Abstract
N-methyl-D-aspartic acid receptor (NMDAR) hypofunction has long been implicated in schizophrenia and NMDARs on γ-aminobutyric acid (GABA)ergic interneurons are proposed to play an essential role in the pathogenesis. However, controversial results have been reported regarding the regulation of NMDAR expression, and direct evidence of how NMDAR antagonists act on specific subpopulations of prefrontal interneurons is missing. We investigated the effects of the NMDAR antagonist dizocilpine (MK-801) on the expression of NMDAR subtypes in the identified interneurons in young adult rat prefrontal cortex (PFC) by using laser microdissection and real-time polymerase chain reaction, combined with Western blotting and immunofluorescent staining. We found that MK-801 induced distinct changes of NMDAR subunits in the parvalbumin-immunoreactive (PV-ir) interneurons vs. pyramidal neurons in the PFC circuitry. The messenger RNA (mRNA) expression of all NMDAR subtypes, including NR1 and NR2A to 2D, exhibited inverted-∪ dose-dependent changes in response to MK-801 treatment in the PFC. In contrast, subunit mRNAs of NMDARs in PV-ir interneurons were significantly down-regulated at low doses, unaltered at medium doses, and significantly decreased again at high doses, suggesting a biphasic dose response to MK-801. The differential effects of MK-801 in mRNA expression of NMDAR subunits were consistent with the protein expression of NR2A and NR2B subunits revealed with Western blotting and double immunofluorescent staining. These results suggest that PV-containing interneurons in the PFC exhibit a distinct responsiveness to NMDAR antagonism and that NMDA antagonist can differentially and dose-dependently regulate the functions of pyramidal neurons and GABAergic interneurons in the prefrontal cortical circuitry.
Keywords: Disinhibition, laser microdissection, neurotoxicity, NMDA antagonist, polymerase chain reaction, schizophrenia
Introduction
Although the mechanisms underlying schizophrenia are not clear, recent advances have indicated that N-methyl-D-aspartic acid receptors (NMDARs) may play a critical role in the pathogenesis of this devastating disorder (Conn et al. 2008; Coyle, 2006; Dracheva et al. 2001; Javitt, 2004; Lisman et al. 2008; Moghaddam, 2003; Mohn et al. 1999; Olney et al. 1999). NMDAR antagonists, such as phencyclidine, ketamine, and dizocilpine (MK-801), are able to produce a pattern of neurochemical and behavioural changes that are similar to those seen in schizophrenia in both animal models and human subjects (Javitt & Zukin, 1991; Krystal et al. 1994; Lahti et al. 1995; Morris et al. 2005). Treatment with NMDAR antagonists produces both positive and negative symptoms in animal models (Pratt et al. 2008) by altering the glutamatergic systems (Abekawa et al. 2007; Behrens et al. 2007; Braun et al. 2007; Eyjolfsson et al. 2006; Mouri et al. 2007; Rujescu et al. 2006; Wilson et al. 1998). In addition, evidence indicates that cortical interneurons, especially parvalbumin-immunoreactive (PV-ir) neurons, are selectively impaired in patients with schizophrenia (Beasley & Reynolds, 1997; Benes & Berretta, 2001; Hashimoto et al. 2003; Lewis et al. 2005). Based on these observations and others, a hypothesis of NMDAR hypofunction has been proposed for the pathological and cognitive features of schizophrenia.
Despite these advances, direct evidence is lacking regarding how NMDAR antagonists selectively act on specific populations of interneurons in the prefrontal cortex (PFC). Some studies suggested that NMDAR subunits localized on GABAergic interneurons might be more sensitive than those on pyramidal cells (Grunze et al. 1996; Kinney et al. 2006; Li et al. 2002; Zhang et al. 2008). Using in-vivo physiological recording, Homayoun and Moghaddam reported that MK-801 produces opposite effects on interneurons and pyramidal neurons in the rat PFC (Homayoun & Moghaddam, 2007). Consistent with these results, we recently found that the development of NMDAR subunits on individual functionally identified inter-neurons is cell-type specific. Compared to pyramidal neurons, fast-spiking interneurons express fewer NMDARs but a significantly higher level of NR2A subunits in the rat PFC (Wang & Gao, 2009). However, it remains unknown how the expression of individual NMDAR subunits in the selected subpopulations of interneurons responds to NMDAR antagonism. To address this question, we examined the changes of NMDAR subunits in the identified PV-ir interneurons in response to MK-801 administration in young adult rats by using laser microdissection (LMD) and quantitative real-time polymerase chain reaction (PCR), combined with Western blotting and immunostaining. We demonstrated that NMDAR subunits in PV-ir interneurons exhibit a distinct pattern of response to MK-801 stimulations compared to pyramidal neurons in the PFC.
Materials and methods
Animal treatment and tissue preparation
Sixty-nine young adult female Sprague–Dawley rats (aged 90±1 d, body weight 246.8±1.57 g) were used in this study. Several reasons prompted us to use young adult female rats as subjects in this study. First, although male rats were used in an animal model of schizophrenia (Jackson et al. 2004), many pharmacological studies indicated that female rats are more sensitive to MK-801 than male rats (Dickerson & Sharp, 2006; Farber et al. 1995; Javitt, 2004; Olney et al. 1989). Second, the symptoms of schizophrenia typically begin to emerge between ages 15 and 25 yr in most of the patients with schizophrenia. This timing coincides with the period of post-adolescent or early adulthood. We therefore choose young adult rats rather than adult animals (Dickerson & Sharp, 2006). The animals were treated under the animal use guidelines of National Institutes of Health and the Institutional Animal Care and Use Committee at Drexel University College of Medicine. Animals were allowed to adapt to a new environment for 1–2 d prior to treatment. Forty-eight rats were divided into control and five groups of treatment with MK-801 (Sigma-Aldrich, USA), i.e. 1.0 ml sterile saline as control and MK-801 at doses of 0.01, 0.033, 0.1, 0.33 and 1.0 mg/kg. The MK-801 was dissolved in 1.0 ml sterile saline and was injected daily [intraperitoneally (i.p.)] for 5 consecutive days. The animals were sacrificed for testing 24 h after the last injection of MK-801 administration. The animals were deeply anaesthetized by Euthasol (0.2 ml/kg i.p., Henry Schein, USA) and the brain regions containing PFC were blocked on dry ice. Some brains were homogenized in lysis buffer (RNAqueous-Micro kit, Applied Biosystems/Ambion, USA) at 4 °C for extraction of total RNA from PFC tissues; while other brains were sectioned for rapid immunostaining of PV-containing interneurons in the PFC (Xi et al. 2009). The brains were cut into 20-μm sections at −20 °C with an UltraPro 5000 Cryostat (Vibratome, USA). Six sections containing the medial PFC were directly mounted on the ribonuclease (RNase)-free polyethylene naphthalate foil slide (Leica Microsystems, Germany) and stored at −80 °C in an airtight box to avoid dehydration. Some of the adjacent sections were used for Nissl staining to identify the laminar architecture of the medial PFC (mPFC).
An additional 21 rats were divided into a control group treated with 0.9% saline and two groups with an injection of MK-801 at a dose of 0.033 mg/kg or 1.0 mg/kg, respectively. Three animals in each group were perfused through the ascending aorta with 0.1 mM phosphate buffer (PB, pH 7.4), followed by 4% paraformaldehyde dissolved in 0.1 mM PB (350 ml, pH 7.4, 4 °C). The brains were removed and stored in 30% sucrose-PB solution (4 °C) overnight, and then cut into 30-μm coronal sections for immunofluorescent staining. The remaining animals (four rats in each group) were sacrificed and the medial prefrontal cortical regions were quickly homogenized in lysis buffer for Western blotting.
RNA extraction, quality control and real-time PCR for PFC tissue samples
Total RNA was extracted from the homogenized lysis mixture directly using an RNAqueous-Micro kit and dissolved in a 40-μl elution buffer for the PFC sample. RNA concentration was measured at wavelengths of 260 nm and 280 nm using the NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, USA). Only the RNA samples with 260/280 ratios of 1.8–2.0 were used for further investigation. RNA extracted from the PFC tissue was directly diluted into RNase-free water at a concentration of 20 ng/μl. The iQ-SYBR Green one-step Supermix kit (Bio-Rad Laboratories, USA) was used to run real-time PCR, which was detailed as follows: 1 μl RNA, 1.5 μl forward primer (4 μM), 1.5 μl backward primer (4 μM), 12.5 μl SYBR Green mix, 8 μl RNase-free water and 0.5 μl iScript. Real-time PCR analysis was performed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin as the housekeeping genes and custom-designed primers for PV and NMDAR subunits (Table 1).
Table 1.
Primers of the genes used in the study
| Gene | Accession no. | Forward primer | Backward primer | Product |
|---|---|---|---|---|
| GAPDH | NM_017008 | ccatcccagaccccataac | gcagcgaactttattgatgg | 78 bp |
| β-actin | NM_031144 | tgacaggatgcagaaggaga | tagagccaccaatccacaca | 104 bp |
| PVALB (PV) | NM_022499 | aagagtgcggatgatgtgaag | agccatcagcgtctttgttt | 150 bp |
| GRIN1 (NR1) | NM_017010 | cttcctccagccactaccc | agaaagcacccctgaagcac | 226 bp |
| GRIN2A (NR2A) | NM_012573 | aggacagcaagaggagcaag | acctcaaggatgaccgaaga | 174 bp |
| GRIN2B (NR2B) | NM_012574 | tgagtgagggaagagagagagg | atggaaacaggaatggtgga | 249 bp |
| GRIN2C (NR2C) | NM_012575 | gggctcctctggcttctatt | gacaacaggacagggacaca | 162 bp |
| GRIN2D (NR2D) | NM_022797 | cccaaatctcacccatcct | gagaggtgtgtctggggcta | 198 bp |
RNA extraction, measurement, reverse PCR, antisense RNA amplification and real-time PCR for identified PV-ir interneurons
NovaRED immunostaining of PV in fresh brain tissue and laser microdissection
We used NovaRED rapid immunostaining (Vector Laboratories, USA) to label the interneurons containing PV. This method uses the avidin–biotin–peroxidase complex (ABC) to amplify the signal and NovaRED as the substrate. The final products appeared as brown/red. The slide was thawed at −20 °C for 1 min and then at room temperature for 30 s. The cortical sections were fixed with 75% ethanol at −20 °C for 2 min and then incubated with mouse anti-PV antibody (500 μl, 1:100, Bioscience Research Reagents, USA) at 40 °C for 8 min, followed by incubation in a universal secondary antibody provided with the NovaRED kit (1:48, 600 μl) at room temperature for 7 min. The sections were then pre-incubated with ABC [solution A and B 1:24 diluted in dielaidoylphosphatidylcholine (DEPC) phosphate-buffered saline] (ABC kit, Vector Laboratories) at room temperature for 5 min and reacted with NovaRED substrate for 8 min. The sections were rinsed three times between each step, dehydrated in 70% and 95% ethanol for 30 s each, then dried at 40 °C for 5 min. LMD was performed immediately using the Leica Microsystems LMD system (Bannockburn, USA). Approximately 100–120 cells were captured from each slide and immersed in 30 μl lysis solution (RNAqueous-Micro kit) (Standaert, 2005). The dissected neurons were stored at −80 °C or directly processed for RNA extraction.
RNA extraction, reverse transcription (RT)-PCR and electrophoresis
RNA was obtained from captured PV-ir cells using the RNAqueous-Micro kit according to the manufacturer’s instructions. Total RNA was extracted by adding 1.25 vol of 100% RNase-free ethanol to the cell lysis mixture. RNA concentration, dissolved in 20-μl elution buffer from the RNAqueous-Micro kit, was measured at 260-nm and 280-nm wavelengths using the NanoDrop Spectrophotometer. RNA integrity was tested with an Agilent Bioanalyzer (Agilent Technologies, USA) (Bustin & Nolan, 2004; Kerman et al. 2006; Schroeder et al. 2006). RT–PCR was performed to ensure the specificity of the LMD products by using primers for PV. RT–PCR was conducted according to the protocol provided with the Qiagen one-step PCR kit (Qiagen, USA) and the PCR product was confirmed with agarose gel electrophoresis as previously described (Xi et al. 2009). Briefly, 10 μl reaction product mixed with 2 μl loading dye was added to gel composed of 30 ml 1% agarose (American Bioanalytical, USA) in 1× Tris (hydroxymethyl) aminomethane acetate–EDTA buffer (Fisher Scientific) and 1.5 μl ethidium bromide (Promega, USA). The agarose gel electrophoresis was performed at 110 V for 30 min and the PCR products in the gel were visualized using ultraviolet transillumination.
Antisense RNA amplification and primer design
Apparently, the concentration (usually <10 ng/μl) was not sufficient for one-step real-time PCR. Therefore, antisense RNA (aRNA) amplification and complementary DNA (cDNA) synthesis were applied to increase the template for two-step real-time PCR (Ginsberg et al. 2004; Hemby et al. 2002). We used the Message Booster cDNA synthesis kit (Epicentre Bio-technologies, USA) for quantitative PCR and the detailed procedures were modified from the protocol provided with the kit. It was essential that the primers used for cDNA synthesized are located within the last 500 bases of the 3′-end of the messenger RNA (mRNA). Primers for sequences more than 500 bases from the 3′-end of the mRNAs may give reduced sensitivity, according to the manufacturer’s instructions. The boundaries of exons and introns in the sequences were checked and the specificity of individual primers was examined using web-based Primer-BLAST at the National Center for Biotechnology Information (NCBI) (Schefe et al. 2006).
Real-time PCR and gene expression
The pre-amplified cDNA was used as the template for real-time PCR at a dilution of 1:20 in DEPC water. We used the iQ-SYBR Green Supermix kit (Bio-Rad Laboratories) for signal detection, which contains 1.5 μl cDNA, 1.0 μl forward primer (4 μM), 1.0 μl backward primer (4 μM), 12.5 μl SYBR Green mix and 9 μl RNase-free water. Real-time PCR analysis was performed with GAPDH and β-actin as the housekeeping genes, and all the primers listed in Table 1 have been confirmed for annealing temperature at 55 °C. There were 40–50 cycles of a two-step PCR protocol used. The threshold cycle (Ct) value for each well and PCR efficiency (E) for each gene were determined. The formula 2−ΔΔCt was used for normalization of gene expression to the control group; i.e. gene expression was calculated as the ratio between the treated group and the control group. Samples from each animal were tested 3–4 times to reduce variability. The data were normalized to reference genes and were expressed as percent change±standard error. Statistical significance (p<0.05) was determined by one-way ANOVA using Tukey’s HSD test for equal variance or Dunnett’s T3 test for unequal variance (SPSS Inc., USA).
Protein expression of NR2A and NR2B subunits with Western blotting
The cortical tissues containing PFC were dissected and homogenized in lysis buffer [20 mM Tris–HCl (pH 7.4), 200 mM NaCl, 1 mM Na3VO4, 10 mM NaF, and cocktail protease inhibitor]. The protein concentrations of the tissue samples were measured using BCA™ protein assay kit (Pierce, USA). Equal amounts of protein (50 μg) were dissolved into 25 μl lysis buffer (which contained 12.5 μl loading buffer and 1.25 β-mercapto-ethanol), boiled at 95 °C for 3 min, and then subjected to Tris–HCl SDS–PAGE and transferred to nitrocellulose membrane (Bio-Rad Laboratories). The membranes were blocked for 1 h in TBST with 5% dry milk and were incubated in anti-NR2A and anti-NR2B at 1:4000 (Zymed Laboratories Inc., USA) and anti-β-actin at 1:20 000 (Sigma-Aldrich) for 1 h. After rinsing with TBST for 3×15 min, the membranes were incubated in HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., USA) at 1:2000 for 2 h. The membrane protein bands were detected with ECL Western Blotting System (Amersham Bioscience, USA). The band densities were measured with Image J (NIH) and were normalized to β-actin. To minimize the inter-blot variability, each sample was analysed 3–4 times. The mean value for each sample was calculated from all the replicates and the results were presented as mean±standard error. Significance was determined using ANOVA for group comparisons.
Protein expression of NR2A and NR2B subunits in PV-ir interneurons and pyramidal neurons with double immunofluorescent labelling
Cortical sections containing mPFC were selected for double immunofluorescent staining. The sections were rinsed with 3% Triton in 0.1 M PBS and then blocked with 3% goat normal serum diluted in 3% Triton-PB for 1 h at room temperature. For double staining of PV and NR2A or NR2B, the slices were incubated in mouse anti-PV IgG1 antibody mixture (Millipore Corp., USA) at 1:3000 and goat anti-NR2A or NR2B IgG antibody (Santa Cruz, USA) at 1:1000 for 36 h at 4 °C. After rinsing with 3% Triton-PB for 3×15 min, the sections were incubated in 1:800 Texas Red-conjugated goat anti-mouse IgG1 and fluorescein-5-isothiocyanate (FITC)-conjugated bovine anti-goat IgG (Santa Cruz) at room temperature for 2 h. The sections were rinsed five times with 0.1 M PB and then mounted with UltraCruz™ mounting medium. All of the primary antibodies used have been well characterized with Western blotting and have been employed in numerous studies in several different species (Fumagalli et al. 2008; Kim et al. 2005; Mauceri et al. 2007; Williams et al. 2007). We also examined the antibody specificity by pre-blocking with serum, excluding primary antibody, or using Western blotting. The specificity of the staining was further evidenced by the fact that only certain cell types showed immunolabelling with these antibodies. In order to quantify the staining, the photographs taken at high magnifications (63×water lens) were converted into greyscale for contrast adjustment of the labelled puncta in individual neurons. Sections from three animals in each group were used for quantitative analysis and we manually counted the puncta localized in the labelled neurons. The immunopositive neurons of both pyramidal neurons and PV-ir interneurons were randomly selected from layers 2/3 to 5, with 50 neurons for each cell type, which include 20–30 cells from layers 2/3 and 20–30 cells from layer 5, respectively. The counting was double checked, with one author blindly counting the puncta and another author, blind to the treatment and animal group, confirming the result. The data were analysed with Student’s t test for statistical significance and the comparisons are expressed as means±standard error.
Results
Relatively high levels of NR2A mRNA in PV interneurons in the PFC
Despite the importance of NMDARs in both normal prefrontal function and in the pathogenesis of schizophrenia, the distribution of individual NMDAR subunits in prefrontal neurons is not clear. To address this issue, we first examined the relative mRNA expression of NMDAR subunits in the PFC tissue, compared to those in the identified PV-ir interneurons. We used PFC tissue because pyramidal neurons in the neocortex constitute ~80% of the cell population and the immunoreactive NMDARs are not exhibited in glia cells. We believe that the levels of NMDARs in the PFC tissue primarily reflect the receptor distribution in pyramidal neurons. The RNA concentrations in the PFC tissue were measured using NanoDrop spectrophotometry at wavelengths of 260 nm and 280 nm, and the averaged 260/280 ratio from the PFC tissue samples was 1.98±0.02. The samples with low 260/280 ratio (<1.6) were discarded from further analyses. As shown in Fig. 1a, all subunits of NMDARs, including NR1 and NR2A–2D, were detected in the PFC tissue, but a significantly higher mRNA expression of NR2B subunits was observed compared to NR2A (p<0.01). This finding is consistent with our recent physiological recording data, which showed a relatively low NR2A:NR2B ratio in the prefrontal pyramidal neurons (Wang et al. 2008b).
Fig. 1.
Relative mRNA expression of NMDAR subunits in adult rat PFC and parvalbumin-immunoreactive (PV-ir) interneurons. (a) mRNA expression of NMDAR subunits in the PFC tissue. NR2B mRNA expression (31.7±3.91, n=8) is significantly higher than that of the NR2A subunit (1.92±0.66, n=8, p=0.0001). (b, c) High-magnification photographs showing the PV-ir interneurons (arrows) labelled with rapid NovaRED immunostaining before (b) and after (c) the laser cut. Scale bar in (c)=50 μm. (d, e) RNA integrity numbers (RIN) were measured with Agilent Bioanalyzer and the electropherogram of RNA extracted from laser microdissection (LMD)-picked PV-ir interneurons is shown in (d), where 5S covers the small rRNA fragments (5S and 5.8S rRNA and tRNA) and 18S and 28S cover the 18S peak and 28S peak (e). RIN=8.63±0.16 (n=9) with 28S/18S=1.90±0.15 and 18S/baseline=7.23±1.17. (f) RT–PCR amplification of PV (150 bp) showing the expression of PV in LMD-captured PV-ir cells compared to that in PV-negative tissue. The left lane was the molecular weight marker with 100-bp intervals. (g) Relative mRNA expression of NMDAR subunits in PV-ir interneurons. The mRNA level of NR2A subunit was significantly higher than that of NR2B subunit (p=0.031). # Indicates that NR2D mRNA was at an undetectable level (* p<0.05, ** p<0.01).
To identify the subunit expression of NMDARs in identified interneurons while at the same time to preserving the integrity of RNA, we applied rapid immunocytochemical analysis in fresh PFC sections. Subpopulations of interneurons expressing PV were visualized with NovaRED staining. The PV-ir interneurons were easily identified under higher magnification (×40), as shown in Fig. 1b, with the final staining product of the PV-ir neurons in brown/red. The PV-ir interneurons in the mPFC were collected with LMD (Fig. 1c) and immediately placed in lysis buffer for analysis of gene expression of NMDAR subunits. The quality of the RNA was assessed by measuring the 260/280 absorbance ratio and the RNA integrity number (RIN) (Fig. 1d, e). The RNA concentration ranged from 2.1 to 10.7 ng/μl and the RIN of the RNA samples obtained from LMD-captured PV-ir interneurons averaged 8.63±0.16 (n=9), suggesting a sufficient quality of RNA (Kerman et al. 2006; Schroeder et al. 2006). Because only GABAergic interneurons express PV in the neocortex, theoretically the LMD-captured neurons should contain a high concentration of PV. Figure 1f shows the PCR products observed with agarose gel electrophoresis as previously described (Xi et al. 2009). We found that all subunits, except the NR2D subunit (which was not detectable), were expressed in the PV-ir interneurons. The mRNA expression of NR2A subunit was significantly higher than that of the NR2B subunit (p<0.05, Fig. 1g). These results indicate that PV-ir interneurons in the rat PFC express a relatively high NR2A/NR2B ratio, in accord with a previous study (Kinney et al. 2006).
Inverted-∪ dose-dependent alterations of NMDAR mRNA in MK-801-treated rat PFC
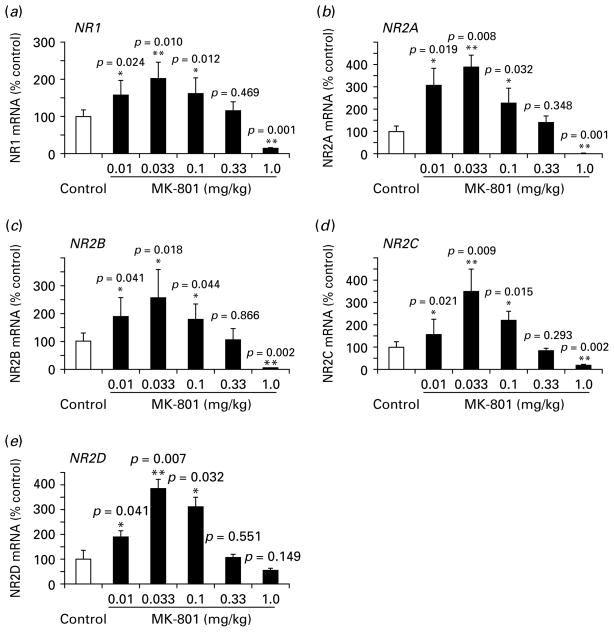
Although MK-801 has been extensively used to study the pathological process of schizophrenia, controversial results have been reported regarding the alterations of NMDAR subunit expression (Eyjolfsson et al. 2006). We believe that the contradictory results are largely attributable to the administration of single doses of NMDAR antagonists in most of the studies. To test this possibility and to examine systematically the alterations of individual NMDAR subunit expression in response to subchronic MK-801 administration, we tested five different doses of MK-801 in a 5-d subchronic treatment, using saline as control. We found that all five subunits, including NR1 and NR2A–NR2D subunits, showed inverted-∪ dose-dependent changes, i.e. increased at low doses but decreased at high doses, although slight differences existed in the five NMDAR subunits examined. As shown in Fig. 2, subchronic MK-801 treatment at doses of 0.01–0.1 mg/kg significantly up-regulated mRNA expression of all subunits in the PFC, with NR1 increased by 1.6- to 2.0-fold, NR2A by as high as 2.3- to 3.9-fold, NR2B by 1.8- to 2.5-fold, NR2C by 1.6- to 3.5-fold, and NR2D by 1.9- to 3.8-fold (p<0.05 for all subunits; Fig. 2). At the higher dose of 0.33 mg/kg, all subunits exhibited no significant changes, whereas at a high dose of 1.0 mg/kg, all subunits significantly decreased to almost undetectable levels (p<0.01 for all) except for NR2D, which showed a 1.9-fold decrease but not statistically different (p=0.149).
Fig. 2.
Effect of subchronic MK-801 administration on mRNA expression of NMDAR subunits in adult rat PFC tissue. (a–c) mRNA levels of NR1, NR2A, and NR2B subunits showed similar changes for different doses of MK-801 treatment. Both were significantly increased at very low doses of 0.01, 0.033 and 0.1 mg/kg, significantly decreased at a high dose of 1.0 mg/kg (p<0.05), and increased but not significantly at 0.33 mg/kg (p>0.05). (d, e) the mRNA levels of NR2C subunits were similar to the NR2B subunit except at 0.01 mg/kg, a dose at which the changes were not significant (p=0.095). (e) the mRNA change of NR2D was not significant at the high dose of 1.0 mg/kg (p=0.149), slightly different from other subunits (* p<0.05, ** p<0.01).
Biphasic pattern of mRNA expression of NMDAR subunits in PV-ir interneurons
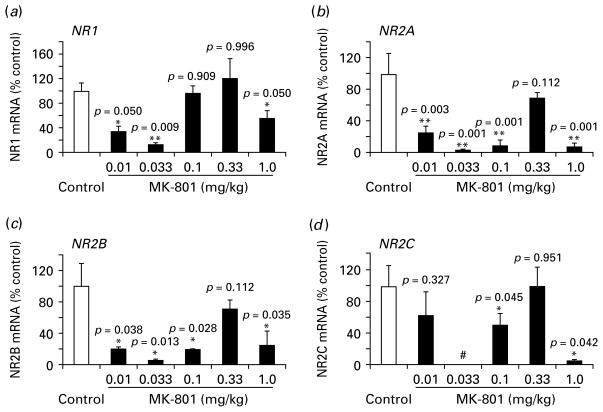
PV-ir interneurons play an essential role in the hypothesis of NMDAR hypofunction (Farber, 2003; Lisman et al. 2008; Moghaddam, 2003), but little is known about how NMDAR subunits located on identified interneurons respond to MK-801 treatment. As described here, NovaRED-stained PV-ir interneurons were selected with the assistance of LMD, and NMDAR subunit genes were tested with real-time PCR. As shown in Fig. 3, we found that although expression of the internal reference genes in the five treated groups was relatively stable without significant difference (e.g. GAPDH), the expression of NMDAR subunits was very responsive to MK-801 stimulation, even at very low concentrations. These receptors responded well to MK-801 in a biphasic pattern for all subunits. mRNA expression of all NMDAR subunits was significantly decreased at low doses of 0.01 and 0.033 mg/kg (p<0.05) except for a slight decrease for NR2C at 0.01 mg/kg (p=0.327) and almost no change for NR1 at 0.1 mg/kg (p=0.909). Similar to mRNA expression in PFC tissue, all subunits exhibited no significant changes at 0.33 mg/kg (p>0.05) but exhibited significant decreases at a high dose of MK-801 (1.0 mg/kg, p<0.05). These results suggest that PV-containing interneurons in the PFC have a distinct responsiveness to NMDAR antagonism.
Fig. 3.
Effect of subchronic MK-801 administration on mRNA expression of NMDAR subunits in parvalbumin-immunoreactive (PV-ir) interneurons. (a) the mRNA levels of NR1 subunit in response to MK-801 treatment at different doses. NR1 was significantly down-regulated at doses 0.01 and 0.033 mg/kg (p<0.05), returned to control levels at 0.1 and 0.33 mg/kg (p>0.05) and significantly decreased at 1.0 mg/kg (p<0.05). (b–d) NR2 subunits A–C showed a similar biphasic pattern of changes in mRNA expression except NR2C, which did not show significance at 0.01 mg/kg following MK-801 administration. # Indicates that the expression of NR2C at the dose of 0.033 mg/kg was undetectable (* p<0.05, ** p<0.01).
Differential protein expression of NR2A and NR2B subunits in response to low and high doses of MK-801 treatment in the PFC tissue
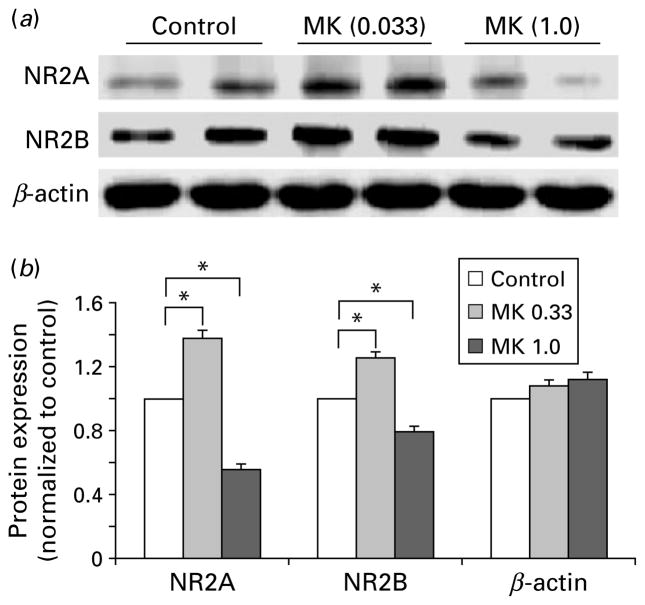
To confirm the changes of mRNA expression, the protein levels of NR2A and NR2B subunits in response to subchronic MK-801 treatment at two typical doses (low dose 0.033 mg/kg, high dose 1 mg/kg) were examined by using two different approaches: Western blotting and double immunofluorescent staining. As shown in Fig. 4, treatment with 0.033 mg/kg MK-801 significantly increased expression of NR2A and NR2B subunit proteins in the PFC by 1.38-fold (n=10, p< 0.05) and 1.25-fold (n=14, p<0.05), respectively. In contrast, protein expression of both NR2A and NR2B subunits was significantly decreased by high-dose MK-801 treatment (1.79-fold for NR2A, n=12, p<0.05; and 1.27-fold for NR2B, n=16, p<0.05), compared to the relatively unchanged β-actin levels. These data were consistent with the relative mRNA expression in the PFC observed above.
Fig. 4.
Differential protein expression of NR2A and NR2B subunits in response to low and high doses of MK-801 treatment in PFC tissue. (a) Representative samples of Western blots showing the relative protein levels of NR2A and NR2B subunits to β-actin in response to treatment with MK-801 at 0.033 and 1.0 mg/kg, respectively. (b) Summary histogram showing the relative changes of NR2A and NR2B subunit proteins that were normalized to β-actin and then to the control with Tukey’s ANOVA test. It was clear that 0.33 mg/kg MK-801 treatment significantly increased protein expression of NR2A and NR2B subunits, whereas treatment with 1.0 mg/kg significantly decreased the expression of NR2A and NR2B subunits. The β-actin levels were stable without clear changes in both low- and high-dose treatments (* p<0.05).
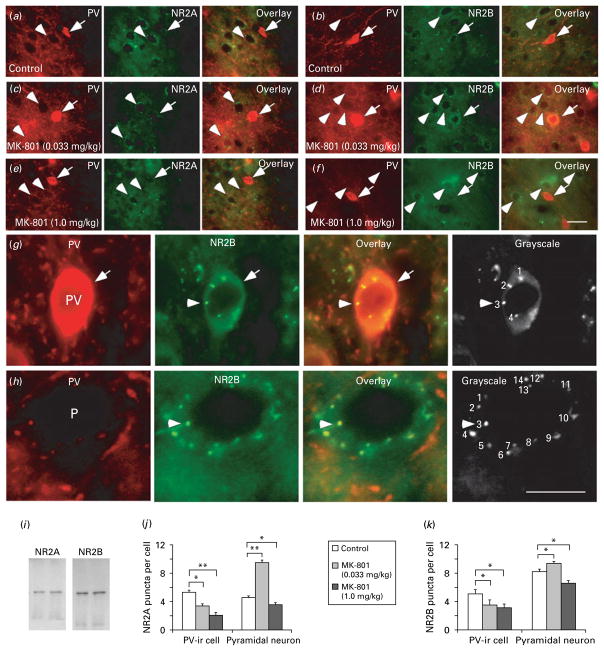
To selectively examine the protein expression of NR2A and NR2B subunits in the PV-ir interneurons in comparison with pyramidal neurons, we applied double immunofluorescent labelling of PV and NR2A or NR2B subunits. As exhibited in Fig. 5, the puncta localized in individual neurons were counted as described in the Materials and methods section. It is clear that low-dose MK-801 treatment (0.033 mg/kg) significantly decreased the puncta numbers of both NR2A and NR2B subunits in PV-ir interneurons by 1.58-fold (p<0.05) and 1.43-fold (p<0.05, Fig. 5), respectively. In contrast, the number of puncta of both NR2A and NR2B subunits around the somas of putative pyramidal neurons was significantly increased by 2.07-fold (p<0.01) and 1.15-fold (p<0.05), respectively. However, the protein expression of NR2A and NR2B subunits in both PV-ir cells and putative pyramidal neurons was consistently and significantly decreased by high-dose MK-801 treatment (1.0 mg/kg: PV-ir cells, 2.53-fold for NR2A, p<0.01 and 1.29-fold for NR2B, p<0.05; pyramidal neurons, 1.32-fold for NR2A, p<0.05 and 1.24-fold for NR2B, p<0.05). These results clearly support the dose-dependent effects of MK-801 in NMDAR subunit mRNA expression in both PV-ir interneurons and PFC tissue.
Fig. 5.
MK-801-induced distinct changes of NR2A and NR2B subunits in parvalbumin (PV)-containing interneurons vs. pyramidal neurons. (a–f) Representative photographs of double immunofluorescent labelling of PV (red) and NMDAR subunits (green) in controls (a, b) and MK-801 treatment at low dose (c, d) and high dose (e, f). The PV-immunoreactive (ir) interneurons (arrows) were double-labelled by PV and NR2A or NR2B, whereas the putative pyramidal neurons (arrowheads) were surrounded by both PV-ir axon terminals (red) and labelled NR2 positive puncta (green). Scale bar in (f)=20 μm for all images in panels (a)–(f). (g, h) Images at high magnification showing the methods used for quantification of NR2A and NR2B puncta in both PV-ir cells (g) and pyramidal neurons (h). Scale bar in (h)=20 μm for all images in panels (g) and (h). (i) Western blot analyses showing the specificities of anti-NR2A and anti-NR2B used for immunostaining. Both antibodies exhibited single molecular weight band. (j, k) Summary histograms showing changes of NR2A and NR2B subunit protein expression (puncta numbers) in the PV-ir interneurons and pyramidal neurons in MK-801-treated rats. Compared to control, 0.033 mg/kg MK-801 treatment significantly decreased the expression of both NR2A and NR2B subunits in PV-ir cells (p<0.05). In contrast, MK-801 significantly increased the puncta numbers of both NR2A and NR2B subunits in pyramidal neurons at low dose (0.033 mg/kg) but dramatically decreased the puncta numbers at high dose (1 mg/kg), suggesting distinct cell-type-specific effects in PFC circuitry (* p<0.05, ** p<0.01).
Discussion
In this study, for the first time we report an inverted-∪ dose-dependent alteration of mRNA levels of NMDAR subunits in the PFC and a biphasic dose response in mRNA expression of NMDAR subunits in PV-ir interneurons in response to MK-801 treatment in young adult rats. These effects were partially confirmed with the changes in protein expression of NR2A and NR2B subunits documented with both Western blotting and double immunostaining. These results suggest that PV-containing interneurons in the PFC are particularly susceptible to NMDAR antagonism and that low doses of NMDA antagonist induce distinctly different effects in PV-ir cells, whereas high doses may disturb interneurons and pyramidal neurons equally in the prefrontal cortical circuitry.
Distinct dose-dependent responses of NMDAR subunits in PV-ir interneurons
In the past decade, numerous attempts have been made to develop animal models of schizophrenia using NMDAR antagonists such as phencyclidine, ketamine, and MK-801. However, many reports on the expression of NMDAR subunits are contradictory. For example, NMDAR subunits were reportedly increased (McDonald et al. 1990; Rujescu et al. 2006; Wilson et al. 1998), decreased (Lindahl & Keifer, 2004; Linden et al. 2001) or unchanged by treatment with MK-801, phencyclidine, or ketamine (Harris et al. 2003). We believe that these discrepancies are probably derived from the differences among the studies in dosing, treatment duration and animal age (Abekawa et al. 2007; Behrens et al. 2007; Braun et al. 2007; Eyjolfsson et al. 2006; Mouri et al. 2007; Wilson et al. 1998). Indeed, our results reconciled many discrepancies reported in previous studies and exhibited a clear inverted-∪ dose-dependent effect in NMDAR subunits in response to MK-801 treatment. Overall, our data are consistent with previous studies that reported no clear alterations in NR1, NR2A and NR2B subunit expression in either the cerebral cortex or the hippocampus after administration of MK-801 at medium doses of 0.25–0.4 mg/kg (Matthews et al. 2000). In other studies, MK-801 treatment increased the subunit expression of NMDARs at relatively low concentrations (Gao & Tamminga, 1995; Rujescu et al. 2006; Wang et al. 1999) but decreased the NMDAR subunits at high doses (Harris et al. 2003; Lindahl & Keifer, 2004). Most importantly, we found that compared to the inverted-∪ dose response in the PFC, the expression of NMDAR subunits in response to MK-801 treatment in the PV-ir interneurons is distinctly different. The NMDAR subunits in PV-ir interneurons are extremely sensitive to MK-801 stimulation, showing significant decreases at very low concentrations. Conversely, at medium and high doses, comparable changes in NMDAR subunit expression were observed in PFC and PV-ir cells. These data suggest a characteristic biphasic pattern of NMDAR subunit expression and an extremely sensitive response of NMDAR subunits to MK-801 stimulation in the PV-ir interneurons, consistent with findings from a previous study conducted in cultured neurons (Kinney et al. 2006).
Although MK-801 has been widely used in animal models of schizophrenia, the dose–response effects have not been established. Our study indicates that NMDAR subunit levels are significantly increased in the PFC at low doses. These alterations peaked at 0.033 mg/kg, with a particular increase of NR2A subunits. In contrast, the expression of NMDAR subunits in PV-ir interneurons was significantly decreased at this concentration. These results suggest that NR2A subunits are extremely responsive to MK-801 administration. However, at higher doses the changes of NMDAR subunits in PFC and PV-ir interneurons appeared to be similar. In all NMDAR subunits, no significant changes were seen at 0.3 mg/kg, but the levels decreased to almost undetectable at 1.0 mg/kg. These interesting results imply that MK-801 evokes opposite NMDAR mRNA alterations in PFC and PV-ir interneurons at low doses but has similar actions in pyramidal neurons and PV-ir interneurons at high doses.
Functional implications
Studies from both animal models and post-mortem brain research in patients with schizophrenia have shown that disrupted NMDAR function may underlie the cognitive deficits in schizophrenia (Coyle, 2006; Dracheva et al. 2001; Kristiansen et al. 2006, 2007; Maxwell et al. 2006; Moghaddam, 2003; Mohn et al. 1999; Olney et al. 1999). A hypothesis of NMDAR hypofunction has been formulated based on characteristic effects of NMDAR antagonism and selective damage of interneurons. According to this hypothesis, NMDAR subunits localized on interneurons might be more sensitive than those on pyramidal cells (Cochran et al. 2002; Grunze et al. 1996; Kinney et al. 2006; Li et al. 2002; Zhang et al. 2008). Despite the extensive study, it is not clear how the NMDARs are distributed on the identified interneurons and how these receptor subtypes respond to NMDAR antagonists in PV-ir interneurons. We found that the NMDAR subunits distributed on PV-ir interneurons are distinctive; they not only contain higher levels of NR2A subunits (Kinney et al. 2006) but they also are more responsive to MK-801. This finding is consistent with our recent physiological data obtained in PFC slices (Wang & Gao, 2009). The increased expression of NMDAR subunits after administration of low doses of MK-801 seems contradictory to the concept of NMDAR hypofunction. In fact, profound increases in cortical excitatory activity after NMDAR blockade have been reported (Pratt et al. 2008). An explanation of this phenomenon has stemmed from recent studies showing that NMDA antagonist-induced excitation of glutamatergic pyramidal cells might be caused by the decreased activity of GABAergic interneurons through blockade of NMDARs present on these cells (Homayoun & Moghaddam, 2007; Lisman et al. 2008; Zhang et al. 2008). In this context, our result provides direct evidence in support of this assumption because NMDARs play a critical role in controlling the firing properties of fast-spiking interneurons.
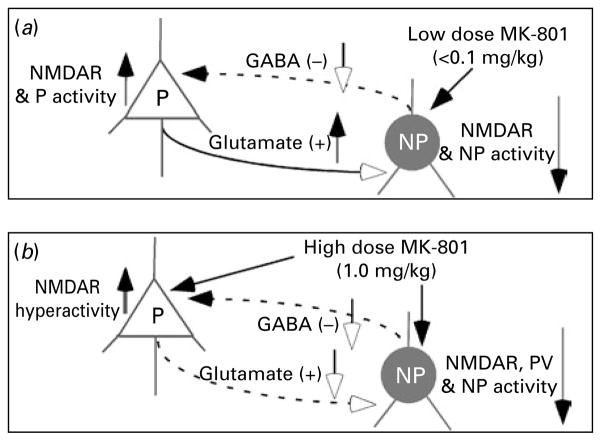
Based on our results and literature review, we propose a heuristic model, as illustrated in Fig. 6, to reconcile the neuropathological and neurocognitive features of the disorder in MK-801-treated rats. Because of higher responsiveness, a low dose of MK-801 directly blocks the NMDARs on GABAergic interneurons, which in turn induces disinhibition of glutamatergic efferents, increased excitatory activity and NMDAR hyperactivity on pyramidal neurons (Ninan et al. 2003), as proposed previously (Lisman et al. 2008; Olney & Farber, 1995). These processes would disrupt the functional integrity of the corticolimbic circuit and cause cognitive impairment and negative symptoms. However, a high dose of MK-801 affects both glutamatergic pyramidal neurons and GABAergic interneurons. Consequently, administraton of a high dose of MK-801 would result in severe dysfunction of both NMDARs and α-amino-3-hydroxy-5-methyl-4-isoxa-zolepropionic acid (AMPA) receptors, followed by neurotoxic cell death (Ikonomidou et al. 1999), particularly in PV-ir interneurons (Wang et al. 2008a). Therefore, NMDAR antagonists do not mimic a single episode of schizophrenia, rather depending on the dose of the drug, the duration of the dosing regimen and the timing of drug administration. Indeed, the different effects of NMDAR antagonist regimens on NMDA and AMPA receptor subunit expression, as well as GABA mechanisms, support this idea (Anastasio & Johnson, 2008; Barbon et al. 2007; Cochran et al. 2002; Newell et al. 2007; Rujescu et al. 2006; Wang et al. 2008a). Therefore, our data indicate that repeated injection of MK-801 at low doses (<0.1 mg/kg) appears to model the effects of NMDA hypofunction, whereas repeated administration of high doses (≥0.3 mg/kg) may mimic the results observed in patients with positive symptoms (Eyjolfsson et al. 2006; Lisman et al. 2008; Mouri et al. 2007). Taken together, this study has provided direct evidence for a distinct responsiveness of PV-ir interneurons to NMDA antagonism and that the low and high doses of treatment may mimic different symptoms of schizophrenia in animal models.
Fig. 6.
Dose-dependent effects in NMDAR and neuronal activity in pyramidal neurons (P) and interneurons (NP) in a MK-801 model. (a) Low dose (<0.1 mg/kg) selectively blocks NMDARs in the interneurons and thus results in increased NMDARs and excitatory activity in P. (b) High-dose MK-801 (1.0 mg/kg) blocks NMDARs in both P and NP, reduces all activities and even induces apoptosis or cell death of parvalbumin-immunoreactive (PV-ir) interneurons.
Acknowledgments
We thank Dr Yue-qiao Huang for instruction in primer design and Dr Lise Rioux for comments on the manuscript. We appreciate the support Dr John Houle and Mr Benjamin Keeler. This study was supported by a grant from Drexel University College of Medicine, a NARSAD Young Investigator award, and National Institutes of Health (NIH) R21 grant MH232307 to W.-J. Gao. The NIH had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Statement of Interest
None.
References
- Abekawa T, Ito K, Nakagawa S, Koyama T. Prenatal exposure to an NMDA receptor antagonist, MK-801 reduces density of parvalbumin-immunoreactive GABAergic neurons in the medial prefrontal cortex and enhances phencyclidine-induced hyperlocomotion but not behavioral sensitization to methamphetamine in postpubertal rats. Psychopharmacology (Berlin) 2007;192:303–316. doi: 10.1007/s00213-007-0729-8. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Johnson KM. Differential regulation of the NMDA receptor by acute and sub-chronic phencyclidine administration in the developing rat. Journal of Neurochemistry. 2008;104:1210–1218. doi: 10.1111/j.1471-4159.2007.05047.x. [DOI] [PubMed] [Google Scholar]
- Barbon A, Fumagalli F, La Via L, Caracciolo L, et al. Chronic phencyclidine administration reduces the expression and editing of specific glutamate receptors in rat prefrontal cortex. Experimental Neurology. 2007;208:54–62. doi: 10.1016/j.expneurol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbuminimmunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophrenia Research. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Braun I, Genius J, Grunze H, Bender A, et al. Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophrenia Research. 2007;97:254–263. doi: 10.1016/j.schres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. Journal of Biomolecular Techniques. 2004;15:155–166. [PMC free article] [PubMed] [Google Scholar]
- Cochran SM, Fujimura M, Morris BJ, Pratt JA. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002;46:206–214. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophrenia: moving beyond monoamine antagonists. Molecular Interventions. 2008;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cellular and Molecular Neurobiology. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson J, Sharp FR. Atypical antipsychotics and a Src kinase inhibitor (PP1) prevent cortical injury produced by the psychomimetic, noncompetitive NMDA receptor antagonist MK-801. Neuropsychopharmacology. 2006;31:1420–1430. doi: 10.1038/sj.npp.1300878. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Marras SAE, Elhakem SL, Kramer FR, et al. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. American Journal of Psychiatry. 2001;158:1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Eyjolfsson EM, Brenner E, Kondziella D, Sonnewald U. Repeated injection of MK801: an animal model of schizophrenia? Neurochemistry International. 2006;48:541–546. doi: 10.1016/j.neuint.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Farber NB. The NMDA receptor hypofunction model of psychosis. Annals of the New York Academy of Science. 2003;1003:119–130. doi: 10.1196/annals.1300.008. [DOI] [PubMed] [Google Scholar]
- Farber NB, Wozniak DF, Price MT, Labruyere J, et al. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biological Psychiatry. 1995;38:788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Frasca A, Racagni G, Riva MA. Dynamic regulation of glutamatergic postsynaptic activity in rat prefrontal cortex by repeated administration of antipsychotic drugs. Molecular Pharmacology. 2008;73:1484–1490. doi: 10.1124/mol.107.043786. [DOI] [PubMed] [Google Scholar]
- Gao XM, Tamminga CA. MK801 induces late regional increases in NMDA and kainate receptor binding in rat brain. Journal of Neural Transmission – General Section. 1995;101:105–113. doi: 10.1007/BF01271549. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Elarova I, Ruben M, Tan F, et al. Single-cell gene expression analysis: implications for neurodegenerative and neuropsychiatric disorders. Neurochemistry Research. 2004;29:1053–1064. doi: 10.1023/b:nere.0000023593.77052.f7. [DOI] [PubMed] [Google Scholar]
- Grunze H, Rainnie D, Hasselmo M, Barkai E, et al. NMDA-dependent modulation of CA1 local circuit inhibition. Journal of Neuroscience. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LW, Sharp T, Gartlon J, Jones DN, et al. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. European Journal of Neuroscience. 2003;18:1706–1710. doi: 10.1046/j.1460-9568.2003.02902.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. Journal of Neuroscience. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Ginsberg SD, Brunk B, Arnold SE, et al. Gene expression profile for schizophrenia: discrete neuron transcription patterns in the entorhinal cortex. Archives of General Psychiatry. 2002;59:631–640. doi: 10.1001/archpsyc.59.7.631. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. Journal of Neuroscience. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proceedings of the National Academy of Sciences USA. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Molecular Psychiatry. 2004;9:984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. American Journal of Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Buck BJ, Evans SJ, Akil H, et al. Combining laser capture microdissection with quantitative real-time PCR: Effects of tissue manipulation on RNA quality and gene expression. Journal of Neuroscience Methods. 2006;153:71–85. doi: 10.1016/j.jneumeth.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, et al. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. Journal of Neuroscience. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Molecular Psychiatry. 2006;11:737–747. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Current Opinion in Pharmacology. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Li Q, Clark S, Lewis DV, Wilson WA. NMDA receptor antagonists disinhibit rat posterior cingulate and retrosplenial cortices: a potential mechanism of neurotoxicity. Journal of Neuroscience. 2002;22:3070–3080. doi: 10.1523/JNEUROSCI.22-08-03070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl JS, Keifer J. Glutamate receptor subunits are altered in forebrain and cerebellum in rats chronically exposed to the NMDA receptor antagonist phencyclidine. Neuropsychopharmacology. 2004;29:2065–2073. doi: 10.1038/sj.npp.1300485. [DOI] [PubMed] [Google Scholar]
- Linden AM, Vasanen J, Storvik M, Lakso M, et al. Uncompetitive antagonists of the N-methyl-D-aspartate (NMDA) receptors alter the mRNA expression of proteins associated with the NMDA receptor complex. Pharmacology and Toxicology. 2001;88:98–105. doi: 10.1034/j.1600-0773.2001.d01-89.x. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neurosciences. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, Kralic JE, Devaud LL, Fritschy JM, et al. Chronic blockade of N-methyl-D-aspartate receptors alters gamma-aminobutyric acid type A receptor peptide expression and function in the rat. Journal of Neurochemistry. 2000;74:1522–1528. doi: 10.1046/j.1471-4159.2000.0741522.x. [DOI] [PubMed] [Google Scholar]
- Mauceri D, Gardoni F, Marcello E, Di Luca M. Dual role of CaMKII-dependent SAP97 phosphorylation in mediating trafficking and insertion of NMDA receptor subunit NR2A. Journal of Neurochemistry. 2007;100:1032–1046. doi: 10.1111/j.1471-4159.2006.04267.x. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, et al. Ketamine produces lasting disruptions in encoding of sensory stimuli. Journal of Pharmacology and Experimental Therapeutics. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Silverstein FS, Johnston MV. MK-801 pretreatment enhances N-methyl-d-aspartate-mediated brain injury and increases brain N-methyl-d-aspartate recognition site binding in rats. Neuroscience. 1990;38:103–113. doi: 10.1016/0306-4522(90)90377-g. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Current Opinion in Pharmacology. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochemistry International. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Huang XF. Short and long term changes in NMDA receptor binding in mouse brain following chronic phencyclidine treatment. Journal of Neural Transmission – General Section. 2007;114:995–1001. doi: 10.1007/s00702-007-0668-x. [DOI] [PubMed] [Google Scholar]
- Ninan I, Jardemark KE, Wang RY. Olanzapine and clozapine but not haloperidol reverse subchronic phencyclidine-induced functional hyperactivity of N-methyl-D-aspartate receptors in pyramidal cells of the rat medial prefrontal cortex. Neuropharmacology. 2003;44:462–472. doi: 10.1016/s0028-3908(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Archives of General Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. Journal of Psychiatric Research. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Winchester C, Egerton A, Cochran SM, et al. Modelling prefrontal cortex deficits in schizophrenia: implications for treatment. British Journal of Pharmacology. 2008;153 (Suppl 1):S465–S470. doi: 10.1038/bjp.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, et al. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biological Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Schefe JH, Lehmann KE, Buschmann IR, Unger T, et al. Quantitative real-time RT-PCR data analysis: current concepts and the novel ‘gene expression’s CT difference’ formula. Journal of Molecular Medicine. 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Molecular Biology. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert DG. Applications of laser capture microdissection in the study of neurodegenerative disease. Archives of Neurology. 2005;62:203–205. doi: 10.1001/archneur.62.2.203. [DOI] [PubMed] [Google Scholar]
- Wang C, Showalter VM, Hillman GR, Johnson KM. Chronic phencyclidine increases NMDA receptor NR1 subunit mRNA in rat forebrain. Journal of Neuroscience Research. 1999;55:762–769. doi: 10.1002/(SICI)1097-4547(19990315)55:6<762::AID-JNR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008a;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Wang H, Stradtman GGr, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proceedings of the National Academy of Sciences USA. 2008b;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Neuropsychopharmacology. 2009. Cell-type specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Published online: 25 February 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Guevremont D, Mason-Parker SE, Luxmanan C, et al. Differential trafficking of AMPA and NMDA receptors during long-term potentiation in awake adult animals. Journal of Neuroscience. 2007;27:14171–14178. doi: 10.1523/JNEUROSCI.2348-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Kinsman SL, Johnston MV. Expression of NMDA receptor subunit mRNA after MK-801 treatment in neonatal rats. Developmental Brain Research. 1998;109:211–220. doi: 10.1016/s0165-3806(98)00084-4. [DOI] [PubMed] [Google Scholar]
- Xi D, Keeler B, Zhang W, Houle JD, et al. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: application of laser micro dissection technique. Journal of Neuroscience Methods. 2009;176:172–181. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Behrens M, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. Journal of Neurophysiology. 2008;100:959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]