Abstract
Faces are salient stimuli for primates that rely predominantly on visual cues for recognizing conspecifics and maintaining social relationships. While previous studies have shown similar face discrimination processes in chimpanzees and humans, data from monkeys are unclear. Therefore, three studies examined face processing in rhesus monkeys using the face inversion effect, a fractured face task, and an individual recognition task. Unlike chimpanzees and humans, the monkeys showed a general face inversion effect reflected by significantly better performance on upright compared to inverted faces (conspecifics, human and chimpanzees faces) regardless of the subjects’ expertise with those categories. Fracturing faces alters first- and second-order configural manipulations whereas previous studies in chimpanzees showed selective deficits for second-order configural manipulations. Finally, when required to individuate conspecific’s faces, i.e., matching two different photographs of the same conspecific, monkeys showed poor discrimination and repeated training. These results support evolutionary differences between rhesus monkeys and Hominoids in the importance of configural cues and their ability to individuate conspecifics’ faces, suggesting a lack of face expertise in rhesus monkeys.
Keywords: face processing, configural cues, inversion effect, rhesus monkey
Research on face recognition in humans has grown into an expansive literature with topics covering visual and perceptual processes to cultural differences. One of the most interesting and well-studied areas of face recognition research concerns the development of face expertise, or the ability to individuate and remember the faces of many different individuals, and its dependence on configural processing (Mondloch, Maurer, & Ahola, 2006). Adult-level face expertise takes many years to develop in humans, some report as long as 10 years (Diamond & Carey, 1986). Although its exact course of development is not well understood, face expertise appears to involve many different stages throughout early infancy and later childhood. Recent data suggests that this expertise becomes tuned early in development, in much the same way as language processes (Kuhl, Williams, Lacerda, & Stevens, 1992). Pascalis and colleagues (Pascalis, de Haan, & Nelson, 2002), for example, showed no viewing preferences for same versus other species’ faces in infants under 6 months, but a perceptual narrowing occurred around 9 months of age when infants developed a species-specific viewing preference. In a later study, newborn babies showed no viewing biases for same versus other race faces, but by 3 months of age, infants preferred the faces of their own species (Kelly et al., 2005).
The importance of configural cues in face processing has been known since the early studies of Yin (1969), in which he demonstrated that inverting faces impaired both the recognition and memory for faces in typical adults. This phenomenon, referred to as the face inversion effect, is now widely used as a cognitive marker for configural face processing in humans, and as a clinical marker for face processing deficits (Carey & Diamond, 1977; Farah, Tanaka, & Drain, 1995; Valentine, 1988). More recently, researchers have further classified configural cues according to their first- and second-order relational features (Diamond & Carey, 1986; Maurer, Le Grand, & Mondloch, 2002; Tanaka & Farah, 1991). First-order relational features refer to the presence and location of specific features on the face, for example, eyes are laterally displaced above the nose, which is centrally located and always above the mouth, and so forth. These first-order configural features are the same in every face and provide information about basic category grouping. Second-order relational features, however, refer to the relative spatial arrangement of these features with regard to one another and their position on the face, resulting in a set of features with a unique spatial configuration, making each face unique (Diamond & Carey, 1986). These second-order cues provide information regarding individual identity and are essential for subordinate-level classifications, or discriminations of faces at the individual level, that is, John versus Ted. It is the ability to utilize these cues that ultimately leads to the development of face expertise (Mondloch, Geldart, Maurer, & Le Grand, 2003; Mondloch et al., 2006). The inversion effect is largely believed to affect second-order configural processing (Collishaw & Hole, 2002; Diamond & Carey, 1986; Freire, Lee, & Symons, 2000; Le Grand, Mondloch, Maurer, & Brent, 2001; Mondloch et al., 2002; Searcy & Bartlett, 1996), although a few recent studies claim these impairments can also extend to isolated facial features (Reisenhuber, Jarudi, Gilad, & Sinha, 2004). The majority of the literature, however, reports configural impairments when faces are inverted, regardless of whether this involves first-order features, second-order features or holistic processing (Maurer et al., 2002). Therefore, the inversion effect appears to affect configural processing globally and may not be capable of addressing the specific mode of configural processing that is impaired.
Although studies of configural face processing in humans are numerous, very few studies have examined this phenomenon from an evolutionary perspective, inquiring whether nonhuman primates or other animal species share these perceptual biases, or whether face expertise might be unique to humans. Several studies, for example, have revealed visual face recognition abilities in birds (Brown & Dooling, 1993; Jitsumori & Makino, 2004) and sheep (for an excellent review, see Tate, Fischer, Leigh, & Kendrick, 2006). Sheep have been found to have excellent long-term memory for familiar conspecifics’ faces (Kendrick, da Costa, Leigh, Hinton, & Peirce, 2001), involving similar neurobiological substrates as humans (Tate et al., 2006), and demonstrate weaker inversion effects for human, a less familiar species, compared to conspecific’s faces, suggesting some role of expertise (Peirce, Leigh, da Costa, & Kendrick, 2001; Peirce, Leigh, & Kendrick, 2000). Studies have even reported basic individual recognition abilities in wasps that appear to depend on variation in pattern markings on the face (Tibbetts, 2002).
Among nonhuman primates, results from face recognition studies are often inconsistent. Some studies, for example, have reported evidence of the face inversion effect in monkeys and apes (cotton-top tamarins: Neiworth, Hassett, & Sylvester, 2007; pigtail macaques: Overman & Doty, 1982; chimpanzees: Parr, Dove, & Hopkins, 1998; Parr & Heintz, 2006; Tomonaga, 2007; Tomonaga, Itakura, & Matsuzawa, 1993; Japanese macaques: Tomonaga, 1994; rhesus monkeys: Vermeire & Hamilton, 1998), although others have failed to find evidence of orientation-specific processing that is exclusive for faces versus other categories of images (longtail macaques: Bruce, 1982; Dittrich, 1990; rhesus monkeys: Gothard, Erickson, & Amaral, 2004; Parr, Winslow, & Hopkins, 1999; Rosenfeld & van Hoesen, 1979; cotton-top tamarins: Weiss, Kralik, & Hauser, 2001). Only a few of these studies have specifically compared face processing for different species’ faces in Old World monkeys and these have failed to support any advantage for expert categories of faces (Phelps & Roberts, 1994; Tomonaga, 1994). However, a recent study of cotton-top tamarins, a New World species, supports expertise effects for face processing. This study used a visual paired comparison task where subjects are first habituated to an image and then are shown this familiarized image and a novel image from the same category. Looking longer at the novel images is used as a marker of discrimination. Tamarins showed novelty effects for viewing conspecific’s faces and human faces, categories for which they had considerable experience, but not for unfamiliar chimpanzee faces. These effects were reduced when the images were inverted, a manipulation affecting configural processing (Neiworth, Hassett, & Sylvester, 2007). This is particularly important because although the inversion effect is often used to demonstrate configural processing for faces, this specificity is lost unless other stimulus categories are used. Moreover, any expertise for conspecifics’ faces compared to other species’ faces is also lost unless several expert and nonexpert categories of faces are also presented.
Parr and colleagues tested both chimpanzees and rhesus monkeys on the face inversion effect using an identical computerized joystick-testing paradigm (chimpanzees: Parr et al., 1998; rhesus monkeys: Parr et al., 1999). Both species were shown photographs of familiar and unfamiliar face categories and nonface objects. The chimpanzees showed clear inversion effects, significantly worse performance on inverted compared to upright orientations, for the expert face categories but not the unfamiliar faces or nonface categories (Parr et al., 1998). The performance of rhesus monkeys, however, failed to support a face-specific inversion effect, or any differences based on stimulus expertise. Rhesus monkeys showed the inversion effect for conspecific’s faces, unfamiliar capuchin monkey faces, and automobiles (Parr et al., 1999).
To determine whether a similar visual processing strategy was involved in the discrimination of upright and inverted faces in these studies, Parr and colleagues adopted the face rotation paradigm of Collishaw and Hole (2002). In this latter study, faces were rotated gradually from 0° to 180° to determine the exact function of the resulting performance deficits. A significant linear deficit with increasing angular disparity would provide evidence for a single processing strategy based on configural cues, whereas a nonlinear decrease in performance would support a shift from one strategy to another. The results in humans revealed a significant linear impairment in performance, supporting a unified configural strategy (Collishaw & Hole, 2002). Parr and Heintz (2006) found a similar linear impairment in chimpanzees for discriminations of rotated faces but not houses, suggesting that houses and faces were processed using different configural strategies. Moreover, a recent study of the rotation effect in rhesus monkeys revealed significant linear decreases with increasing angular disparity for conspecifics’ faces, chimpanzee faces, and houses (Parr & Heintz, in press). Thus, chimpanzees appear to use different cues for processing faces and nonfaces, whereas rhesus monkeys’ performance on previous inversion studies and the face rotation effect suggests a nonface specific strategy.
Recently, Parr and colleagues expanded their studies of configural face processing in chimpanzees by examining the use of first- and second-order relational features. Subjects were presented with faces having undergone several manipulations including faces in which the inner features had been extracted, preserving both first and second-order relational features, faces in which the features had been fractured apart, preserving first-order but impairing second-order relational features, and fractured faces in which the individual features were also rearranged, disrupting both first- and second-order relational features (Parr, Heintz, & Akamagwuna, 2006). Subjects’ performance was significantly impaired when the faces were fractured alone and when they were fractured/rearranged, compared to when inner features alone were presented supporting the importance of second-order relational features in chimpanzee face processing. This raises the very interesting possibility that chimpanzees, like humans, may also have a type of face expertise, which depends on the ability to extract information about the relative spacing and arrangement of facial features. The logical evolutionary question is whether monkeys also share these configural biases or whether expert face processing is something unique to Hominoids and, if so, why.
In this paper, we present the results of three experiments on face processing in rhesus monkeys, the face inversion effect, the fractured face task, and a task of individual recognition. These tasks examined the relationship between configural processing and stimulus expertise, the importance of second-order configural cues in conspecific face processing, and the ability of monkeys to individuate faces presented in different head orientations and view points. Expertise is not quantified in terms of time of familiarity, but is simply whether the subjects had any prior experience with members of the species whose faces were presented. Each task is similar to published experiments run on chimpanzees and, therefore, the results can be compared across species. The first experiment, the face inversion effect, examined monkey’s performance discriminating the faces of familiar and unfamiliar species and nonface categories when presented upright or inverted. Second, the fractured face task manipulated the first-order and second-order configural features in conspecifics faces to determine whether monkeys were selectively impaired for manipulations of second-order relational features. The final task, individual recognition, examined the ability of monkeys to match two different photographs of the same individual. Based on our previous studies (Parr et al., 1999; Parr, Winslow, Hopkins, & da Waal, 2000), we hypothesized that rhesus monkeys would fail to show expertise-dependent inversion effects, show no selective deficits for manipulations of second-order configural cues, and have difficulty when required to individuate faces in different view points.
General Method
Subjects
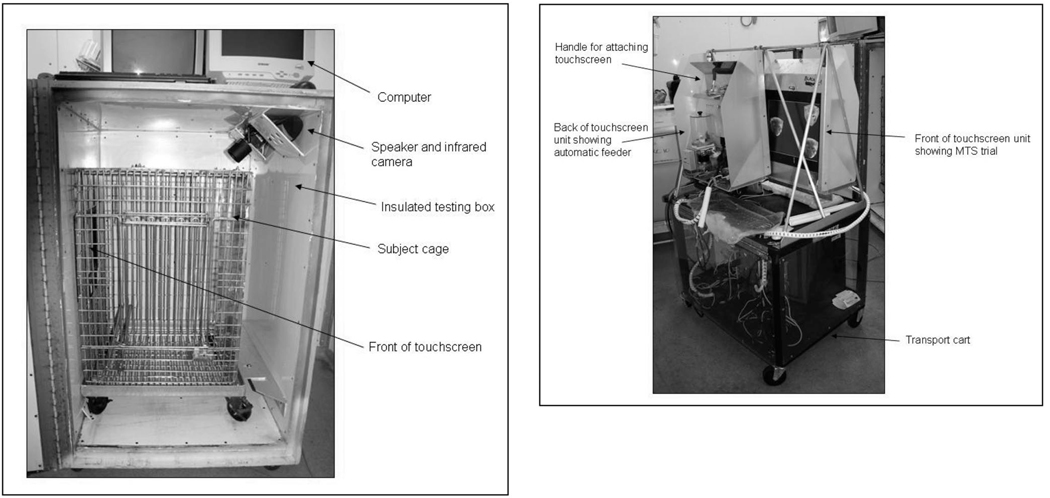
Data were collected from 8 rhesus monkeys (Macaca mulatta), 2 males (Sm and Rk) and 6 females (On, Oi, Cw, Lm, Cl, and Vl) born into large social groups at the Yerkes Primate Center field station, Lawrenceville, GA. Two females were born in the spring of 2001 (On and Oi), whereas the other monkeys were a year older. In Fall 2004, when the subjects were between 3 and 4 years of age, these experimentally naïve monkeys were brought to the main Yerkes campus in Atlanta, GA, for participation in these experiments. There, they were pair-housed and relocated to a large colony room containing approximately 10 to 15 other rhesus monkeys that were used in other projects. Prior to the onset of these experiments, each subject was habituated to leave its home cage in a small transport box and relocated to a dedicated testing lab in an adjacent room. The testing room was equipped with two custom-made, sound-attenuated touchscreen chambers, 85 × 90 × 125 cm, each equipped with a 17 “flat panel touchscreen (Elo secure touch, surface wave monitors, www.elotouch.com), ventilation fan, speakers, and black and white infrared camera (see Figure 1a). Testing on the tasks outlined below took place either in this room, or directly in the home cage using a custom-designed home cage testing system. This was especially useful for conducting testing on multiple subjects at the same time, and for those monkeys who experienced slow testing performance and were given access to the computer tasks for extended periods directly in their home cage. These home-cage devices consisted of a 15” touchscreen built into a metal frame that could be attached directly to the inside of the door opening of subjects’ home cages (see Figure 1b). All computer and reinforcement equipment was held on a transport cart that could be wheeled in front of the home cage and remain out of arms reach of animals living in the home room. These home-cage units were not equipped with external speakers, so primary auditory reinforcement came from the sound of the feeders activating on correct trials. All other aspects of the testing procedures and equipment were identical (see below).
Figure 1.
(a) Dedicated testing box containing cage, touchscreen, speakers and infrared camera. (b). Home cage testing apparatus showing front and back of two touchscreens, automatic feeder, transport cart and attachment handles.
MTS
All subjects were trained in 2005 to perform the computerized matching-to-sample (MTS) task at a high level of proficiency (>80% correct) using simple clip-art and geometric shapes before moving on to assess their skill in matching conspecifics’ faces. Not all subjects completed all tasks, so the subject numbers are listed for each experiment. According to the MTS format, subjects were first required to orient to a single image on the touchscreen, hereafter referred to as the sample, by touching it three times in rapid succession, ensuring that the subject looked at the sample stimulus. The sample stimuli appeared randomly against any of the four walls of the touchscreen, in a central location (top, left, bottom, or right side). After making the orienting response to the sample, two comparison images were presented in each corner of the touchscreen on the wall opposite to the sample, forming a triangular configuration. If, for example, the sample image appeared centrally along the left wall of the touchscreen, the two comparison images would appear on the right side in the upper and lower corners. The delay between the presentation of the sample and the two comparisons was approximately 500 ms. Subjects were then required to select the comparison image that matched the sample. For the experiments presented here, the sample image remained on the screen during the selection period (simultaneous MTS). If the response was correct, the subject would receive a food reward from an automatic feeder followed by an intertrial interval (ITI) of 2 s, whereas an incorrect response was followed by an ITI of 8 s and no food reinforcement. All procedures were approved by the Institutional Animal Care and Use Committee of Emory University.
Initial MTS training with conspecifics’ faces
Prior to the first experiment reported here, monkeys were tested on their face recognition skills using two sets of unfamiliar conspecifics’ faces. Therefore, all monkeys received some exposure to conspecific faces before the onset of these studies. This was to ensure that the monkeys could discriminate between these high quality digitized images. In both tasks, Face Matching 1 and Face Matching 2, subjects were presented with 15 novel MTS trials involving unfamiliar conspecifics’ faces and 10 novel clip-art images. They received two repetitions of each trial per session (N = 50 total trials) and were tested until their performance exceeded 80% on a single session. At this time, they were moved to the second face matching task (Face Matching 2). All 8 monkeys (Sm, Rk, On, Oi, Cw, Lm, Cl, and Vl) were put through this initial evaluation phase before continuing with the experiments outlined below.
Results of initial MTS training with conspecifics’ faces
Mean number of sessions before subjects performed above chance on the first face matching task was 8.00 (SEM = 2.10; 95% CI 3.04 – 12.96) and 30.37 (SEM = 5.99; 95% CI 16.22–44.53) sessions to reach the final criterion of > 80%.1 On the second face matching task, subjects required 5.50 (SEM = 1.84; 95% CI 1.14 –9.86) sessions to exceed chance and 10.00 (SEM = 2.04; 95% CI 5.19 –14.81) sessions to exceed the final criterion. Paired t tests indicated that subjects were significantly faster to reach the final criterion in Face Matching 2 compared to Face Matching 1, t(7) = 3.18, p < .02, Cohen’s d = 1.74, indicating a huge effect, but no significant differences were found in the number of sessions required to exceed chance levels between the two tasks. Thus, subjects overall performance improved with repeated exposure to conspecifics’ faces. After this last task, subjects proceeded to Experiment 1 described below.
Method: Experiment 1
The Face Inversion Effect
Stimuli
Stimuli consisted of 15 examples of digitized photographs depicting neutral faces of unfamiliar rhesus monkeys, humans, and chimpanzees. These were different from any of the stimuli used in the initial conspecific training tasks described above. Also included were photographs of houses and complex clip-art images. Photographs of rhesus monkeys and chimpanzees were acquired from colonies living at the Yerkes Primate Center field station, which contains numerous large colonies of monkeys, while the human stimuli were taken from the Productive Aging Laboratory, University of Illinois at Urbana–Champaign face database (Minear & Park, 2004). House stimuli were taken from realty listings on the Internet, and clip-art images were downloaded from specific libraries. All stimuli were converted to 256 grayscale, placed on a black background, and cropped to a height of 300 pixels using Adobe Photoshop 7.0.®
Procedure
Subjects were tested twice daily, once mid morning and once early afternoon. We used the same testing format as in previous studies with monkeys and chimpanzees. This required subjects to first discriminate the images in each category in their upright orientation before moving on to discriminations of the same stimuli in their inverted orientation (Parr et al., 1998, 1999). Thus, the task itself tested whether subjects could generalize their learned discrimination performance on upright trials to the same trials inverted. During these sessions, 15 different test images from each category were presented along with 5 of the previously learned clip-art images as control trials (N = 20 trials total). Because subjects had already reached a performance criterion for the clip-art, this served as a daily control for whether subjects’ motivation and whether they were consistently performing according to the MTS rule, that is, match the sample. The training on upright images continued until subjects’ performance on the test images exceeded 80%, averaged over two consecutive testing sessions (performance on the clip-art was almost always 100%), where each trial was repeated twice per session for a total of 80 trials daily, or 60 trials of test images. This was to ensure that any differences in the inverted performance could not be attributed to overall differences in performances levels on the original stimuli in their upright orientations. Performance from the home cage, where trial number was not limited, was analyzed in 80 trial blocks so as to be equivalent with the analysis of performance in the testing room.
After reaching this criterion, the same 15 experimental stimuli were added such that the sample remained upright while the two comparison images were presented upside down. This task now contained the same 15 images in both an upright and inverted orientation, totaling 30 trials. Subjects then received four total sessions on the inversion task in which each trial was seen twice per session (N = 60 trials per session). Images were presented pseudorandomly so that one presentation of each trial was randomly presented before any individual trial was repeated. After the four inversion sessions were completed, the subject moved on to the upright training for the next stimulus category. All subjects received the rhesus monkey stimulus set first, due to their initial training on conspecifics’ faces. The presentation order of the remaining stimulus categories was counterbalanced across subjects and the inversion task for each category was completed before advancing to the next category.
Data analysis
Data analyses first examined whether some stimulus categories were more difficult to learn in their upright orientation than others. This was done using a repeated measures analysis of variance (ANOVA) where there were five levels of stimulus type (rhesus faces, human faces, chimpanzee faces, houses, and novel clip-art) as the within-subject factors. To control for multiple comparisons during follow-up tests, alpha was adjusted using Tukey’s studentized range statistic, (Howell, 2002). Paired t tests were used as follow-up comparisons to examine performance on upright compared to inverted trials for each stimulus category during the inverted test sessions. Significantly poorer performance on inverted compared to upright trials indicated a significant inversion effect. All significance values were set at p < .05, two-tailed and all confidence intervals (CI) were listed at 95%. Effect sizes were calculated using Cohen’s d statistic that calculates the difference between two means divided by the standard deviation (Thalheimer & Cook, 2002; www.work-learning.com).
If rhesus monkeys process all faces using the same configural properties, then they should show a significant inversion effect for all face categories. However, if monkeys show face expertise, similar to chimpanzees and humans, then they should only show the inversion effect for face categories for which they have expertise which, in this population of monkeys, includes conspecifics’ and human faces.
Results: Experiment 1
The Face Inversion Effect
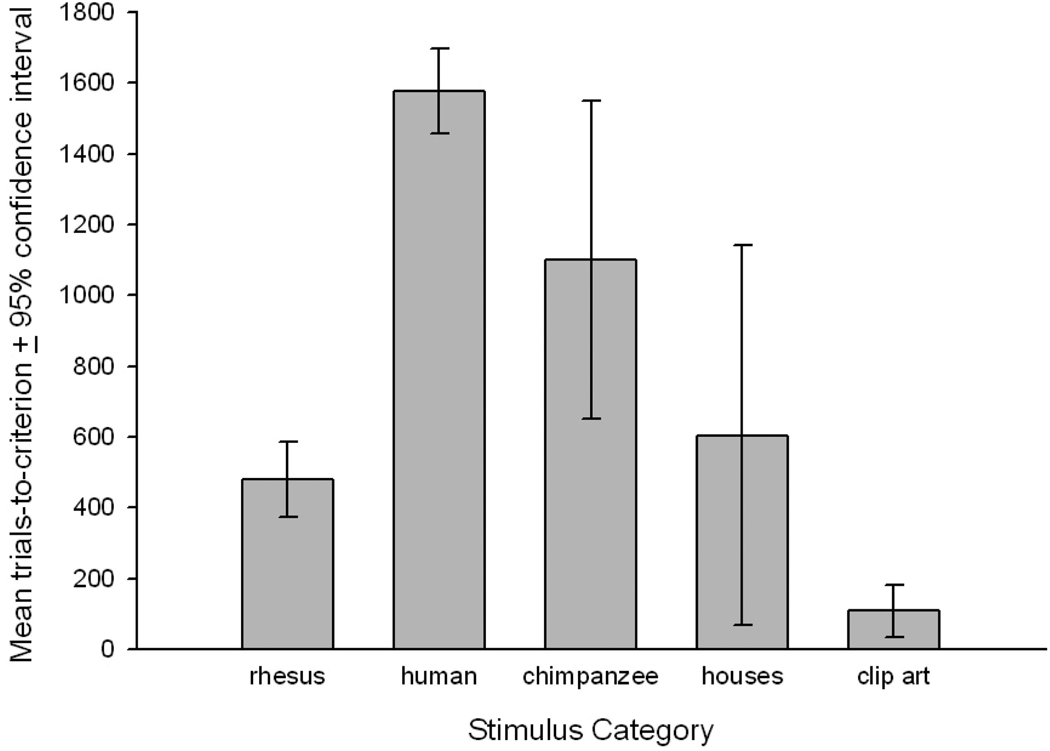
Eight subjects completed testing on the conspecific faces and clip-art (Sm, Rk, On, Oi, Cw, Lm, Vl, and Cl), but Vl did not complete the remaining categories as she never exceeded the criterion during the learning phases. The trials-to-criterion (TTC) for learning all categories of stimuli in their upright orientation can be seen in Figure 2. There was a significant main effect of stimulus type, F(4, 24) = 6.97, p < .001, Cohen’s d = 1.5, indicating a huge effect. Follow-up comparisons, using the Tukey’s studentized range statistic adjusted for 10 comparisons, t = 3.48, revealed that subjects required significantly more trials to reach criterion when learning each face category compared to the clip-art category, rhesus faces t(6) = 4.10, p < .005, Cohen’s d = 2.29; human faces t(6) = 3.52, p < .01, Cohen’s d = 1.85; and chimpanzee faces t(6) = 5.47, p < .002, Cohen’s d = 2.85; all effects sizes indicating huge effects. No other follow-up comparisons reached significance; rhesus versus human, t(6) = 2.76; rhesus versus houses, t(6) = 0.72; rhesus versus chimpanzee, t(6) = 3.15; human versus houses, t(6) = 2.28; human versus chimpanzee, t(6) = 1.21; houses versus clip art, t(6) = 2.55; and houses versus chimpanzee, t(6) = 1.76.
Figure 2.
Mean trials-to-criterion (TTC, ± 95% confidence intervals) for subjects when learning to discriminate each category of stimulus in its upright orientation.
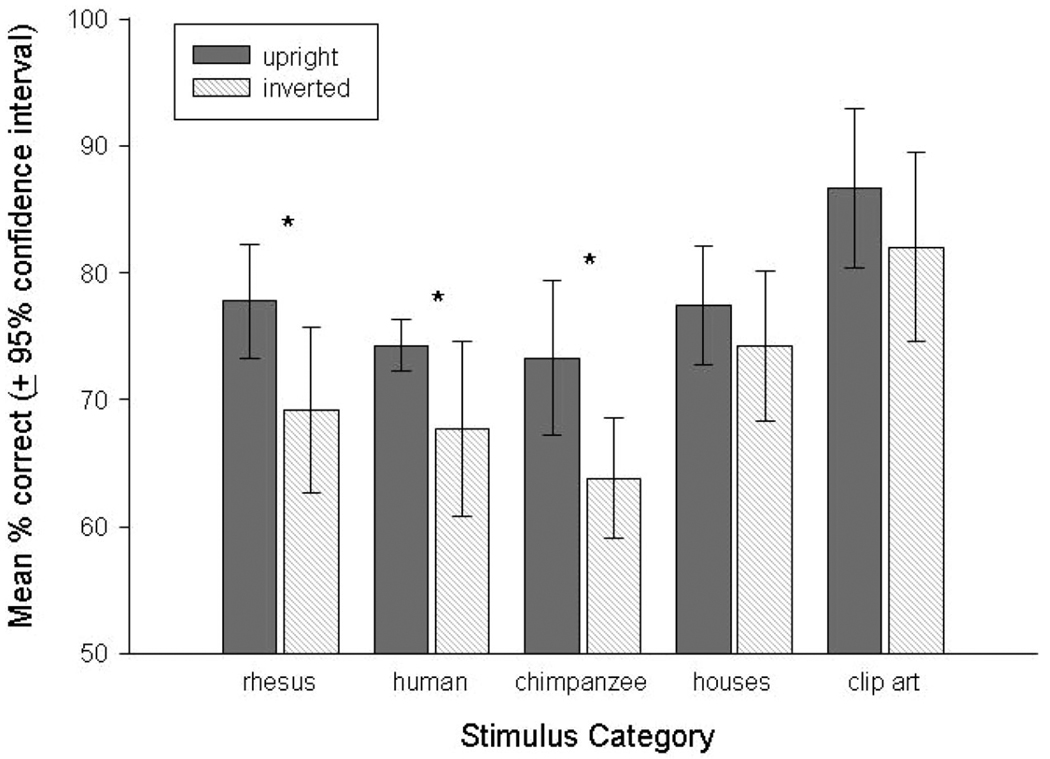
Paired t tests then compared subjects’ performance on upright versus inverted trials for each stimulus type, similar to previous studies in chimpanzees (Parr et al., 1998). This revealed significantly better performance on upright compared to inverted trials for all face categories, rhesus monkey, t(7) = 3.64, p < .01, Cohen’s d = 1.36, indicating a very large effect; chimpanzee, t(6) = 3.09, p < .03, Cohen’s d = 1.74, indicating a huge effect; and human, t(6) = 2.58, p < .05, Cohen’s d = 1.29 indicating a very large effect, but not for either nonface category; houses, t(6) = 1.65, p = .15; or clip-art, t(7) = 2.19, p = .07. Figure 3 shows the mean performance (±95% CI) on the upright and inverted trials for each stimulus category.
Figure 3.
Mean performance (±95% confidence intervals) by subjects when discriminating images in their upright and inverted orientations.
Discussion: Experiment 1
The Face Inversion Effect
Subjects showed differences in their performance when first learning to discriminate the stimuli in their upright orientation, despite reaching the same level of training with MTS tasks before the onset of these experiments. The complex clip-art images were learned significantly faster than any of the face categories, but not significantly faster than the houses. The performance graphed in Figure 2 clearly shows that subjects learned conspecific’s faces faster than the chimpanzee or human face categories, but these were not significantly different from one another when adjusted for multiple comparisons. Moreover, subjects showed significant inversion effects when discriminating each of the face categories, although the effects only just exceeded significance for human faces. In contrast, no significant inversion effects were found for either of the nonface categories, although surprisingly this neared significance for the inverted clip-art. Therefore, the rhesus monkeys showed a general discrimination deficit when face stimuli were inverted, although no such inversion effect was found for the nonface categories, suggesting a general configural processing strategy for faces.
Despite the expertise-dependent inversion effects shown previously for chimpanzees (Parr et al., 1998; Parr & Heintz, 2006), the present results did not confirm any role of expertise in the inversion effects by rhesus monkeys, as they showed a strong inversion effect for the chimpanzee faces for which they had no expertise, and a weak inversion effect for the expert category of human faces. In addition, the present results are somewhat inconsistent with previous studies by the author in a different group of rhesus monkeys using a similar paradigm (Parr et al., 1999). In this previous study, significant inversion effects were demonstrated when rhesus monkeys discriminated their own species face, unfamiliar capuchin monkey faces, and automobiles, but not for the expert category of human faces, or abstract shapes (Parr et al., 1999). It should be noted, however, that these previous data neared significance for the human faces (p < .07), and in the present study the data for human faces was also close to the significance level (p < .05), but this time falling within the range to reject the null hypothesis. Thus, the rhesus in both of these studies showed marginal effects of orientation for discriminating human faces, but strong orientation effects for conspecifics’ faces and other unfamiliar face categories, capuchin monkeys and chimpanzees. Although results from studies of the inversion effect in monkeys have been inconsistent, even from the same laboratory, the data do not support a dedicated system for processing faces of expertise.
To further investigate the role of configural cues in face recognition, a second study was undertaken that presented rhesus monkeys with unfamiliar conspecifics’ faces that had been manipulated in three ways to affect first- and second-order configural cues.
Method: Experiment 2
First- Versus Second-Order Feature Manipulations
Stimuli
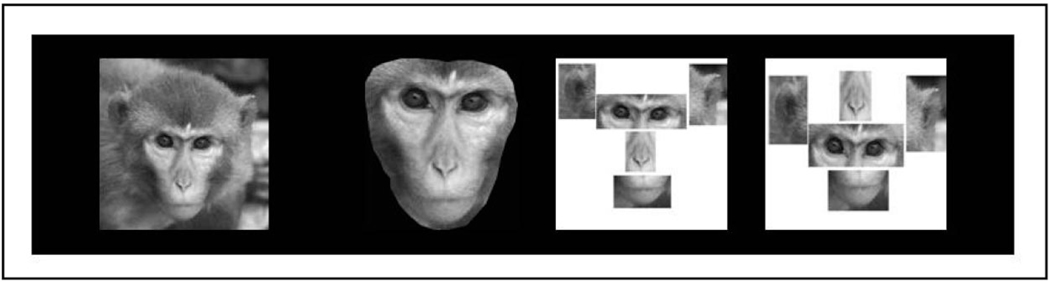
Three different types of manipulations involving unfamiliar rhesus monkey faces were presented; inner features, fractured faces, and rearranged/fractured faces, hereafter referred to as rearranged faces (see Figure 4). There was no overlap in these stimuli with those presented in the inversion studies. Inner feature manipulations were created by extracting the inner features of the face from the outer contour. The fractured faces were created by isolating individual facial features and spacing them apart, preserving the first-order relational configuration but distorting second-order relational properties. Finally, in the rearranged examples, the faces were first fractured as described above but, in addition, the position of the eyes and nose were inverted. This latter manipulation disrupted both first- and second-order relational properties as the location of individual features and their relationship to each other was altered. These manipulations are identical to those used previously in studies of configural face processing in chimpanzees and thus provide the first comparative data on the importance of both first- and second-order relational features for conspecific face processing in these Old World species (Parr, Heintz & Akamagwuna, 2006). All faces were presented as 256 grayscale images and cropped to a height of 300 pixels using Adobe Photoshop 7.0.
Figure 4.
An illustration of the three types of configural manipulations used in Experiment 2: An unaltered face, a face showing inner features (Inner), a fractured face (Fractured), and a fractured and rearranged face (Rearranged).
Procedure
As in the previous experiments, subjects first received training to discriminate 20 novel, unfamiliar conspecific faces without any manipulation before moving on to the experimental trials. For each of the three configural manipulations, a different set of 20 novel, unfamiliar rhesus monkey faces were used, so stimuli were drawn from a set of 120 novel images, 60 for the correct matching pair and 60 for the nonmatching comparison images over the three experiments. Subjects were required to perform above 80% over a 40 trial test session (two repetitions of each novel trial) before moving on to the configural manipulations, again to minimize any potential biases that could result from differences in the ability to discriminate the original images. The manipulated trials were then presented in the following order—fractured faces, rearranged faces, then inner features.
The actual tasks consisted of the original 20 training images, already learned, plus a subset of five of these that were edited to show the configural manipulation (manipulated images 1–5), totaling 25 trials. These are hereafter referred to as Probe1 trials, and in the results below, they are referred to by the type of manipulation, that is, Fractured1, Rearranged1, Inner1. Therefore, subjects were required to generalize their discrimination of previously learned faces to a subset of the same images that contained configural manipulations. Two testing sessions were given that contained these five probe trials (Probe1_1 and Probe1_2), in which two repetitions of each trial were given per session (two sessions of two repetitions of 25 trials = 100 total trials). After this first phase, a second set of five training stimuli were manipulated (manipulated images 6–10, hereafter referred to as Probe2 trials) and a second round of testing was performed using all 30 trials (two sessions of two repetitions of 30 trials = 120 trials). Because the original five Probe1 trials were still included, these were seen eight times over four sessions, whereas Probe2 images were seen four times over final two sessions. This is comparable to two generalization sessions being given in a sequential order. The remaining 10 training images were not manipulated and served as control trials throughout the testing.
Data analysis
Performance was analyzed separately for each of the three configural tasks relative to the original, unmanipulated control trials. First, the influence of learning, or trial repetition, on performance was examined. Repeated measures ANOVAs compared first the four sessions of Probe1 trials, and then the six collective Probe trial sessions (four sessions of Probe1 trials plus the two sessions of Probe2 trials). This was also done for the four sessions of performance on the control images. Polynomial contrasts assessed the impact of trial session, or learning, on performance. There should be no learning effects for control trials as these had already been learned above 80%.
Second, the mean performance on the manipulated images was compared directly to the mean for the control images within each task. Before setting up the contrasts for these analyses, the performance on the first two collective sessions for the Probe1 trials was compared (Probe1_1 vs. Probe1_2), along with the overall performance on all Probe1 versus Probe2 trials. If these comparisons revealed significant differences, then they were assessed versus control trials separately using three planned comparisons, Probe1_1 compared to Controls; Probe1_2 compared to Controls; Probe2 compared to Controls. If, however, performance was not significantly different between these sessions, only two planned comparisons were performed, Probe1 compared to Controls and Probe2 compared to Controls. Alpha was adjusted for multiple comparisons using Bonferroni’s correction procedure (1α = α/no. of comparisons).
Because the rhesus monkeys showed a general configural processing strategy for faces, it was expected that they would perform well on the inner feature manipulations, but poorly on the first- and second-order manipulations. It was not expected that they would show selective impairments for the second-order feature manipulations due to their lack of face expertise.
Results: Experiment 2
First- Versus Second-Order Feature Manipulations
Fractured faces
Seven subjects completed testing on the fractured face task (Sm, Rk, On, Oi, Cw, Lm, and Cl). No significant effects of learning, trial repetition, were found for the fractured faces. The main effect of trial order failed to reach significance whether it was assessed over the first four sessions of Fractured1 trials (the Probe1 trials), F(3, 18) = 0.85, p = .49; the six overall fractured trial sessions, F(5, 30) = 0.81, p = .55; or for the control trials, F(3, 18) = 1.09, p = .38.
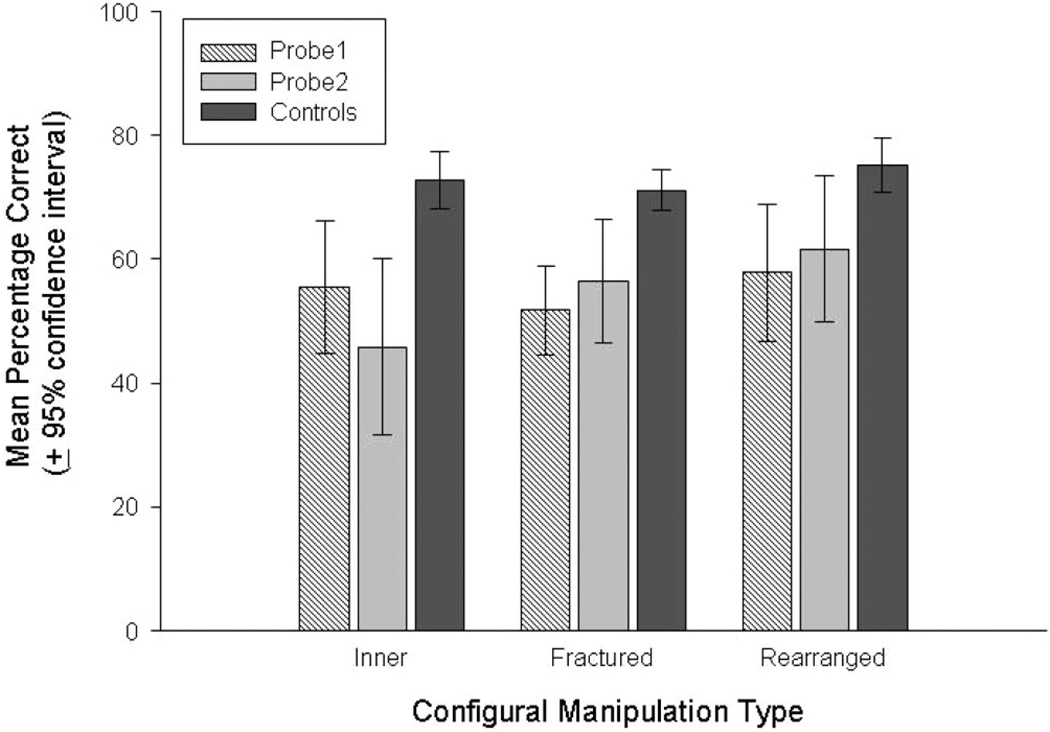
No significant differences were found between the first and second combined sessions of Fractured1 trials (Fractured1_1 vs. Fractured1_2), t(6) = 0.92, p = .40; or the combined performance on each of the two sets of fractured probe trials (Fractured1 vs. Fractured2), t(6) = 1.11, p = .31. Therefore, two planned comparisons were performed between the mean for the two fractured probe trial sessions and the controls using paired t tests. These comparisons revealed significantly worse performance on the fractured probe trials compared to controls, Fractured1 × Controls, t(6) = 7.16, p < .001, Cohen’s d = 3.47; and Fractured2 × Controls, t(6) = 4.50, p < .004, Cohen’s d = 1.99; both indicating huge effects. Figure 5 illustrates the means for each set of fractured probe trials and their controls.
Figure 5.
Subjects’ mean performance (±95% confidence intervals) on each of the configural Probe trials and their control.
Rearranged faces
Six subjects completed testing on the rearranged face task (Sm, Rk, On, Oi, Cw, and Lm). No effect of trial repetition was found for performance on the rearranged probe trials, whether this was assessed for the first four presentations of Rearranged1 trials, F(3, 15) = 1.64, p = .22, or the overall six sessions of rearranged probe trials, F(5, 25) = 1.62, p = .19. Subjects’ performance on repeated sessions of the control trials, however, did reveal a significant main effect, F(3, 15) = 4.62, p < .02, Cohens d = 1.36, indicating a very large effect, which was unexpected as these trials had been learned previously. However, polynomial contrasts revealed that this effect was significantly quadratic, F(1, 5) = 15.07, p < .02, and thus not suggestive of an overall learning effect that would have predicted a significant linear increase in performance over testing sessions.
Follow-up comparisons revealed no significant differences between the first two presentations of Rearranged1 trials (Rearranged1_1 × Rearranged1_2), t(5) = 1.76, p = .14, or the combined performance on each of the two sets of rearranged probe trials (Rearranged1 × Rearranged2), t(5) = 1.06, p = .34. Thus, two main t tests compared performance on each of the rearranged probe trials versus the control images revealing a significant difference between Rearranged1 Trials × Controls, t(5) = 4.37, p < .007, Cohen’s d = 2.37, and a borderline significant difference between Rearranged2 × Control trials, t(5) = 2.57, p = .05, Cohen’s d = 1.74, both indicating huge effects. Figure 5 illustrates the means for each set of rearranged probe trials and their controls.
Inner feature faces
Six subjects completed performance on the inner feature task. Repeated measures ANOVAs failed to find any effect of repetition on the inner probe trial performance, whether it was analyzed for the first four sessions of inner probe trials (Inner1), F(3, 15) = 0.41, p = .75, or the combined 6 sessions of both sets of inner probe trials, F(5, 25) = 1.54, p = .21. Moreover, performance on the four sessions of control trials during the inner feature task also remained stable, F(3, 15) = 0.48, p = .70.
The follow-up comparisons revealed no significant differences between the first two presentations of Inner1 trials (Inner1_1 × Inner1_2), t(5) = 0.15, p = .89; or the combined performance on each of the two sets of inner probe trials (Inner1 × Inner2), t(5) = 2.37, p = .06. Paired t tests then compared performance on each of the sets of inner probe trials and their controls. These revealed significant differences for each comparison, Inner1 × Controls, t(5) = 3.55, p < .02, Cohen’s d = 2.42; and Inner2 × Controls, t(5) = 5.15, p < .005, Cohen’s d = 2.92; both indicating huge effects. Figure 5 illustrates the mean performance for subjects on Inner feature probes and their controls.
Discussion: Experiment 2
First- Versus Second-Order Feature Manipulations
Previous studies demonstrated a preferential use of second-order configural cues by chimpanzees when discriminating conspecifics’ faces. Performance was worse matching unfamiliar conspecifics’ faces that had been fractured and fractured/rearranged compared to controls, but no significant impairments were found when subjects matched faces using only inner features (Parr, Heintz, Akamagwuna, 2006). In contrast, rhesus monkeys in the present study were significantly impaired on all of the configural manipulations. These results suggest that rhesus monkeys have trouble generalizing their face matching performance to images that contained configural changes, but they were not particularly sensitivity to second-order relational features, as has been shown for humans and chimpanzees.
In humans, a developmental preference for external versus internal facial features has been shown since birth (Pascalis, de Schonen, Morton, Deruelle, & Fabre-Grenet, 1995; although, see Simion, Macchi Cassia, Turati, & Valenza, 2003; Valenza, Simion, Cassia, & Umilta, 1996) and remarkably seems persists into early childhood. Human children under 9 years of age still show this infant pattern and are better at recognizing familiar classmates from external facial features, whereas after 9 years, children demonstrate the more adult-like pattern and are better at face recognition from inner features (Campbell, Walker & Baron-Cohen, 1995). Recently, authors have verified that this developmental pattern also holds true for unfamiliar faces using a matching task very similar to the one used in the present studies (Want, Pascalis, Coleman, & Blades, 2003). This age range, between 7 and 9 years, is the same range that has been implicated in the shift to the adult-like pattern characterized by face expertise and reliance on second-order relational features (Mondloch et al., 2006). Thus, shifting between external feature preferences to internal features preferences may be necessary for the development of face expertise in monkeys as it is in humans.
Method: Experiment 3
Individual Recognition
Stimuli
Stimuli for this experiment consisted of 20 individual recognition (IR) trials and 10 control trials. These were digitized photographs depicting neutral faces of unfamiliar conspecifics. Each photograph was cropped around the face to a height of 300 pixels and converted to 256 grayscale using Adobe Photoshop 7.0. In an IR trial, the sample and correct match were two different photographs of the same adult individual, thus showing different backgrounds and head orientations, while the nonmatch was another individual. Control trials consisted of two identical photographs as the matching pair, and a second photograph of another individual as the nonmatch. Again, the ages of all individuals were matched as closely as possible. No individuals were repeated in the IR and control trials, so photographs of 60 different monkeys were used in this task. Control photographs were cropped the same way as IR photographs and were also presented in black and white.
Procedure
Subjects were tested twice daily, once mid morning and once early afternoon. They were always given four repetitions of each trial per session, and only in rare cases were subjects unwilling to complete this number of trials. The IR trials were presented in six groups, Trials 1 to 3, 4 to 6, 7 to 9, 10 to 12, 13 to 15, with the last 5 trials presented together, 16 to 20. These were added cumulatively, so Trials 1 to 3 were first presented and then Trials 4 to 6 were added and so forth, until the final session when subjects were discriminating all 20 IR trials in a single session. This was done so that analyses could compare whether subjects’ performance improved after learning consecutive IR discriminations, so the presentation can be though of as repeated generalization phases where new trials were added cumulatively. The 10 control trials were always included to ensure that subjects still conformed to the matching rule. However, during the final session when Trials 16 to 20 were added, only 5 control trials were presented so that the overall number of trials did not exceed 100 (25 trials total × 4 repetitions). During testing, it became clear that some trials were much easier to discriminate than others, so to keep the task moving, an arbitrary criterion of 75% was adopted before the next block of novel trials was added. However, there was a methodological error made after Trials 4 to 6 in which Trials 7 to 9 were added before subjects had met this criterion.
Data analysis
Binomial z scores were used to determine the percentage correct that was required before subjects’ performance exceeded chance on each block of 3, or 5 trials. Because trials were repeated four times per session, this was evaluated per session as 12 trials, > 78.29% or 20 trials, > 71.91% using a two-tailed z value of 1.96. Repeated measures ANOVAs were then used to assess whether performance differed per block of trials, or whether some pairs of individuals were easier to discriminate than others. In this analysis trial block was the within-subject factor. Finally, performance on the first 3 blocks of trials, Trials 1 to 9 were compared to the last 3 blocks of trials, Trials 10 to 20 using paired t tests to determine whether subject’s IR discrimination improved with successive learning and repetition. All p values were set at p < .05, two-tailed.
Based on previous studies (Parr et al., 2000), monkeys were not expected to perform well on these individual recognition tasks. It was expected that monkeys would need many repetitions of each trial before performing above chance and that their performance would not generalize to novel faces when new trials were added.
Results: Experiment 3
IR
Six subjects participated in these experiments (Sm, Rk, On, Oi, Cw, Lm), but the testing of two subjects (Cw, Lm) was terminated before they reached the final criterion on the last block of five trials. They each received 10 testing sessions with the complete set of trials but had not yet exceeded chance performance, > 71.91% for any 20-trial block. The mean number of sessions (where 4 repetitions of each trial were given per session) ± 95% CI required before subjects exceeded chance performance for each block of trials was as follows, Trials 1 to 3: M = 2.00, CI = 0.37; Trials 4 to 6: M = 17.33, CI = 4.23; Trials 7 to 9: M = 3.00, CI = 2.20; Trials 10 to 12: M = 12.5, CI = 11.30; Trials 13 to 15: M = 14.83, CI = 10.48; Trials 16 to 20: M = 8.50, CI = 5.59.
A repeated measures ANOVA revealed a significant main effect of trial block, F(5, 15) = 10.50, p < .001, Cohen’s d = 2.05, indicating a huge effect. Therefore, performance on some trial blocks was better than others, indicating that some individuals were easier to discriminate than others. Figure 6 provides an example of a trial that was learned very quickly by all subjects, that is, Trial 3, and a trial for which subjects performed very poorly, that is, Trial 4. Comparing the mean performance on the first 3 blocks (Trials 1 to 9) with the second three blocks (Trials 10 to 20) revealed a significant difference, where fewer trials were required for the first block (M = 7.44, CI = 1.76) compared to the second block of trials (M = 13.14, CI = 6.44), t(5) = 2.87, p < .04, Cohen’s d = 1.39, indicating a very large effect. Therefore, no cumulative learning occurred.
Figure 6.
An example of two individual recognition trials, one that subjects performed very well on and another which causes difficulty. The correct choice for each panel is the face in the lower left.
After this experiment was finished, three of the subjects (Sm, Rk, Oi), the first three to complete the previous sequence, participated in three additional tasks of individual recognition. Each subsequent task contained 10 novel individual recognition trials, with no repetition of any individual seen in the previous 20 trials, and 10 identity matching control trials (correct pair is identical pictures of unfamiliar conspecifics’ faces). Subjects were given four repetitions of each trial in a single session and were tested until their performance exceeded 80% on a single session, after which they were moved on to the next IR task. Chance was determined to be > 65.5% for a block of 40 trials (excluding control trials). Each of the three new IR tasks used novel photographs of unfamiliar conspecifics’ faces. Results showed that subjects required an average of 2.67 (CI = 2.47) sessions to exceed chance on the first IR task, and 14.00 (CI = 9.24) sessions to reach the 80% criterion, suggesting that they did learn something about individual recognition. However, this jumped to 20.33 (CI = 4.46) sessions before exceeding chance on Task 2, with 31.67 (CI = 4.43) sessions required to reach 80%. Finally, 10.67 (CI = 5.17) sessions were required to exceed chance on Task 3 and 26.67 (CI = 16.54) to reach the 80% mark. Two repeated measures ANOVAs using IR task as the within-subject factor (n = 3) revealed no significant differences for either chance performance or final performance (>80%). Once again, this suggests that subjects’ failed to generalize their face discrimination performance across different view points in these three new individual recognition tasks.
Discussion: Experiment 3
IR
Results from the initial individual recognition task illustrate the difficulty that rhesus monkeys have in discriminating the faces of unfamiliar conspecifics. Although some blocks of trails/individuals were easier than others, that is, Trials 1 to 3 and 7 to 9, others proved to be quite difficult, that is, Trials 4 to 6 and 13 to 15. This was an unexpected result, as the quality of the images, their preparation that is, cropping the face, adjusting lighting conditions, and removing background features, was highly standardized. Moreover, trials presented at the end of the testing session were not easier to discriminate than trials presented at the beginning of the experiment, indicating no overall, or cumulative, learning. The subsequent three individual recognition tasks performed in a subset of the subjects confirmed this. The first task elicited quite good performance, but this dropped considerably in the final two tasks. Visual inspection of the trials on which subjects performed very well compared to those that resulted in poorer performance revealed no obvious indication of what cues were being used. Therefore, monkeys appear to be unable to quickly and accurately extract similarities in the facial appearance of unfamiliar monkeys despite extensive training.
General Discussion
The results of these studies in monkeys suggest interesting species differences in the use of configural cues and the role of expertise during face processing tasks. Previous studies have shown the inversion effect in chimpanzees only for faces for which subjects have had considerable expertise (Parr et al., 1998). The present studies, however, showed a significant inversion effect in monkeys for all face categories (human, chimpanzee, and conspecific), regardless of their familiarity. Colony records verified that although the rhesus subjects in these studies were born and raised in large social groups at the Yerkes Primate Center field station, which also houses chimpanzees, their enclosures were not in visible proximity with the chimpanzee compounds. The rhesus monkeys did, however, have ample experience with conspecifics and humans as they were all born and raised in large social groups and cared for by human caretakers. Therefore, although the inversion effect alone cannot provide information as to the type of visual processing strategy, the inversion effect data from the present experiment confirm that monkeys do not adopt a different strategy for upright and inverted faces of expertise, as do humans and chimpanzees.
Experiment 2 examined the importance of first- and second-order relational features in conspecific face recognition in rhesus monkeys. Previous studies by Parr and colleagues have shown a bias in chimpanzees for the use of second-order configural features when processing unfamiliar conspecifics’ faces (Parr, Heintz, Akamagwuna, 2006). When presented with these same manipulations, the rhesus monkeys were impaired in all discriminations, including the inner face trials, where both first- and second-order features were preserved. This suggests, perhaps, that the rhesus monkeys may have been relying primarily on external facial features to successfully perform these face discriminations. When external features were removed, impairments resulted, and perhaps the general contour of the faces with regard to inner features was also more difficult to extract when faces were inverted. It may also be, however, than any type of manipulation that changes a stimulus from its condition during learning could produce errors for monkeys. Future studies will examine the selective impairments that result when internal and external features are manipulated.
Finally, despite considerable training, monkeys were unable to match two different photographs of the same unfamiliar conspecific, and repeated training on these discriminations did not significantly improve performance. Because the matching pair showed the same individual presented in two different photographs, these discriminations required subjects to utilize similarities in the faces of the individuals presented, whether their strategy focused on extracting similarities in individual features or overall configuration. One characteristic of face expertise is the ability to individuate and remember many different individuals. So, although this experiment does not specifically address what type of face processing strategy monkeys employed, it directly addressed whether monkeys were able detect similarities among individuals’ faces. Some individuals appeared easier than others, as evidenced by rapid learning on some trial blocks, and during the first generalization task, but overall, monkeys failed to show improvement in their performance with additional testing. Future studies will examine the visual properties of facial images in an attempt to determine why some trials were better learned than others. This will involve quantifying the spacing between the facial features of the sample and foil individuals to determine if some individuals that were paired had similar facial configurations, and thus produced more difficult discriminations. However, the ability to extract second-order configural cues would minimize the deficits from these similarities, as every face contains a unique set of second-order relational properties.
The human literature is very instructive in helping to understand these prominent species differences, as numerous studies on configural face processing from birth through childhood have revealed quite remarkable, and in some cases necessary, developmental stages leading to face expertise. First, sensitivity to faces versus other classes of images occurs very early in development, some argue from birth, and continues to become more specialized throughout infancy and early childhood (Bushnell, 1998; Nelson, 2001; Pascalis et al., 2002). Some report that these early preferences involve attention to external facial features, or face contours, compared to information present from inner features (Pascalis et al., 1995). In their classic model, Morton and Johnson (1991) referred to this as the CONSPEC system, which is driven by subcortical processes and functions to focus the infant’s attention toward relevant social stimuli, like faces. Once attention is focused, a later-developing system—CONLEARN—may hone face processing specializations concomitant with the maturation of cortical brain areas (Morton & Johnson, 1991). There is good evidence that nonhuman primates, both monkeys and apes, are also highly attracted to face stimuli from birth. They show early scanning preferences for faces and also display highly comparable forms of early neonatal imitation as demonstrated in human infants (Bard, Platzman, Lester, & Suomi, 1992; Ferrari et al., 2006; Mendelson, Haith, & Goldman-Rakic, 1982; Myowa, 1996; Myowa-Yamakoshi, Tomonaga, Tanaka, & Matsuzawa, 2004; Myowa-Yamakoshi, Yamakoshi, Tomonaga, Tanaka, & Matsuzawa, 2005). Therefore, among primates (and perhaps other mammals) an innate, domain-general mechanism might be in place for early attraction and attention to faces versus other classes of stimuli.
Second, an important development shift occurs when infants are only a few months old when this general attraction to faces becomes more specialized. This has been revealed in several studies of perceptual narrowing, or selective tuning for faces, similar to that described for human speech perception (Kuhl et al., 1992). Young infants around 6 months of age, for example, are better at discriminating the faces of other species than adults, but this pattern becomes more fine tuned around 9 months of age through repeated exposure to conspecifics’ faces (Nelson, 2001; Pascalis et al., 2002). The pattern for this perceptual tuning in nonhuman primates in unknown, although studies have demonstrated selectivity in adult monkeys for attraction to conspecific versus heterospecific faces (Pascalis & Bachevalier, 1998; Pascalis, Petit, Kim & Campbell, 1999). Therefore, experience appears to shape the early predisoposition and attraction to faces, tunes it to be selective to more specific categories of faces, most often same-species faces for which infants have ample exposure from birth, leading ultimately to a more domain-specific specialization. The exact time course for this specialization and how it emerges remains unknown, but some have suggested that with the proper early exposure to faces, continued selective exposure throughout early childhood, and barring any developmental abnormalities, a domain-specific type of face expertise, including sensitivity to second-order relational cues, will, in effect, self-generate (Nelson, 2001).
How can the lack of face expertise in the rhesus monkey be reconciled with previous studies demonstrating face expertise in the chimpanzee reaching levels comparable to those shown in human adults (Parr & de Waal, 1999; Parr & Heintz, 2006; Parr, Heintz, Akamagwuna, 2006; Parr et al., 2000)? Both chimpanzees and rhesus monkeys live in large social groups characterized by multimale/multifemale relationships, they have an elaborated cortex and well-defined extrastriate regions important for visual categorization. Thus, the social and neuroanatomical framework necessary for a general face processing system to self-generate over time into one that may be, at least, analogous to human face expertise would appear to be present in both species. Several potential explanations are possible, with the overarching caveat that comparative data on the cognitive mechanisms underlying these processes in nonhuman primates are sorely lacking, with perhaps some exception for chimpanzees (Parr et al., 1998; Parr & Heintz, 2006; Parr, Heintz, Akamagwuna, 2006; Parr et al., 2000). Monkeys, for example, perceive direct stares as threatening and thus may avoid gazing directly at one another, limiting information that could be gained from the face (Hinde & Rowell, 1962). However, others have shown that gaze aversion is a learned behavior, occurring after 7 months of age, long after the time that human infants begin to specialize their face processing skills (Mendelson et al., 1982). Moreover, monkeys constantly monitor one another and visual information modifies other signals in important and meaningful ways (Partan, 2002).
The most plausible explanation for why chimpanzees show more elaborate face expertise than monkeys may be due to an overall level social complexity that has led to evolutionary specializations in social cognition, similar to the specializations proposed by Dunbar (1998) for neocortical elaboration. Chimpanzees, unlike most other primates, are characterized by a fission-fusion society in which members of a community associate most often in small units that frequently intermix, but only rarely come together at the same time (Nishida, 1979). This is similar to the social organization of humans, but quite different from rhesus monkeys where females and youngsters socialize primarily within their matriline and the group as a whole is almost always together (Dunbar, 1988). It has been argued that chimpanzee society is more socially complex compared to monkeys, as social complexity is best explained not simply by the size of the social group, but by the number of individual relationships that are possible (Barrett, Henzi & Dunbar, 2003; Dunbar, 1998). Thus, evolution may have favored a more elaborate face processing system for fission-fusion species in which information about specific individuals must be initially stored, updated, and retrieved continually over time (Parr, Waller & Fugate, 2006). Information about individual identity that is relative to other individuals, or the environment, such as matriline composition, grooming partners, geographic location within the home range, would neither be available nor effective for these species. They must depend on a strategy that is stable and able to generate the same representations regardless of environmental or social conditions. Second-order configurations may play as critical role in these skills in chimpanzees as they do in humans.
Acknowledgments
This investigation was supported by RR-00165 from the NIH/NCRR to the Yerkes National Primate Research Center, and R01-MH068791 to L. A. Parr. The Yerkes National Primate Research Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. Thanks to Kristy Schlenker, Caitlin Parry, and Daniel Brubaker for assistance with animal testing, and the animal care staff at the Yerkes National Primate Research Center. Helpful comments were provided by three anonymous reviewers.
Footnotes
After 58 sessions, Vl had still not exceeded 80% Face Matching 1. She was moved to the second face matching task anyway and her Face Matching Task 1 data were analyzed using 58 sessions for her final criterion. She reached the final criterion on Face Matching Task 2 in 16 sessions.
Contributor Information
Lisa A. Parr, Division of Psychiatry and Behavioral Sciences, Emory University, and Yerkes National Primate Research Center
Matthew Heintz, Department of Biology, University of Chicago.
Gauri Pradhan, Department of Clinical Psychology, Albert Einstein College of Medicine, Yeshiva University.
References
- Bard KA, Platzman KA, Lester BM, Suomi SJ. Orientation to social and nonsocial stimuli in neonatal chimpanzees and humans. Infant Behavior and Development. 1992;15:43–56. [Google Scholar]
- Barrett L, Henzi P, Dunbar RIM. Primate cognition: From “what now?” to “what if?” Trends in Cognitive Science. 2003;7:494–497. doi: 10.1016/j.tics.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Brown SD, Dooling RJ. Perception of conspecific faces by budgerigars (Melopsittacus undulatus): II. Synthetic models. Journal of Comparative Psychology. 1993;107:48–60. doi: 10.1037/0735-7036.107.1.48. [DOI] [PubMed] [Google Scholar]
- Bruce C. Face recognition by monkeys: Absence of an inversion effect. Neuropsychologia. 1982;20:515–521. doi: 10.1016/0028-3932(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Bushnell IWR. The origins of face perception. In: Simion FBGF, Butterworth G, editors. The development of sensory, motor and cognitive capacities in early infancy: From perception to cognition. E. Sussex, UK: Psychology Press; 1998. pp. 69–86. [Google Scholar]
- Campbell R, Walker J, Baron-Cohen S. The development of differential use of inner and outer face features in familiar face identification. Journal of Experimental Child Psychology. 1995;59:196–210. [Google Scholar]
- Carey S, Diamond R. From piecemeal to configurational representation of faces. Science. 1977;195:312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- Collishaw SM, Hole GJ. Is there a linear or a nonlinear relationship between rotation and configural processing of faces? Perception. 2002;31:287–296. doi: 10.1068/p3195. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. Journal of Experimental Psychology. 1986;115:107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Dittrich W. Representation of faces in longtailed macaques (Macaca fascicularis) Ethology. 1990;85:265–278. [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology. 1998;6:178–190. [Google Scholar]
- Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? Journal of Experimental Psychologyz. 1995;21:628–634. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K, Symons LA. The face-inversion effect as a deficit in the encoding of configural information: Direct evidence. Perception. 2000;29:159–170. doi: 10.1068/p3012. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLOS: Biology. 2006;4:1501–1508. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys scan faces in a visual paired comparison task? Animal Cognition. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- Hinde RA, Rowell TE. Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta) Proceedings of the Royal Society of London B. 1962;138:1–21. [Google Scholar]
- Howell DC. Statistical Methods for Psychology. 5th Edition. Belmont, CA: Duxbury Press; 2002. [Google Scholar]
- Jitsumori M, Makino H. Recognition of static and dynamic images of depth-rotated human faces in pigeons. Learning and Behavior. 2004;32:145–156. doi: 10.3758/bf03196016. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, Smith M, et al. Three-month olds, but not newborns, prefer own-race faces. Developmental Science, 8. 2005:F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM, da Costa AP, Leigh AE, Hinton MR, Peirce JW. Sheep don’t forget a face. Nature. 2001;414:165–166. doi: 10.1038/35102669. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerda F, Stevens KN. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992;255:606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP. Early visual experience and face processing. Nature. 2001;410:890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Science. 2002;6:255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Mendelson MJ, Haith MM, Goldman-Rakic PS. Face scanning and responsiveness to social cues in infant rhesus monkeys. Developmental Psychology. 1982;18:222–228. [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, and Computers. 2004;36:630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Le Grand R, Maurer D. Configural face processing develops more slowly than featural face processing. Perception. 2002;31:553–566. doi: 10.1068/p3339. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Geldart S, Maurer D, Le Grand R. Developmental changes in face processing skills. Journal Experimental Child Psychology. 2003;86:67–84. doi: 10.1016/s0022-0965(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Mondloch CJ, Maurer D, Ahola S. Becoming a face expert. Psychological Science. 2006;17:930–934. doi: 10.1111/j.1467-9280.2006.01806.x. [DOI] [PubMed] [Google Scholar]
- Morton J, Johnson MH. CONSPEC and CONLEARN: A two-process theory of infant face recognition. Psychological Review. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Myowa M. Imitation of facial gestures by an infant chimpanzee. Primates. 1996;37:207–213. [Google Scholar]
- Myowa-Yamakoshi M, Tomonaga M, Tanaka M, Matsuzawa T. Imitation in neonatal chimpanzees. Developmental Science. 2004;7:437–442. doi: 10.1111/j.1467-7687.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- Myowa-Yamakoshi M, Yamaguchi M, Tomonaga M, Tanaka M, Matsuzawa T. Development of face recognition in infant chimpanzees (Pan troglodytes) Cognitive Development. 2005;20:49–63. [Google Scholar]
- Neiworth JJ, Hassett JM, Sylvester CJ. Face processing in humans and new world monkeys: The influence of experiential and ecological factors. Animal Cognition. 2007;10:125–134. doi: 10.1007/s10071-006-0045-4. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3018. [Google Scholar]
- Nishida T. The social structure of chimpanzees of the Mahale mountains. In: Hamburg DA, McCowen ER, editors. The great apes. Menlo Park, CA: Benjamin-Cummings; 1979. pp. 73–121. [Google Scholar]
- Overman WH, Doty RW. Hemispheric specialization displayed by man but not macaques for analysis of faces. Neuropsychologia. 1982;20:113–128. doi: 10.1016/0028-3932(82)90002-1. [DOI] [PubMed] [Google Scholar]
- Parr LA, de Waal FBM. Visual kin recognition in chimpanzees. Nature. 1999;399:647–648. doi: 10.1038/21345. [DOI] [PubMed] [Google Scholar]
- Parr LA, Dove T, Hopkins WD. Why faces may be special: Evidence for the inversion effect in chimpanzees (Pan troglodytes) Journal of Cognitive Neuroscience. 1998;10:615–622. doi: 10.1162/089892998563013. [DOI] [PubMed] [Google Scholar]
- Parr LA, Heintz M. The perception of unfamiliar faces and houses by chimpanzees: Influence of rotational angle. Perception. 2006;35:1473–1483. doi: 10.1068/p5455. [DOI] [PubMed] [Google Scholar]
- Parr LA, Heintz M. The role of stimulus expertise and rotation angle on the discrimination of faces and houses by Rhesus monkeys. Animal Cognition. 2008 doi: 10.1007/s10071-008-0137-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Heintz M, Akamagwuna U. Three studies of configural face processing in chimpanzees (Pan troglodytes) Brain & Cognition. 2006;62:30–42. doi: 10.1016/j.bandc.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Waller BM, Fugate J. Emotional communication in primates: Implications for neurobiology. Current Opinion in Neurobiology. 2006;15:716–720. doi: 10.1016/j.conb.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD. Is the inversion effect in rhesus monkeys face specific? Animal Cognition. 1999;2:123–129. [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD, de Waal FBM. Recognizing facial cues: Individual recognition in chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta) Journal of Comparative Psychology. 2000;114:47–60. doi: 10.1037/0735-7036.114.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partan S. Single and multichannel signal composition: Facial expressions and vocalizations of rhesus macaques (Macaca mulatta) Behaviour. 2002;139:993–1027. [Google Scholar]
- Pascalis O, Bachevalier J. Face recognition in primates: A cross-species study. Behavioural Processes. 1998;43:87–96. doi: 10.1016/s0376-6357(97)00090-9. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296:1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S, Morton J, Deruelle C, Fabre-Grenet M. Mother’s face recognition by neonates: A replication and an extension. Infant Behavior and Development. 1995;18:79–85. [Google Scholar]
- Pascalis O, Petit O, Kim JH, Campbell R. Picture perception in primates: The case of face perception. Cahiers de Psychologie Cognitive. 1999;18:889–921. [Google Scholar]
- Peirce JW, Leigh AE, da Costa AP, Kendrick KM. Human face recognition in sheep: Lack of configural coding and right-hemisphere advantage. Behavioral Processes. 2001;55:13–26. doi: 10.1016/s0376-6357(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Peirce JW, Leigh AE, Kendrick KM. Configural coding, familiarity and the right-hemisphere advantage for face recognition in sheep. Neuropsychologia. 2000;38:475–483. doi: 10.1016/s0028-3932(99)00088-3. [DOI] [PubMed] [Google Scholar]
- Phelps MT, Roberts WA. Memory for pictures of upright and inverted primate faces in humans (Homo sapiens), squirrel monkeys (Saimiri sciureus), and pigeons (Columba livia) Journal of Comparative Psychology. 1994;108:114–125. doi: 10.1037/0735-7036.108.2.114. [DOI] [PubMed] [Google Scholar]
- Reisenhuber M, Jarudi I, Gilad S, Sinha P. Face processing in humans is compatible with a simple shape-based model of vision. Proceedings of Biological Science. 2004;271 Suppl. 6:S448–S450. doi: 10.1098/rsbl.2004.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld SA, Van Hoesen GW. Face recognition in the rhesus monkey. Neuropsychologia. 1979;17:503–509. doi: 10.1016/0028-3932(79)90057-5. [DOI] [PubMed] [Google Scholar]
- Searcy JH, Bartlett JC. Inversion and processing of component and spatial-relational information in faces. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:904–915. doi: 10.1037//0096-1523.22.4.904. [DOI] [PubMed] [Google Scholar]
- Simion F, Macchi Cassia V, Turati C, Valenza E. Non-specific perceptual biases at the origins of face processing. In: Pascalis O, Slater A, editors. The development of face processing in infancy and early childhood. New York: Nova Science; 2003. pp. 13–25. [Google Scholar]
- Tanaka JW, Farah MJ. Second-order relational properties and the inversion effect: Testing a theory of face perception. Perception & Psychophysics. 1991;50:367–372. doi: 10.3758/bf03212229. [DOI] [PubMed] [Google Scholar]
- Tate AJ, Fischer H, Leigh AE, Kendrick KM. Behavioural and neurophysiological evidence for face identity and face emotion processing in animals. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2006;361:2155–2172. doi: 10.1098/rstb.2006.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalheimer W, Cook S. How to calculate effect sizes from published research articles: A simplified methodology. 2002 August; Retrieved December 17, 2007 from http://work-learning.com/effect_sizes.html. [Google Scholar]
- Tibbetts AE. Visual signals of individual identity in the wasp Polistes fuscatus. Proceedings of Biological Science. 2002;269:1423–1428. doi: 10.1098/rspb.2002.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga M. How laboratory-raised Japanese monkeys (Macaca fuscata) perceive rotated photographs of monkeys: Evidence for an inversion effect in face perception. Primates. 1994;35:155–165. [Google Scholar]
- Tomonaga M. Visual search for orientation of faces by a chim-panzee (Pan troglodytes): Face-specific upright superiority and the role of facial configural properties. Primates. 2007;48:1–12. doi: 10.1007/s10329-006-0011-4. [DOI] [PubMed] [Google Scholar]
- Tomonaga M, Itakura S, Matsuzawa T. Superiority of con-specific faces and reduced inversion effect in face perception by a chimpanzee. Folia Primatologica. 1993;61:110–114. doi: 10.1159/000156737. [DOI] [PubMed] [Google Scholar]
- Valentine T. Upside-down faces: A review of the effects of inversion upon face recognition. British Journal of Psychology. 1988;79:471–491. doi: 10.1111/j.2044-8295.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Valenza E, Simion F, Cassia VM, Umilta C. Face preference at birth. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:892–903. doi: 10.1037//0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- Vermeire BA, Hamilton CR. Inversion effect for faces in split-brain monkeys. Neuropsychologia. 1998;36:1003–1014. doi: 10.1016/s0028-3932(98)00054-2. [DOI] [PubMed] [Google Scholar]
- Want SC, Pascalis O, Coleman M, Blades M. Recognizing people from the inner or outer parts of their faces: Developmental data concerning “unfamiliar” faces. British Journal of Developmental Psychology. 2003;21:125–135. [Google Scholar]
- Weiss DJ, Kralik JD, Hauser MD. Face processing in cotton-top tamarins (Saguinus oedipus) Animal Cognition. 2001;4:191–205. [Google Scholar]
- Yin RK. Looking at upside-down faces. Journal of Experimental Psychology. 1969;81:141–145. [Google Scholar]