Abstract
Stress affects the brain differently depending on the timing, duration and intensity of the stressor. Separation from the dam for 3 hours per day is a potent stressor for rat pups which causes activation of the hypothalamic-pituitary-adrenal (HPA) axis, evidenced by increased plasma levels of adrenocorticotropin (ACTH) and glucocorticoids. Behaviourally, animals display anxiety-like behaviour while structurally, changes occur in neuronal dendrites and spines in the hippocampus and prefrontal regions involved in emotion and behaviour control. The aim of the present study was to determine whether maternal separation alters expression of synaptic markers, synaptophysin and calcium/calmodulin-dependent protein kinase II, CaMKII, in rat hippocampus and prefrontal cortex. A second aim was to determine whether voluntary exercise had a beneficial effect on the expression of these proteins in rat brain. Maternal separation occurred from postnatal day 2 (P2) to P14 for 3 hours per day. Exercised rats were housed in cages with attached running wheels from P29 to P49. At P65, the prefrontal cortex and hippocampus were removed for protein quantification. Maternal separation did not have any effect while exercise increased synaptophysin and CaMKII in the ventral hippocampus but not in the dorsal hippocampus or prefrontal cortex. Since the ventral hippocampus is associated with anxiety-related behaviour, these findings are consistent with the fact that voluntary exercise increases anxiety-like behaviour and improves learning and memory.
Keywords: Maternal separation, stress, exercise, synaptophysin, CaM kinase, CaMKII
INTRODUCTION
The effect of stress on the brain depends on the timing, duration and intensity of the stressor as well as genetic predisposition and prior experience (Lupien et al. 2009). One of the most potent stressors for rat pups is separation from the dam during the early postnatal period (Lupien et al. 2009). Separation for 3 hours or more per day activates the hypothalamic-pituitary-adrenal (HPA) axis, as evidenced by increased plasma levels of adrenocorticotropin (ACTH) and glucocorticoids (Daniels et al. 2004; Lupien et al. 2009). The interplay between the elements of the HPA axis acts to maintain a balanced homeostatic environment. In humans alterations to the HPA axis accompany psychiatric disorders such as depression and anxiety (Kathol et al. 1989; Lupien et al. 2009). In maternally separated animals, separation stress causes anxiety-like behaviour as well as cognitive impairment and structural changes in neuronal dendrites and spines in the hippocampus and prefrontal regions of the brain involved in emotion and behaviour control (Aisa et al. 2007; Andersen et al., 2004; Bock et al. 2005; Pascual and Zamora-León, 2007; Lupien et al. 2009).
Voluntary exercise has been shown enhance neurogenesis and improve cognitive performance (Clark et al. 2008; Duman et al. 2008; Stranahan et al. 2007). One reason may be the increase in hippocampal brain-derived neurotrophic factor (BDNF) levels seen in exercised rats (Vaynman et al. 2004). BDNF has been shown to be positively correlated with nerve terminal proteins suggesting that BDNF increases synaptogenesis (Ying et al. 2008).
The aim of the present study was to determine whether maternal separation alters expression of synaptic markers in rat hippocampus and prefrontal cortex. Synaptophysin, a vesicle-associated glycoprotein, was used as a presynaptic marker of synaptic proliferation while calcium/calmodulin-dependent protein kinase II (CaMKII) was used as a postsynaptic marker of glutamate activation of NMDA receptors required for learning and memory formation. CaMKII phosphorylates and up-regulates the number of AMPA receptors in the postsynaptic cell by either phosphorylation to increase the conductance of sodium ions and/or by promoting the movement of intracellular AMPA receptors into the membrane, making more receptors available for glutamate to stimulate the postsynaptic neuron (Fukunanga et al. 1996). Long-term potentiation (LTP), an activity-dependent strengthening of synapses, considered to be an electrophysiological correlate of learning and memory, induces the activation of CaMKII (Colbran et al. 2004; Vaynman et al. 2007). A second aim was to determine whether voluntary exercise had a beneficial effect on synaptic function in maternally separated rats.
MATERIALS AND METHODS
Animals
Forty-five male Sprague-Dawley rats were obtained from the University of Cape Town Animal Unit. On postnatal day two (P2) all litters were sexed and culled to 8 (with preference given to males) in order to standardize litter size. Rats were divided into 4 groups, maternally separated/non-runners (MSnR, n = 10), maternally separated/runners (MSR, n = 11), non-separated/non-runners (nMSnR, n = 13) and non-separated/runners (nMSR, n = 11). Dams and pups were housed in a 12-hour light/dark cycle, lights on at 06h00. On P21 rats were transferred to a new 12-hour light/dark cycle with the lights off at 11h00. Ambient temperatures were maintained at 23–25°C. Food and water was available ad libitum. Rats were weighed on P49, P52, P55, P63 and P65. All experiments were approved by the Research Ethics Committee of the University of Cape Town.
Maternal Separation
Starting on P2, the dams of the maternally separated pups were physically removed from the home cage and the pups were transferred in their home cage to a different room in order to prohibit communication via ultra-sound vocalizations. The room temperature was maintained at 32 ± 1 °C to prevent hypothermia. Pups were separated for 3 hours per day, until P14 after which normal interaction with the dam was resumed. The control pups (nMSR and nMSnR) were not separated from the dams. On P21 all rats were weaned. Males were housed communally until P29.
Voluntary Exercise
On P29 rats were given free access to running wheels attached to their cages. The number of revolutions was recorded on a counter mechanically activated by the wheel. The distance run was calculated from the revolution count and circumference of the wheel, where one revolution was equal to 1 m. Recordings were taken daily 1 hour before the dark cycle began (when the rats became active) in order to get an accurate reading of daily running distances. Non-exercised rats (MSnR and nMSnR) were housed singly in standard plastic cages from P29 – P49.
On P49 rats were removed from running wheels and all groups were then housed in standard plastic cages in two’s or three’s, until termination of the experiment.
Behavioural Testing
Behavioural tests (open field, elevated plus maze, Morris water maze and object recognition tests) were performed between P49 and P63 as described in Grace et al. (2009).
Protein Quantification
At P65, rats were lightly anaesthetized by placing them in a container filled with halothane vapor and then decapitated using a guillotine. The brain was rapidly removed from the cranium, placed on an ice-cold plate and the prefrontal cortex dissected out, removing the olfactory bulbs. The cerebral hemispheres were separated at the longitudinal cerebral fissure using a blade. The right half of the brain was placed on the frontal surface and the cortex separated from the cerebellum. This exposed the hippocampus which was then rolled away from the cortex and cut in half, separating the dorsal and ventral sub-regions. Samples were placed in small plastic vials, frozen in liquid nitrogen and transferred to a −80°C freezer where they were stored until analysis.
For analysis samples were thawed, weighed, homogenized in 19 volumes extraction buffer (1 M Tris.HCl, Nonidet, 10% SDS, aprotonin, 0.1 M phenylmethylsulphonyl fluoride) and stored at 4°C overnight. Samples were then centrifuged at 4°C for 15 minutes at 12,000 g and the supernatant removed. A bicinchoninic acid (BCA) assay kit (Pierce) was used for quantification of total proteins.
SDS-PAGE and Western Blot analysis
Hippocampal samples were diluted in extraction buffer and proteins denatured in boiling water for 2 minutes. Proteins were then resolved in a sodium dodecyl sulphate (SDS)-polyacrylamide gel; a 12% Separating gel (30% Acrylamide:bis, 1.5 M Tris, 10% SDS, 10% Ammonium persulphate, 10 drops of Temed) and 5% stacking gel (30% Acrylamide:bis, 0.5 M Tris, 10% SDS, 10% Ammonium persulphate, 10 drops of Temed) was used. Approximately 20 µg of proteins in each sample were loaded individually in the following order: MSR, nMSR, MSnR, nMSnR. Either prefrontal cortex samples were loaded onto a gel in duplicate (prefrontal cortex obtained from 2 different rats) or both ventral and dorsal hippocampal samples of the same rats (MSR, nMSR, MSnR, nMSnR) were loaded onto a gel giving a total of 45 rats, 11 × MSR, 10 × MSnR, 11 × nMSR, 13 × nMSnR. Each rat brain tissue sample was analyzed individually. A peqGOLD prestained marker (Octamer) was loaded. Gels were run at 180V for 1.25 hours. Proteins from the gel were then electrophoretically transferred to a Hybond-enhanced electrochemiluminescence (ECL) nitrocellulose membrane at 100 V for 1 hour.
Immunodetection
Membranes were blocked in 5% non-fat powdered milk in Tris-buffered saline-Tween (TBS-T) for 1 hour. A mouse monoclonal primary antibody to Synaptophysin (Abcam Inc) was diluted (1:40,000) in 5% non-fat powdered milk in TBS-T and incubated with the nitrocellulose membrane at 4°C overnight. Membranes were washed with TBS-T and then incubated for 1 hour with the secondary antibody, goat anti-mouse horseradish-peroxidase conjugate (HRP) conjugate (Biorad, 1:1,500 dilution in non-fat powdered milk). The reaction was visualized by using a Supersignal West Pico chemiluminescence (ECL) detection kit (Pierce). X-ray film (AGFA) was used to detect the reaction with the membranes. The X-ray film was placed on the membrane in the dark room. The film was then placed in developer and fixed.
A similar procedure was used to detect and analyze CaMKII (Abcam Inc.) except that membranes were incubated with primary (rabbit-monoclonal antibody to CaMKII (1:1000 dilution) and then with secondary antibody, goat-anti-rabbit HRP conjugate 1:1500. For the analysis of CaMKII only the density of the lower band was quantified.
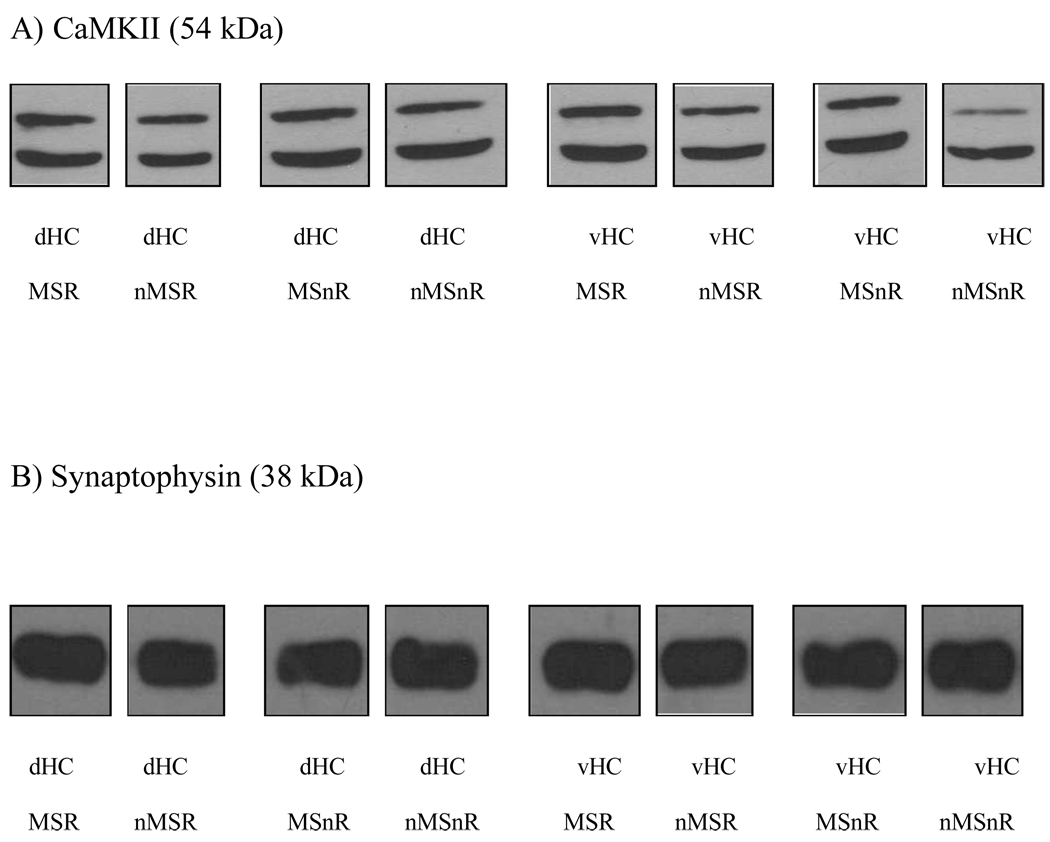
The density of each band was measured using a densitometer (Synergy) and expressed as a percentage of the mean density of all the bands representing that protein (either synaptophysin or CaMKII) on the X-ray film (Figure 1).
Figure 1.
A representative Western blot analysis of CaMKII (A) and Synaptophysin (B) levels in dorsal hippocampus (dHC) and ventral hippocampus (vHC) of a maternally separated runner (MSR), a non-maternally separated runner (nMSR), a maternally separated non-runner (MSnR) and a non-maternally separated non-runner (nMSnR) rat.
Statistical Analysis
All statistical analyses were performed using Statistica version 8. Graphs were plotted using Graph Pad Prism 5. Data are presented as the means ± SEM. Statistically significant differences between groups were determined by 2-way ANOVA with maternal separation and exercise as factors. Post-hoc analysis was performed using Fisher LSD test.
RESULTS
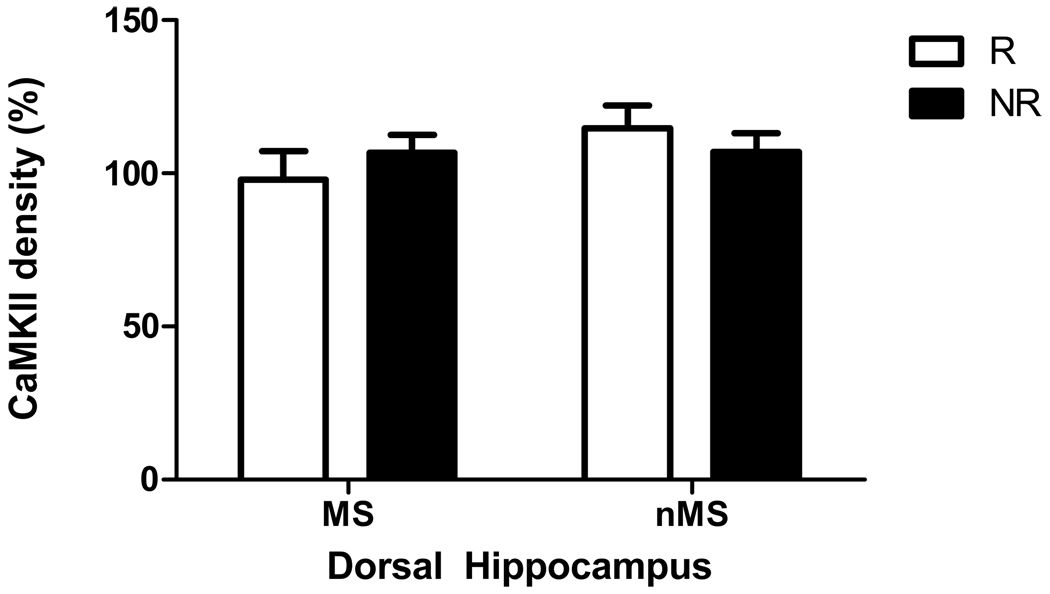
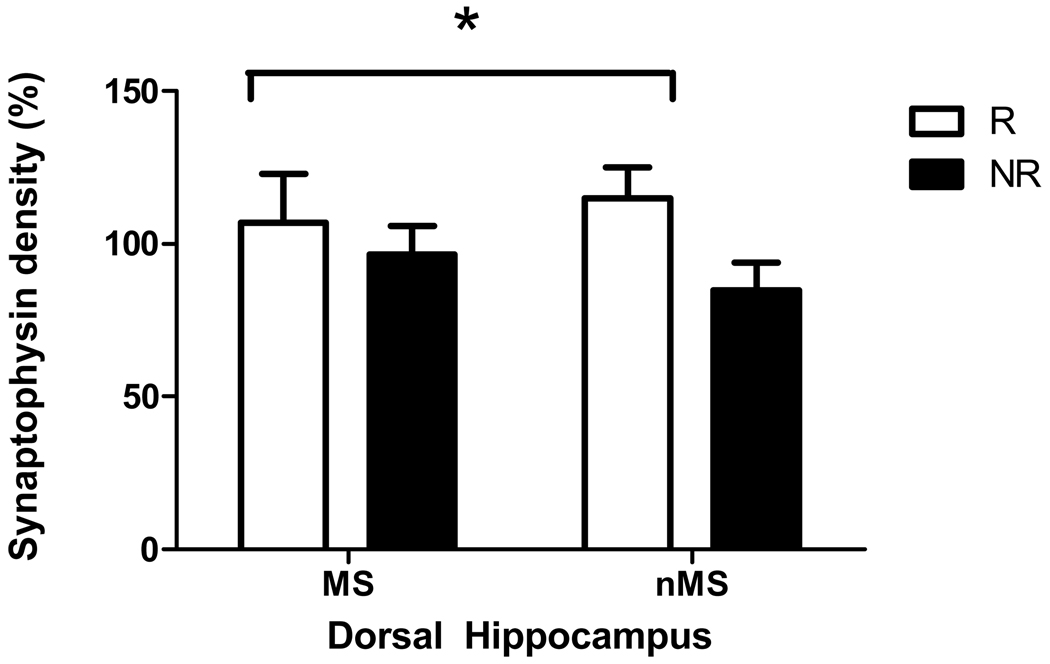
There was no significant difference in either synaptophysin or CaMKII levels in the prefrontal cortex of maternally separated and non-separated rats or between exercised and non-exercised rats. However, exercise caused a trend towards an increase in synaptophysin levels (but not CaMKII) in the dorsal hippocampus (F(1,41) = 3.29, p = 0.07, Figures 2 and ).
Figure 2.
Effect of maternal separation and exercise on CaMKII levels in dorsal hippocampus of maternally separated rats (MS) and normally reared rats (nMS) with significant overall effect of exercise. R = runners, NR = non-runner rats that did not have access to a running wheel. Results are mean ± SEM. No significant difference was found.
Figure 3.
Effect of maternal separation and exercise on synaptophysin levels in dorsal hippocampus of materrnally separated rats (MS) and normally reared rats (nMS). R = runners, NR = non-runner rats that did not have access to a running wheel. Results are mean ± SEM. *Exercise caused a tendency for synaptophysin levels to be increased (F(1,41) = 3.29, p = 0.07)
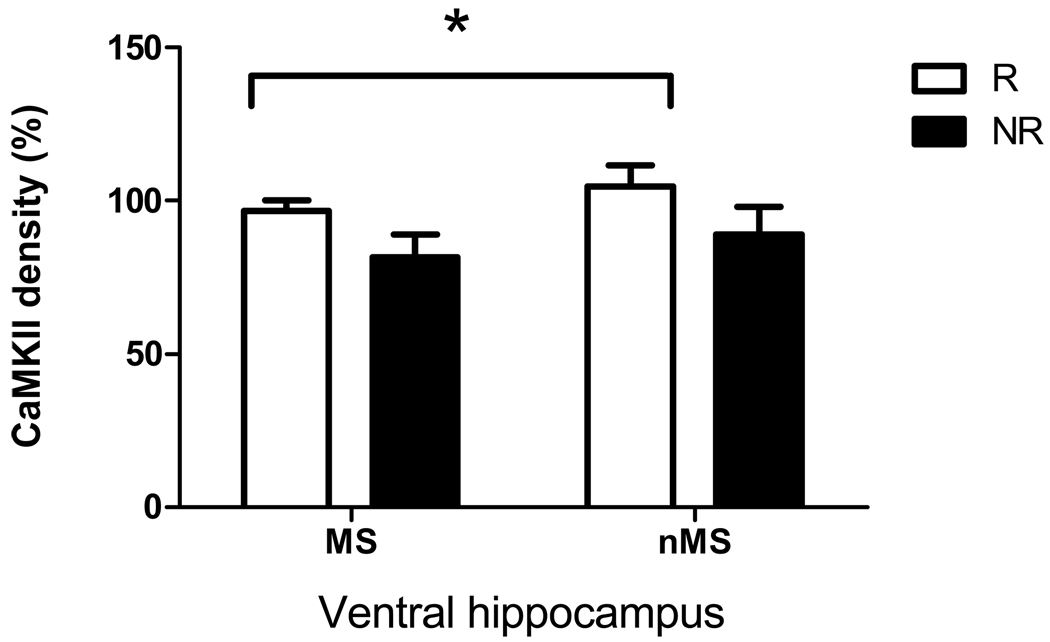
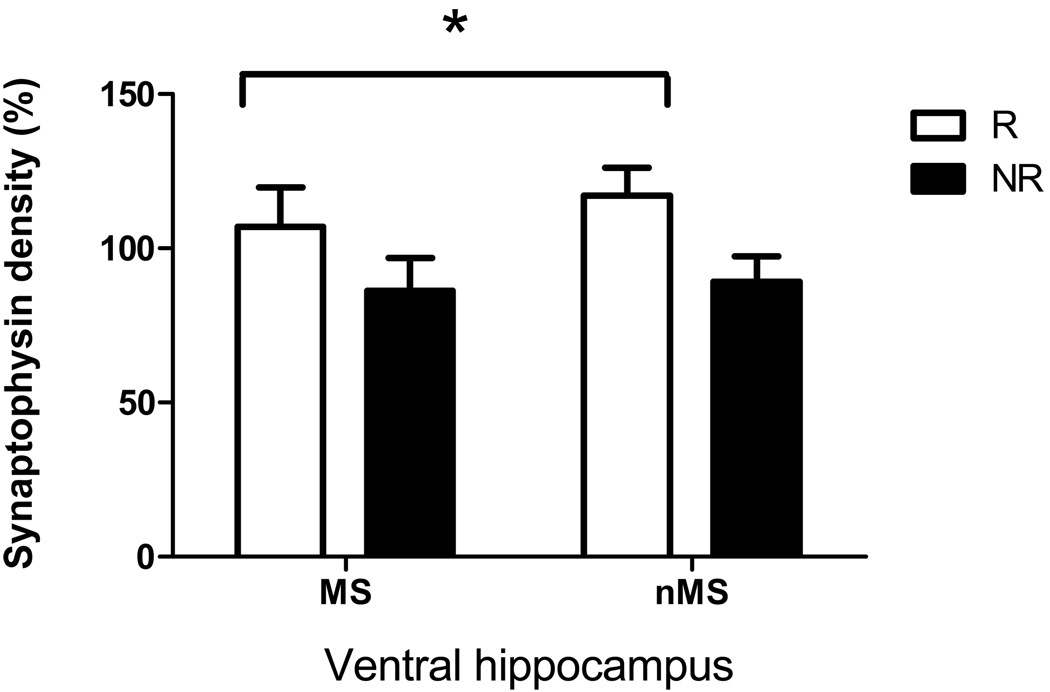
Exercise significantly increased CaMKII levels in the ventral hippocampus of exercised rats (F(1,41) = 4.5, p < 0.05, Figure 4). A post-hoc Fisher LSD test revealed a significant difference between the nMSR and MSnR groups (p < 0.05). Similar differences between exercised and non-exercised rats were observed for synaptophysin levels in the ventral hippocampus with a significant overall effect of exercise (F(1,41) = ,5.6, p < 0.05, Figure 5). Post-hoc Fisher LSD tests revealed a significant difference between the nMSR and MSnR groups (p < 0.05).
Figure 4.
Effect of maternal separation and exercise on CaMKII levels in the ventral hippocampus of maternally separated rats (MS) and normally reared rats (nMS) with significant overall effect of exercise. R = runners, NR = non-runner rats that did not have access to a running wheel. Results are mean ± SEM. *Significantly different from non-runners (F(1,41) = 4.5, p < 0.05).
Figure 5.
Effect of maternal separation and exercise on synaptophysin levels in the ventral hippocampus of maternally separated rats (MS) and normally reared rats (nMS), with significant overall effect of exercise. R = runners, NR = rats that did not have access to a running wheel. Results are mean ± SEM. *Significantly different from non-runners (F(1,41) = ,5.6, p < 0.05).
DISCUSSION
Chronic stress experienced at an early stage of development can have a profound influence on the brain in adulthood, including effects on prefrontal cortex and hippocampal structure and function, neuroplasticity, cell survival and neurogenesis as well as producing fear and anxiety-like behaviour (Pittenger et al. 2007; McEwen, 1999; Bock et al. 2005; Pascual and Zamora-León, 2007; Lupien et al. 2009). Although the prefrontal cortex is critical for stress regulation and anxiety, no changes were observed in synaptophysin and CaMKII in the prefrontal cortex of maternally separated rats compared to their non-separated controls. In fact, maternal separation did not alter synaptophysin or CaMKII levels in the prefrontal cortex or hippocampus of exercised and/or non-exercised rats.
Voluntary exercise caused a significant increase in CaMKII and synaptophysin levels in the ventral hippocampus which is critically involved in learning and memory of anxiety-related experiences (Kjelstrup et al. 2002). This is in agreement with the finding that exercised rats display increased anxiety-like behaviour when removed from their cages with attached running wheels (Grace et al. 2009). The hippocampus is a critical brain area involved in learning and memory whose plasticity is significantly affected by exercise (Vaynman et al. 2004). Long-term potentiation (LTP), an activity dependent strengthening of synapses believed to be an electrophysiological correlate of learning and memory, induces the activation of CaMKII (Vaynman et al. 2007). Pharmalogical and genetic manipulations have demonstrated the importance of CaMKII for memory processes (Silva et al. 1992). CaMKII inhibitors prevented the enhancement of learning and memory produced by exercise (Vaynman et al. 2007).
Synaptophysin levels were increased in the ventral hippocampus which reinforces the suggestion that exercise enhances learning and memory processes (Vaynman et al. 2004). Exercise also tended to increase the dorsal hippocampal synaptophysin levels, an area of the brain that is critically involved in spatial learning and memory formation (Hunsaker et al. 2008, Lee 2005). This may explain the increased ability of the exercised rats to find the platform in the Morris Water maze test of spatial working memory (Grace et al. 2009). The exercised rats also performed better than non-exercised rats in temporal order tasks, consistent with increased hippocampal function relative to non-exercised rats (Grace et al. 2009). The present findings are consistent with the suggestion that stress is an important factor in the development of the individual’s response to future stress.
6. Conclusion
No effect of maternal separation was seen whereas running significantly increased expression of protein markers of pre- and postsynaptic activity in the ventral hippocampus, an area associated with learning of anxiety-related behaviour. This is consistent with the finding that exercised rats display increased anxiety-like behaviour in the elevated plus maze and open field apparatus following removal from their cages with the attached running wheels (Grace et al. 2009).
Running has been shown to activate the HPA axis (Coleman et al. 1998). Stranahan et al. (2006) found that running increased the levels of glucocorticoids, which were significantly higher in the rats running in isolation compared to the socially grouped rats. It was therefore suggested by Stranahan et al. (2006) that social interaction buffers the exercised rats from negative actions of high glucocorticoid levels. This increase in glucocorticoid levels seen in isolated runners could account for the increased anxiety-like behaviour observed in the exercised rats (Grace et al. 2009).
ACKNOWLEDGMENTS
This material is based upon work supported financially by the National Research Foundation (NRF), the National Institutes of Health (NIH) Fogarty International Center grant R01TW008040 to Michael J. Zigmond, and the University of Cape Town. Any opinion, findings and conclusions, or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereto
References
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Bock J, Gruss M, Becker S, Braun K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cereb Cortex. 2005;15:802–808. doi: 10.1093/cercor/bhh181. [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiology. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Coleman MA, Garland T, Marler CA, Newton SS, Swallow JG, Carter PA. Glucocorticoid response to forced exercise in laboratory house mice (Mus domesticus) Physiology and Behavior. 1998;63:279–285. doi: 10.1016/s0031-9384(97)00441-1. [DOI] [PubMed] [Google Scholar]
- Daniels WMU, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metabolic Brain Disease. 2004;19:1/2. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Muller D, Miyamoto E. CaM kinase II in long-term potentiation. Neurochem. Int. 1996;28:343–358. doi: 10.1016/0197-0186(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Grace L, Hescham S, Kellaway LA, Bugarith K, Russell VA. Effect of exercise on learning and memory in a rat model of developmental stress. Metab Brain Dis. 2009 doi: 10.1007/s11011-009-9162-5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Lee B, Kesner RP. Evaluating the temporal context of episodic memory: The role of CA3 and CA1. Behavioural Brain Research. 2008;188:310–315. doi: 10.1016/j.bbr.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathol RG, Jaeckle RS, Lopez JF, Meller WH. Pathophsiology of HPA axis abnormalities in patients with major depression: an update. Am J Psychiatry. 1989;146:311–317. doi: 10.1176/ajp.146.3.311. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach H, Murison R, Moser EI, Moser M. Reduced fear expression after lesions of the ventral hippocampus. PNAS. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. The role of hippocampal subregions in detecting spatial novelty. Behavioral Neuroscience. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and the aging hippocampus. Frontiers in Neuroendocrinology. 1999;20(1):49–70. doi: 10.1006/frne.1998.0173. [DOI] [PubMed] [Google Scholar]
- Pascual R, Zamora-León SP. Effects of neonatal maternal deprivation and postweaning environmental complexity on dendritic morphology of prefrontal pyramidal neurons in the rat. Acta Neurobiol Exp (Wars) 2007;67:471–479. doi: 10.55782/ane-2007-1663. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology. 2007;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and the entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF - exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]