Abstract
OBJECTIVES
The goals of this study were to describe nutritional practices in the first month of life for a large cohort of extremely low gestational age newborns and to determine the impact of these nutritional practices on growth velocity over the same period.
METHODS
The sample included 1187 infants born at 23 weeks to 27 weeks of gestation, at 14 institutions, between 2002 and 2004. Inclusion criteria included survival until day 28 and weight information for both day 7 and day 28. Growth velocity, expressed as grams per kilogram per day (g/kg/day), was calculated for the interval between days 7 and 28. Nutritional practices during the first week and on days 14, 21, and 28 were compared to current nutritional guidelines in the literature. Multivariable logistic regression models estimated the contribution of limited nutrition to limited growth velocity.
RESULTS
Protein and fat delivery approximated current nutritional recommendations while carbohydrate and total caloric delivery did not. Despite this, growth velocity of our study infants exceeded the current guideline of 15 g/kg/day. Nevertheless, we found extrauterine growth restriction (i.e., weight for gestational age below the 10th centile) in 75% of infants at 28 days, as compared to only 18% at birth. A growth velocity of 20-30 g/kg/day was associated with infants' maintaining or exceeding their birth weight Z-score, with rates in the upper range for the gestationally youngest infants. Early (day 7) nutritional practices were positively associated with growth velocity measured between days 7 and 28.
CONCLUSION
The early provision of nutrients is an important determinant of postnatal growth. Extrauterine growth restriction remains high in extremely premature infants even when they achieve a growth velocity rate within current guidelines.
Keywords: Infant, premature; nutrition; growth velocity
INTRODUCTION
Most extremely low gestational age newborns (ELGANs, infants born before 28 weeks of gestation) remain in the neonatal intensive care unit (NICU) during the time that would be equivalent to their third trimester. In that period, one of the goals of the NICU team is to provide nutrition to the infant to achieve a growth velocity (GV) similar to intrauterine GV. However, most infants gain less weight than third trimester fetuses in utero. As a result of this growth deficit, a large proportion of infants exhibit extrauterine growth restriction (weight below the 10th centile for postmenstrual age) 1-5 and this subnormal growth often persists into early childhood 6-8.
Implementation of early parenteral and enteral nutrition to low birth weight infants during the first 24 hours of life results in a rapid regain of weight lost, improved overall weight gain, and earlier achievement of full enteral feedings 9. Early and high protein delivery also has been associated with improved weight gain and head growth 10. In addition, intersite differences in postnatal growth are partially explained by variations in neonatal nutrition practices 11, 12. Higher calorie and protein intake accounted for significant intersite differences in GV of premature infants at six NICUs even after adjusting for case mix and medical characteristics 12.
Yet, the advancement of nutritional intake is often limited by the preterm infant's ability to metabolize the nutrients offered 13-15 and the competing priority of the neonatologist to minimize potential complications with strategies that limit nutrition delivery (e.g. fluid restriction to decrease likelihood of patent ductus arteriosus or chronic lung disease) 16-18. In addition, the ELGAN Study population, like other low gestational age samples19, has a disproportionate number of infants who are growth restricted before birth perhaps limiting the potential for postnatal growth. Further, many ELGANs have increased energy requirements associated with severe illness.20 In view of these factors, some have suggested that postnatal growth failure in the preterm infant is inevitable 21, 22.
However, there are consequences of postnatal growth failure: persistent growth failure in childhood and potential adverse effects on later neurodevelopment23, 24. Thus, it remains important to understand the nutritional factors that influence growth in the early neonatal period to identify factors that might maximize growth. In this study, we examine the impact of nutritional practices on growth velocity during the first month of life in a large cohort of ELGANs.
METHODS
Sample
Our sample is comprised of infants enrolled in the ELGAN Study, a prospective cohort study designed to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in preterm infants. During the years 2002-2004, women delivering between 23 and 27 6/7 weeks of gestation at one of 14 participating institutions in 11 cities in 5 states were asked to enroll in the study. The enrollment and consent processes were approved by the individual institutional review boards. Mothers were approached for consent either upon antenatal admission or shortly after delivery, depending on clinical circumstance and institutional preference. 1249 mothers consented (83%) and approximately 260 women were either missed or did not consent to participate (17%). A total of 1506 infants enrolled in the ELGAN Study. For our analysis of GV through the first 28 days of life, we excluded infants who died before the 28th postnatal day (n=209) and those infants who did not have a weight for both days 7 and 28 (n=110), resulting in a sample of 1187 infants.
Data Collection
Infant Characteristics
Gestational age (GA) estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week of gestation (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks of gestation (29%), last menstrual period without fetal ultrasound (7%), and GA recorded in the log of the NICU (1%). The birth weight Z-score represents the number of standard deviations the infant's birth weight is above or below the median weight of infants at the same GA in a referent sample 25.
Nutritional Practices
Detailed nutritional data were collected daily for the first 7 days of life, then weekly on days 14, 21, and 28. Type and volume of intravenous solutions including composition of parenteral nutrition (PN) and type and volume of enteral feedings including caloric additives were recorded. All nutritional practice variables summarize nutritional intake from both parenteral and enteral routes.
Growth Velocity
Serial weights were measured for each day in the first postnatal week, then on days 14, 21 and 28. GV or the rate in growth expressed as g/kg/day is the primary outcome. Because of the expected weight loss during the first postnatal week 2 and the likelihood that the impact of early nutritional management would not be seen until at least day 7, we calculated GV for the interval between days 7 and 28 [GV in g/kg/day: 1000 × ((wt28-wt7)/wt7)/(28-7)].
Statistical Analyses
We evaluated the following hypotheses, expressed in their null form:
The daily amount of each nutritional offering (i.e., protein, carbohydrate, fat and total calories) does not differ from current recommended guidelines.
The amount of each nutrient offered on selected days does not vary with gestational age or birth weight Z-score.
GV does not vary with gestational age or birth weight Z-score.
GV does not vary with the amount of each nutritional offering on each of the selected days.
The amount of each nutritional offering on each of the selected days was categorized into quartiles. Thus, the lowest quartile reflects the bottom 25% of intake for each nutrient. Among infants who survived to 28 days and were weighed then, as well as on day 7, the upper bound of the lowest GV quartile was 13.8 g/kg/day, the median was 18.3 g/kg/day, and the lower bound of the highest quartile was 22.6 g/kg/day.
We created multivariable logistic regression models to estimate the contribution of limited nutrition on days 7, 14, and 21 to limited GV. In early sets of analyses, we adjusted for GA in two ways, by both week of gestation (23, 24, 25, 26, 27), and by groups of weeks (23-24, 25-26, 27). Each provided almost identical results. Here we present data adjusted for GA in groups of weeks. In addition, the models were adjusted for all other nutrient variables for that day, total fluid intake, birth weight Z-scores, and severity of illness as measured by the Score for Neonatal Acute Physiology-II (SNAP-II) 26. We present the contributions of nutritional practices to GV in the lowest quartile as odds ratios with 95% confidence intervals.
RESULTS
Nutritional practices
Our findings should be viewed in light of the amounts of nutrients currently recommended for premature infants 27-29. One recommendation is that protein delivery be initiated within 24 hours of birth at 1.5 – 3.0 g/kg/day with daily advancement in 0.5 – 1.0 g/kg/day increments to a goal of 3.5 to 4.0 g/kg/day. In our cohort of infants, the median protein intake provided by both parenteral and enteral routes was 1.0 g/kg/day on day 1 (Table 1) and 3.5 g/kg/day by day 4 (data not shown). Median protein intake remained 3.5 g/kg/day between days 3 and 28.
Table 1.
Median values of nutrient intakes (from both parenteral and enteral routes) in quartiles of GV and by postnatal day.
| Nutritional characteristic | Centiles | |||
|---|---|---|---|---|
| 25th | 50th | 75th | ||
| Protein (g/kg/day) | ||||
| Day 1 | 1.0 | 1.0 | 0 | |
| Day 3 | 2.7 | 3.0 | 3.0 | |
| Day 7 | 3.3 | 3.5 | 3.9 | |
| Day 14 | 3.5 | 3.4 | 3.6 | |
| Day 21 | 3.1 | 3.2 | 3.4 | |
| Day 28 | 3.1 | 3.2 | 3.5 | |
| Fat (g/kg/day) | ||||
| Day 1 | 0 | 0 | 0 | |
| Day 3 | 1.2 | 1.4 | 1.2 | |
| Day 7 | 2.7 | 3.1 | 3.0 | |
| Day 14 | 3.5 | 4.1 | 3.5 | |
| Day 21 | 4.3 | 4.8 | 4.0 | |
| Day 28 | 5.1 | 5.4 | 4.7 | |
| Carbohydrate (g/kg/day) | ||||
| Day 1 | 4.3 | 4.4 | 4.5 | |
| Day 3 | 7.1 | 7.8 | 7.6 | |
| Day 7 | 8.0 | 9.3 | 9.6 | |
| Day 14 | 9.2 | 10.0 | 10.5 | |
| Day 21 | 10.1 | 10.6 | 10.9 | |
| Day 28 | 10.8 | 10.9 | 11.0 | |
| Total calories (kcal/kg/day) | ||||
| Day 1 | 25 | 26 | 26 | |
| Day 3 | 55 | 59 | 57 | |
| Day 7 | 70 | 79 | 84 | |
| Day 14 | 84 | 92 | 92 | |
| Day 21 | 93 | 101 | 100 | |
| Day 28 | 103 | 105 | 104 | |
GV = [1000 × ((wt28 − wt7) / wt7) / 21]
Fat and lipid administration is recommended at 0.5 to 1.0 g/kg/day in the first or second day after birth, with incremental advancement to a goal of 3.0 to 3.5 g/kg/day. The majority of out infants did not receive any fat on day 1 (median intake 0 g/kg/day) but by day 3 the median intake provided by both parenteral and enteral routes was 1.4 g/kg/day. By the end of the first postnatal week the median fat intake was 3.1 g/kg/day. For all quartiles of fat intake, the total increased with advancing postnatal age.
The median carbohydrate intakes include both intravenous glucose infusions and complex carbohydrate sources in formulas and breast milk. However, the majority of infants received only minimal amounts of enteral nutrition during the first week of life, so these values mostly reflect intravenous glucose infusions. Glucose infusions are recommended at an initial rate 6 mg/kg/min (8.6 g/kg/day) increasing to 10 mg/k/min (14.4 g/kg/day) by day 7. In our cohort, the median carbohydrate initially offered was 4.4 g/kg/day and intake increased progressively to 9.3 g/kg/day on day 7 and to almost 11 g/kg/day on day 28.
The generally recommended range for caloric intake considering both parenteral and enteral routes is 115-130 kilocalories per kilogram per day (kcal/kg/day), with the understanding that some severely ill preterm infants (e.g. with sepsis, necrotizing enterocolitis) may require an even higher number of calories. This goal was rarely achieved in our sample. The median energy intake on day 3 was 59 kcal/kg/day, which advanced progressively to 105 kcal/kg/day on day 28. Throughout the first month, the 75th centile did not exceed 104 kcal/kg/day.
The association of nutrient delivery during the first 28 days with birth weight and gestational age is shown in Table 2. The range for protein was narrow and did not vary with GA. On the other hand, gestationally older newborns tended to receive more fat, carbohydrates, and total calories than infants born at earlier GAs. The median carbohydrate, fat, protein, calories, and fluids varied minimally with birth weight Z-score.
Table 2.
Median values of nutrient intakes (from both parenteral and enteral routes) in strata defined by GA and birth weight Z-score.
| Nutritional characteristic | Gestational age (weeks) | Birth weight Z-score | |||||
|---|---|---|---|---|---|---|---|
| 23-24 | 25-26 | 27 | <−2 | ≥2, <−1 | ≥1 | ||
| Protein (g/kg/day) | |||||||
| Day 3 | 3.0 | 3.0 | 3.1 | 3.0 | 2.7 | 3.0 | |
| Day 7 | 3.5 | 3.5 | 3.7 | 3.5 | 3.5 | 3.6 | |
| Day 14 | 3.5 | 3.5 | 3.4 | 3.5 | 3.5 | 3.5 | |
| Day 21 | 3.4 | 3.3 | 3.2 | 3.5 | 3.2 | 3.2 | |
| Day 28 | 3.4 | 3.2 | 3.2 | 3.5 | 3.2 | 3.2 | |
| Fat (g/kg/day) | |||||||
| Day 3 | 1.0 | 1.3 | 1.5 | 1.1 | 1.2 | 1.4 | |
| Day 7 | 2.6 | 3.0 | 3.2 | 2.8 | 2.7 | 3.1 | |
| Day 14 | 3.1 | 3.7 | 4.5 | 3.2 | 3.6 | 3.8 | |
| Day 21 | 3.2 | 4.6 | 5.3 | 4.0 | 4.3 | 4.6 | |
| Day 28 | 4.3 | 5.0 | 5.8 | 4.6 | 5.3 | 5.2 | |
| Carbohydrate (g/kg/day) | |||||||
| Day 3 | 6.9 | 7.8 | 7.8 | 6.8 | 6.9 | 7.8 | |
| Day 7 | 7.8 | 9.0 | 10.6 | 8.0 | 8.2 | 9.3 | |
| Day 14 | 8.6 | 9.9 | 10.6 | 10.0 | 9.4 | 10.0 | |
| Day 21 | 9.6 | 10.6 | 10.8 | 10.8 | 10.4 | 10.5 | |
| Day 28 | 10.6 | 10.8 | 11.1 | 11.2 | 10.8 | 10.9 | |
| Total calories (kcals/kg/day) | |||||||
| Day 3 | 54 | 58 | 61 | 53 | 53 | 58 | |
| Day 7 | 67 | 77 | 85 | 70 | 71 | 79 | |
| Day 14 | 78 | 89 | 99 | 86 | 86 | 91 | |
| Day 21 | 83 | 99 | 104 | 95 | 97 | 100 | |
| Day 28 | 99 | 103 | 108 | 96 | 104 | 104 | |
Growth Velocity
The recommended GV of 15 g/kg/day is intended to approximate intrauterine growth rates (though this remains controversial)30. For almost all GA groups and birth weight Z-score categories, GV was greater that 15 g/kg/day with the highest growth velocities (33 and 34 g/kg/day) among the gestationally youngest whose birth weight was more than one standard deviation below the expected median (Table 3).
Table 3.
Median GV within strata of GA and birth weight.
| Gestational age | ||||||
|---|---|---|---|---|---|---|
| 23 | 24 | 25 | 26 | 27 | N | |
| Birth weight | ||||||
| ≤ 750 | 19 | 18 | 18 | 20 | 19 | 443 |
| 751 - 1000 | 20 | 17 | 17 | 18 | 20 | 517 |
| 1001 - 1250 | -- | -- | 18 | 18 | 18 | 209 |
| > 1250 | -- | -- | -- | 17 | 15 | 18 |
| Birth weight Z-score | ||||||
| < −2 | 33 | 15 | 22 | 21 | 18 | 65 |
| < −1 | 34 | 22 | 19 | 19 | 18 | 154 |
| −1 to 1 | 18 | 18 | 17 | 18 | 19 | 832 |
| > 1 | 22 | 15 | 17 | 18 | 19 | 117 |
| > 2 | 15 | -- | 18 | 20 | 13 | 19 |
| N | 57 | 192 | 250 | 301 | 387 | 1187 |
GV = [1000 × ((wt28 − wt7) / wt7) / 21]
Median GV remained in the 15-20 g/kg/day range for infants who received between the 25th and 75th centile of every nutritional factor on all 5 of the days assessed (Table 4). The GV approximated 17g/kg/day for infants at the 25th centile of most nutrients, 18 for infants at the 50th centile and 19 for infants at the 75th centile.
Table 4.
Median GV associated with quartiles of each nutrient intake.
| Nutritional characteristic | Quartiles of each nutritional practice | |||
|---|---|---|---|---|
| 25th | 50th | 75th | ||
| Protein (g/kg/day) | ||||
| Day 3 | 17 | 18 | 20 | |
| Day 7 | 17 | 18 | 20 | |
| Day 14 | 18 | 18 | 19 | |
| Day 21 | 17 | 19 | 19 | |
| Day 28 | 18 | 18 | 20 | |
| Carbohydrate (g/kg/day) | ||||
| Day 3 | 17 | 18 | 19 | |
| Day 7 | 16 | 18 | 20 | |
| Day 14 | 17 | 18 | 20 | |
| Day 21 | 17 | 18 | 20 | |
| Day 28 | 18 | 18 | 19 | |
| Fat (g/kg/day) | ||||
| Day 3 | 19 | 18 | 18 | |
| Day 7 | 17 | 18 | 19 | |
| Day 14 | 18 | 18 | 18 | |
| Day 21 | 17 | 18 | 17 | |
| Day 28 | 17 | 18 | 18 | |
| Total calories (kcals/kg/day) | ||||
| Day 3 | 17 | 18 | 20 | |
| Day 7 | 16 | 18 | 20 | |
| Day 14 | 17 | 18 | 19 | |
| Day 21 | 17 | 18 | 19 | |
| Day 28 | 18 | 18 | 19 | |
GV = [1000 × ((wt28 − wt7) / wt7) / 21]
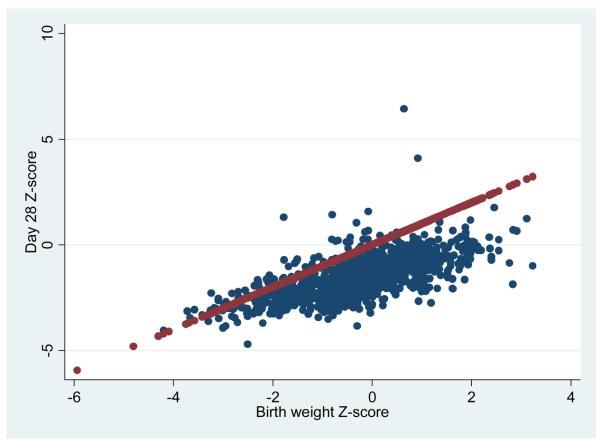
Despite a GV above the current recommended rate of 15 g/kg/day, the weight Z-score for the vast majority of ELGANs on day 28 was lower than at birth (Figure 1). The rising straight line in the figure lies where an infant's dot would be expected to fall if the day 28 weight Z-score was identical to the birth weight Z-score. Dots below the line indicate a lower weight Z-score on day 28 than at birth. At birth, 18% of our cohort had a weight Z-score below -1; in contrast, at 28 days of life, 75% of our cohort had a weight Z-score below -1 (data not shown).
Figure 1.
Scatterplot of weight Z-scores at birth (X-axis) and at 28 days (Y-axis). The vast majority of infants have a lower weight Z-score on day 28 compared to their weight Z-score at birth.
The median growth velocity of infants who maintained or exceeded their birth weight Z-score at 28 days ranged between 20 and 30 gm/kg/day with the highest GV rates seen among the gestationally youngest infants (Table 5).
Table 5.
Median GV for infants who maintained or exceeded their birth weight Z-score at 28 days of life within strata of GA and birth weight.
| Gestational age | ||||||
|---|---|---|---|---|---|---|
| Birth weight | 23 | 24 | 25 | 26 | 27 | N |
| ≤ 750 | 26 | 30 | 24 | 23 | 21 | 142 |
| 751 - 1000 | --- | 29 | 27 | 21 | 20 | 36 |
| 1001 - 1250 | --- | --- | --- | --- | 24 | 2 |
| > 1250 | --- | --- | --- | --- | --- | 0 |
| N | 18 | 24 | 32 | 67 | 39 | 180 |
GV = [1000 × ((wt28 − wt7) / wt7) / 21]
We created multivariable logistic regression models predicting the lowest quartile of GV in light of information about nutrition characteristics on days 7, 14, and 21 while controlling for GA, birth weight Z-score, SNAP-II, total fluid intake, and all other nutritional variables on that day (Table 6). Infants who received protein, fat, and carbohydrate amounts in the lowest quartile on day 7 were more likely than others to have a GV in the lowest quartile. In contrast, these associations were not evident for nutrients on day 14 and only total carbohydrate delivery was important at day 21.
Table 6.
Odds ratios (95% confidence intervals) for the lowest quartile of GV predicted by the lowest quartiles of nutrient intakes at each week adjusted for birth weight Z-score (< −2, ≥ −2 to < −1, ≥ −1), GA (23-24, 25-26, 27 weeks), SNAP-II score (< 30, ≥ 30), total fluid intake (mL/kg/day), and all the other nutrition variables for that day.
| Lowest quartile | OR (95% CI) for lowest quartile of GV | ||
|---|---|---|---|
| Day 7 | Day 14 | Day 21 | |
| Total protein (g/kg/day) | 1.4 (1.02, 2.0) | 1.0 (0.7, 1.4) | 1.3 (0.9, 1.7) |
| Total carbohydrate (g/kg/day) |
2.1 (1.4, 3.1) | 1.5 (0.97, 2.2) | 1.5 (1.02, 2.2) |
| Total fat (g/kg/day) | 1.7 (1.2, 2.3) | 1.1 (0.8, 1.6) | 0.8 (0.6, 1.2) |
| Total calories (kcals/kg/day) | 1.1 (0.7, 1.7) | 1.2 (0.7, 1.9) | 1.2 (0.8, 1.9) |
GV = [1000 × ((wt28 − wt7) / wt7) / 21]
DISCUSSION
Although the nutritional goal is to have ELGANs gain weight in the NICU at the rate they would have if they remained in utero, this is often not achieved 1-4. In our multi-center study of the nutritional practices at 14 NICUs we, too, found that a substantial proportion of infants did not maintain their position in the weight Z-score distribution despite GV rates that exceed the current recommendation.
Nutritional practices
The initiation of protein just fell below recommendations with the median intake on the first day of life at 1.0 versus 1.5 g/kg/day. However, the advancement goal was reached by day 4. Likewise, although fat was unlikely to be initiated as recommended, goal fat intakes were achieved by day 6. In contrast, the remaining nutritional recommendations were not achieved. Our study did not have the ability to determine the reasons for suboptimal carbohydrate and total calories delivery such as limited central line access or the presence of metabolic abnormalities (acidosis, hyperglycemia, hypertriglyceridemia).
Given the different summary measures of nutritional intake in different studies, it is difficult to compare the nutritional attainment of our cohort with previously published studies. However, given these limitations, our cohort does appear to demonstrate a more rapid advancement of protein delivery than has been described in the past in similar cohorts. In a study of six NICUs and for infants < 30 weeks of gestation, the mean protein intake was 0.6 g/kg/day on day 3 and only 2.1 g/kg/day on day 7.12 Similarly, in another study, infants ≤ 30 weeks of gestation received less than 2 g/kg/day of protein at day 7.22
Growth velocity
The median GVs in strata of our population almost always surpassed the recommended 15 g/kg/day. Interestingly, this was also true in all quartiles of nutrient delivery despite the fact that current recommendations for all nutritional parameters were not met. However, despite GVs above 15 g/kg/day and protein and fat delivery reaching goal amounts as recommended, a vast majority of the infants failed to maintain the weight Z-score they had at birth. We consider two explanations for this observation: (1) our definition of GV (chosen interval and calculation) does not reflect growth accurately; and, (2) current recommendations to approximate intrauterine growth rates are too low to achieve the desired effect in extremely low GA newborns.
In our cohort, GV in the first week ranged from −8 to −15 g/kg/day, while the GV from birth to 28 days ranged from 10-11 g/kg/day. We ultimately decided to calculate GV from day 7 to day 28, rather than from birth to day 28, because we felt that weight changes after the first week would more accurately reflect nutritional practices instituted during the first week. However, in the literature, GV has been defined in many different ways. Calculated GVs vary in having different starting points (birth weight, nadir, time regained birth weight; and different time intervals (birth to 28 days, birth to discharge, nadir to discharge, time regained birth weight to discharge, etc.) 31. That GV has not been defined consistently makes comparisons between studies difficult. In an effort to standardize and accurately measure growth velocity, Patel et al proposed that GV be calculated with an exponential model using weights from two time points. Using Patel's exponential equation the median growth velocity for our cohort was 15.5 g/kg/day, only slightly higher than recommended, versus 18.3 g/kg/day using our calculation.
The failure of our cohort to achieve the desired growth despite a GV of greater than 15 g/kg/day may reflect the current inadequacy of this recommendation. As previously discussed, the GV recommendation comes from intrauterine growth curves. Although intrauterine growth charts are useful in determining an infant's status at birth, they do not reflect expected growth patterns in the extrauterine environment after premature delivery. The healthy fetus in utero does not encounter an interrupted nutritional supply, a depletion of nutritional stores, a deprivation of growth factors provided by the mother and placenta, or increased energy consumption as seen in the preterm infant ex utero.32 Collectively, these factors result in weight loss during the first week of life (as much as 15% in our cohort) which is not appreciated in intrauterine growth curves and this deficit may be too much to overcome in the early postnatal period. As a result, it may not be possible to emulate the intrauterine growth curves and postnatal growth failure may be inevitable 21, 22. Growth velocity alone is not a sufficient indicator of nutritional attainment. Other measures need to be considered, including other anthropometric measures or measures of body composition. However, the desirable curves or ranges for these outcomes have yet to be established.
For our infants to stay above their birth weight centile, GVs of 20 – 30 gm/kg/day were necessary, with GVs in the higher end of this range for the gestationally youngest infants. Infants who demonstrated a growth velocity greater than 20 g/kg/day were more likely to be growth restricted and more likely to receive greater amounts of protein and carbohydrates by day 21. The physician's decision to offer more nutrition to the newborn with severe intrauterine growth restriction conveys information about the infant, the physician (and institutional practices), and the perceived level of illness of the infant. These issues are so closely intertwined that we cannot determine with confidence the relative contribution of each to growth velocity.
Although the nutritional practices we observed in our study are an improvement over what has been reported previously in the literature, we still fell short of meeting all recommendations. However, we still do not know to what extent extrauterine growth failure can be minimized with earlier provision and advancement of nutrition. Perhaps more important, the assumed benefits of catch-up growth on developmental outcomes should be weighed against the possibility that it might be detrimental to long-term health.33, 34
Our study supports the importance of providing early nutrition to promote postnatal growth. Even though recommended nutritional intakes were not achieved, early delivery of nutrients remained important to overall GV during the first month of life. We found that GV in the lowest quartile is predicted by the lowest quartile of protein, carbohydrate, and fat intake on day 7 of life. No nutritional characteristics on day 14 had a significant impact on GV; and only total carbohydrate at day 21 was associated with GV. The influence of early nutrition on GV remained important even after we adjusted for birth weight Z-score, SNAP-II, GA, and total fluid intake. This observation supports the findings of other authors who have demonstrated that early nutritional practices influence postnatal growth and reinforces the call for early provision and advancement of nutrients during the NICU stay in order to promote optimal postnatal growth. The optimal composition of nutrients, however, remains undetermined 35, 36 and barriers (practice or clinical) to achieving recommended intakes need to be studied further.
CONCLUSION
In this large, multicenter, prospective study of extremely preterm infants we demonstrate improved protein and fat delivery compared to previous reports and a GV, as defined, that exceeds current recommendations. Despite these improvements, the majority of our infants failed to maintain the weight Z-score they had at birth. It is likely that intrauterine growth rates might not be achievable ex utero for most ELGANs and new standards need to be developed with the goal of establishing growth patterns that promote optimal long-term growth and neurodevelopmental outcomes. The early provision of nutrients remains an important determinant of postnatal growth and will be a critical component to these standards.
Acknowledgments
This study was supported by a cooperative agreement with the National Institute of Neurological Diseases and Stroke (5U01NS040069-05) and a program project grant from the National Institute of Child Health and Human Development (NIH-P30-HD-18655). The authors gratefully acknowledge the contributions of our subjects and their families, as well as those of our colleagues.
Abbreviations
- ELGAN
extremely low gestational age newborn
- g/kg/day
grams per kilogram per day
- GA
gestational age
- GV
growth velocity
- kcal/kg/day
kilocalories per kilogram per day
- NICU
neonatal intensive care unit
- PN
parenteral nutrition
- SNAP
Score for Neonatal Acute Physiology
Footnotes
None of the authors has any financial issue or conflict of interest to disclose
References
- 1.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111:986–90. doi: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–9. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 3.Hay WW, Jr., Lucas A, Heird WC, et al. Workshop summary: nutrition of the extremely low birth weight infant. Pediatrics. 1999;104:1360–8. doi: 10.1542/peds.104.6.1360. [DOI] [PubMed] [Google Scholar]
- 4.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 5.Ernst KD, Radmacher PG, Rafail ST, Adamkin DH. Postnatal malnutrition of extremely low birth-weight infants with catch-up growth postdischarge. J Perinatol. 2003;23:477–82. doi: 10.1038/sj.jp.7210974. [DOI] [PubMed] [Google Scholar]
- 6.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003;27:302–10. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 7.Hack M, Weissman B, Borawski-Clark E. Catch-up growth during childhood among very low-birth-weight children. Arch Pediatr Adolesc Med. 1996;150:1122–9. doi: 10.1001/archpedi.1996.02170360012002. [DOI] [PubMed] [Google Scholar]
- 8.Steward DK, Pridham KF. Growth patterns of extremely low-birth-weight hospitalized preterm infants. J Obstet Gynecol Neonatal Nurs. 2002;31:57–65. doi: 10.1111/j.1552-6909.2002.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 9.Donovan R, Puppala B, Angst D, Coyle BW. Outcomes of early nutrition support in extremely low-birth-weight infants. Nutr Clin Pract. 2006;21:395–400. doi: 10.1177/0115426506021004395. [DOI] [PubMed] [Google Scholar]
- 10.Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148:300–5. doi: 10.1016/j.jpeds.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell MT, Eichenwald EC, McAlmon K, et al. Interneonatal intensive care unit variation in growth rates and feeding practices in healthy moderately premature infants. J Perinatol. 2005;25:478–85. doi: 10.1038/sj.jp.7211302. [DOI] [PubMed] [Google Scholar]
- 12.Olsen IE, Richardson DK, Schmid CH, Ausman LM, Dwyer JT. Intersite differences in weight growth velocity of extremely premature infants. Pediatrics. 2002;110:1125–32. doi: 10.1542/peds.110.6.1125. [DOI] [PubMed] [Google Scholar]
- 13.Brans YW, Andrew DS, Carrillo DW, Dutton EB, Menchaca EM, Puelo-Scheppke BA. Tolerance of fat emulsions in very low birthweight neonates: effect of birthweight on plasma lipid concentrations. Am J Perinatol. 1990;7:114–7. doi: 10.1055/s-2007-999459. [DOI] [PubMed] [Google Scholar]
- 14.Kairamkonda VR, Khashu M. Controversies in the management of hyperglycemia in the ELBW infant. Indian Pediatr. 2008;45:29–38. [PubMed] [Google Scholar]
- 15.Periera GR, Fox WW, Stanley CA, Baker L, Schwartz JG. Decreased oxygenation and hyperlipemia during intravenous fat infusions in premature infants. Pediatrics. 1980;66:26–30. [PubMed] [Google Scholar]
- 16.Oh W, Poindexter BB, Perritt R, et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr. 2005;147:786–90. doi: 10.1016/j.jpeds.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 17.Stephens BE, Gargus RA, Walden RV, et al. Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2008;28:123–8. doi: 10.1038/sj.jp.7211895. [DOI] [PubMed] [Google Scholar]
- 18.Van Marter LJ, Leviton A, Allred EN, Pagano M, Kuban KC. Hydration during the first days of life and the risk of bronchopulmonary dysplasia in low birth weight infants. J Pediatr. 1990;116:942–9. doi: 10.1016/s0022-3476(05)80658-4. [DOI] [PubMed] [Google Scholar]
- 19.Cooke RW. Conventional birth weight standards obscure fetal growth restriction in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F189–92. doi: 10.1136/adc.2005.089698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay WW., Jr Assessing the effect of disease on nutrition of the preterm infant. Clin Biochem. 1996;29:399–417. doi: 10.1016/0009-9120(96)00062-8. [DOI] [PubMed] [Google Scholar]
- 21.Cooke R. Postnatal growth in preterm infants: have we got it right? J Perinatol. 2005;25(Suppl 2):S12–4. doi: 10.1038/sj.jp.7211310. [DOI] [PubMed] [Google Scholar]
- 22.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001;107:270–3. doi: 10.1542/peds.107.2.270. [DOI] [PubMed] [Google Scholar]
- 23.Lucas A, Morley R, Cole TJ. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ. 1998;317:1481–7. doi: 10.1136/bmj.317.7171.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 25.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 26.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 27.Pediatric Nutrition Handbook. Fifth ed American Academy of Pediatrics; 2004. [Google Scholar]
- 28.Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol. 2007;31:48–55. doi: 10.1053/j.semperi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Tsang RC, Uauy R, Koletzko B. In: Nutrition of the Preterm Infant. ZS H, editor. Digital Educating Publishing, Inc.; Cincinnati: 2005. [Google Scholar]
- 30.Anderson DM. Nutritional assessment and therapeutic interventions for the preterm infant. Clin Perinatol. 2002;29:313–26. doi: 10.1016/s0095-5108(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 31.Patel AL, Engstrom JL, Meier PP, Kimura RE. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics. 2005;116:1466–73. doi: 10.1542/peds.2004-1699. [DOI] [PubMed] [Google Scholar]
- 32.Sauer PJ. Can extrauterine growth approximate intrauterine growth? Should it? Am J Clin Nutr. 2007;85:608S–13S. doi: 10.1093/ajcn/85.2.608S. [DOI] [PubMed] [Google Scholar]
- 33.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 34.Thureen PJ. The neonatologist's dilemma: catch-up growth or beneficial undernutrition in very low birth weight infants-what are optimal growth rates? J Pediatr Gastroenterol Nutr. 2007;45(Suppl 3):S152–4. doi: 10.1097/01.mpg.0000302962.08794.62. [DOI] [PubMed] [Google Scholar]
- 35.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008;359:1873–84. doi: 10.1056/NEJMoa0803725. [DOI] [PubMed] [Google Scholar]
- 36.Clark RH, Chace DH, Spitzer AR. Effects of two different doses of amino acid supplementation on growth and blood amino acid levels in premature neonates admitted to the neonatal intensive care unit: a randomized, controlled trial. Pediatrics. 2007;120:1286–96. doi: 10.1542/peds.2007-0545. [DOI] [PubMed] [Google Scholar]