol. 2007;101:187–193. doi: 10.1179/136485907X157004. [DOI] [PubMed] [Google Scholar]
- 3.Newton CR, Warrell DA. Neurological manifestations of falciparum malaria. Ann. Neurol. 1998;43:695–702. doi: 10.1002/ana.410430603. [DOI] [PubMed] [Google Scholar]
- 4.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans. R. Soc. Trop. Med. Hyg. 2000;94(Suppl. 1):S1–S90. [No authors listed] [PubMed] [Google Scholar]
- 5.Idro R, et al. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA. 2007;297:2232–2240. doi: 10.1001/jama.297.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohanty S, Mishra SK, Pati SS, Pattnaik J, Das BS. Complications and mortality patterns due to Plasmodium falciparum malaria in hospitalized adults and children, Rourkela, Orissa, India. Trans. R. Soc. Trop. Med. Hyg. 2003;97:69–70. doi: 10.1016/s0035-9203(03)90027-7. [DOI] [PubMed] [Google Scholar]
- 7.Leder K, et al. Malaria in travelers: a review of the GeoSentinel surveillance network. Clin. Infect. Dis. 2004;39:1104–1112. doi: 10.1086/424510. [DOI] [PubMed] [Google Scholar]
- 8.Ochola LB, Vounatsou P, Smith T, Mabaso ML, Newton CR. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect. Dis. 2006;6:582–588. doi: 10.1016/S1473-3099(06)70579-5. [DOI] [PubMed] [Google Scholar]
- 9.Taylor TE, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 10.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am. J. Trop. Med. Hyg. 2006;75:790–797. [PMC free article] [PubMed] [Google Scholar]
- 11.Berkley JA, Mwangi I, Mellington F, Mwarumba S, Marsh K. Cerebral malaria versus bacterial meningitis in children with impaired consciousness. QJM. 1999;92:151–157. doi: 10.1093/qjmed/92.3.151. [DOI] [PubMed] [Google Scholar]
- 12.Barry E, Hauser WA. Pleocytosis after status epilepticus. Arch. Neurol. 1994;51:190–193. doi: 10.1001/archneur.1994.00540140100019. [DOI] [PubMed] [Google Scholar]
- 13.Cordoliani YS, et al. MR of cerebral malaria. Am. J. Neuroradiol. 1998;19:871–874. [PMC free article] [PubMed] [Google Scholar]
- 14.Newton CR, et al. Brain swelling and ischaemia in Kenyans with cerebral malaria. Arch. Dis. Child. 1994;70:281–287. doi: 10.1136/adc.70.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patankar TF, Karnad DR, Shetty PG, Desai AP, Prasad SR. Adult cerebral malaria: prognostic importance of imaging findings and correlation with postmortem findings. Radiology. 2002;224:811–816. doi: 10.1148/radiol.2243010588. [DOI] [PubMed] [Google Scholar]
- 16.Turner G. Cerebral malaria. Brain Path. 1997;7:569–582. doi: 10.1111/j.1750-3639.1997.tb01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gimenez F, de Lagerie S. Barraud, Fernandez C, Pino P, Mazier D. Tumor necrosis factor α in the pathogenesis of cerebral malaria. Cell. Mol. Life Sci. 2003;60:1623–1635. doi: 10.1007/s00018-003-2347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day NP, et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J. Infect. Dis. 1999;180:1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 19.John CC, Park GS, Sam-Agudu N, Opoka RO, Boivin MJ. Elevated serum levels of IL-1ra in children with Plasmodium falciparum malaria are associated with increased severity of disease. Cytokine. 2008;41:204–208. doi: 10.1016/j.cyto.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain V, et al. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in india. Malar. J. 2008;7:83. doi: 10.1186/1475-2875-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown H, et al. Evidence of blood–brain barrier dysfunction in human cerebral malaria. Neuropathol. Appl. Neurobiol. 1999;25:331–340. doi: 10.1046/j.1365-2990.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- 22.Clark IA, Rockett KA, Cowden WB. Possible central role of nitric oxide in conditions clinically similar to cerebral malaria. Lancet. 1992;340:894–896. doi: 10.1016/0140-6736(92)93295-x. [DOI] [PubMed] [Google Scholar]
- 23.Gitau EN, Newton CR. Blood–brain barrier in falciparum malaria. Trop. Med. Int. Health. 2005;10:285–292. doi: 10.1111/j.1365-3156.2004.01366.x. [DOI] [PubMed] [Google Scholar]
- 24.Mturi N, et al. Cerebrospinal fluid studies in Kenyan children with severe falciparum malaria. The Open Tropical Medicine Journal. 2008:56–62. doi: 10.2174/1874315300801010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newton CR, et al. Intracranial hypertension in Africans with cerebral malaria. Arch. Dis. Child. 1997;76:219–226. doi: 10.1136/adc.76.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grau GE, et al. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J. Infect. Dis. 2003;187:461–466. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- 27.Pongponratn E, et al. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 2003;69:345–359. [PubMed] [Google Scholar]
- 28.Patnaik JK, et al. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am. J. Trop. Med. Hyg. 1994;51:642–647. [PubMed] [Google Scholar]
- 29.Ferreira A, Balla J, Jeney V, Balla G, Soares MP. A central role for free heme in the pathogenesis of severe malaria: the missing link? J. Mol. Med. 2008;86:1097–1111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 30.Francischetti IM. Does activation of the blood coagulation cascade have a role in malaria pathogenesis? Trends Parasitol. 2008;24:258–263. doi: 10.1016/j.pt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassmer SC, et al. Platelet-induced clumping of Plasmodium falciparum-infected erythrocytes from Malawian patients with cerebral malaria—possible modulation in vivo by thrombocytopenia. J. Infect. Dis. 2008;197:72–78. doi: 10.1086/523761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toure FS, et al. Apoptosis: a potential triggering mechanism of neurological manifestation in Plasmodium falciparum malaria. Parasite Immunol. 2008;30:47–51. doi: 10.1111/j.1365-3024.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 33.Medana IM, et al. Cellular stress and injury responses in the brains of adult Vietnamese patients with fatal Plasmodium falciparum malaria. Neuropathol. Appl. Neurobiol. 2001;27:421–433. doi: 10.1046/j.0305-1846.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 34.Crawley J, et al. Seizures and status epileticus in childhood cerebral malaria. QJM. 1996;89:591–597. doi: 10.1093/qjmed/89.8.591. [DOI] [PubMed] [Google Scholar]
- 35.Crawley J, Smith S, Muthinji P, Marsh K, Kirkham F. Electroencephalographic and clinical features of cerebral malaria. Arch. Dis. Child. 2001;84:247–253. doi: 10.1136/adc.84.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–840. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 37.Wright PW, Avery WG, Ardill WD, McLarty JW. Initial clinical assessment of the comatose patient: cerebral malaria vs. meningitis. Pediatr. Infect. Dis. J. 1993;12:37–41. doi: 10.1097/00006454-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q. J. Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 39.Waruiru CM, et al. Epileptic seizures and malaria in Kenyan children. Trans. R. Soc. Trop. Med. Hyg. 1996;90:152–155. doi: 10.1016/s0035-9203(96)90120-0. [DOI] [PubMed] [Google Scholar]
- 40.Carter JA, et al. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–981. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- 41.Newton CR, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol. Ther. 1998;79:1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 42.Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039–1043. doi: 10.1016/0140-6736(90)92498-7. [DOI] [PubMed] [Google Scholar]
- 43.van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J. Pediatr. 1997;131:125–129. doi: 10.1016/s0022-3476(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 44.Warrell DA, et al. Dexamethasone proves deleterious in cerebral malaria. A double-blind trial in 100 comatose patients. N. Engl. J. Med. 1982;306:313–319. doi: 10.1056/NEJM198202113060601. [DOI] [PubMed] [Google Scholar]
- 45.Bajiya HN, Kochar DK. Incidence and outcome of neurological sequelae in survivors of cerebral malaria. J. Assoc. Physicians India. 1996;44:679–681. [PubMed] [Google Scholar]
- 46.Jain V, et al. Burden of cerebral malaria in central India (2004–2007) Am. J. Trop. Med. Hyg. 2008;79:636–642. [PMC free article] [PubMed] [Google Scholar]
- 47.Kihara M, Carter JA, Newton CR. The effect of Plasmodium falciparum on cognition: a systematic review. Trop. Med. Int. Health. 2006;11:386–397. doi: 10.1111/j.1365-3156.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 48.Boivin MJ, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119:e1–e7. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.John CC, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–e99. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.John CC, et al. Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am. J. Trop. Med. Hyg. 2008;78:198–205. [PMC free article] [PubMed] [Google Scholar]
- 51.Abdulla MN, Sokrab TE, Zaidan ZA, Siddig HE, Ali ME. Post-malarial cerebellar ataxia in adult sudanese patients. East Afr. Med. J. 1997;74:570–572. [PubMed] [Google Scholar]
- 52.Senanayake N, de Silva HJ. Delayed cerebellar ataxia complicating falciparum malaria: a clinical study of 74 patients. J. Neurol. 1994;241:456–459. doi: 10.1007/BF00900965. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen TH, et al. Post-malaria neurological syndrome. Lancet. 1996;348:917–921. doi: 10.1016/s0140-6736(96)01409-2. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization . Guidelines For The Treatment Of Malaria. World Health Organization; Geneva: 2006. [Google Scholar]
- 55.Centers for Disease Control and Prevention Treatment of Malaria Part 3: Alternatives for Pregnant women § Treatment: severe Malaria. [accesssed 10 February 2009]. 2008. http://www.cdc.gov/malaria/diagnosis_treatment/clinicians3.htm.
- 56.Dondorp A, Nosten F, Stepniewska K, Day N, White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 57.Welcome to Current Controlled Trials. 2008. http://www.controlled-trials.com.
- 58.Mishra SK, Panigrahi P, Mishra R, Mohanty S. Prediction of outcome in adults with severe falciparum malaria: a new scoring system. Malar. J. 2007;6:24. doi: 10.1186/1475-2875-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. Bacteraemia complicating severe malaria in children. Trans. R. Soc. Trop. Med. Hyg. 1999;93:283–286. doi: 10.1016/s0035-9203(99)90024-x. [DOI] [PubMed] [Google Scholar]
- 60.Kawo NG, et al. Hypoglycaemia and cerebral malaria. Lancet. 1990;336:1128–1129. doi: 10.1016/0140-6736(90)92602-e. [DOI] [PubMed] [Google Scholar]
- 61.Kochar DK, Thanvi I, Kumawat BL, Shubhakaran, Agarwal N. Importance of blood glucose level at the time of admission in severe and complicated malaria. J. Assoc. Physicians India. 1998;46:923–925. [PubMed] [Google Scholar]
- 62.Phillips RE, Looareesuwan S, Molyneux ME, Hatz C, Warrell DA. Hypoglycaemia and counterregulatory hormone responses in severe falciparum malaria: treatment with sandostatin. Q. J. Med. 1993;86:233–240. [PubMed] [Google Scholar]
- 63.Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121:e58–e64. doi: 10.1542/peds.2007-0930. [DOI] [PubMed] [Google Scholar]
- 64.Muchohi SN, et al. Pharmacokinetics and clinical efficacy of midazolam in children with severe malaria and convulsions. Br. J. Clin. Pharmacol. 2008;66:529–538. doi: 10.1111/j.1365-2125.2008.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogutu BR, et al. Pharmacokinetics and anticonvulsant effects of diazepam in children with severe falciparum malaria and convulsions. Br. J. Clin. Pharmacol. 2002;53:49–57. doi: 10.1046/j.0306-5251.2001.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikumi ML, Muchohi SN, Ohuma EO, Kokwaro GO, Newton CR. Response to diazepam in children with malaria induced seizures. Epilepsy Res. 2008;82:215–218. doi: 10.1016/j.eplepsyres.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kokwaro GO, Ogutu BR, Muchohi SN, Otieno GO, Newton CR. Pharmacokinetics and clinical effect of phenobarbital in children with severe falciparum malaria and convulsions. Br. J. Clin. Pharmacol. 2003;56:453–457. doi: 10.1046/j.1365-2125.2003.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogutu BR, et al. Pharmacokinetics and clinical effects of phenytoin and fosphenytoin in children with severe malaria and status epilepticus. Br. J. Clin. Pharmacol. 2003;56:112–119. doi: 10.1046/j.1365-2125.2003.01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riddle MS, Jackson JL, Sanders JW, Blazes DL. Exchange transfusion as an adjunct therapy in severe Plasmodium falciparum malaria: a meta-analysis. Clin. Infect. Dis. 2002;34:1192–1198. doi: 10.1086/339810. [DOI] [PubMed] [Google Scholar]
- 70.Enwere G. A review of the quality of randomized clinical trials of adjunctive therapy for the treatment of cerebral malaria. Trop. Med. Int. Health. 2005;10:1171–1175. doi: 10.1111/j.1365-3156.2005.01505.x. [DOI] [PubMed] [Google Scholar]
- 71.Hoffman SL, et al. High-dose dexamethasone in quinine-treated patients with cerebral malaria: a double-blind, placebo-controlled trial. J. Infect. Dis. 1988;158:325–331. doi: 10.1093/infdis/158.2.325. [DOI] [PubMed] [Google Scholar]
- 72.Rampengan TH. Cerebral malaria in children. Comparative study between heparin, dexamethasone and placebo. Paediatr. Indones. 1991;31:59–66. [PubMed] [Google Scholar]
- 73.Gordeuk VR, et al. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. N. Engl. J. Med. 1992;327:1473–1477. doi: 10.1056/NEJM199211193272101. [DOI] [PubMed] [Google Scholar]
- 74.Thuma PE, et al. Effect of iron chelation therapy on mortality in zambian children with cerebral malaria. Trans. R. Soc. Trop. Med. Hyg. 1998;92:214–218. doi: 10.1016/s0035-9203(98)90753-2. [DOI] [PubMed] [Google Scholar]
- 75.Treeprasertsuk S, et al. N-acetylcysteine in severe falciparum malaria in Thailand. Southeast Asian J. Trop. Med. Public Health. 2003;34:37–42. [PMC free article] [PubMed] [Google Scholar]
- 76.Lehman LG, Vu-Quoc B, Carlson J, Kremsner PG. Plasmodium falciparum: inhibition of erythrocyte rosette formation and detachment of rosettes by pentoxifylline. Trans. R. Soc. Trop. Med. Hyg. 1997;91:74–75. doi: 10.1016/s0035-9203(97)90402-8. [DOI] [PubMed] [Google Scholar]
- 77.Hemmer CJ, et al. Supportive pentoxifylline in falciparum malaria: no effect on tumour necrosis factor alpha levels or clinical outcome: a prospective, randomized, placebo-controlled study. Am. J. Trop. Med. Hyg. 1997;56:397–403. doi: 10.4269/ajtmh.1997.56.397. [DOI] [PubMed] [Google Scholar]
- 78.Di Perri G, et al. Pentoxifylline as a supportive agent in the treatment of cerebral malaria in children. J. Infect. Dis. 1995;171:1317–1322. doi: 10.1093/infdis/171.5.1317. [DOI] [PubMed] [Google Scholar]
- 79.Das BK, et al. Pentoxifylline adjunct improves prognosis of human cerebral malaria in adults. Trop. Med. Int. Health. 2003;8:680–684. doi: 10.1046/j.1365-3156.2003.01087.x. [DOI] [PubMed] [Google Scholar]
- 80.van Hensbroek MB, et al. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J. Infect. Dis. 1996;174:1091–1097. doi: 10.1093/infdis/174.5.1091. [DOI] [PubMed] [Google Scholar]
- 81.Taylor TE, et al. Intravenous immunoglobulin in the treatment of paediatric cerebral malaria. Clin. Exp. Immunol. 1992;90:357–362. doi: 10.1111/j.1365-2249.1992.tb05851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Havlik I, et al. Curdlan sulphate in human severe/cerebral Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 2005;99:333–340. doi: 10.1016/j.trstmh.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Dondorp AM, et al. Levamisole inhibits sequestration of infected red blood cells in patients with falciparum malaria. J. Infect. Dis. 2007;196:460–466. doi: 10.1086/519287. [DOI] [PubMed] [Google Scholar]
- 84.Okoromah CAN, Afolabi BB. Mannitol and other osmotic diuretics as adjuncts for treating cerebral malaria. Cochrane Database of Systematic Reviews. 2004;(issue 4) doi: 10.1002/14651858.CD004615.pub2. Art. No. CD004615. doi:10.1002/14651858. CD004615.pub2. [DOI] [PubMed] [Google Scholar]
- 85.Namutangula B, Ndeezi G, Byarugaba JS, Tumwine JK. Mannitol as adjunct therapy for childhood cerebral malaria in Uganda: a randomized clinical trial. Malar. J. 2007;6:138. doi: 10.1186/1475-2875-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maitland K, et al. Randomized trial of volume expansion with albumin or saline in children with severe malaria: preliminary evidence of albumin benefit. Clin. Infect. Dis. 2005;40:538–545. doi: 10.1086/427505. [DOI] [PubMed] [Google Scholar]
- 87.Agbenyega T, et al. Population kinetics, efficacy, and safety of dichloroacetate for lactic acidosis due to severe malaria in children. J. Clin. Pharmacol. 2003;43:386–396. doi: 10.1177/0091270003251392. [DOI] [PubMed] [Google Scholar]
- 88.Casals-Pascual C, Idro R, Picot S, Roberts DJ, Newton CR. Can erythropoietin be used to prevent brain damage in cerebral malaria? Trends Parasitol. 2009;25:30–36. doi: 10.1016/j.pt.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Casals-Pascual C, et al. High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc. Natl Acad. Sci. USA. 2008;105:2634–2639. doi: 10.1073/pnas.0709715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White NJ, Looareesuwan S, Phillips RE, Chanthavanich P, Warrell DA. Single dose phenobarbitone prevents convulsions in cerebral malaria. Lancet. 1988;2:64–66. doi: 10.1016/s0140-6736(88)90002-5. [DOI] [PubMed] [Google Scholar]
- 91.Kochar DL, Kumawat BL, Bajiya HN, Chauhan S, Kochar SK. Prophylactic role of a single dose of phenobarbitone in preventing convulsions in cerebral malaria. J. Assoc. Physicians India. 1997;45:123–124. [Google Scholar]
- 92.Crawley J, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000;355:701–706. doi: 10.1016/S0140-6736(99)07148-2. [DOI] [PubMed] [Google Scholar]
- 93.Weinberg JB, Lopansri BK, Mwaikambo E, Granger DL. Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria. Curr. Opin. Infect. Dis. 2008;21:468–475. doi: 10.1097/QCO.0b013e32830ef5cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Penet MF, et al. Protection against cerebral malaria by the low-molecular-weight thiol pantethine. Proc. Natl Acad. Sci. USA. 2008;105:1321–1326. doi: 10.1073/pnas.0706867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pradines B, et al. Atorvastatin is 10-fold more active in vitro than other statins against Plasmodium falciparum. Antimicrob. Agents Chemother. 2007;51:2654–2655. doi: 10.1128/AAC.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Golenser J, McQuillan J, Hee L, Mitchell AJ, Hunt NH. Conventional and experimental treatment of cerebral malaria. Int. J. Parasitol. 2006;36:583–593. doi: 10.1016/j.ijpara.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 97.Hemmer CJ, et al. Malaria and bacterial sepsis: similar mechanisms of endothelial apoptosis and its prevention in vitro. Crit. Care Med. 2008;36:2562–2568. doi: 10.1097/CCM.0b013e31818441ee. [DOI] [PubMed] [Google Scholar]
- 98.von Zur MC, et al. A contrast agent recognizing activated platelets reveals murine cerebral malaria pathology undetectable by conventional Mri. J. Clin. Invest. 2008;118:1198–1207. doi: 10.1172/JCI33314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mohanty D, Ghosh K, Pathare AV, Karnad D. Deferiprone (L1) as an adjuvant therapy for Plasmodium falciparum malaria. Indian J. Med. Res. 2002;115:17–21. [PubMed] [Google Scholar]
- 100.Looareesuwan S, et al. Pentoxifylline as an ancillary treatment for severe falciparum malaria in Thailand. Am. J. Trop. Med. Hyg. 1998;58:348–353. doi: 10.4269/ajtmh.1998.58.348. [DOI] [PubMed] [Google Scholar]