Abstract
Aim
To obtain correct evaluation of the prevalence rate of diabetes mellitus and impaired fasting glycemia (IFG) in Hungary.
Method
The study was performed on a nationally representative sample covered by the Hungarian General Practitioners’ Morbidity Sentinel Stations Program. The source population consisted of all persons aged 20-69 years who were registered with the participating centers. The prevalence rates were adjusted to age and sex distribution of the total Hungarian population. Fasting blood samples of 1803 participants were evaluated. Response rate was 90.7%.
Results
In addition to 130 (7.21%) patients with established diabetes, 26 participants with newly diagnosed diabetes were found, resulting in a total crude diabetes prevalence of 8.65% (men: 11.16%; women: 6.41%; P < 0.001). After weighting for age and sex, the prevalence rate of diabetes in participants aged 20-69 years should be set at 7.47% (95% confidence interval [CI], 6.26-8.69) (men: 9.49%; 95% CI, 7.52-11.46; women: 5.58%; 95% CI, 4.12-7.04). In addition to 41 (2.27%) patients with established IFG (fasting blood glucose: 6.1-6.9 mmol/L), 47 participants with newly diagnosed IFG were found, resulting in a total crude IFG prevalence of 4.88% (men: 6.11%; women: 3.78%; P = 0.022). After making corrections for weighting age and sex, the total IFG prevalence rate in participants aged 20-69 years should be set at 4.39% (95% CI, 3.44-5.34) (men: 5.52%; 95% CI, 3.99-7.06; women: 3.33%; 95% CI, 2.19-4.47).
Conclusion
The prevalence rate of diabetes and IFG in Hungary is higher than previously estimated by experts and authorities. The present data may serve as a base for comparative investigations in the future.
Conflict of interest
None declared.
The importance of diabetes mellitus and other forms of glucose intolerance in public health has dramatically increased worldwide (1-3). Not only the recent prevalence rate but the estimated increase in incidence may create a global health problem in the near future (4). In order to establish effective prevention and control programs, the exact number of the affected people should be known.
Type 2 diabetes is the predominating form of diabetes mellitus. Typically, type 2 diabetes evolves without major clinical symptoms and prediabetes or intermediate hyperglycemia (impaired fasting glycemia [IFG] and impaired glucose tolerance) precedes the manifestation (5-7). Accordingly, screening for identifying asymptomatic participants with diabetes or prediabetes is of great clinical importance (8). Several screening procedures with different methods in various settings were applied in order to detect participants with type 2 diabetes, prediabetes, or metabolic syndrome among adult people in Hungary. Nevertheless, only a representative survey should be considered reliable for determining the real prevalence rate of diabetes in a given population.
Regarding the prevalence rate of diabetes in Hungary, instead of confirmed data, at present only estimations from experts and authorities are available. In previous and current versions of Diabetes Atlas (3rd edition, 2006; 4th edition, 2009) published by the International Diabetes Federation (9,10), diabetes prevalence in Hungary was determined by extrapolating data from Polish studies (11,12), due to the lack of reliable data.
In 2005-2006, a representative survey was initiated by the Hungarian Diabetes Association in cooperation with the national health monitoring program established by the School of Public Health, Medical University of Debrecen (13). The aim of the study was to determine the prevalence rate of the metabolic syndrome, diabetes, and IFG in a representative cohort of people aged 20-69 years in Hungary. Data about diabetes and IFG are presented here.
Methods
We performed a cross-sectional study in the population covered by the Hungarian General Practitioners’ Morbidity Sentinel Stations Program. The design of the sentinel stations program has already been described (14). Briefly, participating general practitioners (GP) from different counties continuously report new cases of non-communicable diseases of major public health importance diagnosed using standardized diagnostic criteria. In the present study, 8 out of 19 counties were selected in order to represent different geographical and socio-economical regions (urban/rural, western/eastern, higher/lower economical status areas). Finally, 59 GPs from 8 counties (Baranya, Bács-Kiskun, Győr-Moson-Sopron, Hajdú-Bihar, Heves, Komárom-Esztergom, Szabolcs-Szatmár-Bereg, Zala) participated voluntarily in this survey. GPs and their nurses were trained in advance by members of the Department of Preventive Medicine (Faculty of Public Health, University of Debrecen). During the entire screening procedure, quality control and supervision were provided by county medical officers.
Study sample
The source population of this study consisted of all persons aged 20-69 years who were registered with the participating GPs on December 31, 2005. As the proportion of the population registered with GPs in Hungary is virtually 100%, it enabled us to provide an accurate population denominator. Using an initial calculation, the total number of participants for screening was set at 2006. This number enabled us to detect high-density lipoprotein (HDL)-cholesterol, the component of the metabolic syndrome with the lowest prevalence rate (4.8%) and a margin of 20%-25% (15). According to the criteria of representativeness, participants living in the 8 counties and corresponding to the structure of the Hungarian adult population were considered. In other words, the population was representative for the overall Hungarian population in terms of age and sex. The particular participants were randomly selected from the files of residents in the catchment area. With an overall response rate of 90.7%, 1819 participants were screened but due to lacking samples, 1803 participants were finally analyzed (Table 1). As some misrepresentations occurred in the screened population (lower than expected participation in younger age groups and higher participation in older age groups), a weighted estimation of age and sex was used in order to correct the disproportional representativeness of some age- and sex- specific strata in the sample. Accordingly, crude rate (results of initial evaluation of data) and corrected rate (weighted estimation by age and sex) are reported. In addition, the absolute number of patients with diabetes or IFG was assessed based on the total number (n=6 872 843) of the Hungarian population aged 20-69 years on December 31, 2006 (Table 1).
Table 1.
Age and sex distribution in the sample, in the investigated 8 counties, and in the Hungarian population aged 20-69 years (December 31, 2006)*
| Age-groups (years) |
Percentage of participants |
||
|---|---|---|---|
| sample assessed (n = 1803) | population of the investigated counties (n = 2 331 587) | Hungarian population aged 20-69 y (n = 6 872 843) | |
| Men: |
n = 851 |
n = 1 143 173 |
n = 3 344 572 |
| 20-29 |
18.0 |
21.9 |
21.6 |
| 30-39 |
18.9 |
23.9 |
24.2 |
| 40-49 |
20.9 |
19.9 |
19.2 |
| 50-59 |
24.3 |
21.3 |
21.5 |
| 60-69 |
17.9 |
12.9 |
13.5 |
| Women: |
n = 952 |
n = 1 188 414 |
n = 3 528 271 |
| 20-29 |
14.8 |
20.3 |
19.8 |
| 30-39 |
16.9 |
21.9 |
22.2 |
| 40-49 |
22.1 |
19.1 |
18.4 |
| 50-59 |
25.3 |
22.3 |
22.6 |
| 60-69 | 20.9 | 16.4 | 17.0 |
*Source: Central Office for Administrative and Electronic Public Services.
Data collection and evaluation
Participants were invited by their GPs to join the survey. The relevant data on medical history, presence of diseases, and mode of treatment were registered by the GPs. A physical examination including measurement of weight, height, waist circumference, and blood pressure was carried out by the GPs. Fasting venous blood samples were taken to determine serum triglycerides, HDL-cholesterol, and plasma glucose levels. Laboratory measurements of all samples were performed by the same institute (Department of Clinical Chemistry, University of Debrecen). Waist circumference >88 cm in women and >102 cm in men was considered abnormal (16).
Diabetes (type 1 or 2) and IFG were considered known if the diagnosis was documented in the patient’s file. The diagnosis of previously unknown (newly diagnosed) diabetes or IFG was based on the criteria of the World Health Organization (WHO) (17). Accordingly, diabetes was diagnosed if fasting blood glucose values reached or exceeded 7.0 mmol/L. IFG was determined with fasting blood glucose values ranging between 6.1 and 6.9 mmol/L. In addition, IFG was also assessed according to the American Diabetes Association (ADA) criteria (fasting blood glucose, 5.6-6.9 mmol/L) (18). In this epidemiological survey, no oral glucose tolerance test was performed and confirmatory fasting blood glucose values were not available.
The survey was approved by the Ethics Committee of University of Debrecen and participants gave informed consent. The results of the survey were reported to the GPs and therapeutic suggestions were also made if needed.
Statistical analysis was performed using the Stata program (StataCorp LP, College Station, Tx, USA). Data are presented as mean values and standard deviation (x±SD). The corrected prevalence rates are expressed as mean and 95% confidence interval (CI). Continuous variables were compared by using unpaired t-test while categorical variables were compared by χ2 test. The level of significance was set at P < 0.05.
Results
The overall response rate in this survey was as high as 90.7% (1819 out of 2006 randomly selected and invited participants). Due to technical difficulties (insufficient number of questionnaires, lacking blood samples), data of 1803 participants (851 men and 952 women) were finally analyzed. Women had a higher age (46.7 ± 13.5 vs 45.0 ± 13.7 years; P < 0.010) and a lower body mass index (27.5 ± 5.8 vs 27.9 ± 5.0 kg/m2; P = 0.043), as well as a smaller waist circumference (93.1 ± 15.6 vs 99.4 ± 14.0 cm; P < 0.0001) than men. Abnormal waist circumference was more often found in women than men (57.1 vs 37.9%; P < 0.001). On the other hand, the prevalence rate of obesity (body mass index >30 kg/m2) was nearly the same in women and men (30.9 vs 30.3%; P = 0.764).
There were 130 participants (7.21%) with known diabetes. Previously unknown (newly diagnosed) diabetes was found in 26 participants (1.44%), resulting in a total crude prevalence rate of diabetes as high as 8.65% (men: 11.16%, women: 6.41%; P < 0.001). After making corrections, the prevalence rate of diabetes in participants aged 20-69 years should be set at 7.47% (95% CI, 6.26-8.69) (men: 9.49%; 95% CI, 7.52-11.46; women 5.58%; 95% CI, 4.12-7.04). In this way, the absolute number of patients with diabetes in the total Hungarian population aged 20-69 years was established to be 513 455.
There were 41 participants (2.27%) with known IFG (fasting blood glucose: 6.1-6.9 mmol/L). Previously unknown (newly diagnosed) IFG was found in 47 participants (2.61%), resulting in a total crude prevalence rate of IFG as high as 4.88% (men: 6.11%, women: 3.78%; P = 0.022). After making corrections, the prevalence rate of IFG in participants aged 20-69 years was set at 4.39% (95% CI, 3.44-5.34) (men: 5.52%; 95% CI, 3.99-7.06; women: 3.33%; 95% CI, 2.18-4.47). When the ADA criterion was used for IFG determination (fasting blood glucose 5.6-6.9 mmol/L), the total crude prevalence rate of IFG was 8.82% (men: 11.28%, women: 6.62%; P < 0.001); corrected total prevalence rate 8.00% (95% CI, 6.74-9.25) (men: 10.27%; 95% CI, 8.23-12.32; women: 5.86%; 95% CI, 4.37-7.36). In this way, the absolute number of participants with IFG in the total Hungarian population aged 20-69 years was established to be 301 936 (WHO criteria) or 549 551 (ADA criteria).
The prevalence rate of diabetes (known and newly diagnosed) increased with age, while that of IFG (known and newly diagnosed) increased up to the 50-59 years age group and declined beyond (Figure 1).
Figure 1.
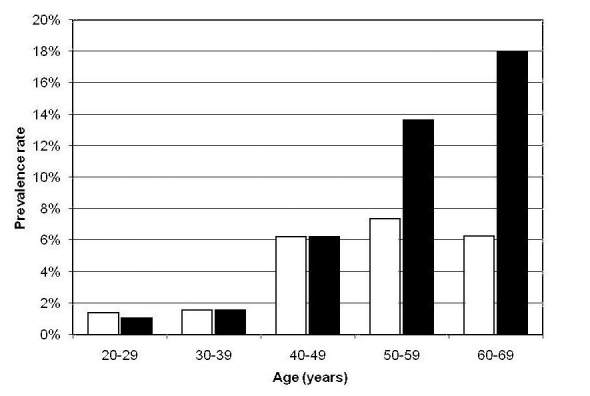
Prevalence rate of diabetes mellitus and impaired fasting glycemia (IFG) in different age groups of participants screened (n = 1803). Open bars – IFG; closed bars – diabetes.
No significant variation in the prevalence of diabetes was observed between the counties (it ranged from 6.90% in Baranya county to 12.28% in Zala county; P = 0.464).
There was a wide variation in the prevalence of IFG (WHO criteria) between the counties (it ranged from 1.75% in Baranya county to 8.94% in Heves county; P = 0.018).
Discussion
Our study showed that the prevalence of diabetes in Hungarian population aged 20-69 years was 7.47% (95% CI, 6.26-8.69) and the prevalence of IFG was 4.39% (95% CI, 3.44-5.34). These rates are higher than previously estimated by Hungarian experts and authorities.
Obviously, the results of a screening procedure depend on the participants selected, the response rate achieved, and the method used. In our study, the size and the structure of the sample population were determined well in advance. The selection followed the epidemiological rules for obtaining a sample of 2006 participants who should be considered representative of a population aged 20-69 years in Hungary. The response rate of 90.7% in our screening procedure was appropriate. Although oral glucose tolerance test with fasting and 2-hour glucose values has been widely used in the clinical practice for detecting glucose intolerance in asymptomatic subjects, an epidemiological survey should be based on fasting blood glucose values only. In addition, the use of confirmatory blood glucose values should not be considered mandatory in epidemiological studies (17). Clearly, by using fasting blood glucose values for screening, participants with impaired glucose tolerance could not be identified.
Classification of diabetes was not performed in this study although it is generally known that the predominant type of diabetes is type 2 (10).
The prevalence rate of diabetes and IFG was greater in men than in women. Our results did not provide explanation for the difference. The age-dependency in the prevalence rate of diabetes in our study corresponds to the results of other investigations (18).
The number of participants with IFG nearly doubled when we used ADA criteria as compared with WHO criteria. Undoubtedly, a pandemic of prediabetes can be created by lowering the diagnostic threshold of IFG from 6.1 to 5.6 mmol/L (19).
In our study, the prevalence of known diabetes was higher than that of the newly diagnosed diabetes (previously unknown). The proportion of undiagnosed persons with diabetes proved to be 0.3 or 0.7 in surveys, depending on the method used (20,21). The low proportion (0.2) of undiagnosed persons with diabetes in our study can partly be explained by the fact that no oral glucose tolerance test was performed.
This survey provided new epidemiological data about diabetes and IFG in Hungary. Although a former study also investigated some epidemiological characteristics of diabetes in Hungary (14), the number of counties involved was smaller, no nation-wide data were provided, IFG was not studied, and the diagnostic criteria for diabetes were based on former diagnostic criteria. In this way, the epidemiology of diabetes and IFG in Hungary was better characterized in the present study.
The results of our study are in accordance with other studies performed recently in Central Europe, despite some differences in the structure of the population and the screening method used. Namely, the prevalence rate of diabetes in Slovakia was 7.0% and the prevalence rate of type 2 diabetes in Croatia was 6.1% (20,21). Similar results have been found recently in southern Poland (22). The prevalence rate of IFG in our study was much lower (4.39%) than that (11.3%) reported in Croatia (20).
Our study has some limitations. Although it was designed as a representative survey, some misrepresentations occurred in the screened population and, therefore, corrections should be made. In addition, no oral glucose tolerance test was performed and, accordingly, it can be assumed that some patients with diabetes could not be identified and participants with impaired glucose tolerance could not be detected. Nevertheless, epidemiological surveys could be based on measuring fasting glucose values only. Strength of our study is the high overall response rate, centrally measured laboratory parameters, and the quality control performed.
Taken altogether, our study provided reliable data about the prevalence of diabetes and IFG in Hungarian population aged 20-69 years. All prevalence rates proved to be higher than previously estimated. The present data may serve as a base for comparative investigations in the future.
Acknowledgments
The study was supported by a grant from the Hungarian Diabetes Association and Ányos Jedlik Foundation (NKFP1-0003/2005).
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Colagiuri S, Borch-Johnsen K, Glumer C, Vistisen D. There really is an epidemic of type 2 diabetes. Diabetologia. 2005;48:1459–63. doi: 10.1007/s00125-005-1843-y. [DOI] [PubMed] [Google Scholar]
- 3.Kolb H, Mandrup-Poulsen T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia. 2010;53:10–20. doi: 10.1007/s00125-009-1573-7. [DOI] [PubMed] [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–9. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 6.U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–58. doi: 10.2337/diabetes.44.11.1249. [DOI] [PubMed] [Google Scholar]
- 7.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–21. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med. 2007;24:451–63. doi: 10.1111/j.1464-5491.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- 9.International Diabetes Federation. Diabetes atlas. 3rd edition. Brussels: International Diabetes Federation; 2006. [Google Scholar]
- 10.International Diabetes Federation. Diabetes atlas. 4th edition. Brussels: International Diabetes Federation; 2009. [Google Scholar]
- 11.Szurkowska M, Szybiński Z, Nazim A, Szafraniec K, Jedrychowski W. Prevalence of type II diabetes mellitus in population of Krakow. Pol Arch Med Wewn. 2001;106:771–9. [in Polish] [PubMed] [Google Scholar]
- 12.Łopatyński J, Mardarowicz G, Nicer T, Szcześniak G, Król H, Matej A, et al. The prevalence of type II diabetes mellitus in rural urban population over 35 years of age in Lublin region (Eastern Poland) Pol Arch Med Wewn. 2001;106:781–6. [in Polish] [PubMed] [Google Scholar]
- 13.Szigethy E, Voko Z, Jermendy G, Nadas J, Paragh G, Blasko G, et al. The epidemiology of metabolic syndrome in the Hungarian adult population. Diab Vasc Dis Res. 2007;4(Suppl 1):S94. [abstract] [Google Scholar]
- 14.Szeles G, Voko Z, Jenei T, Kardos L, Pocsai Z, Bajtay A, et al. A preliminary evaluation of a health monitoring programme in Hungary. Eur J Public Health. 2005;15:26–32. doi: 10.1093/eurpub/cki107. [DOI] [PubMed] [Google Scholar]
- 15.Verschuren WM. Serum cholesterol and coronary heart disease – a public health perspective. Neth J Med. 1997;51:1–9. doi: 10.1016/S0300-2977(97)00019-3. [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO consultation. Geneva (Switzerland): WHO; 2006. [Google Scholar]
- 18.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 19.Borch-Johnsen K, Colagiuri S, Balkau B, Glumer C, Carstensen B, Ramachandran A, et al. Creating a pandemic of prediabetes: the proposed new diagnostic criteria for impaired fasting glycaemia. Diabetologia. 2004;47:1396–402. doi: 10.1007/s00125-004-1468-6. [DOI] [PubMed] [Google Scholar]
- 20.Metelko Z, Pavlic-Renar I, Poljicanin T, Szirovitza L, Turek S. Prevalence of diabetes mellitus in Croatia. Diabetes Res Clin Pract. 2008;81:263–7. doi: 10.1016/j.diabres.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Mokan M, Galajda P, Pridavkova D, Tomaskova V, Sutarik L, Krucinska L, et al. Prevalence of diabetes mellitus and metabolic syndrome in Slovakia. Diabetes Res Clin Pract. 2008;81:238–42. doi: 10.1016/j.diabres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Wittek A, Sokalski B, Grzeszczak W, Strojek K. Prevalence of diabetes and cardiovascular risk factors of industrial area in southern Poland. Exp Clin Endocrinol Diabetes. 2009;117:350–3. doi: 10.1055/s-0029-1220689. [DOI] [PubMed] [Google Scholar]


