Abstract
Available studies indicate that both genetic background and aging influence collateral growth capacity, but it is not known how their combination affects collateral growth. We evaluated collateral growth induced by ileal artery ligation in Fischer 344 (F344), Brown Norway (BN), and the first generation hybrid of F344 × BN (F1) rats available for aging research from the National Institute on Aging. Collateral growth was determined by paired diameter measurements in anesthetized rats immediately and 7 days postligation. In 3-mo-old rats, significant collateral growth occurred only in BN (35% ± 11%, P < 0.001). The endothelial cell number in arterial cross sections was also determined, since this precedes shear-mediated luminal expansion. When compared with the same animal controls, the intimal cell number was increased only in BN rats (92% ± 21%, P < 0.001). The increase in intimal cell number and the degree of collateral luminal expansion in BN rats was not affected by age from 3 to 24 mo. Immunohistochemical studies demonstrated that intimal cell proliferation was much greater in the collaterals of BN than of F1 rats. The remarkable difference between these three strains of rats used in aging research and the lack of an age-related impairment in the BN rats are novel observations. These rat strains mimic clinical observations of interindividual variation in collateral growth capacity and the impact of age on arteriogenesis and should be useful models to investigate the molecular mechanisms responsible for such differences.
Keywords: strain dependent, endothelial proliferation, macrophage recruitment
Advancing age is considered a major risk factor for vascular disease (22), including peripheral arterial disease (32). Arteriosclerosis, the most common arterial disease, results in vascular stenosis/occlusion and reduced tissue perfusion. Natural compensation to arterial occlusion includes the enlargement of preexisting bypass vessels (collaterals) and the formation of new vessels (angiogenesis). Of the two processes, collateral growth is the more efficient in restoring tissue perfusion (38, 55, 60). Recent human (31) and preclinical (36, 51) studies have shown that the ability to compensate for arterial occlusion by collateral growth is impaired with advancing age. Yet, the specific mechanisms by which aging suppresses collateral growth remain poorly understood.
Previous work in our laboratory has demonstrated an impairment in collateral growth in retired breeder Wistar rats (~8 to 10 mo of age) (51). This impairment was associated with abnormal protein expression in collateral vessels relative to the same animal controls. The first generation cross between the Fischer 344 (F344) and Brown Norway (BN) rats (F344 × BN or F1) is a rodent model promoted by the National Institute on Aging (43) for aging research. Several studies have investigated the effects of aging on conduit vessels in this model. These studies have shown increased expression of matrix metalloproteinases (MMPs) and growth modulators (57, 58) associated with aberrant age-related aortic remodeling. However, the extent to which these observations extend into the distal vasculature and influence compensation to arterial occlusion has not been investigated. The present study was planned to investigate the impairment of collateral growth in the F1 model of aging.
Relative to our previous studies in Wistar and Wistar-Kyoto (WKY) rats (51–54), initial studies demonstrated a dramatic suppression of collateral growth in young F1 rats. Because the F344, BN, and F1 rats have all been used for vascular and aging studies and because previous studies have shown strain-related or genetic differences in response to arterial occlusion and myocardial ischemia (4, 17, 38), an investigation of collateral growth in the parent strains seemed appropriate. When only the young BN rats exhibited the ability to grow mesenteric collaterals, additional studies evaluated the effect of age in this strain, as originally planned for the F1 rats. Unexpectedly, collateral growth in this strain was not suppressed with age. Additional experiments demonstrated that impaired collateral growth in the F1 rat was associated with suppressed intimal cell proliferation. Together, these observations indicate that even young F344 and F1 rats have limited capacity to enlarge collaterals subsequent to arterial occlusion. In contrast, the BN rat does not show impairment in collateral growth, even at an advanced age. These strains provide unique rat models to investigate genetic determinants of collateral growth capacity.
MATERIALS AND METHODS
Male BN, F344, and F1 rats were obtained through the National Institute on Aging from Harlan Industries (Indianapolis, IN). The body weights, in grams, of young (3 mo) F1, BN, and F344 rats were 217 (SD 19), 203 (SD 5), and 248 (SD 9), respectively. Older BN rats had body weights of 339 (SD 32) and 473 g (SD 26) at 6 and 24 mo, respectively. All animal procedures were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. For both survival surgery and final experimentation, rats were anesthetized with pentobarbital sodium (50 mg/kg ip) and supplemented as needed. Preexisting ileal arteries were made to function as collaterals, as we have previously described (53). Briefly, the abdomen of the rat was shaved and prepped for attachment of a heated tissue support chamber. With the use of aseptic techniques, a midline abdominal incision was made and the terminal ileum exteriorized into the chamber. Extreme care was taken to prevent desiccation and distension of the mesentery, which result in angiogenesis. Three or four sequential arteries were ligated such that a segment of terminal ileum consisting of ~50 microvascular perfusion units (first-order arterioles when viewed from one side of the bowel) was perfused from a collateral on each side of the ligations. While observed under a dissecting microscope, monofilament suture (8-0) was used to ligate these arteries, leaving the accompanying vein intact. The intestines were returned to the abdominal cavity and the incision closed in two layers with a 4-0 suture. The animals were again anesthetized 1 to 7 days later, laparotomy was performed, and vessels were harvested as described in Tissue harvest for morphometric and immunohistochemical analysis.
Diameter measurements
Digital images were taken of experimental (collateral) arteries and nonadjacent control arteries using intravital microscopy techniques. The images were obtained immediately after arterial ligation and 1 wk later under maximally dilated conditions (topical suffusion with 0.1 mM adenosine and 0.01 mM sodium nitroprusside). Diameters were measured at each time by the width of the red cell column, and the percent change in control and collateral arteries was calculated.
Tissue harvest for morphometric and immunohistochemical analysis
The caudal aorta was cannulated with a double lumen catheter. After ligation of both renal arteries and the aorta cephalad to the superior mesenteric artery, the vena cava was transected and the mesenteric vasculature perfused with 30 ml of warm saline containing dilator (0.1 mM adenosine and nitroprusside), followed by freshly prepared paraformaldehyde (4%, pH 7.4). During this perfusion fixation, the aortic pressure was monitored through one catheter port and maintained at the mean arterial pressure of the animal. The terminal ileum and mesenteric vasculature were then excised and placed in paraformaldehyde. After 24 h, individual arteries were isolated and placed in physiological saline for tissue processing.
Immunohistochemical staining and evaluation
To assess macrophage recruitment in response to collateral development, paraffin-embedded vascular cross sections of both control and collateral arteries were reacted with a rat macrophage-specific antibody. After paraffin removal, rehydration, antigen retrieval, and peroxide and nonspecific protein blocking, sections were incubated overnight with mouse anti-rat ED-2 (CD163, 1:200; Serotec). This was followed by a 60-min incubation with rat adsorbed horse anti-mouse Biotinylated IgG (1:100; Vector), amplification with the Elite ABC Kit, and detection by diaminobenzidine staining (Vector). Sections were counterstained with Mayer’s hematoxylin, dehydrated, coverslipped, and imaged on a Leica DM 5000B fluorescent microscope with a Spot RT digital camera. Sections of rat spleen were used as positive controls. The number of ED-2 positive cells, as indicated by a dark brown color in the digitized images, in the intimal-medial layers and within 50 μm of the outer elastic lamina separating the media and adventitia were then counted via MetaMorph 6.1 software. The same procedure was then performed on control and collateral cross sections utilizing an antibody to von Willebrand factor (1:200; Chemicon) and to the cell proliferation antigen Ki-67 (1:50; Novocastra). The secondary antibody was the biotinylated anti-mouse IgG previously described. Sections of rat intestine were used as positive controls for Ki-67.
Morphometric analysis of wall areas and cell density
Vessel segments were dehydrated through a graded series of alcohols and embedded in JB-4 plastic (Polysciences). For morphological analysis of these small arteries, 3-μm-thick cross sections were made at 10-μm intervals and stained with methylene blue-basic fuchsin. Digital images of the entire arterial cross sections were acquired using a Leica microscope and assigned an alphanumeric code. Wall areas were measured from multiple sections of each artery using manual tracing or gray level thresholding techniques with MetaMorph image analysis software, as previously described (53). The total number of nuclei in the intima of each section was manually counted from these digital images. After these measurements were entered into a spreadsheet, the appropriate information for statistical analyses was added [i.e., strain and age and vessel type (control or collateral)].
Statistical analyses
ANOVA with SigmaStat 3.0 was used to evaluate all data. The Holm-Sidak method was used for pairwise multiple comparisons. Data are expressed as means ± SE except for body mass, where SD is reported.
RESULTS
Preliminary experiments performed to evaluate the effect of age on collateral growth in the F1 rat suggested a profound impairment in the capacity for collateral growth at ages 3, 12, and 18 mo (data not shown). This surprising finding is similar to what we have observed previously in spontaneously hypertensive rats (SHRs) (52). We next determined whether the capacity for mesenteric collateral artery growth differed between the F1 strain and both parent strains in young rats. These results are summarized in Fig. 1A. Repeated-measures (RM) ANOVA indicated no overall difference in percentage diameter change between strains but a significant effect between vessel types (control vs. collateral, P < 0.001) and an interaction between strain and vessel type. Control arteries of all strains displayed no significant change in diameter between observations. Similar to the F1 rats, F344 rats did not exhibit significant collateral enlargement (P = 0.142). In contrast, the BN rat exhibited a highly significant increase (35 ± 10.8%, P < 0.001) in collateral diameter compared with control arteries of the same animal. The percentage diameter increase in the BN collateral was also significantly greater than that observed in either F344 or F1 collaterals (P = 0.008 and 0.003, respectively).
Fig. 1.
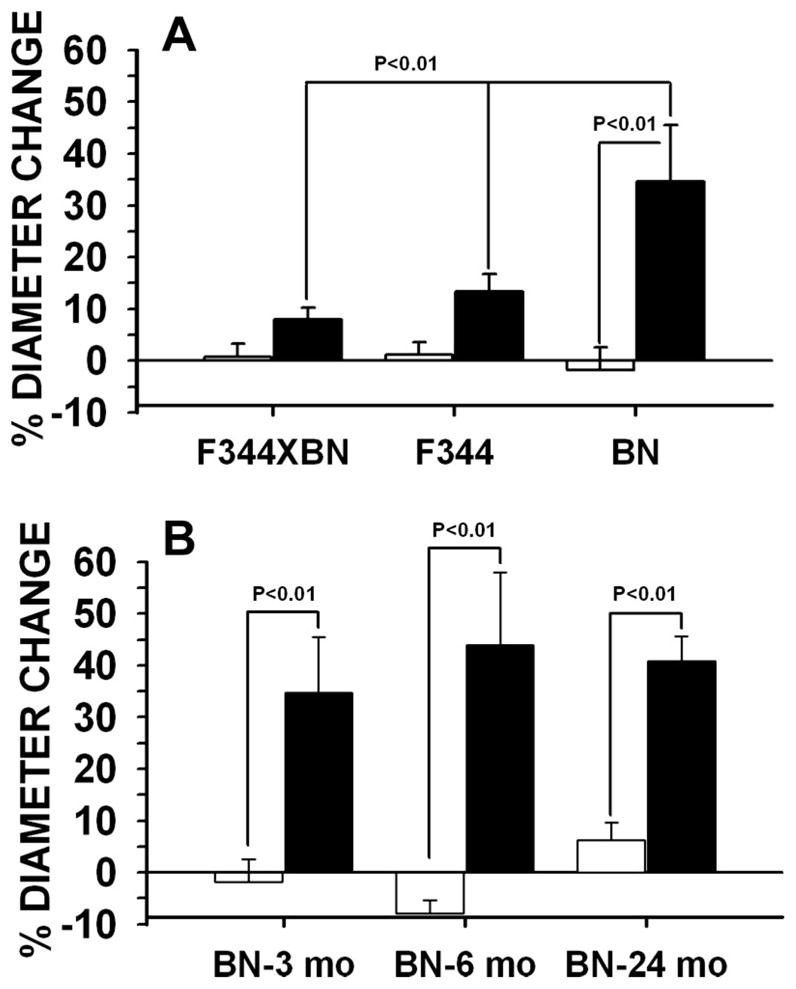
Percent change in passive diameter from immediately to 7 days postligation in control (white bars) and collateral (black bars) arteries of first-generation cross hybrid between Fischer 344 (F344) and Brown Norway (BN) (F1), F344, and BN rats at 3 mo (A) and BN rats at 3, 6, and 24 mo (B) (n = 6 for all groups).
Having established that collateral growth occurred in the young BN rats similar to what we have previously observed in Wistar and WKY rats, we sought to evaluate the effect of age on collateral growth in this strain. We had previously found that collateral growth was remarkably suppressed in retired breeder Wistar rats at age ~8 to 10 mo (51). Fig. 1B summarizes the results for 3-, 6-, and 24-mo-old BN rats. RM ANOVA indicated statistical significance between vessel type (P < 0.001) but no age effect (P = 0.703) or interaction between age and vessel type (P = 0.471).
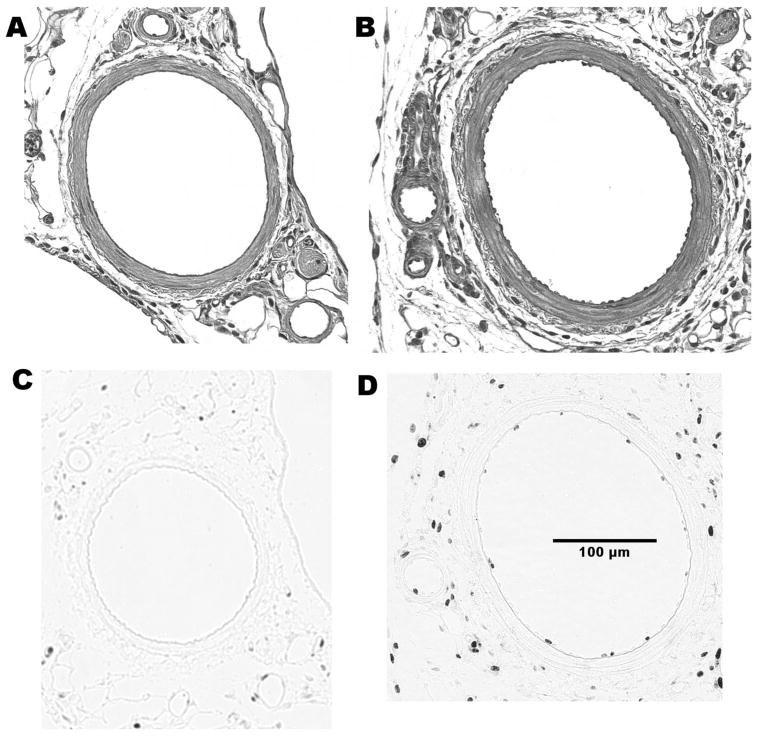
Representative cross sections of control and collateral arteries from F1, F344, and BN rats are illustrated in Fig. 2. Morphometric analyses were performed on cross sections of young (3 mo) F1, F344, and BN rats and older BN rats to identify differences between control and collateral arteries. These data are reported in Table 1. In young rats, luminal area was increased in the collateral relative to the same animal control arteries only in the BN rats (P < 0.001). This increase of luminal area in collaterals was observed for all ages of BN rats. These results are consistent with the in vivo diameter measurements reported in Fig. 1. Medial area was increased in collaterals of BN and F1 but not F344 rats. RM ANOVA of the intimal cell number in the arterial cross sections for 3-mo-old rats revealed differences between strains (P < 0.001) and vessel type (P < 0.001), with a significant interaction between strain and vessel type (P = 0.015). Multiple pairwise comparisons (Holm-Sidak method) indicated increased intimal cell number in collaterals relative to control arteries only for the BN rats (92 ± 21%, P < 0.001); however, cross-sectional endothelial cell number between collaterals and controls in the F1 and F344 rats tended to be different (P = 0.070 and 0.052, respectively). Between strains, there was a difference in intimal cell number in collaterals but not in control arteries of BN rats compared with the F1 and F344 rats (P < 0.001). Histological sections showed significant intimal changes at day 2 in BN collaterals, and the cell proliferation marker Ki-67 demonstrated significant immunoreactivity (Fig. 3). The number of Ki-67-positive nuclei in arterial cross sections was much greater in the BN than in the F1 collaterals (10.3 ± 1.08 vs. 2.5 ± 1.32, P = 0.004, n = 3).
Fig. 2.
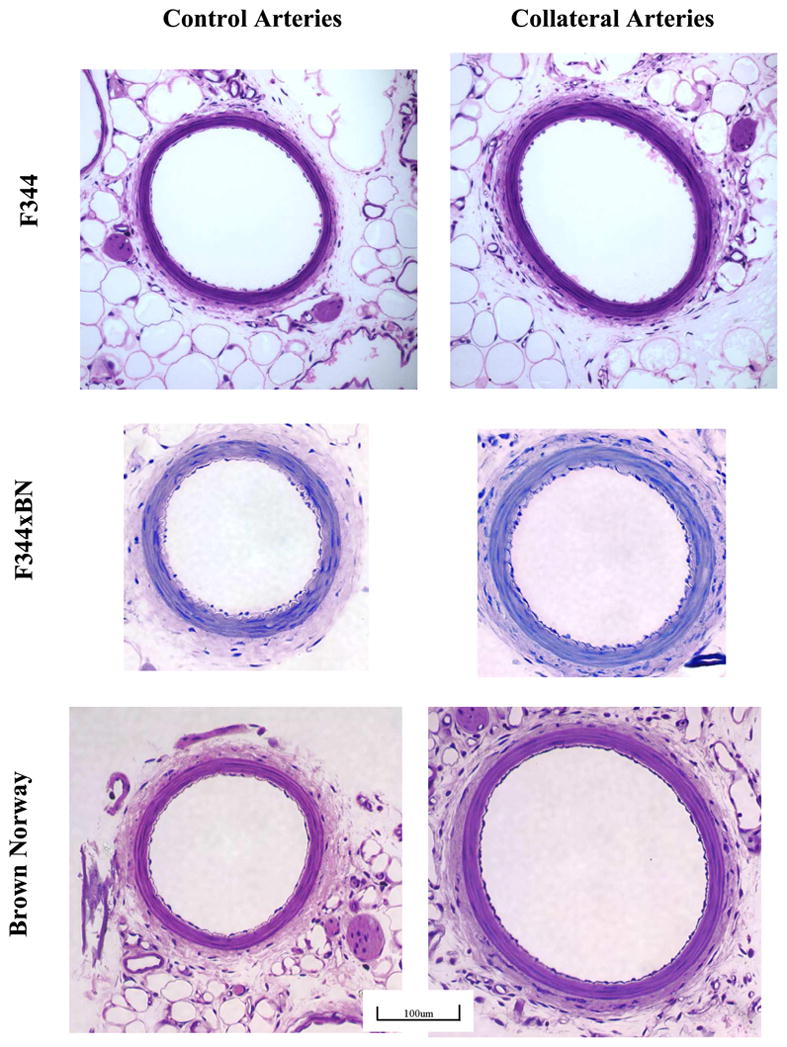
Representative micrographs of control and collateral artery cross sections after plastic embedding. Only the BN artery exhibited significantly larger lumens and greater intimal cell number in collaterals vs. controls. The scaling bar indicates magnification for all images. The calibration bar represents 100 μm.
Table 1.
Wall areas and intimal cell numbers
| Luminal Area × 103 μm |
Medial Area × 103 μm |
Intimal Cell Number |
||||
|---|---|---|---|---|---|---|
| Control | Collateral | Control | Collateral | Control | Collateral | |
| F1, 3 mo | 42.3±7.92 | 45.5±9.62 | 16.0±2.34 | 23.7±0.94* | 20.9±0.86 | 30.5±1.08† |
| F344, 3 mo | 43.8±2.78 | 52.1±5.82 | 16.5±2.04 | 18.7±0.77 | 22.7±1.08 | 30.5±1.25† |
| BN, 3 mo | 32.1±5.89 | 65.4±9.99* | 14.9±0.99 | 19.0±1.12* | 31.0±0.80 | 56.3±6.59* |
| BN, 6 mo | 59.4±4.35 | 89.3±13.90* | 16.4±1.20 | 21.5±1.42* | 22.8±0.97 | 38.3±3.88* |
| BN, 24 mo | 64.9±9.92 | 95.0±14.10*† | 20.7±1.51†‡ | 28.1±1.40* | 30.1±2.44 | 51.4±6.85* |
Values are means ± SE; n = 6 rats per group. F1, first-generation cross hybrid between Fischer 344 (F344) and Brown Norway (BN) rats. P < 0.01 for collateral vs.
same animal control,
3-mo BN, and
6-mo BN.
Fig. 3.
Representative micrographs of BN control (A and C) and collateral (B and D) artery cross sections after paraffin embedding and staining with hematoxylin-eosin (A and B) and the proliferation marker Ki-67 (C and D). Intimal changes were very apparent at 2 days postligation in the collaterals since nuclei were seen protruding much farther into the lumen than that in the control. Immunostaining verifies that this response includes intimal proliferation. There was no significant Ki-67 staining in BN or F1 controls, and the response was much greater in BN than in F1 collaterals. Immunostaining for von Willebrand factor was continuous and covered the entire intima in collateral vessels consistent with an endothelial phenotype.
Lastly, immunohistochemical staining with the macrophage-specific antibody ED-2 was performed to determine whether recruitment of macrophages occurred within or around the collateral wall of BN arteries. Figure 4 shows typical results for BN control and collateral arteries. ED-2-positive cells were observed in perivascular tissue but only rarely seen within the vascular wall itself. No differences were apparent in any of the paired micrographs of the same animal control and collateral vessels at either 1 or 3 days. The number of ED-2-positive cells in cross sections of control and collateral arteries, respectively, at 3 days postligation averaged 19 ± 0.6 and 18 ± 5.3 for BN and 14 ± 2.4 and 15 ± 2.8 for F1 (n = 4–6). There were no statistical differences between strains or vessel type (control vs. collateral).
Fig. 4.
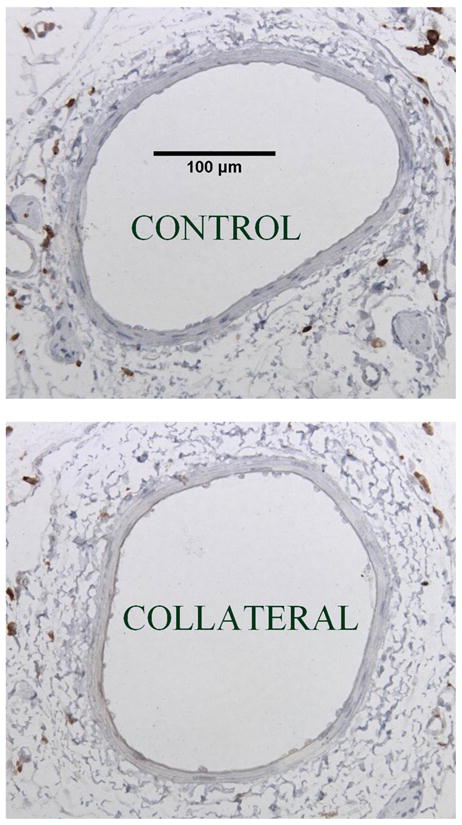
Representative micrographs of ED-2 immunostaining (brown) for macrophages in BN control and collateral arteries. The calibration scale is for both images. No differences were observed between control and collateral arteries in BN or F1 rats at 3 days postligation.
DISCUSSION
A genetic component of human vascular occlusive disease is well established (1, 56). At least for the coronary circulation, tremendous variability exists between individuals in the formation of collateral vessels (8, 21). In support of a genetic contribution to human collateral growth capacity, recent studies by Simons and collegues (8, 39) have identified humoral and macrophage markers for both extensive and poorly developed collaterals. Because aging is a major risk factor of vascular disease, it is especially important to understand how the combination of genetic background and aging influences vascular disease and collateral growth capacity. The identification of rat models of differing capacity for collateral growth could provide powerful models to expand our understanding of how genetic regulation determines remodeling capacity within the collateral vessels themselves as well as to identify potential plasma or circulating cell markers of collateral growth capacity. Four salient and novel observations of this study include 1) the remarkable difference in the capacity for collateral growth between these three rat models of aging, 2) the impaired ability of mesenteric collaterals to enlarge in the F344 and the F1 model of aging promoted by the National Institute on Aging, 3) collateral growth capacity in the BN rat is not suppressed by age, and 4) the differences in the capacity for outward expansion of the collateral lumen are associated with differences in endothelial cell proliferation and not macrophage recruitment.
We have previously developed a mesenteric model to evaluate collateral growth (53, 54). This model offers a number of advantages including a well-defined collateral pathway that is essentially two dimensional. The dissection of collateral and control arteries for morphometric and biochemical analyses is relatively easy. Relative to small mouse models, much larger amounts of blood and tissue are obtained for analyses. While variations in hindlimb vasculature exist between strains, and even in individuals within a strain, which can confound interpretation of results (61), our mesenteric model provides for repeated diameter measurements of an equivalent, preexisting collateral network between strains and individuals. Another advantage of the mesenteric model is that surgery is not performed directly on or immediately adjacent to the organ or tissues to be studied, resulting in the potential for local inflammation that could influence results.
Our past and present studies reveal three different responses in the growth and remodeling of mesenteric collaterals subsequent to abrupt arterial occlusion. In young male Wistar (54), WKY (52), and BN rats (Fig. 1 and Table 1), there is ~30–35% enlargement of the inner passive diameter within the first week after ligation. In the male Wistar (51) and WKY rats (unpublished observations), this capacity is essentially abolished by 8 to 10 mo of age (retired breeders). In the BN rats, the enlargement remains similar from 3 to 24 mo of age (Figure 1B). In contrast, in F344, F1 (Fig. 1 and Table 1), and SHRs (52), there is no significant growth even in young animals.
We believe that change in flow/shear rate in our model is important as a molding force if not a primary stimulus for collateral growth. In anesthetized animals, we have calculated wall shear rate to be elevated ~175% initially after arterial occlusion, to remain elevated at 1 wk, and to be near preocclusion levels by 4 wk postocclusion (53, 54). It is well documented by cell culture and in vivo studies that shear level influences gene expression in endothelial cells. Elevated laminar shear is atheroprotective and anti-inflammatory and increases endothelial NOS (eNOS) expression and nitric oxide production (50). Nitric oxide can have autocrine and paracrine effects on gene expression, cell growth, and matrix metabolism. Miyashiro et al. (29) have presented evidence that the shear-mediated induction of eNOS expression is diminished with age. We have previously shown that the protein expression pattern in collaterals relative to the same animal controls is different between young and retired breeder Wistar rats (51). Similar results were obtained between BN and F1 strains (unpublished observations). Differences in shear level between strains or sensitivity to the stimuli could explain these results. However, attempts to increase the size of the collateral-dependent region in the F1 resulted in a dusky-colored bowel acutely and subsequent ileus formation indicating that the insult was as great as could be tolerated. Other studies have reported differences in gene expression or nitric oxide production resulting from equivalent stimuli for these strains (4, 9). As we did not measure collateral flow in this study, it is possible that the flow-related stimulus could be less in the F344 and F1 rats than in the BN rats. However, even when the model was modified so that flow increased about half as much, significant alteration in gene expression was observed and luminal expansion occurred in young Wistar rats (50). Regardless, the results indicate that collateral growth for equivalent arterial ligation in terms of the number of microvascular perfusion units (first-order arterioles) is impaired in the F1 and F344 relative to BN rats and is not suppressed by age in the BN rat.
Successful shear-mediated remodeling requires an intact endothelium (23, 25), and endothelial activation and proliferation precede shear-mediated luminal expansion (26, 50). Collaterals of the BN but not the F344 or F1 rats show a significant increase in endothelial cell number (Table 1). The increase in endothelial cell number involves proliferation (Fig. 3) but could also involve recruitment of endothelial progenitor cells or migration of upstream endothelial cells, as proposed by Sho et al. (41). Differences in endothelial proliferation could result from either differences in levels of growth modulators or cell responsiveness. Studies that have administered growth factors to young and aged rodents have shown equivalent results (36, 59), thus implying that there is not an age-related reduction in cell responsiveness. Alternatively, macrophage recruitment during collateral growth may provide delivery of growth factors (2), and diminished recruitment has been associated with reduced collateral growth (16). However, we did not observe a difference in macrophage number in the BN or F1 collaterals relative to control arteries at 1 or 3 days postocclusion (Fig. 4). These results are consistent with the lack of MMP-9 that is expressed by macrophages in our model of collateral growth (14).
The lack of evidence for macrophage recruitment is surprising in that substantial evidence of a role for monocytes and inflammatory cytokines in modulating collateral growth exists, as reviewed by Buschmann et al. (7). However, in at least some cases, inflammatory mediators influence the rate of growth after an abrupt occlusion but not the final outcome (vascular conductance and collateral number) (18). We performed our assessment of macrophage number at day 3 postligation based on earlier studies that have shown a maximum recruitment after abrupt arterial ligation (2, 47). It is interesting that in some, (47) but not all, cases (45) the macrophages have disappeared by day 7. Tang et al. (46) have demonstrated that there is limited recruitment of inflammatory cells when arterial occlusion is produced gradually by an ameroid constrictor as opposed to abrupt ligation, yet compensation occurs. Together with the current study, this suggests that not all collateral growth is mediated by inflammatory cells. This concept is supported by recent work by Ishida et al. (20), who have shown that pathological but not physiological angiogenesis is mediated by inflammatory cells.
It seems likely that there are multiple and redundant mechanisms that modulate collateral growth, and the involved mechanisms may vary between organs and vascular beds and with stimulus level (e.g., abrupt vs. acute occlusion). We believe our model is definitely relevant to the clinical situation of mesenteric ischemia, which may develop as result of an embolis. But we also believe that this model serves as a general model for collateral growth. In support of this are studies that show a similar impairment in collateral growth in both the hindlimb and mesentery of SHRs (10, 27). In addition, we have observed similar histological results including a well-defined intima with increased endothelial cell density in vessels we have identified as primary collaterals in the hindlimbs of mice, rats, and pigs (unpublished observations). Such similarities suggest common mechanisms. However, vessels surrounded by ischemic tissue may respond differently than the bypass collaterals we have investigated.
Even though aging is a major risk factor for vascular disease, there are relatively few studies that have investigated age-related effects on the ability of the resistance vessels to compensate for vascular occlusion. Existing studies have consistently shown age-related impairment of endothelial function in mesenteric arteries and skeletal muscle arterioles (30, 44). However, studies of age-related effects on collateral growth have been inconsistent. Limited, if any, compensation to arterial occlusion in aged rodents has been reported in the hindlimb of mice and rabbits (36) and rat mesentery (51). These results, which demonstrate an age-related impairment in the enlargement of vessels exposed to elevated flow conditions, are consistent with results in larger vessels that have shown age-related suppression of shear-mediated outward remodeling (6, 29). However, aging is not always associated with impaired compensation, including collateral growth, as indicated in this study of BN rats. Also, Yang and Feng (59) did not observe age-related impairment of compensation to femoral artery ligation in the F344 strain from 11 to 23 mo (59).
Independent studies in the year 2000 presented compelling evidence that genetic background influences flow-mediated vascular remodeling (15) and growth factor-mediated angiogenesis (37). Since that time, additional studies in mice and rats have provided further evidence that mechanisms mediating vascular remodeling are under genetic control. Studies with mice (11, 17, 38, 61) have demonstrated that genetic background has a profound effect on both preexisting collateral vessels and the vascular growth that occurs in response to arterial occlusion in the mouse hindlimb. The results of this current study, which found striking differences in collateral growth between the BN and the F344 and F1 hybrid, are consistent with these observations. Dramatic differences between these three strains commonly used for aging research have also been reported for tumor growth and associated angiogenesis (9, 35).
Several studies have demonstrated unique responses involving cardiovascular structure and function within the BN rat. The BN rats have dramatic resistance to acute myocardial ischemia relative to four other strains (4). Of the six rat strains studied, the BN rat exhibited by far the greatest angiogenic response in the skeletal muscle after chronic electrical stimulation of the peroneal nerve (13). Relative to the Sprague-Dawley rat, the BN rat is more resistant to acute ischemic renal injury (5) and has greater capacity for retinal neovascularization (12). In terms of intimal remodeling, the BN rat ranked second in neointimal formation of 11 strains in response to iliac artery balloon injury (3) but does not develop age-related pathology within the aortic intima as observed in the F1 rat (28).
There are several distinctive characteristics of the BN rat, which may partly explain these responses and could also impact the capacity of the BN rat for collateral growth. The response to myocardial ischemia is correlated with increased nitric oxide production and the association of heat shock protein 90 with eNOS (40). Available studies have demonstrated that nitric oxide and the eNOS system are very important, if not essential, for collateral growth and flow-mediated outward remodeling (24, 49). Shear-mediated remodeling requires activation of MMPs (42, 48), and the BN rat vasculature is characterized by reduced elastin levels and elevated elastinolytic activity (34). ANG II can promote angiogenesis and collateral growth (19) and, in the BN, plasma ANG I-converting enzyme activity and ANG II levels are elevated and ANG 1–7 levels are reduced (33). Enhanced ischemia-induced retinal neovascularization in the BN rat relative to the Sprague-Dawley rat is associated with greater suppression and augmentation, respectively, of an inhibitor (pigment epithelium-derived factor) and promoter (vascular endothelial growth factor) of angiogenesis (12).
In conclusion, these results demonstrate major differences in the capacity for collateral growth in rat strains available for aging research from the National Institutes of Health. Together with our previous work, we have identified rat strains that display different effects of age on collateral growth (i.e., BN vs. Wistar rats). These rodent strains mimic clinical observations of interindividual variation in collateral growth capacity and impact of age on compensation. Future investigations are warranted to identify specific genetic, molecular, and cellular differences within the collateral vessels and surrounding tissues and organs as well as the whole animal, which mediate these differences in collateral growth capacity.
Acknowledgments
The excellent technical assistance from Randal G. Bills and P. Melanie Pride is gratefully acknowledged as are the excellent histological services provided by Jennifer Stashevsky.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-42898 (to J. L. Unthank) and AG-22691 (to S. J. Miller) and a dissertation support grant from the National Institute on Aging (to M. J. Ferguson).
References
- 1.Aronson DC, Ruys T, van Bockel JH, Briet E, Brommer EJ, Gevers Leuven JA, Kempen HJ, Feuth JD, Giesberts MA. A prospective survey of risk factors in young adults with arterial occlusive disease. Eur J Vasc Surg. 1989;3:227–232. doi: 10.1016/s0950-821x(89)80087-8. [DOI] [PubMed] [Google Scholar]
- 2.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assadnia S, Rapp JP, Nestor AL, Pringle T, Cerilli GJ, Gunning WT, III, Webb TH, Kligman M, Allison DC. Strain differences in neointimal hyperplasia in the rat. Circ Res. 1999;84:1252–1257. doi: 10.1161/01.res.84.11.1252. [DOI] [PubMed] [Google Scholar]
- 4.Baker JE, Konorev EA, Gross GJ, Chilian WM, Jacob HJ. Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardioprotection? Am J Physiol Heart Circ Physiol. 2000;278:H1395–H1400. doi: 10.1152/ajpheart.2000.278.4.H1395. [DOI] [PubMed] [Google Scholar]
- 5.Basile DP, Donohoe D, Cao X, Van Why SK. Resistance to ischemic acute renal failure in the Brown Norway rat: a new model to study cytoprotection. Kidney Int. 2004;65:2201–2211. doi: 10.1111/j.1523-1755.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee RD, Langille BL. Arterial adaptations to altered blood flow. Can J Physiol Pharmacol. 1991;69:978–983. doi: 10.1139/y91-147. [DOI] [PubMed] [Google Scholar]
- 7.Buschmann I, Heil M, Jost M, Schaper W. Influence of inflammatory cytokines on arteriogenesis. Microcirculation. 2003;10:371–379. doi: 10.1038/sj.mn.7800199. [DOI] [PubMed] [Google Scholar]
- 8.Chittenden TW, Sherman JA, Xiong F, Hall AE, Lanahan AA, Taylor JM, Duan H, Pearlman JD, Moore JH, Schwartz SM, Simons M. Transcriptional profiling in coronary artery disease: indications for novel markers of coronary collateralization. Circulation. 2006;114:1811–1820. doi: 10.1161/CIRCULATIONAHA.106.628396. [DOI] [PubMed] [Google Scholar]
- 9.Cracchiolo D, Swick JW, McKiernan L, Sloan E, Raina S, Sloan C, Wendell DL. Estrogen-dependent growth of a rat pituitary tumor involves, but does not require, a high level of vascular endothelial growth factor. Proc Soc Exp Biol Med. 2002;227:492–499. doi: 10.1177/153537020222700714. [DOI] [PubMed] [Google Scholar]
- 10.Emanueli C, Salis MB, Stacca T, Pinna A, Gaspa L, Spano A, Madeddu P. Ramipril improves hemodynamic recovery but not microvascular response to ischemia in spontaneously hypertensive rats. Am J Hypertens. 2002;15:410–415. doi: 10.1016/s0895-7061(01)02332-9. [DOI] [PubMed] [Google Scholar]
- 11.Fukino K, Sata M, Seko Y, Hirata Y, Nagai R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun. 2003;310:143–147. doi: 10.1016/j.bbrc.2003.08.134. [DOI] [PubMed] [Google Scholar]
- 12.Gao G, Li Y, Fant J, Crosson CE, Becerra SP, Ma JX. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium-derived factor in Brown Norway and Sprague Dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes. 2002;51:1218–1225. doi: 10.2337/diabetes.51.4.1218. [DOI] [PubMed] [Google Scholar]
- 13.Greene AS. Application of physiological genomics to the microcirculation. Microcirculation. 2002;9:3–12. doi: 10.1038/sj.mn.7800117. [DOI] [PubMed] [Google Scholar]
- 14.Haas TL, Doyle JL, Distasi MR, Norton LE, Sheridan KM, Unthank JL. Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am J Physiol Heart Circ Physiol. 2007;292:H2429–H2437. doi: 10.1152/ajpheart.00100.2007. [DOI] [PubMed] [Google Scholar]
- 15.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156:1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking cc-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 17.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 18.Hoefer IE, van Royen N, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–617. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 19.Ichiki T. Role of renin angiotensin system in angiogenesis: it is still elusive. Arterioscler Thromb Vasc Biol. 2004;24:622–624. doi: 10.1161/01.ATV.0000125707.94116.a4. [DOI] [PubMed] [Google Scholar]
- 20.Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng YS, D’Amore PA, Shima DT, Adamis AP. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–489. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koerselman J, van der Graaf Y, de Jaegere PPT, Grobbee DE. Coronary collaterals: an important and underexposed aspect of coronary artery disease. Circulation. 2003;107:2507–2511. doi: 10.1161/01.CIR.0000065118.99409.5F. [DOI] [PubMed] [Google Scholar]
- 22.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 23.Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2001;281:H2528–H2538. doi: 10.1152/ajpheart.2001.281.6.H2528. [DOI] [PubMed] [Google Scholar]
- 25.Masuda H, Kawamura K, Sugiyama T, Kamiya A. Effects of endothelial denudation in flow-induced arterial dilatation. Front Med Biol Eng. 1993;5:57–62. [PubMed] [Google Scholar]
- 26.Masuda H, Kawamura K, Tohda K, Shozawa T, Sageshima M, Kamiya A. Increase in endothelial cell density before artery enlargement in flow-loaded canine carotid artery. Atherosclerosis. 1989;9:812–823. doi: 10.1161/01.atv.9.6.812. [DOI] [PubMed] [Google Scholar]
- 27.Miller SJ, Norton LE, Murphy MP, Dalsing MC, Unthank JL. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat mesenteric collateral growth impairment. Am J Physiol Heart Circ Physiol. 2007;292:H2523–H2531. doi: 10.1152/ajpheart.01296.2006. [DOI] [PubMed] [Google Scholar]
- 28.Miller SJ, Sheridan KM, Ferguson MJ, Labarrere CA, Unthank JL. Development of rat aorta intimal pathology is age and strain dependent (Abstract) FASEB J. 2004;18:A1192. [Google Scholar]
- 29.Miyashiro JK, Poppa V, Berk BC. Flow-induced vascular remodeling in the rat carotid artery diminishes with age. Circ Res. 1997;81:311–319. doi: 10.1161/01.res.81.3.311. [DOI] [PubMed] [Google Scholar]
- 30.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 31.Nakae I, Fujita M, Miwa K, Hasegawa K, Kihara Y, Nohara R, Miyamoto S, Ueda K, Tamaki S, Sasayama S. Age-dependent impairment of coronary collateral development in humans. Heart Vessels. 2000;15:176–180. doi: 10.1007/pl00007269. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB. Peripheral arterial disease: insights from population studies of older adults. J Am Geriatr Soc. 2000;48:1157–1162. [PubMed] [Google Scholar]
- 33.Ocaranza MP, Palomera C, Roman M, Bargetto J, Lavandero S, Jalil JE. Effect of hypertension on angiotensin-(1–7) levels in rats with different angiotensin-I converting enzyme polymorphism. Life Sci. 2006;78:1535–1542. doi: 10.1016/j.lfs.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Osborne-Pellegrin MJ, Farjanel J, Hornebeck W. Role of elastase and lysyl oxidase activity in spontaneous rupture of internal elastic lamina in rats. Arteriosclerosis. 1990;10:1136–1146. doi: 10.1161/01.atv.10.6.1136. [DOI] [PubMed] [Google Scholar]
- 35.Pandey J, Cracchiolo D, Hansen FM, Wendell DL. Strain differences and inheritance of angiogenic versus angiostatic activity in oestrogen-induced rat pituitary tumours. Angiogenesis. 2002;5:53–66. doi: 10.1023/a:1021550211921. [DOI] [PubMed] [Google Scholar]
- 36.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 37.Rohan RM, Fernandez A, Udagawa T, Yuan J, D’Amato RJ. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14:871–876. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- 38.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 39.Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M. Humoral and cellular factors responsible for coronary collateral formation. Am J Cardiol. 2006;98:1194–1197. doi: 10.1016/j.amjcard.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y, Hutchins W, Ogawa H, Chang CC, Pritchard J, Zhang C, Khampang P, Lazar J, Jacob HJ. Increased resistance to myocardial ischemia in the Brown Norway vs. Dahl S rat: role of nitric oxide synthase and Hsp90. J Mol Cell Cardiol. 2005;38:625–635. doi: 10.1016/j.yjmcc.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Sho E, Komatsu M, Sho M, Nanjo H, Singh TM, Xu C, Masuda H, Zarins CK. High flow drives vascular endothelial cell proliferation during flow-induced arterial remodeling associated with the expression of vascular endothelial growth factor. Exp Mol Pathol. 2003;75:1–11. doi: 10.1016/s0014-4800(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 42.Sho E, Sho M, Singh TM, Nanjo H, Komatsu M, Xu C, Masuda H, Zarins CK. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp Mol Pathol. 2002;73:142–153. doi: 10.1006/exmp.2002.2457. [DOI] [PubMed] [Google Scholar]
- 43.Sprott RL, Ramirez I. Current inbred and hybrid rat and mouse models for gereontological research. Ins Anim Res J. 1997;38:104–109. doi: 10.1093/ilar.38.3.104. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan JC, Loomis ED, Collins M, Imig JD, Inscho EW, Pollock JS. Age-related alterations in NOS and oxidative stress in mesenteric arteries from male and female rats. J Appl Physiol. 2004;97:1268–1274. doi: 10.1152/japplphysiol.00242.2004. [DOI] [PubMed] [Google Scholar]
- 45.Tang G, Charo DN, Wang R, Charo IF, Messina L. CCR2−/− knockout mice revascularize normally in response to severe hindlimb ischemia. J Vasc Surg. 2004;40:786–795. doi: 10.1016/j.jvs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005;41:312–320. doi: 10.1016/j.jvs.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(p)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 48.Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20:E120–E126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 49.Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996;16:1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- 50.Tuttle JL, Condict KW, Bhuller AS, Nachreiner RD, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear-level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281:H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- 51.Tuttle JL, Hahn TL, Sanders BM, Witzmann FA, Miller SJ, Dalsing MC, Unthank JL. Impaired collateral development in mature rats. Am J Physiol Heart Circ Physiol. 2002;283:H146–H155. doi: 10.1152/ajpheart.00766.2001. [DOI] [PubMed] [Google Scholar]
- 52.Tuttle JL, Sanders BM, Burkhart HM, Fath SW, Herring BP, Dalsing MC, Unthank JL. Impaired collateral artery development in spontaneously hypertensive rats. Microcirculation. 2002;9:343–351. doi: 10.1038/sj.mn.7800151. [DOI] [PubMed] [Google Scholar]
- 53.Unthank JL, Fath SW, Burkhart HM, Miller S, Dalsing MC. Wall remodeling during luminal expansion of mesenteric arterial collaterals in the rat. Circ Res. 1996;79:1015–1023. doi: 10.1161/01.res.79.5.1015. [DOI] [PubMed] [Google Scholar]
- 54.Unthank JL, Nixon JC, Burkhart HM, Fath SW, Dalsing MC. Early collateral and microvascular adaptations to intestinal artery occlusion in the rat. Am J Physiol Heart Circ Physiol. 1996;271:H914–H923. doi: 10.1152/ajpheart.1996.271.3.H914. [DOI] [PubMed] [Google Scholar]
- 55.Unthank JL, Nixon JC, Lash JM. Early adaptations in collateral and microvascular resistances after ligation of the rat femoral artery. J Appl Physiol. 1995;79:73–82. doi: 10.1152/jappl.1995.79.1.73. [DOI] [PubMed] [Google Scholar]
- 56.Valentine RJ, Guerra R, Stephan P, Scoggins E, Clagett GP, Cohen J. Family history is a major determinant of subclinical peripheral arterial disease in young adults. J Vasc Surg. 2004;39:351–356. doi: 10.1016/j.jvs.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 58.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-β1 (TGF- β1) and TGF- β1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 59.Yang HT, Feng Y. bFGF increases collateral blood flow in aged rats with femoral artery ligation. Am J Physiol Heart Circ Physiol. 2000;278:H85–H93. doi: 10.1152/ajpheart.2000.278.1.H85. [DOI] [PubMed] [Google Scholar]
- 60.Yang HT, Ren J, Laughlin MH, Terjung RL. Prior exercise training produces NO-dependent increases in collateral blood flow after acute arterial occlusion. Am J Physiol Heart Circ Physiol. 2002;282:H301–H310. doi: 10.1152/ajpheart.00160.2001. [DOI] [PubMed] [Google Scholar]
- 61.Zbinden S, Clavijo LL, Kantor B, Morsli H, Cortes GA, Andrews JA, Jang JG, Burnett MS, Epstein SE. Inter-animal variability in preexisting collaterals is a major factor determining outcome in experimental angiogenesis trials. Am J Physiol Heart Circ Physiol. 2007;292:H1891–H1897. doi: 10.1152/ajpheart.00537.2006. [DOI] [PubMed] [Google Scholar]