Abstract
Calbindin-D28k has been reported to be a facilitator of calcium diffusion and to protect against apoptotic cell death. Most recently we found that the presence of calbindin-D28k results in reduced calcium influx through voltage-dependent L-type Ca2+ channels and enhanced sensitivity of the channels to calcium dependent inactivation. Co-immunoprecipitation and GST pull down assays indicate that calbindin-D28k interacts with the C-terminus of the L-type calcium channel alpha1c subunit (Cav1.2). This is the first report of the binding of calbindin to a calcium channel and provides new insight concerning mechanisms by which calbindin acts to modulate intracellular calcium. Besides calbindin, another major target of 1,25(OH)2D3 is 24(OH)ase, which is involved in the catabolism of 1,25(OH)2D3. We reported that C/EBPβ is a major transcriptional activator of 24(OH)ase that cooperates with CBP/p300 in regulating VDR mediated 24(OH)ase transcription. Recently we found, in addition to p160 coactivators, that SWI/SNF complexes (that facilitate transcription by remodeling chromatin using the energy of ATP hydrolysis) are also involved in VDR mediated 24(OH)ase transcription and functionally cooperate with C/EBPβ in regulating 24(OH)ase. These findings define novel mechanisms that may be of fundamental importance in understanding how 1,25(OH)2D3 mediates its multiple biological effects.
Keywords: calbindin-D28k, L type calcium channels, 25-hydroxyvitamin D3 24-hydroxylase, CCAAT enhancer binding protein, SWI/SNF chromatin remodeling complex
1. Introduction
Vitamin D is a principal factor that maintains calcium homeostasis and is required for bone development and maintenance [1]. Vitamin D is currently recommended as a dietary supplement for all patients with osteoporosis or decreased bone mass and has been reported to prevent bone loss and decrease fracture incidence [2, 3]. In addition, numerous studies have indicated an interrelationship between vitamin D and health beyond bone including effects on preventing or at least partially protecting against certain autoimmune diseases and inhibition of proliferation of cancer cells [1, 4, 5]. However, in spite of the importance of vitamin D, our understanding of vitamin D action has remained incomplete. It is known that the hormonally active form of vitamin D, 1,25dihydroxyvitamin D3 (1,25(OH)2D3), heterodimerizes with the retinoid X receptor and interacts with the vitamin D response element (VDRE) in the promoter of target genes [1,4,5]. The mechanisms involved in VDR mediated transcription are now being defined. TFIIB, several TATA binding protein associated factors (TAFs) as well as the p160 coactivators that include SRC-1, SRC-2 and SRC-3 that have histone acetylase (HAT) activity have been reported to be involved in VDR mediated transcription [1, 4–6]. VDR mediated transcription is also mediated by the coactivator complex DRIP (vitamin D receptor interacting protein) that acts through recruitment of RNA polymerase II holoenzyme [6, 7]. In addition, a number of promoter specific transcription factors have been reported by our lab and others to modulate VDR mediated transcription [8–11]. Thus we are only now beginning to understand the multiple factors and mechanisms involved in VDR mediated transcription. Further questions that need to be addressed are 1) what additional cofactors are necessary and sufficient for VDR mediated transcription? 2) what are the cofactor dynamics in VDR mediated transcriptional regulation? 3) what are the mechanisms involved in the integration of extracellular signals and 1,25(OH)2D3 action? This article focuses on research from our laboratory related to a further understanding of the molecular mechanisms of 1,25(OH)2D3 action. In addition our recent findings related to the biological significance of calbindin are also discussed.
2. Materials and methods
For electrophysiological measurements, standard whole cell patch clamp techniques were used as previously described [12, 13]. For the glutathione S-transferase (GST) fusion protein pull-down assay, fusion proteins containing fragments of α11.2 (GST-α1c N-terminus 1–154 and GSTα1c C-terminus (1509–1905) were obtained from Geoffrey Pitt, Columbia Medical School. CBP and C/EBPβ antisera were obtained from Santa Cruz Biotechnology. Phospho-C/EBPβ antibody, Thr188 MAPK site was purchased from Cell Signaling Technology. For transcriptional assays, promoter constructs containing regions of the rat 24(OH)ase promoter linked to chloramphenicol acetyltransferase or the human 24(OH)ase promoter region (−5500/−22) linked to the luciferase reporter gene were used [14, 15]. Transcription assays were performed by standard protocols [10,16]. COS-7 cells, C33A cells and SW13 cells were obtained from American Type Culture Collection. MC3T3-E1 cells were from Riken Cell Bank, Tsukuba, Japan. Stable transfection of RIN-38 cells with calbindin-D28k has been described [13]. pCMV-Brm and pCMV-mutant Brm expression vectors were obtained from M. Yaniv.
3. Results and discussion
3.1 Calbindin
One of the most pronounced effects of 1,25(OH)2D3 known is increased synthesis of the calcium binding protein, calbindin, the first identified target of 1,25(OH)2D3 action in intestine and kidney. It has been suggested that the role of calbindin is to facilitate vitamin D dependent transcellular movement of calcium in the intestinal or renal cell (see review, Christakos et al. [17]). However studies from our lab and others have shown that calbindin also has a major role in protecting against apoptotic cell death in different cell types (see review, [18]). Sustained elevations in intracellular calcium result in damage to the mitochondria and cell death. Calcium dependent proteases and calcium activated endonucleases are also involved in apoptosis. Calbindin, by buffering calcium, can block apoptosis. We have also shown that the antiapoptotic effects of calbindin involve inhibition of caspase 3, a common downstream effector of multiple apoptotic signaling pathways [19, 20]. GST pull down assays indicated that calbindin-D28k directly binds to caspase 3 [19, 20]. Besides the inhibitor of apoptotic proteins, calbindin-D28k is the only other natural endogenous inhibitor of caspase 3.
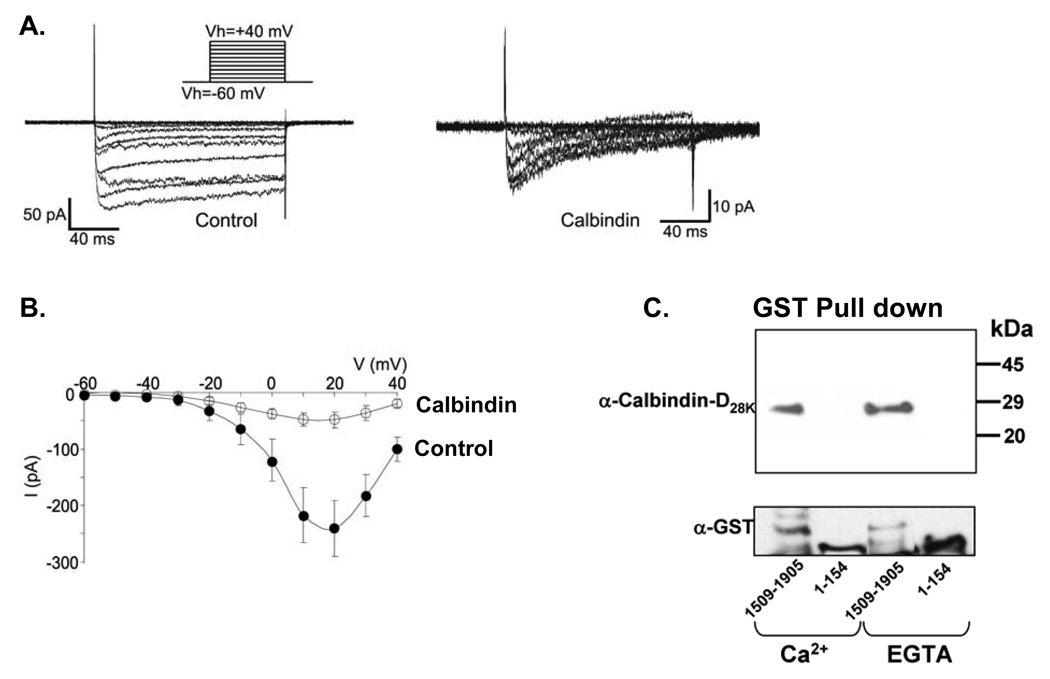
In brain and pancreas, calbindin-D28k is colocalized with L-type calcium channels [21, 22]. In duodenum and jejunum calbindin-D9k is colocalized with the epithelial calcium channel TRPV6 and in the kidney calbindin-D28k is colocalized with the epithelial calcium channel TRPV5 [23]. In previous studies using renal luminal membrane vesicles, we reported that calbindin-D28k enhanced apical calcium entry [24], identifying for the first time a causal link between calbindin-D28k and apical calcium entry. In addition, in neuronal cells and pancreatic beta cells calbindin-D28k has been shown to modulate evoked calcium transients [17]. However the exact mechanisms involved in the modulation of intracellular calcium by calbindin-D28k are not known. Direct binding of calmodulin to L-type calcium channels has been reported to be a key step in the autoregulation of L-type channels [25]. Recent studies have indicated that calmodulin also binds to the C terminus of TRPV6 and modulates TRPV6 activity [26]. In addition, calcium binding proteins other than calmodulin have been shown to bind calcium channels, suggesting differential adjustment of calcium influx through calcium channels by different calcium binding proteins [27, 28]. In our continuing efforts to understand the role of calbindin, we examined mechanisms involved in the modulation of intracellular calcium by calbindin-D28k in the beta cell line RINr104-38 (RIN-38). We found that calbindin-D28k reduces calcium influx through voltage dependent L-type calcium channels and enhances sensitivity of the channels to calcium dependent inactivation (Fig. 1; [13]). Calbindin-D28k was also found to interact with the C-terminus of the α1 subunit (Cav1.2) of the L-type calcium channel (1509–1905) which includes the calmodulin binding site (Fig. 1C). This is the first report of the binding of calbindin to a calcium channel. The calcium independent binding of calbindin to the C-terminus, similar to what has been reported for other calcium binding proteins that bind to a region of the C-terminus that overlaps with the region of calmodulin binding [27,28], suggests that calbindin may be tethered to this region. Multiple calcium binding proteins may be important for fine tuning calcium channel activity. The exact calbindin binding site in the C-terminus and whether calbindin and calmodulin compete for binding regions remain to be determined. In addition, although preliminary studies are suggestive, it will be important to determine whether calbindin can bind to the epithelial calcium channels and affect their activity. Thus we no longer think of calbindin as a calcium binding protein whose principal function is to facilitate calcium transport. Calbindin-D28k has a major role both in modulating calcium channel activity and in protecting against apoptotic cell death.
Fig. 1.
Calbindin-D28k modifies voltage gated calcium currents in RIN-38 cells and binds to the C-terminal domain of the α1 subunit (Cav1.2) of the L-type calcium channel. A. Whole cell recordings from control and calbindin expressing cells. B. Current-voltage relationship illustrating peak current amplitudes from control (filled circles) and calbindin-expressing (open circles) cells. Note in the presence of calbindin Ca2+ current amplitudes are smaller. C. Binding of calbindin-D28k to GST tagged a1.2 fragment 1509–1905 but not to the N terminal fragment 1–154.
3.2 25(OH)D3 24-Hydroxylase (24(OH)ase)
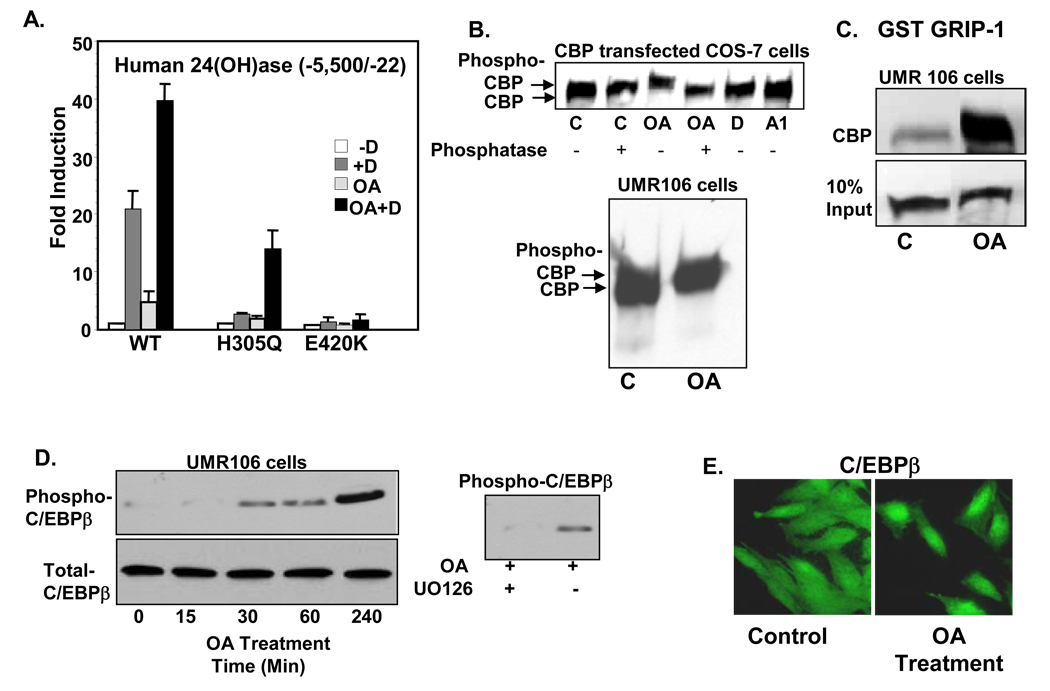
Besides calbindin, the other known pronounced effect of 1,25(OH)2D3 in intestine and kidney is increased synthesis of 24(OH)ase. Charcterization of 24(OH)ase null mutant mice provided the first in vivo evidence for a role for 24(OH)ase in the catabolism of 1,25(OH)2D3 [29]. Since 24(OH)ase is the gene most transciptionally responsive to 1,25(OH)2D3, is regulated by other hormones and signaling pathways as well as by 1,25(OH)2D3 and is present in different tissues, the 24(OH)ase gene serves as a good model to study the genomic mechanism of 1,25(OH)2D3. We recently reported that CCAAT enhancer binding protein beta (C/EBPβ), which is induced by 1,25(OH)2D3 in kidney and osteoblastic cells, is a major transcriptional activator of the 24(OH)ase gene. Our findings indicate functional cooperation between C/EBP proteins, CBP (CREB binding protein) and VDR in regulating 24(OH)ase [10]. We also found cooperation between protein kinase C and VDR in the regulation of 24(OH)ase [30]. Our results indicate that the protein kinase C enhancement of 1,25(OH)2D3 stimulated 24(OH)ase transcription may be due, in part, to an increase in VDR concentration and may also be mediated through changes in phosphorylation of VDR coregulators [30]. In addition, using the human 24(OH)ase promoter, we found that transcriptional activation of VDR from patients with HVDRR (1,25(OH)2D3 resistant rickets; mutant H305Q) can be enhanced by inhibition of phosphatase 1 and 2A (Fig. 2A; [16]). At least partial rescue of the transcriptional activity is correlated with enhanced interaction between the mutant VDR and DRIP205 [16]. In addition, inhibition of phosphatase [treatment with okadaic acid (OA; 50 nM)] induces phosphorylation of CBP and C/EBPβ (Fig. 2B–D). Phosphorylation of CBP by OA was shown by Western blot analysis. Treatment of COS-7 cells, UMR 106 cells (Fig. 2B) and MC3T3-E1 osteoblastic cells (not shown) with OA (50 nM) consistently resulted in the appearance of a slower migrating form of CBP. The slower migrating form was no longer detected after subsequent incubation with phosphatase (Fig. 2B, upper panel), providing evidence for the first time that OA results in the phosphorylation of CBP. OA also results in enhanced interaction between GRIP-1 and CBP as indicated by GST pull down assays (Fig. 2C). Immunoblotting with antibodies against a synthetic phosphopeptide corresponding to residues surrounding Thr 188 (a conserved mitogen activated protein kinase (MAPK) consensus site in C/EBPβ) showed that OA also promotes the phosphorylation of C/EBPβ (Fig. 2D). The MEK inhibitor, UO126 prevented the phosphorylation of C/EBPβ, suggesting that the phosphorylation involves OA activated MAPK signaling (Fig. 2D, right panel). Studies using immunocytochemistry and fluorescence microscopy indicate that OA can also promote the nuclear accumulation of C/EBPβ (Fig. 2E). Thus enhanced transcriptional activity by inhibition of phosphatase may be mediated by increased coactivator binding as well as by nuclear accumulation and phosphorylation of specific VDR cofactors. Further studies examining phosphorylation of VDR coactivators by different cellular signaling pathways and the effects on cofactor dynamics in VDR mediated transcriptional regulation will be important for understanding how coactivators mediate different physiological functions of VDR.
Fig. 2.
Partial activation of mutant VDR (H305Q) by phosphorylation and phosphorylation of coactivators mediated by okadaic acid (OA). A. COS-7 cells were transfected with the h24(OH)ase promoter and WT VDR or mutant VDRs (H305Q, E420K). Cell were treated with vehicle (−D), 10−8M 1,25(OH)2D3 (+D), 50 nM OA or 1,25(OH)2D3 + OA (OA + D). Note partial rescue of transcription of H305Q by phosphorylation. The E420K mutant (mutation in the coactivator binding site) was unresponsive to 1,25(OH)2D3 in the presence or absence of OA. B. Phosphorylation of CBP by OA treatment in COS-7 cells transfected with CBP were treated with vehicle (C), OA (50 nM), 1,25(OH)2D3 (D) or 1,25(OH)2D3 analog RO-262198 (A1) for 4h. UMR 106 cells were also treated with OA for 4h. C. GST pull down assay. Note enhanced interaction between GRIP-1 and CBP after OA treatment. D. Phosphorylation of C/EBPβ by OA and inhibition of C/EBPβ phophorylation by the MEK inhibitor UO126 (10 µM). E. Immunocytochemistry using C/EBPβ antiserum and fluorescence microscopy indicate that OA promotes nuclear accumulation of C/EBPβ in UMR 106 osteoblastic cells 2h after treatment.
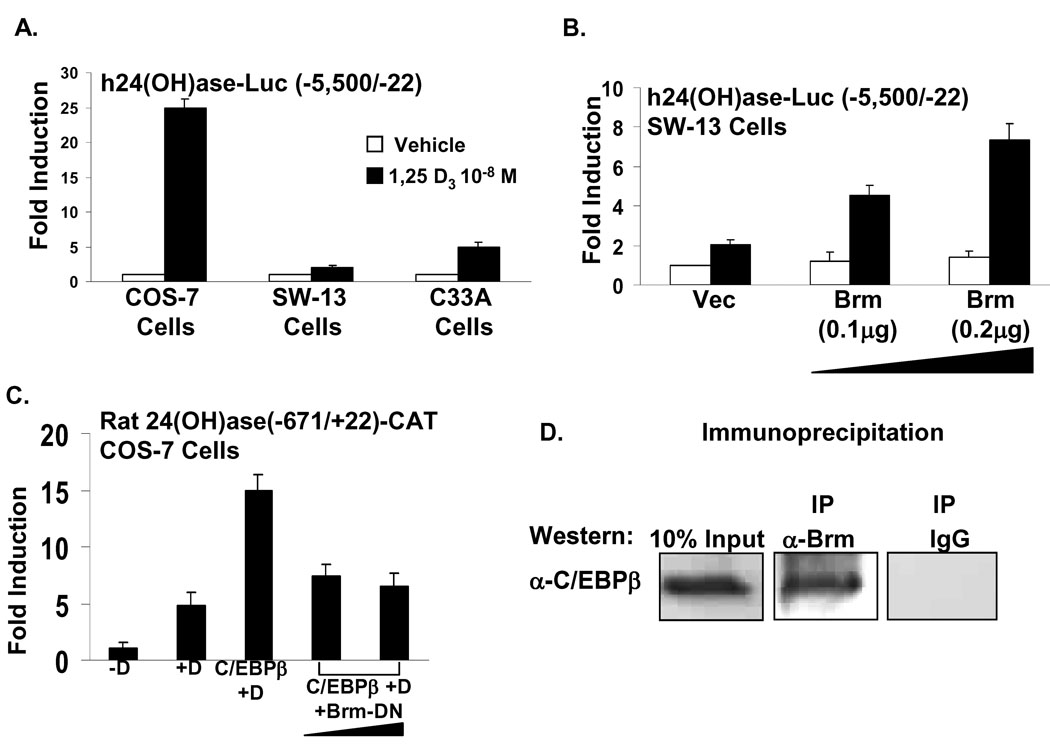
Recently, we found, in addition to p160 activators, that SWI/SNF complexes (that facilitate transcription by remodeling chromatin using the energy of ATP hydrolysis) are also involved in VDR mediated 24(OH)ase transcription and functionally cooperate with C/EBPβ in regulating 24(OH)ase. Each SWI/SNF complex contains one of two homologous ATPases, Brahma (Brm) and Brahma/related gene 1 (Brg-1) [31]. We found using the Brm and Brg-1 deficient cell lines, SW13 and C33A, that VDR mediated activation of 24(OH)ase transcription is markedly reduced but can be restored preferentially by Brm (Fig. 3; A, B). In addition mutant Brm inhibits C/EBPβ mediated enhancement of 1,25(OH)2D3 induced 24(OH)ase transcription and immunoprecipitation experiments using MC3T3-E1 cells indicate that Brm can interact with C/EBPβ (Fig. 3; C, D) . Chromatin immunoprecipitation (ChIP) using MC3T3-E1 cells showed the association of Brm as well as C/EBPβ with the C/EBP site in the 24(OH)ase promoter. The effect of SWI/SNF is not specific for 24(OH)ase since mutant Brm or mutant Brg-1 (that can act as dominant negative inhibitors) can inhibit VDR mediated OPN transcription. Together these findings reveal a role for SWI/SNF in VDR mediated transcription and suggest that the interaction between C/EBPβ and SWI/SNF may also be an important determinant in the C/EBPβ mediated enhancement of 24(OH)ase transcription.
Fig. 3.
SWI/SNF chromatin-remodeling complexes cooperate with VDR in the regulation of 24(OH)ase transcription. A. COS-7 cells were transfected with the same concentration of VDR (0.02 µg). Note the responsiveness of the 24(OH)ase promoter to 1,25(OH)2D3 is markedly reduced in the Brm andBrg-1 deficient cell lines SW13 and C33A. B. 1,25(OH)2D3 induced 24(OH)ase transcription can be restored by Brm. C. Inhibition of C/EBPβ enhancement of 24(OH)ase transcription. D. Immunoprecipitation (ip, Brm antibody; Western blot, C/EBPβ antibody) using MC3T3-E1 cells indicate that Brm can bind to C/EBPβ.
In summary, these findings define novel mechanisms that may be of fundamental importance in understanding how 1,25(OH)2D3 mediates its multiple biological effects. Understanding the function of target proteins as well as the multiple cofactors involved in VDR mediated transcription will lead in the future to the selective modulation of specific 1,25(OH)2D3 responses in specific target tissues.
Acknowledgements
These studies were supported by National Institutes of Health grant DK-38961 (to S.C.). Studies related to calcium channel activity were also supported in part by an AHA NSD grant to A.G.O. and an NIH grant to M.C.N. Studies with mutant vitamin D receptors were done in collaboration with Dr. David Feldman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 2.Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab. 1995;80:1052–1058. doi: 10.1210/jcem.80.4.7714065. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomized double blind controlled trial. Brit. Med. J. 2003;326:469–472. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 5.Sutton AL, MacDonald PN. Vitamin D: more than a "bone-a-fide" hormone. Mol Endocrinol. 2003;17:777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 6.Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene. 2000;246:9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 7.Rachez C, Lemon BD, Suldan Z, Bromleigh V, Gamble M, Naar AM, Erdjument-Bromage H, Tempst P, Freedman LP. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 8.Raval-Pandya M, Dhawan P, Barletta F, Christakos S. YY1 represses vitamin D receptor-mediated 25-hydroxyvitamin D(3)24-hydroxylase transcription: relief of repression by CREB-binding protein. Mol Endocrinol. 2001;15:1035–1046. doi: 10.1210/mend.15.6.0651. [DOI] [PubMed] [Google Scholar]
- 9.Guo B, Aslam F, van Wijnen AJ, Roberts SG, Frenkel B, Green MR, DeLuca HF, Lian JB, Stein GS, Stein JL. YY1 regulates vitamin D receptor/retinoid X receptor mediated transactivation of the vitamin D responsive osteocalcin gene. Proc Natl Acad Sci U S A. 1997;94:121–126. doi: 10.1073/pnas.94.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhawan P, Peng X, Sutton AL, MacDonald PN, Croniger CM, Trautwein C, Centrella M, McCarthy TL, Christakos S. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol Cell Biol. 2005;25:472–487. doi: 10.1128/MCB.25.1.472-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez S, Javed A, Tennant DK, van Rees M, Montecino M, Stein GS, Stein JL, Lian JB. CCAAT/enhancer-binding proteins (C/EBP) beta and delta activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J Biol Chem. 2002;277:1316–1323. doi: 10.1074/jbc.M106611200. [DOI] [PubMed] [Google Scholar]
- 12.Obukhov AG, Nowycky MC. TRPC4 can be activated by G-protein-coupled receptors and provides sufficient Ca(2+) to trigger exocytosis in neuroendocrine cells. J Biol Chem. 2002;277(18):16172–16178. doi: 10.1074/jbc.M111664200. [DOI] [PubMed] [Google Scholar]
- 13.Lee D, Obukhov AG, Shen Q, Liu Y, Dhawan P, Nowycky MC, Christakos S. Calbindin-D(28k) decreases L-type calcium channel activity and modulates intracellular calcium homeostasis in response to K(+) depolarization in a rat beta cell line RINr1046-38. Cell Calcium. 2006 doi: 10.1016/j.ceca.2006.01.010. (in press) [DOI] [PubMed] [Google Scholar]
- 14.Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;22(271):29715–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- 15.Zou A, Elgort MG, Allegretto EA. Retinoid X receptor (RXR) ligands activate the human 25-hydroxyvitamin D3-24-hydroxylase promoter via RXR heterodimer binding to two vitamin D-responsive elements and elicit additive effects with 1,25-dihydroxyvitamin D3. J Biol Chem. 1997;272(30):19027–19034. doi: 10.1074/jbc.272.30.19027. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Shen Q, Malloy PJ, Soliman E, Peng X, Kim S, Pike JW, Feldman D, Christakos S. Enhanced coactivator binding and transcriptional activation of mutant vitamin D receptors from patients with hereditary 1,25-dihydroxyvitamin D-resistant rickets by phosphorylation and vitamin D analogs. J Bone Miner Res. 2005;20(9):1680–1691. doi: 10.1359/JBMR.050410. [DOI] [PubMed] [Google Scholar]
- 17.Christakos S, Liu Y, Dhawan P, Peng X. The Calbindins: Calbindin D9k and Calbindin D28K. Chapter 42. In: Feldman, Pike, Glorieux, editors. Vitamin D. 2nd Edition. San Diego, CA: Academic Press; 2005. pp. 721–735. [Google Scholar]
- 18.Christakos S, Liu Y. Biological actions and mechanism of action of calbindin in the process of apoptosis. J Steroid Biochem Mol Biol. 2004;89–90(1–5):401–404. doi: 10.1016/j.jsbmb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Bellido T, Huening M, Raval-Pandya M, Manolagas SC, Christakos S. Calbindin-D28k is expressed in osteoblastic cells and suppresses their apoptosis by inhibiting caspase-3 activity. J Biol Chem. 2000;275(34):26328–26332. doi: 10.1074/jbc.M003600200. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Porta A, Peng X, Gengaro K, Cunningham EB, Li H, Dominguez LA, Bellido T, Christakos S. Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. J Bone Miner Res. 2004;19(3):479–490. doi: 10.1359/JBMR.0301242. [DOI] [PubMed] [Google Scholar]
- 21.Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35(2):375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 22.Parkash J, Chaudhry MA, Amer AS, Christakos S, Rhoten WB. Intracellular calcium ion response to glucose in beta-cells of calbindin-D28k nullmutant mice and in betaHC13 cells overexpressing calbindin-D28k. Endocrine. 2002;18:221–229. doi: 10.1385/ENDO:18:3:221. [DOI] [PubMed] [Google Scholar]
- 23.Hoenderop JG, Nilius B, Bindels RJ. Epithelial calcium channels: from identification to function and regulation. Pflugers Arch. 2003;446:304–308. doi: 10.1007/s00424-003-1045-8. [DOI] [PubMed] [Google Scholar]
- 24.Bouhtiauy I, Lajeunesse D, Christakos S, Brunette MG. Two vitamin D3-dependent calcium binding proteins increase calcium reabsorption by different mechanisms. I. Effect of CaBP 28K. Kidney Int. 1994;45(2):461–468. doi: 10.1038/ki.1994.60. [DOI] [PubMed] [Google Scholar]
- 25.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399(6732):159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 26.Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ. Regulation of the mouse epithelial Ca2(+) channel TRPV6 by the Ca(2+)-sensor calmodulin. J Biol Chem. 2004;279:28855–28861. doi: 10.1074/jbc.M313637200. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H, Kim SA, Kirk EA, Tippens AL, Sun H, Haeseleer F, Lee A. Ca2+-binding protein-1 facilitates and forms a postsynaptic complex with Cav1.2 (L-type) Ca2+ channels. J Neurosci. 2004;24(19):4698–4708. doi: 10.1523/JNEUROSCI.5523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyers MB, Puri TS, Chien AJ, Gao T, Hsu PH, Hosey MM, Fishman GI. Sorcin associates with the pore-forming subunit of voltage-dependent L-type Ca2+ channels. J Biol Chem. 1998;273(30):18930–18935. doi: 10.1074/jbc.273.30.18930. [DOI] [PubMed] [Google Scholar]
- 29.St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 30.Barletta F, Dhawan P, Christakos S. Integration of hormone signaling in the regulation of human 25(OH)D3 24-hydroxylase transcription. Am J Physiol Endocrinol Metab. 2004;286:E598–E608. doi: 10.1152/ajpendo.00214.2003. [DOI] [PubMed] [Google Scholar]
- 31.Varga-Weisz P. ATP-dependent chromatin remodeling factors: nucleosome shufflers with many missions. Oncogene. 2001;20:3076–3085. doi: 10.1038/sj.onc.1204332. [DOI] [PubMed] [Google Scholar]