Abstract
Arterial stiffness describes the rigidity of the arterial wall. Its significance owes to its relationship with the pulsatile afterload presented to the left ventricle and its implications on ventricular-arterial coupling. In adults, the contention that arterial stiffness as a marker and risk factor for cardiovascular morbidity and mortality is gaining support. Noninvasive methods have increasingly been adopted in both the research and clinical arena to determine local, segmental, and systemic arterial stiffness in the young. With adoption of these noninvasive techniques for use in children and adolescents, the phenomenon and significance of arterial stiffening in the young is beginning to be unveiled. The list of childhood factors and conditions found to be associated with arterial stiffening has expanded rapidly over the last decade; these include traditional cardiovascular risk factors, prenatal growth restriction, vasculitides, vasculopathies associated with various syndromes, congenital heart disease, and several systemic diseases. The findings of arterial stiffening have functional implications on energetic efficiency, structure, and function of the left ventricle. Early identification of arterial dysfunction in childhood may provide a window for early intervention, although longitudinal studies are required to determine whether improvement of arterial function in normal and at-risk paediatric populations will be translated into clinical benefits.
Keywords: Arteriosclerosis, Children
Introduction
Arterial stiffness, in simple terms, describes the rigidity of the arterial wall. It is primarily determined by structural components of the arterial wall, vascular smooth muscle tone, and transmural distending pressure.1) Increasing evidence suggests a role of the endothelium in the regulation of arterial stiffness through the release of vasoactive mediators that affect smooth muscle tone.
The significance of arterial stiffness owes to its direct relationship with the characteristic impedance of the arterial system, which is the pulsatile component of the afterload that is presented to the left ventricle. Furthermore, arterial stiffening increases the velocity at which the pulse wave travels, resulting in an earlier return of the reflected wave from peripheral sites, and hence, suboptimal ventricular-arterial interaction. Given the relationships between arterial stiffness, vascular impedance and wave reflection, it is understandable that arterial stiffness may impact cardiovascular health.
The contention that arterial stiffness is a marker of vascular disease and a risk factor for cardiovascular morbidity and mortality, in adults, is gaining support. In adults, the association of increased arterial stiffness with various pathophysiological conditions has been extensively reviewed.2-5) Importantly, stiffness of central arteries, as assessed by the aortic pulse wave velocity (PWV) and carotid distensibility, has been shown to have an independent predictive value for cardiovascular events in the general adult population,6),7) in elderly,8) and in adults with hypertension,9-11) end-stage renal failure,12-15) and impaired glucose tolerance.16)
Noninvasive methods have been increasingly adopted in both the research and clinical arenas to determine systemic arterial stiffness, and these methods have significantly increased the understanding of the pathophysiological significance. With adoption of these non-invasive methodologies for use in children and adolescents, the phenomenon and significance of arterial stiffening in the young are beginning to unfold. The present article aims to provide an overview of the methods used to assess arterial stiffness in vivo and of the determinants and significance of arterial stiffness in children and adolescents.
Measurement of Arterial Stiffness In Vivo
Noninvasive methods are available for determination of 1) local or cross-sectional stiffness at a particular site in the artery, 2) regional stiffness along the length of an arterial segment, and 3) systemic or whole-body arterial stiffness.
Local arterial stiffness
Local arterial stiffness is ascertained by relating changes in arterial diameter or cross-sectional area to pressure changes at the site of interest. The commonly used indices for quantification of local arterial stiffness are summarized in Table 1.
Table 1.
Commonly used indices of local or cross-sectional arterial stiffness
D: diastolic diameter, ΔD: difference in systolic and diastolic diameter, ln: natural logarithm, ΔP: pressure, Pd: diastolic blood pressure, Ps: systolic blood pressure
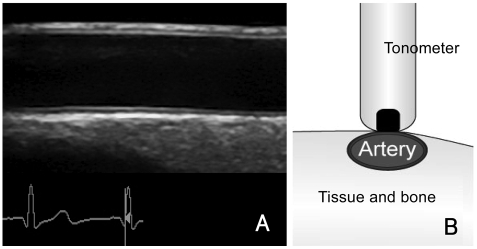
For superficial arteries including the brachial, femoral and carotid arteries, the diameter and diameter change from end-diastole to end-systole can be assessed by ultrasound (Fig. 1A) and echo-tracking techniques. Compared with two-dimensional ultrasound assessment, echo-tracking permits the tracking of displacement of the anterior and posterior arterial walls with a much higher precision.2),17),18) For deeper arteries, such as the aorta, magnetic resonance imaging19) and transesophageal echocardiography with acoustic quantification20) have been used to determine the change in arterial diameter during the cardiac cycle.
Fig. 1.
Derivation of local arterial stiffness by measurement of (A) diameter changes in the cardiac cycle using two-dimensional ultrasound, and (B) arterial pressure using applanation tonometry.
Ideally, local pressure should be measured at the site of diameter measurements. Applanation tonometry (Fig. 1B) allows noninvasive recording of the arterial pressure waveform in the carotid and peripheral conduit arteries.21) The tonometer has the size of a pen, is hand-held and gently compressed against the underlying bone, thus flattening the artery slightly and equalizing the circumferential pressures. The recorded pressure waveform is almost identical to that obtained intra-arterially and can then be calibrated against the cuff mean and diastolic blood pressures of the brachial artery.22),23) Alternatively, the cuff brachial artery pulse pressure has also been commonly used for the calculation of local arterial stiffness indices. Amplification of pulse pressure along the arterial tree, however, constitutes a potential source of error.
Recently, tissue Doppler imaging24) and speckle tracking echocardiography25),26) have been used to assess arterial strain and strain rate as novel indices of cross-sectional arterial stiffness.
Regional arterial stiffness
Measuring the PWV over the segment of interest assesses stiffness along the length of an arterial segment or regional stiffness. PWV is the speed at which the forward pressure or flow wave is transmitted from the aorta through the arterial tree.
The Bramwell and Hill27) equation relates PWV to arterial distensibility: PWV=√(ΔP·V)/ΔVρ=√1/ ρD, where P is pressure, V is volume, ΔP·V/ΔV represents volume elasticity, ρ is density of blood, and D is volume distensibility of the arterial segment. Hence, PWV is related inversely to arterial distensibility; in other words, the stiffer the artery, the faster the PWV. By providing an average stiffness of the arterial segment of interest, PWV may provide a better reflection of the general vascular health.
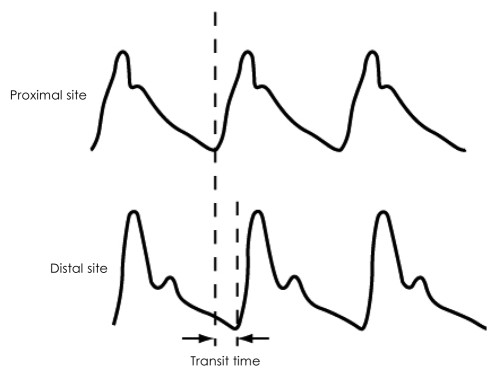
PWV is determined by dividing the distance of pulse travel, between two sites, by the transit time. As the pulse pressure and flow pulse propagate at the same velocity, the arterial pulse may be registered using pressure-sensitive transducers,28) an oscillometric device,29) applanation tonometry,30) Doppler ultrasound31),32) photoplethysmography,33) and magnetic resonance imaging.34),35) The pulse recording at the two sites can be obtained simultaneously (Fig. 2) or by gating separate recordings to the R wave of the electrocardiogram.
Fig. 2.
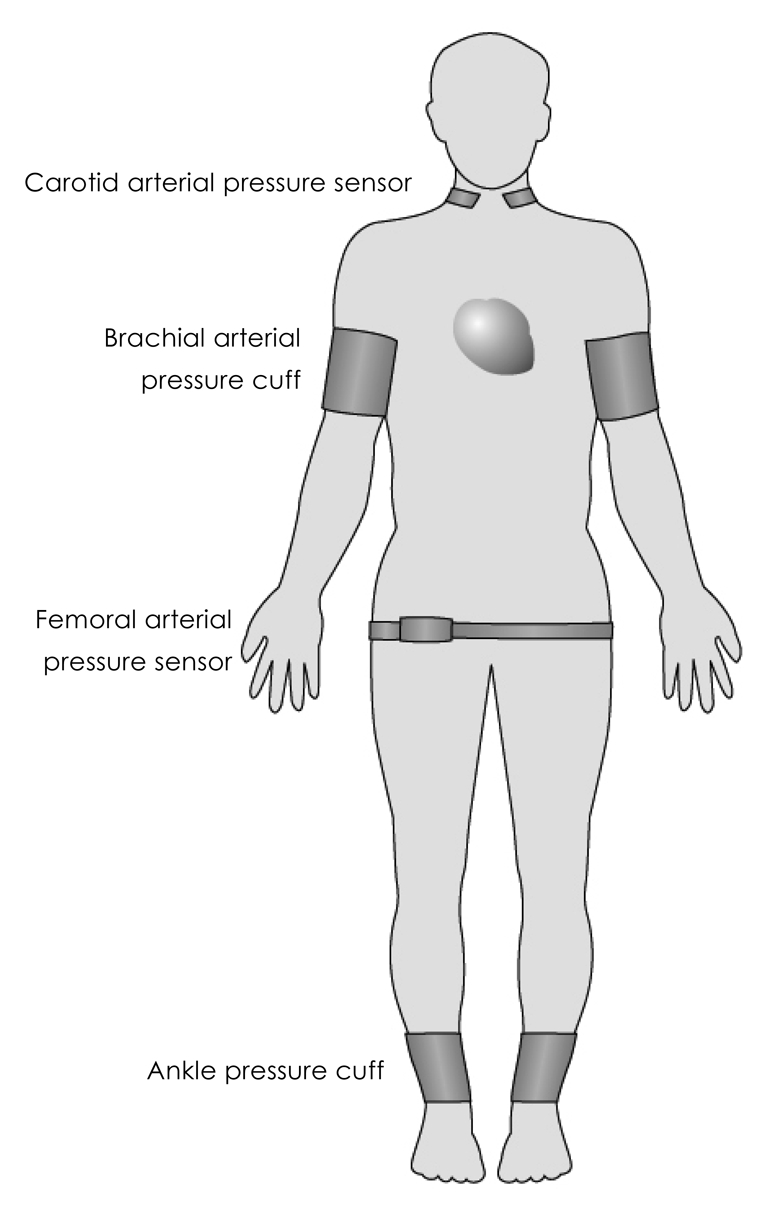
Simultaneous registration of arterial pulse waveforms by pressure sensors placed at different parts of body for calculation of pulse transit time.
The distance along which the pulse travels is usually estimated by direct superficial measurement between the two pressure transducers or other devices used to register the pulse. Transit time is measured as the time delay between the feet of the proximal and distal pulse waves (Fig. 3). The foot of the pulse wave is used as it is relatively unaffected by wave reflections. A potential source of error is the need to use the nearest superficial arteries as a surrogate site for inaccessible central arteries, and the estimation of the actual distance between the recording sites using surface measurements. Despite these limitations, PWV is probably the most widely used technique for assessment of arterial stiffness.
Fig. 3.
Derivation of pulse transit time from the feet of the proximal and distal pulse waves.
Systemic arterial stiffness
Pulse contour analysis has been used to assess systemic or whole-body arterial stiffness noninvasively.36-38) One of the methods focuses on the diastolic pressure decay of the radial pulse contour obtained by tonometry. An algorithm is used to determine the best set of values for matching the diastolic contour to a multi-exponential waveform equation. Based on these values, the lumped compliance of the major arteries and that of the small peripheral arteries is estimated. However, the biologic relevance of the derived lumped compliance remains unclear.
The area method has also been used to determine systemic arterial compliance using the formula: compliance= A/{total vascular resistance×(Pes-Pd)}, where A is area under the diastolic portion of the arterial pressure wave from end-systole to end-diastole, Pes is end-systolic pressure, and Pd is end-diastolic pressure.39),40) The pressure readings and waveform are obtained by applanation tonometry over the common carotid artery. The total vascular resistance is calculated as mean blood pressure divided by mean aortic blood flow, the latter obtained by a velocimeter positioned at the suprasternal notch. The area method nonetheless shares a similar concern as aforementioned.
Arterial Stiffening in the Young
Evolution with age
Aortic, upper limb, and lower limb pulse wave velocities increase with age from child- to adulthood.41-43) Notwithstanding the influence of distending pressure on arterial stiffness, previous data did not suggest that the change in PWV with age is entirely due to differences in systemic blood pressure.41),42) With cyclical mechanical stress, fragmentation of the elastin fibres and transfer of stress to the much stiffer collagen fibres inevitably results in the progressive increase in vascular stiffness.44)
Furthermore, studies of developmental changes in arterial structure during childhood have demonstrated progressive increase in intimal and medial thickness after birth.45) Hence, the observed age-related increase in stiffness is likely related to progressive structural changes in the arterial wall during childhood. Interpretation of results obtained from paediatric populations at risk for arterial dysfunction should therefore take into account age-related evolution.
Cardiovascular risk factor
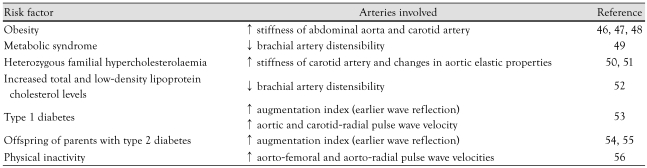
Traditional cardiovascular risk factors46-56) have been associated with arterial stiffening in children and are summarized in Table 2.
Table 2.
Traditional cardiovascular risk factors associated with arterial stiffening in childhood
Prenatal growth restriction
The 'fetal origins hypothesis'57) proposes that cardiovascular disease originates through adaptation to an adverse environment in utero. These adaptations have been suggested to cause permanent alterations in cardiovascular structure and physiology through the process of programming. Indeed, there is evidence that individuals who are born small may be at risk for arterial dysfunction in child- and adulthood.
In very low birth weight, premature infants, reduced aortic wall distensibility and whole-body compliance have been shown as early as the neonatal period.58) Other studies have demonstrated inverse relationships between systemic arterial stiffness and gestational age59) and birth weight standardized for gestational age.60) In fetuses with umbilical placental insufficiency, an increase in afterload has been shown to result in a decrease in aortic distensibility during the neonatal period.61) Furthermore, reduced compliance of the aorta and conduit arteries of the legs has been shown to occur in adults born small.62)
In monozygotic twins with twin-twin transfusion syndrome, the growth-restricted donor twin has been reported to have increased peripheral conduit arterial stiffness during infancy.63) Such vascular programming has been shown to be ameliorated, albeit not completely abolished, by intrauterine endoscopic laser ablation of placental anastomoses.64) Even in monozygotic twins without twin-twin transfusion syndrome, the twin with the lower birthweight has been found to have higher systolic blood pressure and pulse pressure in childhood.65)
The mechanism whereby low birth weight is associated with increased arterial stiffness in child- and adulthood remains unclear. The reported endothelial dysfunction in individuals born preterm and small-for-gestational age62),66-69) suggests that functional alteration of arterial tone may contribute to an increase in systemic arterial stiffness. Altered haemodynamics in intrauterine growth retardation, which result in preferential perfusion of upper part of body,70) may affect the mechanical properties of the large arteries. Another proposed mechanism is impaired synthesis of elastin in the arterial wall.44)
Vasculitides
Vasculitis is the predominant feature in several childhood diseases. The acute inflammation and subsequent reparative process may lead to replacement of elastic tissue by fibrous scar, thereby potentially altering the mechanical properties of the vessels.
Kawasaki disease, a systemic vasculitis with predilection for children in the East, is the most commonly acquired heart disease in children in developed countries. Apart from the well-documented long-term structural alteration and functional disturbance of coronary arteries,71-75) systemic arterial dysfunction is increasingly documented. Indeed, concerns have been raised regarding the possibility of its predisposition to premature atherosclerosis in adulthood.76-80)
Increased stiffness of the carotid artery81),82) and brachioradial artery80) has been documented in the long-term follow-up of patients with a history of Kawasaki disease. Measurement of aortic input impedance during cardiac catheterization showed reduction in both characteristic impedance and total peripheral arterial compliance, regardless of the persistence of coronary artery aneurysms, suggesting an increase in both central and peripheral arterial stiffness.83) The magnitude of chronic low-grade inflammation, as reflected by elevated high-sensitivity C-reactive protein,84),85) in patients with coronary aneurysm formation has been associated positively with carotid arterial stiffness.84) Additionally, mannose-binding lectin,86) C-reactive protein,87) and tumour necrosis factor-α87) genotype polymorphisms have recently been found to exert modulating effects on long-term systemic arterial stiffness in Kawasaki patients.
In another type of childhood vasculitis, polyarteritis nodosa, the recurrent inflammatory cycles result in multiple stages comprised of acute fibrinoid necrosis and healing fibrotic lesions. While the chronic phenomenon, with recurrent episodes of inflammatory exacerbations, contrasts with the acute vasculitis in Kawasaki disease, stiffening of the peripheral conduit arterial stiffness and its amplification, during episodes of inflammatory exacerbation, are similarly found in polyarteritis nodosa.42)
Vasculopathies
Abnormalities of the arterial vasculature have been described in Marfan and Williams syndromes and in association with a bicuspid aortic valve. Marfan syndrome is caused by a mutation in the gene that encodes fibrillin-1,88) a matrix glycoprotein that is the principal constituent of microfibrils. On the other hand, haploin-sufficiency of the elastin gene has been implicated in the arteriopathy of Williams syndrome.89) Increased aortic stiffness is well documented in patients with Marfan syndrome, as shown by the decreased distensibility and increased stiffness index,90-97) increased PWV,98) and decreased tissue Doppler-derived systolic and diastolic velocities of the aortic wall.99) Importantly, aortic stiffness has been shown to be an independent predictor of progressive aortic dilation100),101) and aortic dissection.101) Beta-blocker therapy98) and angiotensin-converting enzyme inhibition102) appear to reduce aortic stiffness, which may, in turn, slow aortic dilation and delay aortic root replacement.102) Despite a biological basis for abnormal elastic fibres, results of studies exploring arterial elastic properties in patients with Williams syndrome are controversial.103-106) In both children and adults,107) an isolated bicuspid aortic valve is associated with progressive dilation of the ascending aorta and increased aortic stiffness.108),109)
Congenital heart disease
In a variety of congenital heart lesions, medial abnormalities with elastic fibre fragmentation have been identified in intraoperative biopsies and necropsy aortic specimens.110) These congenital heart lesions include tetralogy of Fallot with or without pulmonary atresia, truncus arteriosus, complete transposition of the great arteries, coarctation of the aorta, double-outlet ventricles, and univentricular hearts.
In children and adolescents with tetralogy of Fallot, aortic stiffness has been shown to be increased and related to the aortic root dimensions.111) Additional data suggest that there is preferential stiffening of the central, over peripheral, conduit arteries.112) Importantly, the heart-femoral PWV has been found to be a significant size determinant of the sinotubular junction, suggesting that central arterial stiffening may contribute to progressive aortic root dilation in these patients.
In transposition of the great arteries, patients undergoing two-stage anatomic correction were found to have decreased distensibility of the neoaorta, and this is thought to be related to pulmonary arterial banding.113) Nonetheless, even after one-stage arterial switch operation, impaired distensibility of the neoaorta has similarly been documented.114) Recent studies documented an increased stiffness index of the carotid artery in patients both after atrial and arterial switch operations,115),116) suggesting that impaired elastogenesis may be an intrinsic component of this congenital anomaly.
In the aortic segment proximal to the site of aortic coarctation, increase in collagen and decrease in smooth muscle content have been described.117) Functionally, distensibility of the aortic arch has been shown to be significantly lower than that of the distal thoracic aorta.118) The importance of early coarctation repair on possible prevention of late vascular dysfunction is highlighted by the inverse relationships found between age at repair and stiffness and vascular reactivitiy of the precoarctation arterial segments.118-120) Interestingly, the results of a recent study suggest that impaired elastic properties of the prestenotic aorta may be a primary abnormality as evidenced by an increased ascending aortic stiffness index, even preoperatively, in neonates with coarctation.121)
Systemic childhood diseases
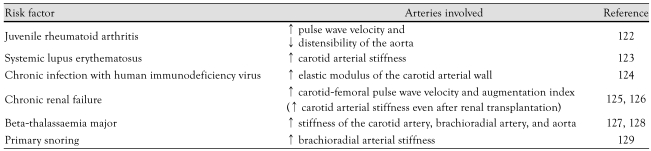
Apart from diseases of the heart and blood vessels, several chronic systemic diseases in childhood have been found to be associated with arterial stiffening (Table 3).122-129)
Table 3.
Systemic childhood conditions associated with arterial stiffening
Functional Implications on Cardiac Performance
Ventricular afterload is increased in the presence of systemic arterial stiffening. To generate the same stroke volume against a stiffened arterial tree, with increased afterload, the systemic ventricle has to generate a higher end-systolic pressure at the expense of greater myocardial oxygen consumption.
Structural adaptation of the left ventricle to increased afterload is also evident in the presence of arterial stiffening. In an otherwise healthy population of adults, measures of arterial stiffness including elastic modulus, distensibility, and PWV have been shown to be significant determinants of left ventricular mass.130)
Arterial stiffening is also associated with alteration of phasic coronary flow pattern.131),132) Early return of the reflected pressure wave, due to a faster PWV, augments central systolic pressure and lowers diastolic coronary perfusion pressure.
The increased myocardial oxygen consumption, left ventricular hypertrophy, and decreased diastolic coronary perfusion pressure predispose the conditions of subendocardial ischemia and interstitial fibrosis, which in turn can impair myocardial relaxation and reduce ventricular compliance.133),134) Indeed, associations between arterial stiffness and left ventricular diastolic dysfunction in adults with hypertension134-137) and diabetes mellitus135),137),138) are recognized. Associations between arterial stiffening and left ventricular systolic function in adults with139) and without135) coronary artery disease have also been found.
Clinical implications on management
Early identification of arterial dysfunction in childhood may provide a window for early intervention. Amelioration of endothelial dysfunction may reduce arterial stiffness through the lowering of smooth muscle tone. The potential beneficial effects on endothelial function of folic acid in children with renal failure,140),141) antioxidant vitamins and statins in those with familial hypercholesterolemia,142-144) vitamin C in those with Kawasaki disease,145) and exercise training in obese children146),147) have been reported. In patients with Marfan syndrome, beta-blocker therapy98) and angiotensin-converting enzyme inhibition102) appear to reduce aortic stiffness.
Lifestyle modification early in life may prevent premature stiffening of arteries. In this regard, there is evidence to suggest a beneficial impact on arterial stiffness by sodium restriction,148) regular exercise,149) intake of fish oil150) and isoflavone,151) and smoking cessation.152) Longitudinal studies are, however, required to determine whether improvement of arterial function in the young will be translated into clinical benefits in adulthood.
Conclusions
With availability of noninvasive technologies for determination of arterial stiffness in children, the significance of the phenomenon of arterial stiffening in the young is becoming better understood. Importantly, even in children and adolescents, accumulating evidence suggests that clinical conditions associated with abnormal functioning of the arterial system may have long-term clinical implications. Further studies to elucidate the underlying mechanisms of arterial stiffening in the young are warranted. Additionally, longitudinal studies are required to clarify whether systemic arterial stiffening tracks from child- to adulthood and whether early implementation of strategies to reduce arterial stiffness may have an impact on long-term cardiovascular health in both healthy and at-risk paediatric populations.
References
- 1.Laurent S, Hayoz D, Trazzi S, et al. Isobaric compliance of the radial artery is increased in patients with essential hypertension. J Hypertens. 1993;11:89–98. doi: 10.1097/00004872-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie IS, Wilkinson IB, Cockcroft JR. Assessment of arterial stiffness in clinical practice. QJM. 2002;95:67–74. doi: 10.1093/qjmed/95.2.67. [DOI] [PubMed] [Google Scholar]
- 4.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–566. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- 5.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 6.Shokawa T, Imazu M, Yamamoto H, et al. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J. 2005;69:259–264. doi: 10.1253/circj.69.259. [DOI] [PubMed] [Google Scholar]
- 7.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 8.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 9.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 12.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998;32:570–574. doi: 10.1161/01.hyp.32.3.570. [DOI] [PubMed] [Google Scholar]
- 13.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 14.Shoji T, Emoto M, Shinohara K, et al. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol. 2001;12:2117–2124. doi: 10.1681/ASN.V12102117. [DOI] [PubMed] [Google Scholar]
- 15.Barenbrock M, Kosch M, Joster E, Kisters K, Rahn KH, Hausberg M. Reduced arterial distensibility is a predictor of cardiovascular disease in patients after renal transplantation. J Hypertens. 2002;20:79–84. doi: 10.1097/00004872-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 17.Tardy Y, Meister JJ, Perret F, Brunner HR, Arditi M. Non-invasive estimate of the mechanical properties of peripheral arteries from ultrasonic and photoplethysmographic measurements. Clin Phys Physiol Meas. 1991;12:39–54. doi: 10.1088/0143-0815/12/1/003. [DOI] [PubMed] [Google Scholar]
- 18.Hoeks AP, Brands PJ, Smeets FA, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16:121–128. doi: 10.1016/0301-5629(90)90139-4. [DOI] [PubMed] [Google Scholar]
- 19.Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL. Direct magnetic resonance determination of aortic distensibility in essential hypertension: relation to age, abdominal visceral fat, and in situ intracellular free magnesium. Hypertension. 1997;30:654–659. doi: 10.1161/01.hyp.30.3.654. [DOI] [PubMed] [Google Scholar]
- 20.Kang SM, Ha JW, Chung N, et al. Assessment of elastic properties of the descending thoracic aorta by transesophageal echocardiography with acoustic quantification in patients with a stroke. Echocardiography. 2000;17:713–720. doi: 10.1111/j.1540-8175.2000.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 21.Benetos A, Laurent S, Hoeks AP, Boutouyrie PH, Safar ME. Arterial alterations with aging and high blood pressure: a noninvasive study of carotid and femoral arteries. Arterioscler Thromb. 1993;13:90–97. doi: 10.1161/01.atv.13.1.90. [DOI] [PubMed] [Google Scholar]
- 22.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 23.Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM. Noninvasive assessment of local pulse pressure: importance of brachial-to-radial pressure amplification. Hypertension. 2005;46:244–248. doi: 10.1161/01.HYP.0000166723.07809.7e. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki T, Fukuda S, Shimada K, et al. Direct measurement of wall stiffness for carotid arteries by ultrasound strain imaging. J Am Soc Echocardiogr. 2009;22:1389–1395. doi: 10.1016/j.echo.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Oishi Y, Mizuguchi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. A novel approach to assess aortic stiffness related to changes in aging using a two-dimensional strain imaging. Echocardiography. 2008;25:941–945. doi: 10.1111/j.1540-8175.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim KH, Park JC, Yoon HJ, et al. Usefulness of aortic strain analysis by velocity vector imaging as a new echocardiographic measure of arterial stiffness. J Am Soc Echocardiogr. 2009;22:1382–1388. doi: 10.1016/j.echo.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Bramwell JC, Hill AV. Velocity of transmission of the pulse wave. Lancet. 1992;1:891–892. [Google Scholar]
- 28.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement: validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 29.Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol. 2003;91:1519–1522. doi: 10.1016/s0002-9149(03)00416-8. A9. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 31.Kontis S, Gosling RG. On-line Doppler ultrasound measurement of aortic compliance and its repeatability in normal subjects. Clin Phys Physiol Meas. 1989;10:127–135. doi: 10.1088/0143-0815/10/2/002. [DOI] [PubMed] [Google Scholar]
- 32.Wright JS, Cruickshank JK, Kontis S, Dore C, Gosling RG. Aortic compliance measured by non-invasive Doppler ultrasound: description of a method and its reproducibility. Clin Sci. 1990;78:463–468. doi: 10.1042/cs0780463. [DOI] [PubMed] [Google Scholar]
- 33.Loukougeorgakis S, Dawson R, Phillips N, et al. Validation of a device to measure arterial pulse wave velocity by a photoplethysmographic method. Physiol Meas. 2002;23:581–596. doi: 10.1088/0967-3334/23/3/309. [DOI] [PubMed] [Google Scholar]
- 34.Mohiaddin RH, Firmin DN, Longmore DB. Age-related changes of human aortic flow wave velocity measured noninvasively by magnetic resonance imaging. J Appl Physiol. 1993;74:492–497. doi: 10.1152/jappl.1993.74.1.492. [DOI] [PubMed] [Google Scholar]
- 35.Stevanov M, Baruthio J, Gounot D, Grucker D. In vitro validation of MR measurements of arterial pulse-wave velocity in the presence of reflected waves. J Magn Reson Imaging. 2001;14:120–127. doi: 10.1002/jmri.1161. [DOI] [PubMed] [Google Scholar]
- 36.Cohn JN, Finkelstein S, McVeigh G, et al. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–508. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- 37.McVeigh GE, Bratteli CW, Morgan DJ, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999;33:1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 38.McVeigh GE. Pulse waveform analysis and arterial wall properties. Hypertension. 2003;41:1010–1011. doi: 10.1161/01.HYP.0000069006.98113.22. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol. 1986;251:H588–H600. doi: 10.1152/ajpheart.1986.251.3.H588. [DOI] [PubMed] [Google Scholar]
- 40.Marcus RH, Korcarz C, McCray G, et al. Noninvasive method for determination of arterial compliance using Doppler echocardiography and subclavian pulse tracings: validation and clinical application of a physiological model of the circulation. Circulation. 1994;89:2688–2699. doi: 10.1161/01.cir.89.6.2688. [DOI] [PubMed] [Google Scholar]
- 41.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 42.Cheung YF, Brogan PA, Pilla CB, Dillon MJ, Redington AN. Arterial distensibility in children and teenagers: normal evolution and the effect of childhood vasculitis. Arch Dis Child. 2002;87:348–351. doi: 10.1136/adc.87.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senzaki H, Akagi M, Hishi T, et al. Age-associated changes in arterial elastic properties in children. Eur J Pediatr. 2002;161:547–551. doi: 10.1007/s00431-002-1025-6. [DOI] [PubMed] [Google Scholar]
- 44.Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–955. doi: 10.1016/s0140-6736(96)10508-0. [DOI] [PubMed] [Google Scholar]
- 45.Glukhova MA, Frid MG, Koteliansky VE. Phenotypic changes of human aortic smooth muscle cells during development and in the adult vessel. Am J Physiol. 1991;261(4 Suppl):78–80. doi: 10.1152/ajpheart.1991.261.4.78. [DOI] [PubMed] [Google Scholar]
- 46.Levent E, Goksen D, Ozyurek AR, et al. Stiffness of the abdominal aorta in obese children. J Pediatr Endocrinol Metab. 2002;15:405–409. doi: 10.1515/JPEM.2002.15.4.405. [DOI] [PubMed] [Google Scholar]
- 47.Iannuzzi A, Licenziati MR, Acampora C, et al. Preclinical changes in the mechanical properties of abdominal aorta in obese children. Metabolism. 2004;53:1243–1246. doi: 10.1016/j.metabol.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 48.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 49.Whincup PH, Gilg JA, Donald AE, et al. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112:1789–1797. doi: 10.1161/CIRCULATIONAHA.104.532663. [DOI] [PubMed] [Google Scholar]
- 50.Aggoun Y, Bonnet D, Sidi D, et al. Arterial mechanical changes in children with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;20:2070–2075. doi: 10.1161/01.atv.20.9.2070. [DOI] [PubMed] [Google Scholar]
- 51.Virkola K, Pesonen E, Akerblom HK, Siimes MA. Cholesterol and carotid artery wall in children and adolescents with familial hypercholesterolaemia: a controlled study by ultrasound. Acta Paediatr. 1997;86:1203–1207. doi: 10.1111/j.1651-2227.1997.tb14847.x. [DOI] [PubMed] [Google Scholar]
- 52.Leeson CP, Whincup PH, Cook DG, et al. Cholesterol and arterial distensibility in the first decade of life: a population-based study. Circulation. 2000;101:1533–1538. doi: 10.1161/01.cir.101.13.1533. [DOI] [PubMed] [Google Scholar]
- 53.Haller MJ, Samyn M, Nichols WW, et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care. 2004;27:2911–2917. doi: 10.2337/diacare.27.12.2911. [DOI] [PubMed] [Google Scholar]
- 54.Hopkins KD, Lehmann ED, Jones RL, Turay RC, Gosling RG. A family history of NIDDM is associated with decreased aortic distensibility in normal healthy young adult subjects. Diabetes Care. 1996;19:501–503. doi: 10.2337/diacare.19.5.501. [DOI] [PubMed] [Google Scholar]
- 55.McEleavy OD, McCallum RW, Petrie JR, et al. Higher carotidradial pulse wave velocity in healthy offspring of patients with Type 2 diabetes. Diabet Med. 2004;21:262–266. doi: 10.1111/j.1464-5491.2004.01127.x. [DOI] [PubMed] [Google Scholar]
- 56.Schack-Nielsen L, Molgaard C, Larsen D, Martyn C, Michaelsen KF. Arterial stiffness in 10-year-old children: current and early determinants. Br J Nutr. 2005;94:1004–1011. doi: 10.1079/bjn20051518. [DOI] [PubMed] [Google Scholar]
- 57.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tauzin L, Rossi P, Giusano B, et al. Characteristics of arterial stiffness in very low birth weight premature infants. Pediatr Res. 2006;60:592–596. doi: 10.1203/01.pdr.0000242264.68586.28. [DOI] [PubMed] [Google Scholar]
- 59.Oren A, Vos LE, Bos WJ, et al. Gestational age and birth weight in relation to aortic stiffness in healthy young adults: two separate mechanisms? Am J Hypertens. 2003;16:76–79. doi: 10.1016/s0895-7061(02)03151-5. [DOI] [PubMed] [Google Scholar]
- 60.Cheung YF, Wong KY, Lam BC, Tsoi NS. Relation of arterial stiffness with gestational age and birth weight. Arch Dis Child. 2004;89:217–221. doi: 10.1136/adc.2003.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akira M, Yoshiyuki S. Placental circulation, fetal growth, and stiffness of the abdominal aorta in newborn infants. J Pediatr. 2006;148:49–53. doi: 10.1016/j.jpeds.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 62.Martyn CN, Barker DJ, Jespersen S, Greenwald S, Osmond C, Berry C. Growth in utero, adult blood pressure, and arterial compliance. Br Heart J. 1995;73:116–121. doi: 10.1136/hrt.73.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheung YF, Taylor MJ, Fisk NM, Redington AN, Gardiner HM. Fetal origins of reduced arterial distensibility in the donor twin in twin-twin transfusion syndrome. Lancet. 2000;355:1157–1158. doi: 10.1016/s0140-6736(00)02068-7. [DOI] [PubMed] [Google Scholar]
- 64.Gardiner HM, Taylor MJ, Karatza A, et al. Twin-twin transfusion syndrome: the influence of intrauterine laser photocoagulation on arterial distensibility in childhood. Circulation. 2003;107:1906–1911. doi: 10.1161/01.CIR.0000060543.64250.80. [DOI] [PubMed] [Google Scholar]
- 65.Halvorsen CP, Andolf E, Hu J, Pilo C, Winbladh B, Norman M. Discordant twin growth in utero and differences in blood pressure and endothelial function at 8 years of age. J Intern Med. 2006;259:155–163. doi: 10.1111/j.1365-2796.2005.01593.x. [DOI] [PubMed] [Google Scholar]
- 66.Martin H, Gazelius B, Norman M. Impaired acetylcholine-induced vascular relaxation in low birth weight infants: implications for adult hypertension? Pediatr Res. 2000;47:457–462. doi: 10.1203/00006450-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Leeson CP, Whincup PH, Cook DG, et al. Flow-mediated dilation in 9- to 11-year-old children: the influence of intrauterine and childhood factors. Circulation. 1997;96:2233–2238. doi: 10.1161/01.cir.96.7.2233. [DOI] [PubMed] [Google Scholar]
- 68.Leeson CP, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103:1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- 69.Singhal A, Kattenhorn M, Cole TJ, Deanfield J, Lucas A. Preterm birth, vascular function, and risk factors for atherosclerosis. Lancet. 2001;358:1159–1160. doi: 10.1016/S0140-6736(01)06276-6. [DOI] [PubMed] [Google Scholar]
- 70.Akalin-Sel T, Campbell S. Understanding the pathophysiology of intra-uterine growth retardation: the role of the 'lower limb reflex' in redistribution of blood flow. Eur J Obstet Gynecol Reprod Biol. 1992;46:79–86. doi: 10.1016/0028-2243(92)90250-3. [DOI] [PubMed] [Google Scholar]
- 71.Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease: a 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 72.Sugimura T, Kato H, Inoue O, Takagi J, Fukuda T, Sato N. Vasodilatory response of the coronary arteries after Kawasaki disease: evaluation by intracoronary injection of isosorbide dinitrate. J Pediatr. 1992;121:684–688. doi: 10.1016/s0022-3476(05)81893-1. [DOI] [PubMed] [Google Scholar]
- 73.Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–311. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sugimura T, Kato H, Inoue O, et al. Intravascular ultrasound of coronary arteries in children: assessment of the wall morphology and the lumen after Kawasaki disease. Circulation. 1994;89:258–265. doi: 10.1161/01.cir.89.1.258. [DOI] [PubMed] [Google Scholar]
- 75.Furuyama H, Odagawa Y, Katoh C, et al. Assessment of coronary function in children with a history of Kawasaki disease using (15) O-water positron emission tomography. Circulation. 2002;105:2878–2884. doi: 10.1161/01.cir.0000018652.59840.57. [DOI] [PubMed] [Google Scholar]
- 76.Kato H, Inoue O, Kawasaki T, Fujiwara H, Watanabe T, Toshima H. Adult coronary artery disease probably due to childhood Kawasaki disease. Lancet. 1992;340:1127–1129. doi: 10.1016/0140-6736(92)93152-d. [DOI] [PubMed] [Google Scholar]
- 77.Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253–257. doi: 10.1016/0735-1097(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 78.Ishiwata S, Fuse K, Nishiyama S, Nakanishi S, Watanabe Y, Seki A. Adult coronary artery disease secondary to Kawasaki disease in childhood. Am J Cardiol. 1992;69:692–694. doi: 10.1016/0002-9149(92)90168-x. [DOI] [PubMed] [Google Scholar]
- 79.Silva AA, Maeno Y, Hashmi A, Smallhorn JF, Silverman ED, McCrindle BW. Cardiovascular risk factors after Kawasaki disease: a case-control study. J Pediatr. 2001;138:400–405. doi: 10.1067/mpd.2001.111430. [DOI] [PubMed] [Google Scholar]
- 80.Cheung YF, Yung TC, Tam SC, Ho MH, Chau AK. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J Am Coll Cardiol. 2004;43:120–124. doi: 10.1016/j.jacc.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 81.Noto N, Okada T, Yamasuge M, et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics. 2001;107:1095–1099. doi: 10.1542/peds.107.5.1095. [DOI] [PubMed] [Google Scholar]
- 82.Cheung YF, Wong SJ, Ho MH. Relationship between carotid intima-media thickness and arterial stiffness in children after Kawasaki disease. Arch Dis Child. 2007;92:43–47. doi: 10.1136/adc.2006.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Senzaki H, Chen CH, Ishido H, et al. Arterial hemodynamics in patients after Kawasaki disease. Circulation. 2005;111:2119–2125. doi: 10.1161/01.CIR.0000162483.51132.25. [DOI] [PubMed] [Google Scholar]
- 84.Cheung YF, Ho MH, Tam SC, Yung TC. Increased high sensitivity C reactive protein concentrations and increased arterial stiffness in children with a history of Kawasaki disease. Heart. 2004;90:1281–1285. doi: 10.1136/hrt.2003.018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mitani Y, Sawada H, Hayakawa H, et al. Elevated levels of high-sensitivity C-reactive protein and serum amyloid-A late after Kawasaki disease: association between inflammation and late coronary sequelae in Kawasaki disease. Circulation. 2005;111:38–43. doi: 10.1161/01.CIR.0000151311.38708.29. [DOI] [PubMed] [Google Scholar]
- 86.Cheung YF, Ho MH, Ip WK, Fok SF, Yung TC, Lau YL. Modulating effects of mannose binding lectin genotype on arterial stiffness in children after Kawasaki disease. Pediatr Res. 2004;56:591–596. doi: 10.1203/01.PDR.0000139406.22305.A4. [DOI] [PubMed] [Google Scholar]
- 87.Cheung YF, Huang GY, Chen SB, et al. Inflammatory gene polymorphisms and susceptibility to Kawasaki disease and its arterial sequelae. Pediatrics. 2008;122:e608–e614. doi: 10.1542/peds.2008-0646. [DOI] [PubMed] [Google Scholar]
- 88.Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 89.Tassabehji M, Metcalfe K, Donnai D, et al. Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum Mol Genet. 1997;6:1029–1036. doi: 10.1093/hmg/6.7.1029. [DOI] [PubMed] [Google Scholar]
- 90.Yin FC, Brin KP, Ting CT, Pyeritz RE. Arterial hemodynamic indexes in Marfan's syndrome. Circulation. 1989;79:854–862. doi: 10.1161/01.cir.79.4.854. [DOI] [PubMed] [Google Scholar]
- 91.Jeremy RW, Huang H, Hwa J, McCarron H, Hughes CF, Richards JG. Relation between age, arterial distensibility, and aortic dilatation in the Marfan syndrome. Am J Cardiol. 1994;74:369–373. doi: 10.1016/0002-9149(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 92.Haouzi A, Berglund H, Pelikan PC, Maurer G, Siegel RJ. Heterogeneous aortic response to acute beta-adrenergic blockade in Marfan syndrome. Am Heart J. 1997;133:60–63. doi: 10.1016/s0002-8703(97)70248-5. [DOI] [PubMed] [Google Scholar]
- 93.Hirata K, Triposkiadis F, Sparks E, Bowen J, Wooley CF, Boudoulas H. The Marfan syndrome: abnormal aortic elastic properties. J Am Coll Cardiol. 1991;18:57–63. doi: 10.1016/s0735-1097(10)80218-9. [DOI] [PubMed] [Google Scholar]
- 94.Reed CM, Fox ME, Alpert BS. Aortic biomechanical properties in pediatric patients with the Marfan syndrome, and the effects of atenolol. Am J Cardiol. 1993;71:606–608. doi: 10.1016/0002-9149(93)90522-e. [DOI] [PubMed] [Google Scholar]
- 95.Sonesson B, Hansen F, Lanne T. Abnormal mechanical properties of the aorta in Marfan's syndrome. Eur J Vasc Surg. 1994;8:595–601. doi: 10.1016/s0950-821x(05)80597-3. [DOI] [PubMed] [Google Scholar]
- 96.Adams JN, Brooks M, Redpath TW, et al. Aortic distensibility and stiffness index measured by magnetic resonance imaging in patients with Marfan's syndrome. Br Heart J. 1995;73:265–269. doi: 10.1136/hrt.73.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franke A, Muhler EG, Klues HG, et al. Detection of abnormal aortic elastic properties in asymptomatic patients with Marfan syndrome by combined transoesophageal echocardiography and acoustic quantification. Heart. 1996;75:307–311. doi: 10.1136/hrt.75.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Groenink M, de Roos A, Mulder BJ, Spaan JA, van der Wall EE. Changes in aortic distensibility and pulse wave velocity assessed with magnetic resonance imaging following beta-blocker therapy in the Marfan syndrome. Am J Cardiol. 1998;82:203–208. doi: 10.1016/s0002-9149(98)00315-4. [DOI] [PubMed] [Google Scholar]
- 99.Harada K, Yasuoka K, Shimada Y. Usefulness of tissue doppler imaging for assessing aortic wall stiffness in children with the Marfan syndrome. Am J Cardiol. 2004;93:1072–1075. doi: 10.1016/j.amjcard.2003.12.067. [DOI] [PubMed] [Google Scholar]
- 100.Nollen GJ, Groenink M, Tijssen JG, Van Der Wall EE, Mulder BJ. Aortic stiffness and diameter predict progressive aortic dilatation in patients with Marfan syndrome. Eur Heart J. 2004;25:1146–1152. doi: 10.1016/j.ehj.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 101.Vitarelli A, Conde Y, Cimino E, et al. Aortic wall mechanics in the Marfan syndrome assessed by transesophageal tissue Doppler echocardiography. Am J Cardiol. 2006;97:571–577. doi: 10.1016/j.amjcard.2005.09.089. [DOI] [PubMed] [Google Scholar]
- 102.Yetman AT, Bornemeier RA, McCrindle BW. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am J Cardiol. 2005;95:1125–1127. doi: 10.1016/j.amjcard.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 103.Wessel A, Pankau R, Berdau W, Lons P. Aortic stiffness with the Williams-Beuren syndrome. Pediatr Cardiol. 1997;18:244. doi: 10.1007/s002469900165. [DOI] [PubMed] [Google Scholar]
- 104.Salaymeh KJ, Banerjee A. Evaluation of arterial stiffness in children with Williams syndrome: does it play a role in evolving hypertension? Am Heart J. 2001;142:549–555. doi: 10.1067/mhj.2001.116763. [DOI] [PubMed] [Google Scholar]
- 105.Aggoun Y, Sidi D, Levy BI, Lyonnet S, Kachaner J, Bonnet D. Mechanical properties of the common carotid artery in Williams syndrome. Heart. 2000;84:290–293. doi: 10.1136/heart.84.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lacolley P, Boutouyrie P, Glukhova M, et al. Disruption of the elastin gene in adult Williams syndrome is accompanied by a paradoxical reduction in arterial stiffness. Clin Sci. 2002;103:21–29. doi: 10.1042/cs1030021. [DOI] [PubMed] [Google Scholar]
- 107.Holmes KW, Lehmann CU, Dalal D, et al. Progressive dilation of the ascending aorta in children with isolated bicuspid aortic valve. Am J Cardiol. 2007;99:978–983. doi: 10.1016/j.amjcard.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 108.Nistri S, Sorbo MD, Basso C, Thiene G. Bicuspid aortic valve: abnormal aortic elastic properties. J Heart Valve Dis. 2002;11:369–373. discussion 373-4. [PubMed] [Google Scholar]
- 109.Schaefer BM, Lewin MB, Stout KK, Byers PH, Otto CM. Usefulness of bicuspid aortic valve phenotype to predict elastic properties of the ascending aorta. Am J Cardiol. 2007;99:686–690. doi: 10.1016/j.amjcard.2006.09.118. [DOI] [PubMed] [Google Scholar]
- 110.Niwa K, Perloff JK, Bhuta SM, et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393–400. doi: 10.1161/01.cir.103.3.393. [DOI] [PubMed] [Google Scholar]
- 111.Chong WY, Wong WH, Chiu CS, Cheung YF. Aortic root dilation and aortic elastic properties in children after repair of tetralogy of Fallot. Am J Cardiol. 2006;97:905–909. doi: 10.1016/j.amjcard.2005.09.141. [DOI] [PubMed] [Google Scholar]
- 112.Cheung YF, Ou X, Wong SJ. Central and peripheral arterial stiffness in patients after surgical repair of tetralogy of Fallot: implications for aortic root dilatation. Heart. 2006;92:1827–1830. doi: 10.1136/hrt.2006.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sievers HH, Lange PE, Arensman FW, et al. Influence of two-stage anatomic correction on size and distensibility of the anatomic pulmonary/functional aortic root in patients with simple transposition of the great arteries. Circulation. 1984;70:202–208. doi: 10.1161/01.cir.70.2.202. [DOI] [PubMed] [Google Scholar]
- 114.Murakami T, Nakazawa M, Momma K, Imai Y. Impaired distensibility of neoaorta after arterial switch procedure. Ann Thorac Surg. 2000;70:1907–1910. doi: 10.1016/s0003-4975(00)01837-3. [DOI] [PubMed] [Google Scholar]
- 115.Mersich B, Studinger P, Lenard Z, Kadar K, Kollai M. Transposition of great arteries is associated with increased carotid artery stiffness. Hypertension. 2006;47:1197–1202. doi: 10.1161/01.HYP.0000218826.72592.e9. [DOI] [PubMed] [Google Scholar]
- 116.Pinter A, Laszlo A, Mersich B, Kadar K, Kollai M. Adaptation of baroreflex function to increased carotid artery stiffening in patients with transposition of great arteries. Clin Sci. 2007;113:41–46. doi: 10.1042/CS20060363. [DOI] [PubMed] [Google Scholar]
- 117.Sehested J, Baandrup U, Mikkelsen E. Different reactivity and structure of the prestenotic and poststenotic aorta in human coarctation: implications for baroreceptor function. Circulation. 1982;65:1060–1065. doi: 10.1161/01.cir.65.6.1060. [DOI] [PubMed] [Google Scholar]
- 118.Brili S, Dernellis J, Aggeli C, et al. Aortic elastic properties in patients with repaired coarctation of aorta. Am J Cardiol. 1998;82:1140–1143. doi: 10.1016/s0002-9149(98)00575-x. A10. [DOI] [PubMed] [Google Scholar]
- 119.de Divitiis M, Pilla C, Kattenhorn M, et al. Vascular dysfunction after repair of coarctation of the aorta: impact of early surgery. Circulation. 2001;104(12 Suppl 1):I165–I170. doi: 10.1161/hc37t1.094900. [DOI] [PubMed] [Google Scholar]
- 120.Heger M, Willfort A, Neunteufl T, et al. Vascular dysfunction after coarctation repair is related to the age at surgery. Int J Cardiol. 2005;99:295–299. doi: 10.1016/j.ijcard.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 121.Vogt M, Kuhn A, Baumgartner D, et al. Impaired elastic properties of the ascending aorta in newborns before and early after successful coarctation repair: proof of a systemic vascular disease of the prestenotic arteries? Circulation. 2005;111:3269–3273. doi: 10.1161/CIRCULATIONAHA.104.529792. [DOI] [PubMed] [Google Scholar]
- 122.Argyropoulou MI, Kiortsis DN, Daskas N, et al. Distensibility and pulse wave velocity of the thoracic aorta in patients with juvenile idiopathic arthritis: an MRI study. Clin Exp Rheumatol. 2003;21:794–797. [PubMed] [Google Scholar]
- 123.Chow PC, Ho MH, Lee TL, Lau YL, Cheung YF. Relation of arterial stiffness to left ventricular structure and function in adolescents and young adults with pediatric-onset systemic lupus erythematosus. J Rheumatol. 2007;34:1345–1352. [PubMed] [Google Scholar]
- 124.Bonnet D, Aggoun Y, Szezepanski I, Bellal N, Blanche S. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS. 2004;18:1037–1041. doi: 10.1097/00002030-200404300-00012. [DOI] [PubMed] [Google Scholar]
- 125.Covic A, Mardare N, Gusbeth-Tatomir P, et al. Increased arterial stiffness in children on haemodialysis. Nephrol Dial Transplant. 2006;21:729–735. doi: 10.1093/ndt/gfi196. [DOI] [PubMed] [Google Scholar]
- 126.Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR. Abnormal carotid artery structure and function in children and adolescents with successful renal transplantation. Circulation. 2004;110:97–101. doi: 10.1161/01.CIR.0000133412.53089.26. [DOI] [PubMed] [Google Scholar]
- 127.Cheung YF, Chan GC, Ha SY. Arterial stiffness and endothelial function in patients with beta-thalassemia major. Circulation. 2002;106:2561–2566. doi: 10.1161/01.cir.0000037225.92759.a7. [DOI] [PubMed] [Google Scholar]
- 128.Ulger Z, Aydinok Y, Gurses D, Levent E, Ozyurek AR. Stiffness of the abdominal aorta in beta-thalassemia major patients related with body iron load. J Pediatr Hematol Oncol. 2006;28:647–652. doi: 10.1097/01.mph.0000212987.18694.5a. [DOI] [PubMed] [Google Scholar]
- 129.Kwok KL, Ng DK, Cheung YF. BP and arterial distensibility in children with primary snoring. Chest. 2003;123:1561–1566. doi: 10.1378/chest.123.5.1561. [DOI] [PubMed] [Google Scholar]
- 130.Chen CH, Ting CT, Lin SJ, et al. Which arterial and cardiac parameters best predict left ventricular mass? Circulation. 1998;98:422–428. doi: 10.1161/01.cir.98.5.422. [DOI] [PubMed] [Google Scholar]
- 131.Saeki A, Recchia F, Kass DA. Systolic flow augmentation in hearts ejecting into a model of stiff aging vasculature: influence on myocardial perfusion-demand balance. Circ Res. 1995;76:132–141. doi: 10.1161/01.res.76.1.132. [DOI] [PubMed] [Google Scholar]
- 132.Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail Rev. 2002;7:51–62. doi: 10.1023/a:1013749806227. [DOI] [PubMed] [Google Scholar]
- 133.O'Rourke MF. Diastolic heart failure, diastolic left ventricular dysfunction and exercise intolerance. J Am Coll Cardiol. 2001;38:803–805. doi: 10.1016/s0735-1097(01)01452-8. [DOI] [PubMed] [Google Scholar]
- 134.Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vinereanu D, Nicolaides E, Boden L, Payne N, Jones CJ, Fraser AG. Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart. 2003;89:449–450. doi: 10.1136/heart.89.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yambe M, Tomiyama H, Hirayama Y, et al. Arterial stiffening as a possible risk factor for both atherosclerosis and diastolic heart failure. Hypertens Res. 2004;27:625–631. doi: 10.1291/hypres.27.625. [DOI] [PubMed] [Google Scholar]
- 137.Eren M, Gorgulu S, Uslu N, Celik S, Dagdeviren B, Tezel T. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90:37–43. doi: 10.1136/heart.90.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loimaala A, Groundstroem K, Majahalme S, Nenonen A, Vuori I. Impaired myocardial function in newly onset type 2 diabetes associates with arterial stiffness. Eur J Echocardiogr. 2006;7:341–347. doi: 10.1016/j.euje.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 139.Sakuragi S, Iwasaki J, Tokunaga N, Hiramatsu S, Ohe T. Aortic stiffness is an independent predictor of left ventricular function in patients with coronary heart disease. Cardiology. 2005;103:107–112. doi: 10.1159/000082472. [DOI] [PubMed] [Google Scholar]
- 140.Bennett-Richards K, Kattenhorn M, Donald A, et al. Does oral folic acid lower total homocysteine levels and improve endothelial function in children with chronic renal failure? Circulation. 2002;105:1810–1815. doi: 10.1161/01.cir.0000014417.95833.1d. [DOI] [PubMed] [Google Scholar]
- 141.Bennett-Richards KJ, Kattenhorn M, Donald AE, et al. Oral Larginine does not improve endothelial dysfunction in children with chronic renal failure. Kidney Int. 2002;62:1372–1378. doi: 10.1111/j.1523-1755.2002.kid555.x. [DOI] [PubMed] [Google Scholar]
- 142.Mietus-Snyder M, Malloy MJ. Endothelial dysfunction occurs in children with two genetic hyperlipidemias: improvement with antioxidant vitamin therapy. J Pediatr. 1998;133:35–40. doi: 10.1016/s0022-3476(98)70174-x. [DOI] [PubMed] [Google Scholar]
- 143.de Jongh S, Lilien MR, Bakker HD, Hutten BA, Kastelein JJ, Stroes ES. Family history of cardiovascular events and endothelial dysfunction in children with familial hypercholesterolemia. Atherosclerosis. 2002;163:193–197. doi: 10.1016/s0021-9150(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 144.Engler MM, Engler MB, Malloy MJ, et al. Antioxidant vitamins C and E improve endothelial function in children with hyperlipidemia: Endothelial Assessment of Risk from Lipids in Youth (EARLY) Trial. Circulation. 2003;108:1059–1063. doi: 10.1161/01.CIR.0000086345.09861.A0. [DOI] [PubMed] [Google Scholar]
- 145.Deng YB, Li TL, Xiang HJ, Chang Q, Li CL. Impaired endothelial function in the brachial artery after Kawasaki disease and the effects of intravenous administration of vitamin C. Pediatr Infect Dis J. 2003;22:34–39. doi: 10.1097/00006454-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 146.Woo KS, Chook P, Yu CW, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109:1981–1986. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- 147.Watts K, Beye P, Siafarikas A, et al. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol. 2004;43:1823–1827. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 148.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 149.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 150.Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76:326–330. doi: 10.1093/ajcn/76.2.326. [DOI] [PubMed] [Google Scholar]
- 151.Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23:1066–1071. doi: 10.1161/01.ATV.0000072967.97296.4A. [DOI] [PubMed] [Google Scholar]
- 152.Jatoi NA, Jerrard-Dunne P, Feely J, Mahmud A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension. 2007;49:981–985. doi: 10.1161/HYPERTENSIONAHA.107.087338. [DOI] [PubMed] [Google Scholar]