Abstract
Vitamin D is a principal regulator of calcium homeostasis. However, recent evidence has indicated that vitamin D can have numerous other physiological functions including inhibition of proliferation of a number of malignant cells including breast and prostate cancer cells and protection against certain immune mediated disorders including multiple sclerosis (MS). The geographic incidence of MS indicates an increase in MS with a decrease in sunlight exposure. Since vitamin D is produced in the skin by solar or UV irradiation and high serum levels of 25-hydroxyvitamin D (25(OH)D) have been reported to correlate with a reduced risk of MS, a protective role of vitamin D is suggested. Mechanisms whereby the active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3 ) may act to mediate this protective effect are reviewed. Due to its immunosuppressive actions, it has been suggested that 1,25(OH)2D3 may prevent the induction of MS.
Keywords: Vitamin D; Sunlight; Multiple Sclerosis; Immune Function; 1,25-Dihdyroxyvitamin D3; Review
INTRODUCTION
Multiple sclerosis (MS) is an inflammatory, demyelinating and neurodegenerative disease of the central nervous system (Fontoura et al., 2006). While the exact etiology of MS remains unknown, it is thought that many different genetic as well as environmental factors play a role (Hauser and Oksenberg, 2006). Numerous epidemiological studies have indicated a negative correlation between increased sun exposure, which would result in a greater vitamin D synthetic rate, and diets rich in vitamin D (fortified dairy products and fish oils) and MS prevalence (Freedman et al., 2000; Goldberg, 1974; Kurtzke, 1967; Kurtzke, 2000; van der Mei et al., 2001). For example, MS is, for the most part, unknown in equatorial regions. The prevalence of MS increases with latitude. In Norway, although there is high MS prevalence inland, on the Norwegian coast, where there is high fish consumption, there is lower prevalence of MS. The mechanisms by which vitamin D may decrease the risk for MS, which have been suggested to involve normalization of the T-cell response, are only now beginning to be defined.
VITAMIN D
Vitamin D metabolism and mechanism of action
Vitamin D3 (cholecalciferol) is taken in the diet or is synthesized in the skin from 7-dehydrocholesterol by ultraviolet irradiation. For biological activity vitamin D must be converted to its active form. Vitamin D is transported in the blood by the vitamin D binding protein (DBP; a specific binding protein for vitamin D and its metabolites in the serum) to the liver where it is hydroxylated by the 25-hydroxylase enzyme resulting in the formation of 25-hydroxyvitamin D3 (25(OH)D3), the major circulating form of vitamin D. 25(OH)D3 is transported by the DBP to the kidney. In the proximal convoluted and straight tubules of the kidney 25(OH)D3 is converted to the hormonally active form of vitamin D 1,25-dihydroxyvitamin D3 (1,25(OH)2D3 ) by the 25-hydroxyvitamin D3 1-alpha-hydroxylase enzyme (1- alpha(OH)ase (Christakos et al., 2003; DeLuca, 2004; Sutton and MacDonald, 2003). Besides the proximal tubule of the kidney, placenta and macrophages are also sites of synthesis of 1- alpha(OH)ase. The presence of 1-alpha(OH)ase and the role for the local production of 1,25(OH)2D3 in the function of additional cell types are still a matter of debate. Although the phenotype of the 1- alpha(OH)ase null mutant mice is identical to human vitamin D dependent rickets type I, it is of interest that immune dysfunction has also been noted in these mice, suggesting local production of 1,25(OH)2D3 in cells in the immune system (Panda et al., 2001). In an auto-regulatory mechanism 1,25(OH)2D3 induces its own degrading enzyme, 25-hydroxyvitamin D3 24-hydroxylase (24(OH)ase), thereby preventing vitamin D toxicity. 1,25(OH)2D3 is a principal factor that regulates calcium and phosphate homeostasis. The classical target tissues are bone, kidney and intestine. However, 1,25(OH)2D3 has been reported to have numerous other physiological functions including inhibition of proliferation of a number of malignant cell, including breast and prostate cancer cells, effects on epidermal differentiation and effects on differentiation and function of cells in the immune system. 1,25(OH)2D3, similar to other steroid hormones, regulates gene expression in target cells by binding stereospecifically to a high affinity, low capacity nuclear receptor (vitamin D receptor or VDR) resulting in the concentration of the 1,25(OH)2D3 receptor complex in the nucleus and the activation or repression of target genes. Liganded VDR acts as a heterodimer with the retinoid X receptor (RXR), binds to vitamin D response elements (VDREs) within the promoter of target genes and, together with coactivators, affects target gene transcription (Christakos et al., 2003; DeLuca, 2004; Rachez and Freedman, 2000; Rachez et al., 1999; Sutton and MacDonald, 2003), Coactivators include the p160 coactivators, SRC-1, SRC-2 and SRC-3 that have histone acetylase (HAT) activity. The HAT activity of the p160 coactivators is thought to destabilize the interaction between DNA and the histone core, liberating DNA for transcription. Recent studies have indicated that cooperativity between histone methyltransferases and p160 coactivators may also play a fundamental role in VDR mediated transcriptional activation (Christakos et al., 2007). VDR mediated transcription is also mediated by the coactivator complex DRIP (vitamin D receptor interacting protein (Christakos et al., 2003; Rachez and Freedman, 2000; Rachez et al., 1999). This complex functions by recruitment of RNA polymerase II. It has been suggested that cell and promoter specific functions of VDR may be mediated through differential recruitment of coactivators.
Sunlight and vitamin D sufficiency
Total body sun exposure can provide an equivalent of 10,000 IU vitamin D per day (Holick, 1995; Holick, 2002). However, the vitamin D3 produced from 7-dehydrocholesterol depends on the intensity of UV irradiation which varies with latitude and season. For example in San Juan (18deg N) the skin produces vitamin D3 all year. In Boston (42.2deg N) no vitamin D is produced from sun exposed skin from November to February and in Edmonton (52deg N) none is produced from October until April (Webb et al., 1988). Clothing as well as sunscreen have been reported to prevent the conversion of 7-dehydrocholesterol to vitamin D3 in the covered areas (Matsuoka et al., 1987; Matsuoka et al., 1992). Serum 25(OH)D3 levels, the most reliable indicator of vitamin D sufficiency, have been shown to vary with sunlight levels (there is a two month lag period between the highest and lowest level of sunlight and the respective peak and troughs in 25(OH)D3 levels) (Hine and Roberts, 1994; Holick, 2002). Although there is still some controversy as to the normal and optimal blood levels of 25(OH)D3, most experts define vitamin D deficiency as levels of 25(OH)D3 below 30 ng/ml (Holick, 2007). With this definition, a significant proportion of the world’s population is vitamin D deficient. Due to the concerns regarding the association of sunlight and skin cancer and when enough sunlight is not available, vitamin D supplements are recommended. The general consensus is that the current recommended dosage 400 IU is inadequate (Vieth, 1999). It is estimated that 1,000 IU vitamin D3/day is needed to maintain 25(OH)D3 level at the desired level (Heaney et al., 2003). It has been reported that vitamin D toxicity with hypercalcemia has not been observed at doses lower than 10,000 IU per day (Hathcock et al., 2007).
VITAMIN D AND IMMUNE FUNCTION
Mechanism of vitamin D action in the immune system
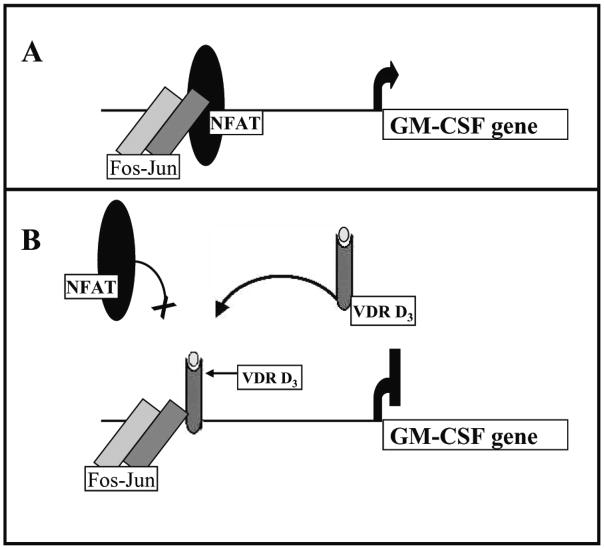
Early studies indicating the presence of VDR in activated T-cells led to the suggestion that 1,25(OH)2D3 may play a role in the regulation of the immune response. 1,25(OH)2D3 can inhibit T-lymphocyte proliferation and activation (Bhalla et al., 1983). This immunosuppressive effect is correlated with a decrease in IL-2 mRNA (one of the first genes to be expressed post activation) as well as with a decrease in IFN-gamma and GM-CSF mRNA levels (Bhalla et al., 1986; Reichel et al., 1987; Rigby, 1988; Tobler et al., 1987). For IL-2, IFN-gamma and GM-CSF, it has been reported that the mechanism involves VDR mediated inhibition of gene transcription (Alroy et al., 1995; Cippitelli and Santoni, 1998; Towers and Freedman, 1998). The mechanism of the transcriptional repression of the human IL-2 gene by 1,25(OH)2D3 involves block of the formation of the NFATp/AP-1 complex by the VDR/RXR heterodimer and stable association of VDR/RXR with the NFAT1 element in the IL-2 promoter (Alroy et al., 1995). For inhibition of GM-CSF, VDR binds to the GM550 element that contains binding sites for NFAT1, Jun and Fos. Unlike the mechanism of IL-2 repression, VDR binds to this element as a monomeric species, independent of RXR. VDR competes with NFAT for binding to the composite site, positioning itself adjacent to Jun-Fos, stabilizing the Jun-Fos complex (Towers and Freedman, 1998). The selective interaction is between VDR and c-Jun (Fig. 1). Over-expression of c-Jun but not c-Fos is able to rescue the VDR repression. For GM-CSF repression, the model is that VDR would lock AP-1 in an off state. For the repression of IFN-gamma transcription, direct binding of VDR/RXR to a silencer region BED (−212/−183) in the human IFN-gamma promoter was suggested (Cippitelli and Santoni, 1998). 1,25(OH)2D3 has also been reported to up-regulate IL-4 under non-polarizing conditions (Cantorna et al., 1998b). In addition, 1,25(OH)2D3 has been shown to inhibit the differentiation and survival of dendritic cells, resulting in impaired alloreactive T-cell activation (Griffin et al., 2001; Penna and Adorini, 2000). The inhibition of maturation and differentiation of dendritic cells results in a decrease in IL-12 and an increase in IL-10 secretion (Penna and Adorini, 2000). The repressive effect of 1,25(OH)2D3 on IL-12 production was reported to be at the level of transcription and required binding of the VDR/RXR heterodimer to the binding site for NF-kappaB in the IL-12 p40 promoter (p40-kB) (D’Ambrosio et al., 1998). It is possible that the effects of 1,25(OH)2D3 on IL-10, IL-4 and perhaps other cytokines may be also be indirect, resulting from an effect of 1,25(OH)2D3 on other cells and genes resulting in a net change in cytokine expression.
Figure 1.
Mechanism of repression of GM-CSF activated transcription by 1,25(OH)2D3. Unlike most mechanisms involved in 1,25(OH)2D3 mediated effects on transcription that require the VDR/RXR heterodimer, VDR bound to 1,25(OH)2D3 (VDRD3 in B) acts as a monomer on the GM-CSF promoter and competes with NFAT for binding to a composite NFAT AP1 site (composite site is shown in A). VDR bound to 1,25(OH)2D3 also stabilizes the binding of Fos-Jun heterodimer by direct interaction with cJun (B). These two events result in 1,25(OH)2D3 mediated transcriptional repression of GM-CSF (Adapted from Towers and Freedman, 1998)
Recent studies have indicated that 1,25(OH)2D3 regulates not only adaptive but also innate immunity. 1,25(OH)2D3 has been shown to induce the antimicrobial peptide cathelicidin with subsequent killing of bacteria including mycobacterium tuberculosis (Liu et al., 2007). Thus, with regard to innate immunity, 1,25(OH)2D3 may promote the host’s response to a pathogen. The effect on adaptive immunity, on the other hand, may limit the immune response and may function in a process representative of tolerance.
Autoimmune diseases and vitamin D
Previous studies have indicated that 1,25(OH)2D3 can at least partially protect against a number of experimental autoimmune diseases. The experimental autoimmune diseases suppressed by 1,25(OH)2D3 include experimental allergic encephalitis (EAE, the murine model of multiple sclerosis), experimental lupus erythematosus and autoimmune thyroiditis (Abe et al., 1990; Fournier et al., 1990; Lemire and Archer, 1991). 1,25(OH)2D3 has also been reported to prevent the progression of arthritis in murine models of human arthritis (infection of mice with Borrelia burgdorferi or immunization of mice with type II collagen) (Cantorna et al., 1998a). 1,25(OH)2D3 was also effective in collagen induced arthritis in inhibiting progression of arthritis when given to mice with early symptoms (Cantorna et al., 1998a).
In addition, 1,25(OH)2D3 prevents autoimmune diabetes in nonobese diabetic (NOD) mice (Mathieu et al., 1994). In the NOD mouse model and in the EAE model, it has been reported that an analog of 1,25(OH)2D3, 1,25-dihydroxy-16,23Z-diene-26,27-hexafluoro-19-nor-vitamin D3 and 1,25(OH)2D3 respectively are effective not only in preventing the induction of the disease but also in inhibiting its progression when administered after disease onset (Cantorna et al., 1996; Gregori et al., 2002).
VITAMIN D AND MULTIPLE SCLEROSIS
Incidence of multiple sclerosis varies with sunlight
Numerous epidemiological studies have reported a negative correlation between exposure to sunlight and sufficient dietary vitamin D3 intake and prevalence of MS (Freedman et al., 2000; Goldberg, 1974; Kurtzke, 1967; Kurtzke, 2000; van der Mei et al., 2001). MS prevalence was shown to vary not only with latitude but also with altitude (Kurtzke, 1967). There is lower MS incidence at high altitudes that corresponds to more optimal solar irradiation and increased cutaneous vitamin D synthesis. Also, it has been reported that immigrants, particularly if they move before the age of 15, acquire the MS risk of their new homeland, supporting the concept that early intervention to maintain vitamin D sufficiency may have a protective role against MS (Hammond et al., 2000; Pugliatti et al., 2002; van der Mei et al., 2003).
1,25(OH)2D3 inhibits EAE
Although numerous studies have shown that 1,25(OH)2D3 inhibits EAE, the precise mechanisms involved have not been clearly defined and have been a matter of debate. 1,25(OH)2D3 was found to inhibit EAE in mice lacking CD8+ T-cells but not in Rag-1 null mice (Meehan and DeLuca, 2002; Nashold et al., 2001). The findings in the Rag-1 null mice suggest that 1,25(OH)2D3 acts through a Rag-1 dependent cell to limit the occurrence of Th1 activation in the CNS. It has been suggested that CD4+ T-cells are a target of 1,25(OH)2D3 immunosuppression in EAE and that 1,25(OH)2D3 may sensitize CD4+ T-cells to apoptotic stimuli (Pedersen et al., 2007). Both an increase and no change in IL-4 in lymph nodes in EAE after 1,25(OH)2D3 treatment have been reported (Cantorna et al., 1998b; Nashold et al., 2001). In response to 1,25(OH)2D3, a reduction and no change in interferon gamma have been described (Muthian et al., 2006; Nashold et al., 2001). The lack of consistency may be due to both indirect and direct effects of 1,25(OH)2D3. Further studies need to be done to differentiate between these effects. With regard to IL-4, using IL-4 KO mice it has been reported that EAE is more severe in the absence of IL-4 and IL-4 KO mice are resistant to 1,25(OH)2D3 treatment (Cantorna et al., 2000). 1,25(OH)2D3 is also unable to inhibit EAE in IL-10 or IL-10 receptor KO mice, suggesting IL-10 dependent protective effects of 1,25(OH)2D3 (Spach et al., 2006). It has also been reported that in vivo treatment with 1,25(OH)2D3 or 1,25(OH)2D3 analog inhibits EAE in association with inhibition of IL-12 (Mattner et al., 2000).
Gender differences have also been noted in the effect of 1,25(OH)2D3 or vitamin D on EAE. It has been reported that higher concentrations of 1,25(OH)2D3 are needed to prevent EAE in male mice compared to female mice (Cantorna et al., 1999). In addition, it was found that 5 micrograms/day of vitamin D3 significantly inhibited EAE in female but not male mice (Spach and Hayes, 2005). In ovariectomized mice the protective effect of vitamin D was not observed. In the vitamin D study both male and female mice had equivalent levels of serum 1,25(OH)2D3 (Spach and Hayes, 2005). Further studies examining mechanisms involved in the gender differences in the response to 1,25(OH)2D3 or vitamin D3 are needed.
It has been suggested that the effects of 1,25(OH)2D3 or vitamin D could include effects in the CNS. However, it should be noted, when suggesting central effects of 1,25(OH)2D3, that 1,25(OH)2D3 receptors are present in low levels in the brain and have a very localized distribution in the CNS (Stumpf and O’Brien, 1987; Stumpf et al., 1982). 1,25(OH)2D3 sites of action have been identified by autoradiography within certain structures in the forebrain, hindbrain and spinal cord (Stumpf et al., 1988). The central nucleus of the amygdala and the bed nucleus of the stria terminalis have the densest accumulation of 1,25(OH)2D3. In the spinal cord nuclear retention of 1,25(OH)2D3 is strongest in motor neurons in lamina IX. Nuclear retention is also observed in neurons of lamina II, VIII and X and in cells in the caudal spinal trigeminal nucleus. Immunocytochemical studies are generally in agreement with the autoradiographic findings (Prufer et al., 1999). Also, restricted transport of vitamin D metabolites to the brain has been reported, at least in wild type rodent brain. Unlike estrogens, in which the protein steroid complex freely enters the brain, it has been reported that the vitamin D serum binding protein limits access of 1,25(OH)2D3 into the CNS (Gascon-Barre and Huet, 1983). In addition, although it has been suggested that 1,25(OH)2D3 could be synthesized from 25(OH)D3 in the CNS and thus could account for observed effects of vitamin D, it should be noted that the presence of 1-alpha-(OH)ase at sites other than kidney, placenta and macrophages is a matter of debate (Shultz et al., 1983).
The role of calcium in the 1,25(OH)2D3 suppressive effects in EAE also needs to be considered. Low calcium diets have been reported to result in resistance to treatment with 1,25(OH)2D3 and it was found that calcium is required for the suppressive effect in EAE (Cantorna et al., 2000). It is of interest that in the lupus model MRL/1 mice, low calcium plus 1,25(OH)2D3 accelerated SLE while mice on the normal calcium (0.87%) diet showed reduced SLE with 1,25(OH)2D3 treatment (DeLuca and Cantorna, 2001). In addition, inflammatory bowel disease (IBD) is prevented by 1,25(OH)2D3 administration also under conditions of normal calcium (DeLuca and Cantorna, 2001). Unlike EAE, SLE and IBD, 1,25(OH)2D3 can prevent collagen induced arthritis under low dietary calcium conditions (Cantorna et al., 1996; DeLuca and Cantorna, 2001). Thus low calcium results in resistance to 1,25(OH)2D3 in some but not all autoimmune diseases. These studies should help in the design of analogs that will be effective in vivo. It has recently been reported that the combination of the 1,25(OH)2D3 analog TX527 with interferon beta resulted in protection against EAE that was more effective than each treatment alone, suggesting consideration of a combination treatment for clinical intervention in MS (van Etten et al., 2007).
Does vitamin D or 1,25(OH)2D3 analog have clinical potential for MS?
Although the effects of 1,25(OH)2D3 in the EAE model are suggestive, the question that remains is whether vitamin D3 or 1,25(OH)2D3 analogs have clinical potential. A number of studies have shown that high serum levels of 25(OH)D3 are associated with a decreased risk of MS. The largest of these trials by Munger et al in 2006 searched the US Army and Navy databases and found 257 cases of military personnel with diagnosed MS and stored serum available for testing of 25(OH)D3 levels (Munger et al., 2006). A case matched analysis using 514 matched controls showed that MS incidence was lower in samples from 148 subjects with the highest circulating levels of 25(OH)D3. The inverse relationship between 25(OH)D3 levels and MS was particularly strong for 25(OH)D3 levels measured before age 20, suggesting that vitamin D supplements in adolescents and young adults, particularly those who are at high risk for MS (with a family history, for example), may have protective benefit. A protective effect of cod liver oil and regular fish consumption has also been reported (Kampman et al., 2007). A large clinical trial will be required to determine whether vitamin D or analog, alone or in combination with known treatments, is not only protective but is also effective in patients with active MS. A better understanding of the mechanisms by which 1,25(OH)2D3 acts to affect the immune system is needed in order to aid in the design of 1,25(OH)2D3 analogs with clinical potential for MS.
CONCLUSION
For individuals predisposed to MS, evidence indicates that maintenance of adequate vitamin D has a protective effect. One mechanism of vitamin D action may be to maintain balance in the T-cell response and thus avoid autoimmunity. Further studies are needed with regard to mechanisms involved in 1,25(OH)2D3 mediated immune regulation and to determine whether vitamin D or analog, alone or in combination with other treatments, is not only protective but is also effective in patients with active MS.
Acknowledgments
Studies referenced from the laboratory of S Christakos were supported in part by NIH grant DK38961 to S.C.
Abbreviations
- MS
multiple sclerosis
- EAE
experimental allergic encephalitis
- SLE
systemic lupus erythematosus
- IBD
inflammatory bowel disease
- CNS
central nervous system
- DNA
deoxyribonucleic acid
- RNA
ribonucleic acid
- NFAT
nuclear factor of activated T-cells
- NF-kappaB
nuclear factor kappa B
- IL-2
interleukin 2
- IL-4
interleukin 4
- IL-10
interleukin 10
- IL-12
interleukin 12
- GM-CSF
granulocyte macrophage colony stimulating factor
- DRIP
vitamin D receptor interacting protein complex
- HAT
histone acetylase
- VDR
vitamin D receptor
- RXR
retinoid X receptor
- VDRE
vitamin D response element
- 1-alpha-(OH)ase
25-hydroxyvitamin D3 1-alpha-hydroxylase
- 24(OH)ase
25-hydroxyvitamin D3 24-hydroxylase
- 25(OH)D3
25-hydroxyvitamin D3
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- KO
knock out
- UV
ultraviolet
References
- 1.Abe J, Nakamura K, Takita Y, Nakano T, Irie H, Nishii Y. Prevention of immunological disorders in MRL/l mice by a new synthetic analogue of vitamin D3: 22-oxa-1 alpha,25-dihydroxyvitamin D3. J Nutr Sci Vitaminol (Tokyo) 1990;36:21–31. doi: 10.3177/jnsv.36.21. [DOI] [PubMed] [Google Scholar]
- 2.Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15:5789–99. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla AK, Amento EP, Krane SM. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell Immunol. 1986;98:311–22. doi: 10.1016/0008-8749(86)90291-1. [DOI] [PubMed] [Google Scholar]
- 5.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–4. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998a;128:68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- 7.Cantorna MT, Humpal-Winter J, DeLuca HF. Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr. 1999;129:1966–71. doi: 10.1093/jn/129.11.1966. [DOI] [PubMed] [Google Scholar]
- 8.Cantorna MT, Humpal-Winter J, DeLuca HF. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D(3) Arch Biochem Biophys. 2000;377:135–8. doi: 10.1006/abbi.2000.1765. [DOI] [PubMed] [Google Scholar]
- 9.Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998b;160:5314–9. [PubMed] [Google Scholar]
- 10.Christakos S, Dhawan P, Benn B, Porta A, Hediger M, Oh GT, Jeung EB, Zhong Y, Ajibade D, Dhawan K, Joshi S. Vitamin D: molecular mechanism of action. Ann N Y Acad Sci. 2007;1116:340–8. doi: 10.1196/annals.1402.070. [DOI] [PubMed] [Google Scholar]
- 11.Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 12.Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28:3017–30. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 15.DeLuca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 16.Fontoura P, Steinman L, Miller A. Emerging therapeutic targets in multiple sclerosis. Curr Opin Neurol. 2006;19:260–6. doi: 10.1097/01.wco.0000227035.93199.e0. [DOI] [PubMed] [Google Scholar]
- 17.Fournier C, Gepner P, Sadouk M, Charreire J. In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis. Clin Immunol Immunopathol. 1990;54:53–63. doi: 10.1016/0090-1229(90)90005-b. [DOI] [PubMed] [Google Scholar]
- 18.Freedman DM, Dosemeci M, Alavanja MC. Mortality from multiple sclerosis and exposure to residential and occupational solar radiation: a case-control study based on death certificates. Occup Environ Med. 2000;57:418–21. doi: 10.1136/oem.57.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gascon-Barre M, Huet PM. Apparent [3H]1,25-dihydroxyvitamin D3 uptake by canine and rodent brain. Am J Physiol. 1983;244:E266–71. doi: 10.1152/ajpendo.1983.244.3.E266. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg P. Multiple sclerosis: vitamin D and calcium as environmental determinants of prevalence; (A viewpoint) part 1: sunlight, dietary factors and epidemiology. Int J Environ Stud. 1974;6:19–27. [Google Scholar]
- 21.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–74. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 22.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:6800–5. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond SR, English DR, McLeod JG. The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain. 2000;123(Pt 5):968–74. doi: 10.1093/brain/123.5.968. [DOI] [PubMed] [Google Scholar]
- 24.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 25.Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 27.Hine TJ, Roberts NB. Seasonal variation in serum 25-hydroxy vitamin D3 does not affect 1,25-dihydroxy vitamin D. Ann Clin Biochem. 1994;31( Pt 1):31–4. doi: 10.1177/000456329403100105. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61:638S–645S. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin in Endocrinol Diab. 2002;9:87–98. [Google Scholar]
- 30.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 31.Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol. 2007;254:471–7. doi: 10.1007/s00415-006-0395-5. [DOI] [PubMed] [Google Scholar]
- 32.Kurtzke JF. On the fine structure of the distribution of multiple sclerosis. Acta Neurol Scand. 1967;43:257–82. doi: 10.1111/j.1600-0404.1967.tb05733.x. [DOI] [PubMed] [Google Scholar]
- 33.Kurtzke JF. Epidemiology of multiple sclerosis. Does this really point toward an etiology? Lectio Doctoralis. Neurol Sci. 2000;21:383–403. doi: 10.1007/s100720070055. [DOI] [PubMed] [Google Scholar]
- 34.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–7. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 36.Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37:552–8. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–8. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 38.Matsuoka LY, Wortsman J, Dannenberg MJ, Hollis BW, Lu Z, Holick MF. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol Metab. 1992;75:1099–103. doi: 10.1210/jcem.75.4.1328275. [DOI] [PubMed] [Google Scholar]
- 39.Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, Martino G, Panina-Bordignon P, Adorini L. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3) Eur J Immunol. 2000;30:498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 40.Meehan TF, DeLuca HF. CD8(+) T cells are not necessary for 1 alpha,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2002;99:5557–60. doi: 10.1073/pnas.082100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 42.Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83:1299–309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 43.Nashold FE, Hoag KA, Goverman J, Hayes CE. Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D(3) prevention of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;119:16–29. doi: 10.1016/s0165-5728(01)00360-5. [DOI] [PubMed] [Google Scholar]
- 44.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85:2480–90. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 46.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 47.Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16:135–45. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 48.Pugliatti M, Sotgiu S, Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg. 2002;104:182–91. doi: 10.1016/s0303-8467(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 49.Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene. 2000;246:9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 50.Rachez C, Lemon BD, Suldan Z, Bromleigh V, Gamble M, Naar AM, Erdjument-Bromage H, Tempst P, Freedman LP. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–8. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 51.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84:3385–9. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rigby WF. The immunobiology of vitamin D. Immunol Today. 1988;9:54–8. [PubMed] [Google Scholar]
- 53.Shultz TD, Fox J, Heath H, 3rd, Kumar R. Do tissues other than the kidney produce 1,25-dihydroxyvitamin D3 in vivo? A reexamination. Proc Natl Acad Sci U S A. 1983;80:1746–50. doi: 10.1073/pnas.80.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175:4119–26. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 55.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:6030–7. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 56.Stumpf WE, Clark SA, O’Brien LP, Reid FA. 1,25(OH)2 vitamin D3 sites of action in spinal cord and sensory ganglion. Anat Embryol (Berl) 1988;177:307–10. doi: 10.1007/BF00315837. [DOI] [PubMed] [Google Scholar]
- 57.Stumpf WE, O’Brien LP. 1,25 (OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry. 1987;87:393–406. doi: 10.1007/BF00496810. [DOI] [PubMed] [Google Scholar]
- 58.Stumpf WE, Sar M, Clark SA, DeLuca HF. Brain target sites for 1,25-dihydroxyvitamin D3. Science. 1982;215:1403–5. doi: 10.1126/science.6977846. [DOI] [PubMed] [Google Scholar]
- 59.Sutton AL, MacDonald PN. Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17:777–91. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 60.Tobler A, Gasson J, Reichel H, Norman AW, Koeffler HP. Granulocyte-macrophage colony-stimulating factor. Sensitive and receptor-mediated regulation by 1,25-dihydroxyvitamin D3 in normal human peripheral blood lymphocytes. J Clin Invest. 1987;79:1700–5. doi: 10.1172/JCI113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Towers TL, Freedman LP. Granulocyte-macrophage colony-stimulating factor gene transcription is directly repressed by the vitamin D3 receptor. Implications for allosteric influences on nuclear receptor structure and function by a DNA element. J Biol Chem. 1998;273:10338–48. doi: 10.1074/jbc.273.17.10338. [DOI] [PubMed] [Google Scholar]
- 62.van der Mei IA, Ponsonby AL, Blizzard L, Dwyer T. Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology. 2001;20:168–74. doi: 10.1159/000054783. [DOI] [PubMed] [Google Scholar]
- 63.van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor BV, Butzkueven H, Kilpatrick T. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ. 2003;327:316. doi: 10.1136/bmj.327.7410.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Etten E, Gysemans C, Branisteanu DD, Verstuyf A, Bouillon R, Overbergh L, Mathieu C. Novel insights in the immune function of the vitamin D system: synergism with interferon-beta. J Steroid Biochem Mol Biol. 2007;103:546–51. doi: 10.1016/j.jsbmb.2006.12.094. [DOI] [PubMed] [Google Scholar]
- 65.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 66.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]