Abstract
Objective
Nephrogenic systemic fibrosis (NSF) is a clinical syndrome linked with exposure in renal failure patients to gadolinium-based contrast agents (GBCAs) during magnetic resonance imaging. Recently, we demonstrated that GBCA exposure led to increased matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) levels in human skin fibroblasts. The goals of the present work were to assess the relationship between altered MMP-1 / TIMP-1 expression and collagen production / deposition, and the intracellular signaling events that lead from GBCA stimulation to altered MMP-1 and TIMP-1 production.
Materials and Methods
Human dermal fibroblasts were treated with one of the currently used GBCAs (Omniscan). Proliferation was quantified as were levels of MMP-1, TIMP-1, procollagen type I and collagen type I. Signaling events were concomitantly assessed, and signaling inhibitors were used.
Results
Fibroblasts exposed to Omniscan had increases in both MMP-1 and TIMP-1 levels. Omniscan treatment interfered with collagen turnover, leading to increased type I collagen deposition without an increase in type I procollagen production. U0126, an inhibitor of mitogen-activated protein kinase signaling, and LY294002, a phosphatidylinositol-3 kinase inhibitor, reduced MMP-1 levels. U0126 also reduced TIMP-1 levels, but LY294002 increased TIMP-1.
Conclusion
These data provide evidence for complex regulation of collagen deposition in Omniscan-treated skin. They suggest that the major effect of Omniscan exposure is on an enzyme / inhibitor system that regulates collagen breakdown rather than on collagen production, per se.
Keywords: gadolinium-based contrast agent (GBCA), matrix metalloproteinase-1 (MMP-1), tissue inhibitor of metalloprotienases-1 (TIMP-1), mitogen-activated protein (MAP) kinase, phosphatidylinositol-3 (PI3) kinase
INTRODUCTION
Nephrogenic systemic fibrosis (NSF) is a clinical syndrome linked in individuals with renal failure to exposure to gadolinium-based contrast agents (GBCAs) during magnetic resonance imaging (MRI) procedures (1–8). The disease has been likened to scleroderma based on histopathology, which includes the presence of dense, thickened collagen bundles in lesional skin. However, most pathologists describes the lesions as fibroplastic (1,2,9,10), based on the presence of numerous, “plump” fibroblast-like cells. Some studies have reported only a minor increase in the number of proliferating interstitial cells, while others have reported a florid fibroplasia, resembling the cellular phase of acute wound healing (11).
The mechanistic events that lead from GBCA exposure to NSF are not fully understood. Some investigators have suggested an important role for circulating fibrocytes (12,13) while others have noted the pro-inflammatory changes in kidney disease and suggested that these changes may contribute to disease pathophysiology (14). Delineating the critical pathophysiological events in NSF has been slow (in part, at least) because there are no models that precisely mimic the disease. Recently, Sieber et al (15,16) showed that some of the features of NSF could be seen in healthy rats following repeated injection with various chelated gadolinium moieties. In another study, Edward et al. (17) demonstrated increased proliferation of human dermal fibroblasts following treatment with chelated gadolinium.
Our own recent study used human skin in organ culture as a model for assessing effects of GBCA treatment on human skin (18). Our study demonstrated that exposure of human skin in organ culture (or human dermal fibroblasts in monolayer culture) to the clinically-used GBCAs resulted in increased production of both matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1). Interestingly, while a quantitatively and qualitatively similar response was seen with four different GBCAs, there were significant differences in the concentrations required for response. Specifically, response to Omniscan occurred over a concentration range of 2.5 to 500 µM. With Magnevist and Multihance, responses were observed at 25 to 5000 µM; with Prohance, responses were observed at 25 mM and above. The observed concentration-dependent differences are of interest in that they correlate with apparent differences in stability of the compounds as reported by others (19,20). Based on these observations, it was concluded that resident fibroblasts are themselves a target of GBCA stimulation, and the response to stimulation involves alterations in an enzyme / inhibitor system that regulates collagen turnover in the skin. The present study continues our effort to understand how GBCA exposure influences fibroblast function. Here we demonstrate that inhibiting mitogen-activated protein (MAP) kinase signaling blocks the elevation of both MMP-1 and TIMP-1, resulting from stimulation with one of the clinically used GBCAs (Omniscan). Inhibiting phosphatidylinositol-3 (PI3) kinase signaling also suppresses MMP-1 but further enhances TIMP-1 expression, in contrast to MAP kinase inhibition. Consistent with these results, Omniscan treatment does not stimulate production of type I procollagen – it leads to increased deposition of intact collagen in the cell layer. Our conclusion is that a primary effect of Omniscan treatment is on collagen breakdown rather than on its production.
MATERIALS AND METHODS
Omniscan and other reagents
Omniscan (GE Healthcare) was obtained from In-Patient Pharmacy at the University of Michigan Hospitals. Omniscan is a sterile aqueous solution of gadodiamide at 287 mg per mL (0.5M with respect to gadodiamide) along with 12 mg per mL of sodium caldiamide (same chelator but without gadolinium), suitable for injection. U0126, LY294002, and antibodies against phosphor ERK, total ERK, phosphor AKT and total AKT were obtained from Cell Signaling Technologies. TGF-β was obtained from R&D Systems. Antibodies against human type I collagen and human MMP-1 were obtained from Abcam and Chemicon, respectively. Antibody against β-tubulin was from Santa Cruz Biotechnology.
Human dermal fibroblasts in monolayer culture
Human foreskin tissue was obtained at circumcisions performed at the University of Michigan Hospitals. De-identified tissue samples were obtained under a waver of IRB approval. Fibroblasts were grown using Dulbecco's Modified Minimal Essential Medium supplemented with nonessential amino acids and 10% fetal bovine serum (DMEM-FBS) as culture medium. Maintenance was at 37°C in an atmosphere of 95% air and 5% CO2. Cells were used at passage 2–3. The isolation and handling of dermal fibroblasts has been described previously (21).
Proliferation assay
Fibroblasts were added to wells of a 24-well dish, at 2×104 cells per well in DMEM-FBS, and allowed to attach. The wells were then washed two additional times with 1 mL of keratinocyte growth medium (KGM) (Lonza) supplemented with extracellular Ca2+ to a final concentration of 1.5 mM. KGM is a low-Ca2+ (0.15 mM) modification of MCDB-153 medium supplemented with a mixture of growth factors, including EGF, insulin, and bovine pituitary extract. Our past studies have demonstrated that when KGM is supplemented with extracellular Ca2+ to a final concentration of 1.4 mM, it provides a potent fibroblast growth medium (21). After washing, duplicate wells were counted to provide accurate “zero-time” values. One mL of Ca2+-supplemented KGM was added to each remaining well. Omniscan was added at the desired amount with or without additional inhibitors as indicated in the Results Section. Incubation was for 3 days at 37°C in an atmosphere of 95% air and 5% CO2. At the end of the incubation period, culture fluids were harvested for assessment of MMP-1, TIMP-1, and type I procollagen, as described below. Cells were harvested with trypsin-EDTA and counted.
In other experiments, cells were added to wells of a 6-well dish at 3×105 cells per well. Cell attachment and growth as well as treatment with Omniscan with and without additional agents was exactly as above. However, instead of harvesting and counting cells at the end of the 3-day incubation period, the cell layer was washed one time in PBS and then treated with a “lysis buffer” (Pierce Biotech) according to the manufacturer’s instructions. Type I collagen was assessed in the lysate as described below.
MMP-1 production
Western blotting with a rabbit polyclonal anti-MMP-1 antibody was used to assess MMP-1 levels (22). Briefly, samples were separated in SDS-PAGE under denaturing and reducing conditions and transferred to nitrocellulose membranes. After blocking with a 5% nonfat milk solution in Tris-buffered saline with 0.1% Tween (TTBS) at 4°C overnight, membranes were incubated for one hour at room temperature with the desired antibody, diluted 1:1000 in 5% nonfat milk / TTBS. Thereafter, the membranes were washed with TTBS and bound antibody detected using the Phototope-HRP Western blot detection kit (Cell Signaling Technologies, Inc.). Images were scanned, digitized, and quantified. Prior to loading the gels, protein levels in each sample were determined using the BCA protein determination kit (Pierce Biotechnology) and equal amounts of protein were loaded onto each lane. In some experiments, the nitrocellulose filters were exposed to the Ponceau S reversible staining solution (Pierce Biotechnology) to visualize the transferred proteins and confirm that comparable amounts of total protein were transferred.
TIMP-1 production
Culture fluids were assayed for TIMP-1 by enzyme-linked immunosorbent assay (ELISA) using a commercially-available assay kit (R&D Systems) (22,23).
Type I procollagen
Culture fluids were assayed for type I procollagen by ELISA (Takara Bio, Inc.) as described previously (22). Type I procollagen contains the N- and C-terminal peptide sequences that are present at synthesis and, therefore, provides a measure of newly synthesized collagen precursor.
Type I collagen
To assess type I collagen deposited in the cell layer, cell lysates were prepared using lysis buffer as described above. Total protein content of the cell lysates was determined and 20 µg of total protein was loaded and resolved using acrylamide gels. Gels were resolved in the presence of SDS and reducing buffer. Protein was transferred to nitrocellulose membranes and probed with a rabbit anti-human type I collagen antibody (Abcam) using a procedure similar to that described above for MMP-1.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed to assess transcript levels for the following genes: type I procollagen (α1 chain), lysyl oxidase, procollagen N-propeptidase, procollagen C-proteinase enhancer, MMP-1, and TIMP-1. Preparation of the RNA samples and transcript assessment were carried out as previously described (24–26) using reagents and TaqMan Gene Expression primers and probes from Applied Biosystems.
Cell signaling
Fibroblasts were plated in 6-well tissue culture dishes, with 3×105 cells per well as described above, and allowed to attach. Cells were then washed twice with DPBS and incubated in growth medium with or without Omniscan and additional reagents as described in the Results section. At various times later (5 minutes to 48 hours), cells were lysed as above. After protein determination, equal amounts of protein were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were probed for phosphor- and total-ERK and phosphor- and total-AKT. Images on film were scanned and digitized, and the digital images were quantified. For ERK, which consists of two forms (p44 and p42), both forms were combined in the quantitation. The value for the 5-minute control was arbitrarily set at 1.0 and the other values, including the control values at other time-points, were expressed as a change relative to the 5-minute control value.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni post-test for selected pairs (GraphPad Prism 4.0 for Windows, GraphPad Software). Data were considered significant at p< 0.05. Asterisks have been added to the appropriate bars in each figure to denote values that are significantly higher than the respective control values.
RESULTS
Human dermal fibroblast response to Omniscan stimulation
In a recent study (18), human dermal fibroblasts from both adult skin and neonatal foreskin were examined for response to Omniscan, one of the commonly used GBCAs. Cells from both sources demonstrated a proliferative response over a wide range of Omniscan concentrations (0.25 µM to 25 mM), with a peak response at 25–250 µM. Enhanced proliferation was observed with cells incubated in either of two highly enriched growth media. In conjunction with the proliferative response, there were increased amounts of MMP-1 and TIMP-1 released into the culture fluid (18). In the present studies, we assessed the same three parameters using two concentrations of Omniscan from the middle of the concentration curve (50 and 250 µM). Consistent with our past findings, cell growth, MMP-1 levels, and TIMP-1 levels were increased in fibroblasts exposed to either concentration. Proliferation was increased at the two Omniscan concentrations by 40% and 118% respectively under conditions in which control cells were already rapidly growing. MMP-1 levels were increased by 2.7-fold and 3.3-fold over control levels; TIMP-1 was increased by 1.9-fold and 2.3-fold over control (not shown). The increase in MMP-1 and TIMP-1 likely reflect transcriptional changes, since increased transcript levels for both proteins were seen in the Omniscan-treated cells (Table 1).
Table 1.
Transcripts for type I procollagen, collagen-processing enzymes, MMP-1, and TIMP-1 in Omniscan-treated fibroblasts.
| Transcript | 50 µM Omniscan | 250 µM Omniscan |
|---|---|---|
| MMP-1 | 5.7 ± 0.9* | 8.9 ± 0.8* |
| TIMP-1 | 2.1 ± 0.3* | 2.5 ± 0.1* |
| Type I procollagen | No change | No change |
| Lysyl oxidase | No change | No change |
| Procollagen-N-propeptidase | No change | No change |
| Procollagen C-proteinase enhancer | No change | No change |
Fibroblasts were incubated for 3 days in growth factor (EGF and insulin)-enriched, serum-free culture medium and treated with Omniscan. At the end of the incubation period, RNA was prepared and processed for RT-PCR as described in the Materials and Methods section. The results are presented as fold-change relative to the control and based on three independent assessments for each transcript.
indicates statistically significant increase compared to control at p<0.05 level.
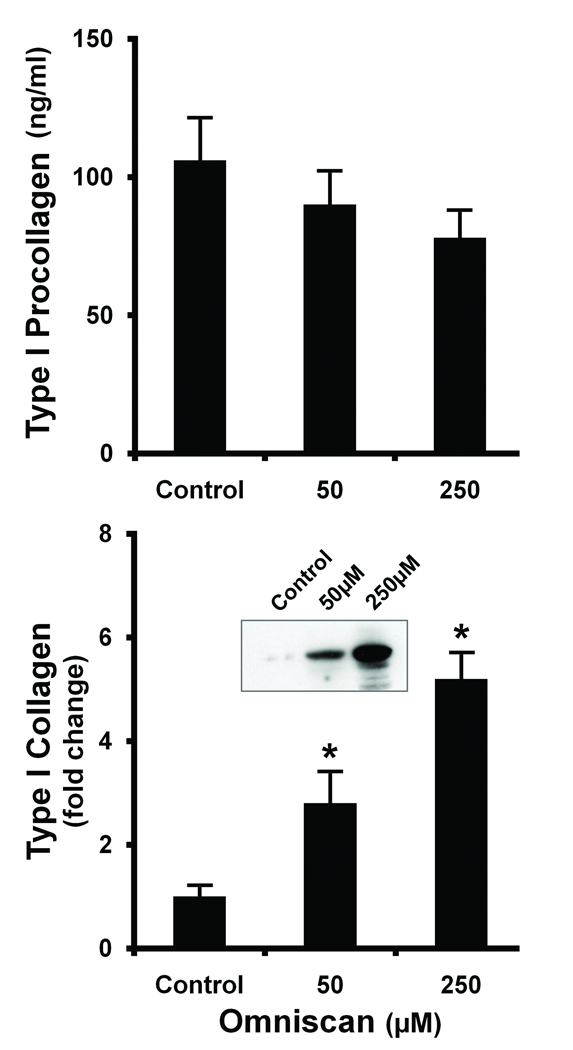
Figure 1 compares elaboration of type I procollagen and deposition of intact type I collagen in the cell layer of fibroblasts exposed to Omniscan (50 and 250 µM). In contrast to the effects on MMP-1 and TIMP-1, there was no increase in the level of type I procollagen with either concentration of Omniscan. In fact, levels of type I procollagen were actually lower in the culture fluid from cells exposed to Omniscan than in culture fluid from the control cells (upper panel). In additional experiments (not shown), we assessed type I procollagen levels over the Omniscan concentration range from 2.5 µM to 5 mM; no increase was detected at any concentration. Since there was no detectable change in the level of type I procollagen protein, RT-PCR was used to assess transcript levels; no increase in the transcript level for type I procollagen (α1 chain) was observed (Table 1). There were also no differences in transcript levels for several of the enzymes needed for processing procollagen into the mature form (Table 1).
Figure 1. Production of type I procollagen and deposition of type I collagen in the cell layer in Omniscan-treated cells.
Cells were incubated under control conditions or in the presence of Omniscan for three days. At the end of the incubation period, cell culture fluid was collected and assayed for type I procollagen by ELISA (Upper panel). Lysates were prepared from the cell layer (Lower panel) and assayed for type I collagen by western blotting. Values shown are means and standard errors based on n=5 separate experiments. The inset showing intact type I collagen is from one of the replicate experiments with β-tubulin (no change) used as control. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant increase compared to control at p<0.05 level.
The lower panel of Figure 1 presents results of western blot studies for intact type I collagen deposited in the cell layer of control and Omniscan-stimulated fibroblasts. An increase in type I collagen deposition was seen in the Omniscan-treated cells under conditions in which there was no increase in the level of type I procollagen. In control experiments, fibroblasts were exposed to TGF-β at 1–10 ng/mL. As expected, TGF-β induced an increase in type I procollagen production (4.1-fold over control) as well as an increase in the level of intact collagen (approximately 2-fold over control). MMP-1 was decreased by greater than 50% in response to TGF-β; there was little change in the TIMP-1 level. These responses to TGF-β stimulation are consistent with findings that are well-established in the literature (27,28). Taken together, the data presented here suggest that fibroblasts respond to Omniscan exposure with altered expression of an enzyme / inhibitor system that regulates collagen turnover in the skin rather than by a change in type I procollagen production, per se. The response to Omniscan stimulation does not appear to involve TGF-β as a critical intermediate.
Intracellular signaling pathways activated in fibroblasts by Omniscan exposure
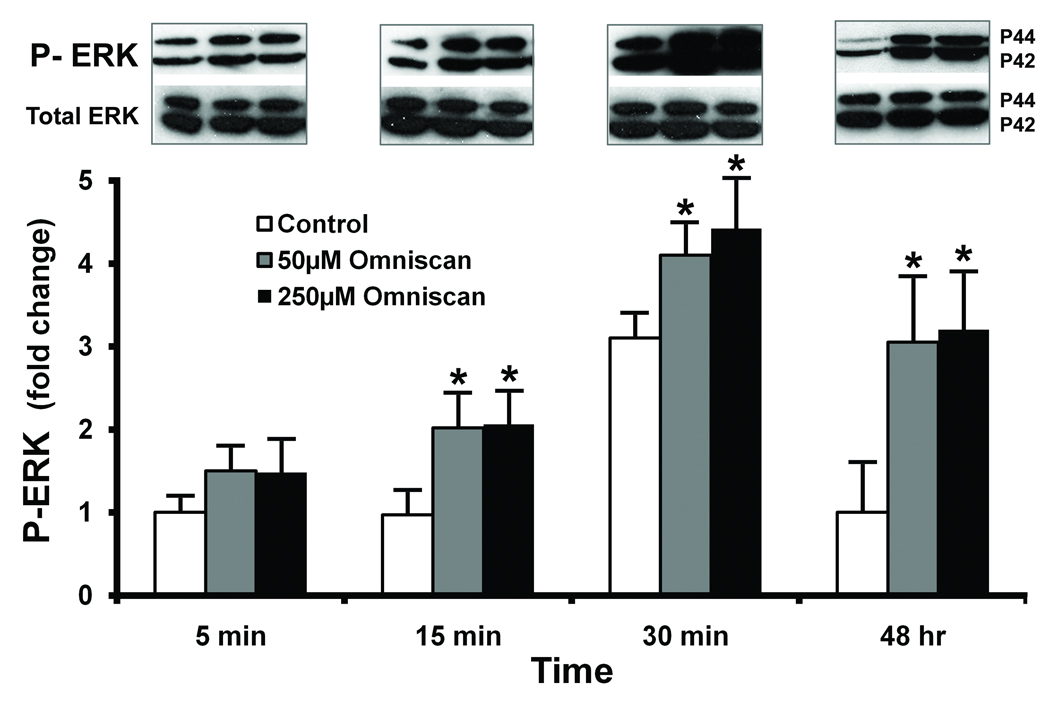
Given the findings presented above, we sought to determine the intracellular signaling pathways activated by Omniscan exposure. Two sets of experiments were carried out. In the first, we assessed ERK phosphorylation as an indicator of MAP kinase activity and AKT phosphorylation as an indicator of PI3 kinase activity. In parallel, we assessed the ability of MAP kinase and PI3 kinase antagonists to interfere with Omniscan stimulation. ERK phosphorylation in treated fibroblasts is presented in Figure 2. An increase in phosphorylation was observed as early as five minutes after exposure, and was increased at 15 and 30 minutes. Parallel increases in both the p42 and p44 forms of ERK occurred. Longer-term studies indicated that increased ERK phosphorylation was still evident 48 hours post-treatment. There was, as expected, no significant change in total ERK protein expression over the same time period. It can also be seen from Figure 2 that there was a transient increase in ERK phosphorylation in control cells (most evident at the 30-minute time-point). This is not surprising since there is EGF and insulin in the culture medium.
Figure 2. ERK phosphorylation in Omniscan-treated cells.
Cells were incubated under control conditions or in the presence of Omniscan for the indicated times. At the end of the incubation period, cell lysates were prepared and assayed for phosphor-ERK and total ERK protein by western blotting. Western blots were scanned, digitized, and quantified. Values shown are means and standard errors based on n=3 separate experiments. The inset shows phosphor-ERK and total-ERK from one of the replicate experiments. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant increase compared to the respective (timepoint-matched) control value at p<0.05 level.
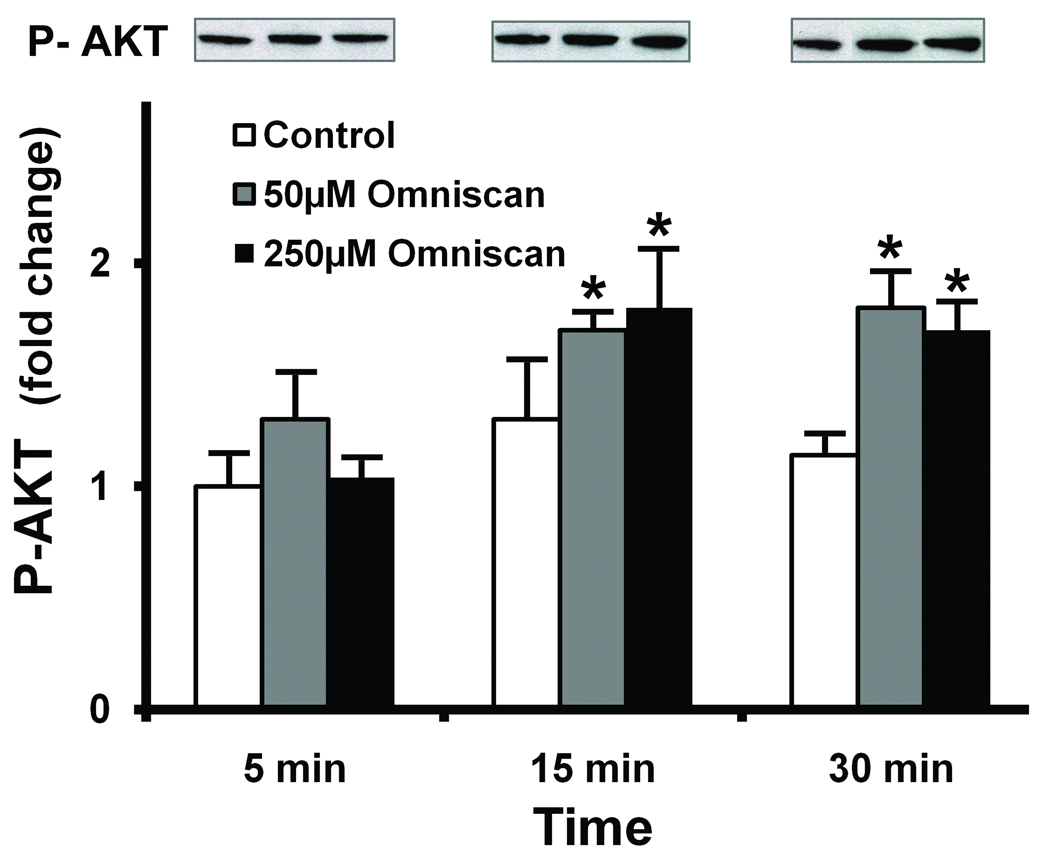
Figure 3 shows phosphor-AKT in fibroblasts at 5, 15, and 30 minutes after exposure to Omniscan. A modest but consistent increase in AKT phosphorylation was apparent. Unlike ERK, the phosphorylation pattern for AKT was not sustained: beyond 30 minutes, there were no differences between the control and Omniscan-treated cells. As expected, there was no change in total AKT protein at any time-point.
Figure 3. AKT phosphorylation in Omniscan-treated cells.
Cells were incubated under control conditions or in the presence of Omniscan for the indicated times. At the end of the incubation period, cell lysates were prepared and assayed for phosphor-AKT and total AKT protein by western blotting. Western blots were scanned, digitized, and quantified. Values shown are means and standard errors based on n=3 separate experiments. The insert shows phosphor-AKT and total-AKT from one of the replicate experiments. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant increase compared to the respective control value at p<0.05 level.
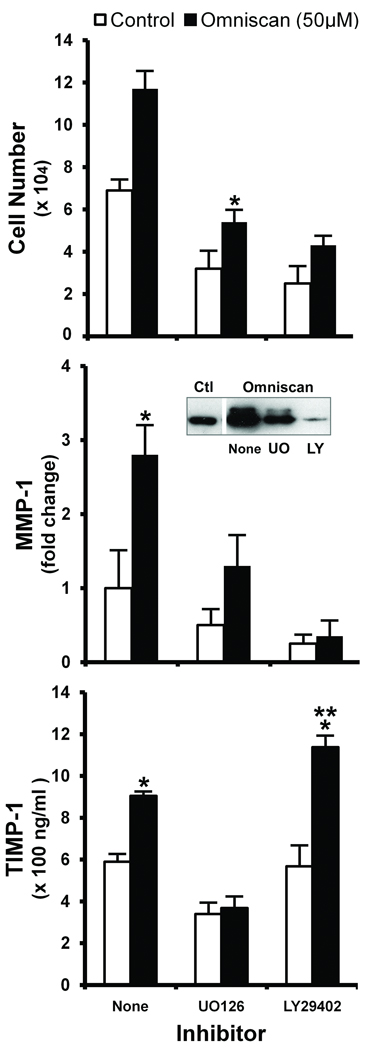
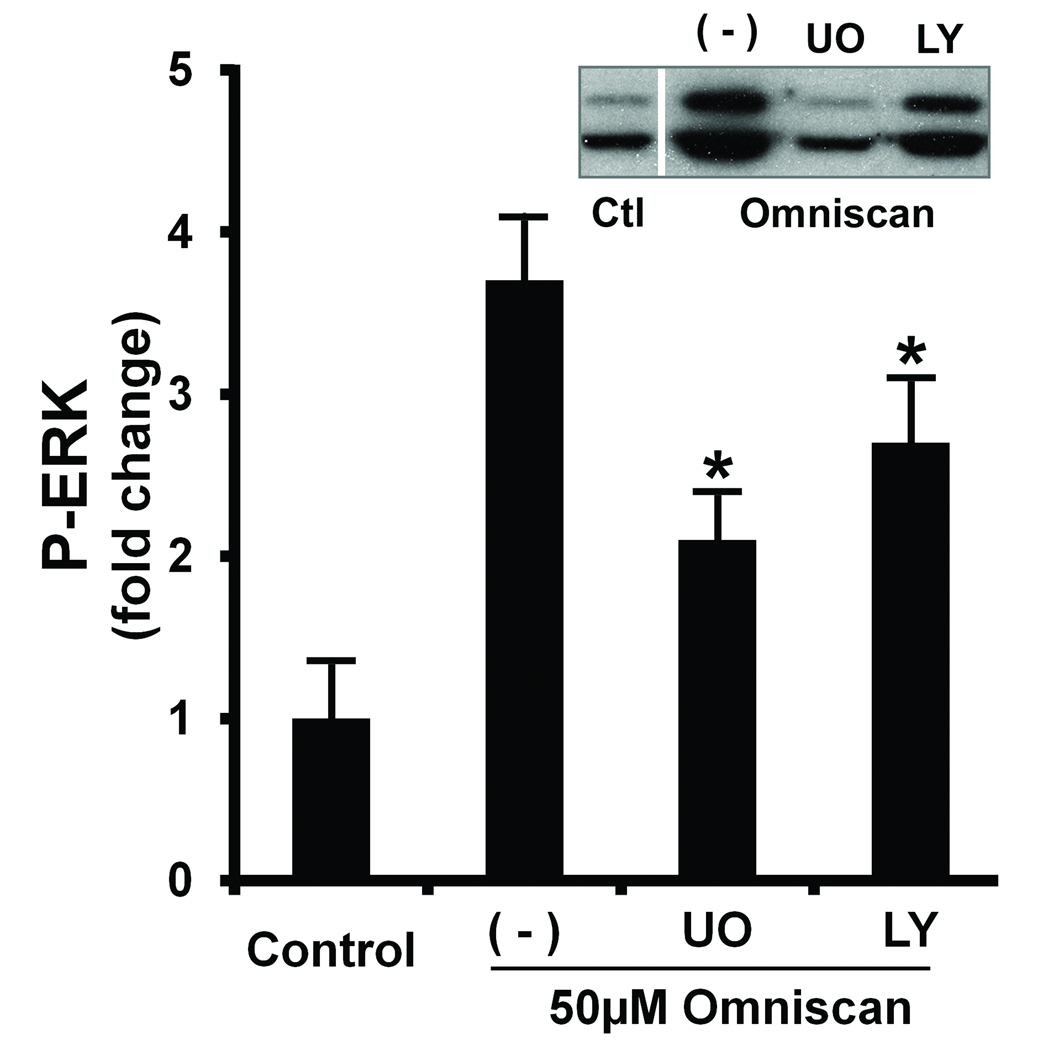
In parallel studies, human dermal fibroblasts were exposed to Omniscan in the presence of U0126 (10 µM) or LY294002 (25 µM). Effects on proliferation, MMP-1, and TIMP-1 were assessed. Figure 4 demonstrates that in the presence of U0126 (inhibitor of ERK activation), all three responses were reduced to near background levels (i.e., levels observed in the absence of stimulator and inhibitor) or slightly below. In the presence of the PI3 kinase inhibitor (LY294002), proliferation and MMP-1 were also inhibited. The MMP-1 level was substantially below background levels. The TIMP-1 level, however, was not reduced. Quite the opposite was found: the TIMP-1 level was substantially up-regulated over the level induced by Omniscan alone (Figure 4). As can also be seen from the figure, when cells were exposed to U0126 or to LY294002 under basal conditions, i.e., in culture medium containing EGF and insulin but no Omniscan, all three responses – proliferation, MMP-1 production, and TIMP-1 production – were substantially reduced in the presence of 10 µM U0126. Proliferation and MMP-1 were also reduced in the presence of 25 µM LY294002, but LY294002 had no effect on the TIMP-1 level in the absence of Omniscan stimulation (Figure 4). Given these data, we wanted to determine whether the two signaling inhibitors would differentially regulate collagen deposition in the cell layer. Unfortunately, both inhibitors directly suppressed the production of type I procollagen in human dermal fibroblasts. Not surprisingly, deposition of type I collagen was concomitantly affected (not shown).
Figure 4. Effects of U0126 and LY294002 on proliferation, MMP-1 production, and TIMP-1 production in control and Omniscan-treated fibroblasts.
Cells were incubated under control conditions or in the presence of Omniscan with or without U0126 or LY294002 for three days. At the end of the incubation period, culture fluid was collected and assayed as follows. Upper panel. Cell proliferation assessed as cell counts. Middle panel. MMP-1 assessed by western blotting. Inset: Western blot from one experiment. Lower panel. TIMP-1 assessed by ELISA. Values shown are means and standard errors based on five separate experiments. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant increase compared to control at p<0.05 level. **indicates statistically significant increase compared to Omniscan alone at p<0.05 level.
In control experiments, the two inhibitors were examined for effects on MAP kinase and PI3 kinase signaling intermediates. As expected, AKT phosphorylation was not affected by U0126 but was completely inhibited in the presence of LY294002 (not shown). ERK phosphorylation was strongly suppressed by U0126 but interestingly was also partially suppressed (approximately 35% inhibition) in the presence of LY294002 (Figure 5). Total ERK was not affected by either agent.
Figure 5. Effects of LY294002 on ERK phosphorylation in Omniscan-treated cells.
Cells were incubated under control conditions or in the presence of Omniscan with or without U0126 or LY294002 for three days for the indicated times. At the end of the incubation period, cell lysates were prepared and assayed for phosphor-ERK by western blotting. Values shown are means and standard errors based on n=3 separate experiments. The inset shows phosphor-ERK and from one of the replicate experiments. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant decrease compared to Omniscan alone at p<0.05 level.
DISCUSSION
MMPs and MMP inhibitors play a critical role in matrix remodeling. MMP-1, in particular, is responsible for degradation of type I collagen in the skin. Our own past studies showed that MMP-1 was dramatically up-regulated in human skin one day after exposure to ultraviolet B (UVB) radiation (29–31). MMP-13 (collagenase 3) was not increased following UVB exposure, and neutralization of MMP-1 suppressed virtually all of the collagen damage in UVB-treated skin (31). In other studies, increased MMP-1 was detected in human skin associated with basal cell carcinomas, and neutralization of this enzyme blocked tumor-associated collagen-damaging activity (32). In addition to these findings demonstrating increased MMP-1 in conjunction with dermal collagen degradation, other previous studies have shown reduced levels of MMP-1 in conjunction with TGF-β-induced fibrotic changes (33,34). Given these past observations, we carried out a series of studies in which human skin in organ culture was exposed to Omniscan or to one of several other GBCAs. The expectation going into the studies was that increased collagen synthesis and decreased MMP-1 production would be seen. In fact, something quite different was observed. MMP-1 was substantially increased in Omniscan-treated skin (18). However, along with increased MMP-1 was an increase in TIMP-1, a major collagenolytic inhibitor in the skin (32). Taken in conjunction with past findings, we hypothesized that the fibrotic changes seen in the skin of individuals with NSF might reflect contrast agent-induced changes in an enzyme / inhibitor system that regulates collagen turnover rather than a direct effect on collagen synthesis. The goals of the present study include determining whether there is a relationship between changes in MMP-1 / TIMP-1 production and altered collagen production / deposition in GBCA-treated fibroblasts and delineating the intracellular signaling pathways that regulate MMP-1 and TIMP-1 production. Two issues are worth discussing.
First, we observed in the present study that the amount of type I collagen recovered in the cell layer was increased in response to Omniscan treatment under conditions in which there was no increase in the production of type I procollagen (or its transcript). Likewise, there was no change in transcript levels for various enzymes involved in the processing of procollagen into the mature form. As a control for these studies, we assessed the same endpoints in TGF-β-treated fibroblasts. The results were significantly different. In dermal fibroblasts treated with TGF-β, there was an increase in type I procollagen production along with increased collagen deposition. Furthermore, MMP-1 was lower following TGF-β treatment than it was in control cells. These effects of TGF-β are well-established in the literature (27,28). Taken together, our findings are consistent with the idea that Omniscan exposure affects an enzyme / inhibitor system that regulates collagen turnover rather than collagen synthesis, per se. Specifically, our data allow us to hypothesize that overproduction of TIMP-1 in Omniscan-treated skin prevents MMP-1-mediated collagen breakdown and allows excess matrix to build up in the tissue. In addition, TIMP-1 is known to be a potent inducer of fibroblast proliferation (at least under some conditions) (35,36). Previous studies by others have linked excess TIMP-1 production with enhanced fibroblast growth in scleroderma (37), and elevated TIMP-1 could contribute to fibrotic changes in NSF by promoting the accumulation of matrix-producing cells in the lesions as well as preventing matrix turnover. This hypothesis is tentative at the moment for a number of reasons. First, while TIMP-1 is clearly elevated in fibroblasts (and in whole skin) following Omniscan exposure, whether the amount of inhibitor is sufficient to neutralize all of the excess MMP-1 has not been definitively shown at present. Second, other matrix metalloproteinases have collagen-degrading activity, and TIMP-1 is a broad-spectrum MMP inhibitor (38). What role these enzymes might play in collagen turnover is yet to be determined. Finally, and most importantly, it is only speculation as to whether these responses of fibroblasts to Omniscan have anything at all to do with NSF. While NSF is clearly linked to GBCA exposure (1–8), very similar disease presentation has been reported in the absence of GBCA exposure (39).
The second issue relates to MAP kinase and PI3 kinase signaling in fibroblast responses to GBCA stimulation. Based on phosphorylation of key elements in each pathway and the effects of specific pathway inhibitors (U0126 and LY294002), both intracellular signaling pathways appear to be activated in Omniscan-treated fibroblasts. Inhibitors of the two signaling pathways reduced Omniscan-stimulated fibroblast proliferation and the increase in MMP-1 seen in the Omniscan-stimulated cells. It is known from past studies that when ERK is activated, it results in the phosphorylation and activation of cell cycle-regulating proteins such as Cyclin D1 (40). At the same time, MAP kinase signaling leads to formation of the activation protein-1 (AP-1) complex and transcription of MMP genes (41). The two responses were also inhibited under basal conditions (i.e., in the absence of Omniscan treatment). Effects under basal conditions are not surprising since the culture medium used in these studies contains EGF and insulin.
A role for PI3 kinase activation in these responses is also evident. AKT, one of the downstream targets in this pathway (42), was activated in Omniscan-treated cells, and LY294002, an inhibitor of PI3 kinase signaling, proved to be a potent inhibitor of Omniscan-stimulated proliferation and Omniscan-induced MMP-1 production. Although these data provide strong support for a PI3 kinase role, the importance of AKT, per se, is less clear. Past studies have suggested a role for AKT in MMP modulation (43,44), perhaps involving NF-κB, and there is no reason to believe, a priori, that this could not happen in Omniscan-treated fibroblasts. Alternatively, a modest but consistent inhibition of ERK phosphorylation in the presence of LY924002 was noted, suggesting that the fibroblast response to Omniscan might reflect, in part at least, PI3 kinase-mediated ERK activation and the downstream effects of ERK activation aforementioned. Increased synthesis of collagen and other matrix components is a major downstream effect of PI3 kinase / AKT activation (45). Since we observed no increase in type I procollagen production following GBCA stimulation, we do not assume a major role for AKT.
Although both signaling pathways appear to play a role in the up-regulation of fibroblast proliferation and MMP-1 production, the regulation of TIMP-1 appears to be more complex. U0126 reduced the level of this protein in Omniscan-treated cells. However, suppression of PI3 kinase signaling with LY294002 did not reduce it. On the contrary, TIMP-1 levels were further increased in Omniscan-stimulated cells that were concomitantly treated with the PI3 kinase inhibitor. Our tentative interpretation of these findings is that both signaling pathways are activated in basal conditions (containing EGF and insulin but not Omniscan) and further up-regulated in response to Omniscan. Both pathways appear to have a stimulatory effect on proliferation and MMP-1 production while the two pathways may function at cross-purposes with regard to TIMP-1. There is precedent for distinct regulation of MMPs and TIMP-1. In an earlier study it was demonstrated that both MMP-1 and TIMP-1 were up-regulated in human skin exposed to ultraviolet (UV) irradiation. When the skin was treated with retinoic acid in conjunction with UV irradiation, up-regulation of MMP-1 was suppressed but TIMP-1 remained elevated (29). In another study involving murine fibroblasts, TIMP-1 up-regulation by oncostatin M was inhibited with U0126 but further up-regulated in conjunction with PI3 kinase inhibition (46). Of note, the human TIMP-1 gene is known to contain repressive elements in the promoter and intron 1 (47). Further complicating the issue is the fact that TIMP-1 itself is capable of stimulating PI3 kinase / AKT activation in some cells (48,49). Additional experimentation will be required to resolve many of these outstanding issues. Suffice to say that the human dermal fibroblast response to chelated Gd3+ is complex and involves multiple interrelated signaling pathways. Presuming that the high level of TIMP-1 is the driving force for increased collagen deposition in Omniscan-treated fibroblasts, it would be generally useful to down-regulate this moiety without compromising fibroblast function. Of course, it must be noted that a rise in TIMP-1 is not the only response to stimulation. In particular, increased fibroblast proliferation also occurs in GBCA stimulation. The intense fibroplasia previously noted in some NSF patients (9–11) may reflect increased fibroblast proliferation more than a change in collagen metabolism, per se.
In summary, our recent study provided evidence that the gadolinium-containing MRI contrast agents used clinically have effects on a fibroblast enzyme / inhibitor system that regulates collagen turnover in the skin (18). The current studies demonstrate that alterations in enzyme / inhibitor expression are associated with an increase in collagen deposition without a change in procollagen synthesis. Furthermore, the current studies show that MMP-1 and TIMP-1 elaboration are regulated through at least two intracellular signaling pathways and these moieties are regulated through overlapping but distinct mechanisms. Taken together with our previous findings, the present studies provide novel insight into mechanisms of collagen regulation in the skin. It must be remembered, of course, that what is seen in healthy human dermal fibroblasts in vitro or with human skin from healthy individuals in organ culture (18) may have little do with what occurs in NSF. It should also be noted that MMPs and their inhibitors have important regulatory influences on the inflammatory response (50). Changes in MMP-1 and TIMP-1 levels brought about by GBCA exposure may (if they have any role in NSF at all) contribute to altered inflammation rather than to fibrotic changes directly. At present we can only state that our findings are intriguing and warrant further study.
Acknowledgments
Supported in part by grants R43 GM077724-01 and R43 GM080779-01A1 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000–1001. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- 2.Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563–572. doi: 10.1016/s0002-9343(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 3.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 4.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 5.Yerram P, Saab G, Karuparthi PR, et al. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure--role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol. 2007;2:258–263. doi: 10.2215/CJN.03250906. [DOI] [PubMed] [Google Scholar]
- 6.Rydahl C, Thomsen HS, Marckmann P. High prevalence of nephrogenic systemic fibrosis in chronic renal failure patients exposed to gadodiamide, a gadolinium-containing magnetic resonance contrast agent. Invest Radiol. 2008;43:141–144. doi: 10.1097/RLI.0b013e31815a3407. [DOI] [PubMed] [Google Scholar]
- 7.Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (omniscan) Invest Radiol. 2007;42:139–145. doi: 10.1097/01.rli.0000253505.88945.d5. [DOI] [PubMed] [Google Scholar]
- 8.Hope TA, Herfkens RJ, Denianke KS, et al. Nephrogenic systemic fibrosis in patients with chronic kidney disease who received gadopentetate dimeglumine. Invest Radiol. 2009 Jan;15 doi: 10.1097/RLI.0b013e31819343ba. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.McNeill AM, Barr RJ. Scleromyxedema-like fibromucinosis in a patient undergoing hemodialysis. Int J Dermatol. 2002;41:364–367. [PubMed] [Google Scholar]
- 10.Neudecker BA, Stern R, Mark LA, et al. Scleromyxedema-like lesions of patients in renal failure contain hyaluronan: a possible pathophysiological mechanism. J Cutan Pathol. 2005;32:612–615. doi: 10.1111/j.0303-6987.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238–249. doi: 10.1016/j.semarthrit.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortonne N, Lipsker D, Chantrel F, et al. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150:1050–1052. doi: 10.1111/j.1365-2133.2004.05900.x. [DOI] [PubMed] [Google Scholar]
- 13.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 14.Stenvinkel P. Inflammation in end-stage renal failure: could it be treated? Nephrol Dial Transplant. 2002;17:33–38. doi: 10.1093/ndt/17.suppl_8.33. [DOI] [PubMed] [Google Scholar]
- 15.Sieber MA, Pietsch H, Walter J, et al. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol. 2008;43:65–75. doi: 10.1097/RLI.0b013e31815e6277. [DOI] [PubMed] [Google Scholar]
- 16.Sieber MA, Lengsfeld P, Walter J, et al. Gadolinium-based contrast agents and their potential role in the pathogenesis of nephrogenic systemic fibrosis: the role of excess ligand. J Magn Reson Imaging. 2008;27:955–962. doi: 10.1002/jmri.21368. [DOI] [PubMed] [Google Scholar]
- 17.Edward M, Quinn JA, Mukherjee S, et al. Gadodiamide contrast agent 'activates' fibroblasts: a possible cause of nephrogenic systemic fibrosis. J Pathol. 2008;214:584–593. doi: 10.1002/path.2311. [DOI] [PubMed] [Google Scholar]
- 18.Varani J, DaSilva M, Warner RL, et al. Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Invest Radiol. 2009;44:74–81. doi: 10.1097/RLI.0b013e31818f76b5. [DOI] [PubMed] [Google Scholar]
- 19.Lin SP, Brown JJ. MR contrast agents: physical and pharmacologic basics. J Magn Reson Imaging. 2007;25:884–899. doi: 10.1002/jmri.20955. [DOI] [PubMed] [Google Scholar]
- 20.Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol. 2008;66:175–179. doi: 10.1016/j.ejrad.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Varani J, Perone P, Griffiths CE, et al. All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. Adult skin from sun-protected and sun-exposed sites responds in an identical manner to RA while neonatal foreskin responds differently. J Clin Invest. 1994;94:1747–1756. doi: 10.1172/JCI117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varani J, Fay K, Perone P. MDI 301, a non-irritating retinoid, induces changes in human skin that underlie repair. Arch Dermatol Res. 2007;298:439–448. doi: 10.1007/s00403-006-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lateef H, Stevens MJ, Varani J. All-trans-retinoic acid suppresses matrix metalloproteinase activity and increases collagen synthesis in diabetic human skin in organ culture. Am J Pathol. 2004;165:167–174. doi: 10.1016/S0002-9440(10)63285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan T, He T, Kang S, et al. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan T, He T, Kang S, et al. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- 26.Quan T, He T, Shao Y, et al. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169:482–490. doi: 10.2353/ajpath.2006.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Laethem JL, Robberecht P, Résibois A, et al. Transforming growth factor beta promotes development of fibrosis after repeated courses of acute pancreatitis in mice. Gastroenterology. 1996;110:576–582. doi: 10.1053/gast.1996.v110.pm8566606. [DOI] [PubMed] [Google Scholar]
- 28.Cutroneo KR, White SL, Phan SH, et al. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol. 2007;211:585–589. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- 29.Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 30.Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 31.Brennan M, Bhatti H, Nerusu KC, et al. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003;78 doi: 10.1562/0031-8655(2003)078<0043:mmitmc>2.0.co;2. 43-38. [DOI] [PubMed] [Google Scholar]
- 32.Varani J, Hattori Y, Chi Y, et al. Collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors in basal cell carcinoma of skin: comparison with normal skin. Br J Cancer. 2000;82:657–665. doi: 10.1054/bjoc.1999.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verrecchia F, Mauviel A, Farge D. Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. 2006;5:563–569. doi: 10.1016/j.autrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Yuan W, Varga J. Transforming growth factor-beta repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J Biol Chem. 2001;276:38502–38510. doi: 10.1074/jbc.M107081200. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa T, Yamashita K, Tanzawa K, et al. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 36.Hayakawa T, Yamashita K, Ohuchi E, et al. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2) J Cell Sci. 1994;107:2373–2379. doi: 10.1242/jcs.107.9.2373. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi K, Kadono T, Furue M, et al. Tissue inhibitor of metalloproteinase 1 (TIMP-1) may be an autocrine growth factor in scleroderma fibroblasts. J Invest Dermatol. 1997;108:281–284. doi: 10.1111/1523-1747.ep12286457. [DOI] [PubMed] [Google Scholar]
- 38.Cawston TE. Protein inhibitors of metalloproteinases. In: Barrett JA, Salveson G, editors. Proteinase Inhibitors. Amsterdam: Elsevier; 1986. pp. 589–610. [Google Scholar]
- 39.Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature. Am J Transplant. 2007;7:2425–2432. doi: 10.1111/j.1600-6143.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- 40.Klein EA, Campbell LE, Kothapalli D, et al. Joint requirement for Rac and ERK activities underlies the mid-G1 phase induction of cyclin D1 and S phase entry in both epithelial and mesenchymal cells. J Biol Chem. 2008;283:30911–30918. doi: 10.1074/jbc.M804537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barchowsky A, Frleta D, Vincenti MP. Integration of the NF-kappaB and mitogen-activated protein kinase/AP-1 pathways at the collagenase-1 promoter: divegence of IL-1 and TNF-dependent signal transduction in rabbit primary synovial fibroblasts. Cytokine. 2000;12:1469–1479. doi: 10.1006/cyto.2000.0743. [DOI] [PubMed] [Google Scholar]
- 42.Stambolic V, Woodgett JR. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol. 2006;16:461–466. doi: 10.1016/j.tcb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Wahl LM. Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinsositol-3 kinase/Akt/IKKalpha/NF-kappaB pathway. J Leukoc Biol. 2005;78:259–265. doi: 10.1189/jlb.0904498. [DOI] [PubMed] [Google Scholar]
- 44.Oh JH, Kim A, Park JM, et al. Ultraviolet B-induced matrix metalloproteinase-1 and -3 secretions are mediated via PTEN/Akt pathway in human dermal fibroblasts. J Cell Physiol. 2006;209:775–785. doi: 10.1002/jcp.20754. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Lee Y, Seo JE, et al. Caveolin-1 increases basal and TGF-beta1-induced expression of type I procollagen through PI-3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cell Signal. 2008;20:1313–1319. doi: 10.1016/j.cellsig.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Tong L, Smyth D, Kerr C, et al. Mitogen-activated protein kinases Erk1/2 and p38 are required for maximal regulation of TIMP-1 by oncostatin M in murine fibroblasts. Cell Signal. 2004;16:1123–1132. doi: 10.1016/j.cellsig.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Dean G, Young DA, Edwards DR, et al. The human tissue inhibitor of metalloproteinase (TIMP) -1 gene contains repressive elements within the promoter and intron 1. J Biol Chem. 2000;275:32664–32671. doi: 10.1074/jbc.275.42.32664. [DOI] [PubMed] [Google Scholar]
- 48.Lambert E, Boudot C, Kadri Z, et al. Tissue inhibitor of metalloproteinases-1 signalling pathway leading to erythroid cell survival. Biochem J. 2003;372:767–774. doi: 10.1042/BJ20030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boulday G, Fitau J, Coupel S, et al. Exogenous tissue inhibitor of metalloproteinase-1 promotes endothelial cell survival through activation of the phosphatidylinositiol 3-kinase/Akt pathway. Ann N Y Acad Sci. 2004;1030:28–36. doi: 10.1196/annals.1329.004. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Chen X, Hong Q, et al. TIMP-1 promotes age-related renal fibrosis through upregulating ICAM-1 in human TIMP-1 transgenic mice. J Gerontol A Biol Sci Med Sci. 2006;61:1130–1143. doi: 10.1093/gerona/61.11.1130. [DOI] [PubMed] [Google Scholar]