Abstract
Axonemal dyneins have been demonstrated to monitor the mechanical state of the axoneme and must also alter activity in response to various signaling pathways. The central pair/radial spoke systems are clearly involved in controlling inner dynein arm function; however, the mechanisms by which the outer dynein arm transduces regulatory signals appear quite distinct at the molecular level. In Chlamydomonas, these regulatory components include thioredoxins involved in response to redox changes, molecules that tether the γ heavy chain motor unit to the A-tubule of the outer doublet and a Ca2+-binding protein that controls the structure of the γ heavy chain N-terminal domain. Together, these studies now suggest that the γ heavy chain acts as a key regulatory node for controlling outer arm function in response to alterations in curvature and ligand binding. Furthermore, they allow us to propose a testable molecular mechanism by which altered Ca2+ levels might lead to a change in ciliary waveform by controlling whether one heavy chain of outer arm dynein acts as a microtubule translocase or as an ATP-dependent brake that limits the amount of inter-doublet sliding.
Keywords: Axoneme, Cilia, Dynein, Flagella, Microtubule
Introduction
Cilia and flagella are required for numerous essential motile processes and are powered by a complex linear array of dynein motors. Current models for ciliary/flagellar motility propose that a wave of dynein activity (usually directed from base to tip) is propagated along the axonemal microtubules resulting in a region of localized sliding that drives the waveform (Brokaw 2009; Hayashi and Shingyogi 2008; Lindemann 2002; Sugino and Naitoh 1982). In addition, as the bend direction is altered, the region undergoing active sliding must switch from one side of the axoneme to the other (Nakano et al. 2003; Satir and Matsuoka 1989; Wargo et al. 2004). As demonstrated in many systems e.g. (Bessen et al. 1980; Gibbons and Gibbons 1972), reactivated demembranated cilia and flagella beat with approximately normal waveform and at wildtype rates upon addition of nucleotide. Thus, all of the control systems necessary to locally activate dyneins on particular doublet microtubules and to translocate the region of active sliding must be physically incorporated within the axonemal superstructure. This requires that some component(s) of the axoneme function to detect curvature and activate both the inner and outer dynein arm motors appropriately. How might this be achieved? Studies in Chlamydomonas over the last approximately ten years have now identified some of the key molecules that signal the dynein motors. Indeed, these experiments have defined two conserved systems that control inner and outer arm function and which appear completely distinct at the molecular level. Additional support for this idea comes from genomic analyses (Merchant et al. 2007) which revealed that during evolution outer arms have been lost in certain organisms (e.g. the bryophyte Physcomitrella) whereas the inner arms, radial spokes and central pair complex have together been lost in others (e.g. the centric diatom Thalassiosira). As the sperm flagella from these outer arm- and inner arm/radial spoke/central pair-less organisms are capable of rhythmic coordinated movement and they must therefore both retain a mechanosensory pathway.
These mechanosensory systems must be integrated with other regulatory pathways to yield appropriate responses to environmental changes such as those signaled by alterations in Ca2+ concentration and redox poise. Here I briefly review the physiological and molecular evidence for regulation of outer arm dynein and propose a molecular mechanism by which this motor complex might integrate Ca2+ signaling with conformational switching.
Mechanical Activation of Mutant Flagella
The initial identification of two separate mechanosensory systems controlling outer and inner dynein arms came from experiments in which the paralyzed flagella of various single and double mutants of Chlamydomonas were activated to beat either by mechanically stimulating the structure with a microneedle or through hydrodynamic forces generated by holding the cell with a pipette and rapidly moving it with a piezoelectric actuator (Hayashibe et al. 1997). When the mutants pf18 and pf19, which lack the central pair microtubule complex, were treated in this way, the flagella beat in an irregular manner such that a single cycle took almost one second (i.e. ∼50 × slower than wildtype). In contrast, a double mutant of ida2 (lacking the dimeric inner arm I1/f) and ida4 strain (lacking three monomeric inner arm dynein species a, c and d) exhibited rhythmic oscillations of up to 10 Hz for extended periods. When these mutations were combined individually with pf18, the resulting strains also beat for a few cycles when mechanically activated. However, mutants lacking the outer dynein arm and docking complex (oda1) in combination with either pf18 or ida2 showed no activation, whereas the ida4 oda1 double mutant propagated the mechanically-activated wave very slowly but did not initiate any further cyclic oscillations. These observations led to the hypothesis that outer arm dynein is activated by some factor that is independent of the central pair complex whereas this latter system is absolutely essential for inner arm function (Hayashibe et al. 1997). Furthermore, the data support the idea that either the outer arm or the dimeric inner arm I1/f is necessary to propagate a flagellar bend; i.e. the monomeric inner arm heavy chains are insufficient for this task even though they are essential for beating under certain solution conditions such as high imposed viscous load (Yagi et al. 2005).
Conformational Switching Through the Central Pair
As described above, mutants that lack the entire central pair apparatus exhibit paralyzed flagella which can be mechanically activated to beat. Furthermore, analysis of inter-doublet sliding in disintegrating axonemes revealed that orientation of the central pair apparatus controls the region of dynein-driven active sliding (Wargo and Smith 2003). Further evidence that this structure is required for conformational switching at the power/recovery stroke transitions came from RNAi experiments in which hydin was down-regulated (Lechtreck and Witman 2007). Hydin defects in mammals cause highly impaired ciliary beating and lead to hydrocephalus (Lechtreck et al. 2008). In Chlamydomonas an ∼80% reduction in hydin expression caused paralysis of most cells, with the two flagella arrested in a novel “hands up, hands down” orientation with one pointing away from the cell whereas the other was aligned along the cell body. This lack of hydin resulted in the reduction/absence of two projections from the C2 microtubule of the central pair. Thus, it is likely that the hydin-containing central pair projection is intimately involved in contacting the radial spokes which represent the signaling conduit to the dynein regulatory complex and inner arm system. Furthermore, in accord with the data of (Hayashibe et al. 1997) these results imply that the control of the inner arms in response to the mechanical state of the axoneme is initiated from the central pair complex.
An Outer Arm Dynein Mechano-sensor
So is the same signaling pathway involved in controlling inner arms propagated across the outer doublet to the outer arms and if not, as implied by the study of Hayashibe and colleagues, how does the latter motor sense flagellar curvature and initiate conformational switching? The outer arm contains three distinct heavy chains (α, β and γ HCs) and is unusual in that these proteins are directly associated with four light chain (LC) components that appear to have regulatory functions and which are not found in other axonemal dyneins (King and Kamiya 2008); an interaction map for the components of the outer arm is shown in Fig 1. These HC-associated components include two thioredoxins (LC3 and LC5) (Patel-King et al. 1996) that are thought to be involved in detecting and responding to alterations in flagellar redox poise (Wakabayashi and King 2006) and a Ca2+-binding calmodulin homologue (LC4) (King and Patel-King 1995) that appears to function as the Ca2+ sensor for waveform conversion from an asymmetric to a symmetric beat (see below); these three LCs associate with the N-terminal domains of the α (LC5), β (LC3) and γ (LC3 and LC4) outer arm HCs. In contrast, the other HC-associated component (LC1) interacts directly with the AAA domains of the γ HC motor unit; one copy is bound to AAA1 and the second likely to AAA3 or AAA4 (Benashski et al. 1999). Furthermore, crosslinking and direct binding assays revealed that LC1 also binds tubulin, and indeed, appears to tether the motor to the outer doublet A-tubule in a nucleotide and Ca2+-independent manner (Benashski et al. 1999; Patel-King and King 2009). The geometry of the ternary system is consistent with this hypothesis as the long axis of LC1 determined by NMR spectroscopy (Wu et al. 2003; Wu et al. 2000) is sufficient to span the distance between the γ HC AAA ring and the A-tubule. Indeed, a linkage between the γ HC motor unit and the A-tubule that may represent this connection has been directly observed by cryoEM tomography (Oda et al. 2007). Furthermore, examination of sea urchin outer arm dynein–microtubule complexes by cryo-positive staining EM revealed a similar connection emanating from the α HC2 (Ueno et al. 2008).
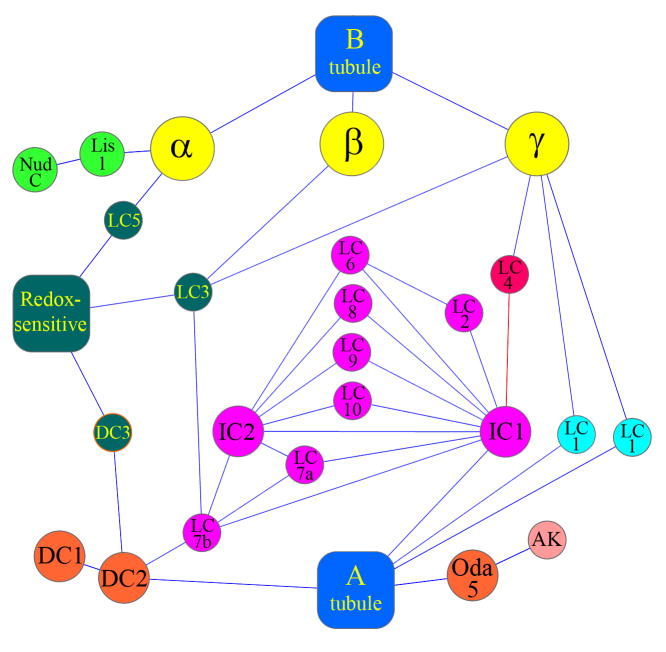
Fig. 1. Interaction Map for Outer Arm Dynein.
This diagram illustrates the interactions that are known to occur within outer arm dynein; only associations for which there is direct experimental evidence in Chlamydomonas are illustrated. The color code is: blue, A and B tubules; yellow, HCs; light green, Lis1/NudC; dark green, redox-sensitive LCs, docking complex component DC3 and unidentified soluble substrates; magenta, IC/LC complex, red, LC4 Ca2+-sensor; cyan, LC1; orange, docking complex and Oda5 proteins required for arm assembly; pink, Oda5-associated adenylate kinase. The Ca2+-sensitive association of LC4 with IC1 is shown in red. Although indicated by single circles, LCs 6, 8, 9 and 10 are actually homodimers. This figure was prepared using Cytoscape v.2.6.3 (http://www.cytoscape.org) (Shannon et al. 2003).
RNAi knockdowns of LC1 in Trypanosoma and the planarian Schmidtea both result in dramatic alterations in ciliary/flagellar beating (Baron et al. 2007; Rompolas et al. 2009). Although no LC1 null strain has been identified in Chlamydomonas, the oda2-t mutant expresses a truncated γ HC that assembles an outer arm lacking both the γ HC motor unit and LC1 (Liu et al. 2008). This mutant exhibits a number of motility phenotypes, including slow forward swimming, reduced beat frequency and a dramatic defect in the ability to generate a symmetric waveform under high Ca2+ conditions. In microtubule gliding assays, lack of the γ HC motor unit and LC1 resulted in an increase in microtubule translocation rate by the remaining outer arm motors further suggesting that the γ HC and/or LC1 play a regulatory role (Furuta et al. 2009).
LC1 is highly asymmetric and consists of an N-terminal helix and a central ββα barrel formed from six leucine-rich repeats followed by a C-terminal helical domain that projects away from the main long axis (Wu et al. 2000). Based on the location of a hydrophobic patch thought to interact with the γ HC, the C-terminal helix was predicted to insert into the motor unit. Expression of mutant forms of LC1, which alter the composition or flexibility of the terminal helix, in a wildtype background had a dominant negative effect yielding dramatic reductions in swimming velocity and in some cases directional control but had minimal impact on beat frequency (Patel-King and King 2009). Furthermore, these mutant proteins had a novel effect on the ability of the cell to generate propulsive force. Under low viscosity conditions (where beat frequency is high) the LC1 mutant strains behaved almost as classic oda mutants which are capable of generating only 1/3-1/2 the propulsive force of wildtype flagella. However, as viscosity was increased (and beat frequency consequently decreased) the force generated tended to the wildtype level; a similar phenotype was observed for oda2-t. These observations implied that the waveform had been altered to a less effective form. High speed imaging subsequently revealed several inter-related defects. During forward swimming, the two flagella continually beat out-of-phase which led to a sideways rolling motion of ∼50° as the cell moved forward. In addition, detailed analysis of the waveform revealed that the flagella randomly stalled for a few msec either at or near the power-recovery stroke transition. Similarly, we have now found that Ca2+ induced backward swimming is also aberrant as the two flagella again are aphasic and thus ineffective at propelling the cell (R.S. Patel-King and S.M. King, unpublished observations). Importantly though, these cells did undergo waveform conversion which implies that the outer arm Ca2+ sensor remains intact.
How Does the Outer Arm Detect and Transduce Ca2+ Signals?
Ca2+ is used to control several distinct axonemal responses and there are multiple Ca2+-and/or Ca2+/calmodulin binding proteins located in various axonemal substructures including the central pair (DiPetrillo and Smith 2009; Wargo et al. 2005) and radial spokes (Patel-King et al. 2004; Yang et al. 2001; Yang et al. 2006). In addition, the outer dynein arm is associated with two Ca2+-binding calmodulin homologues: LC4 interacts with the γ HC N-terminal domain (Sakato et al. 2007) and DC3 is a component of the docking complex required for outer arm assembly at appropriate locations on the axoneme (Casey et al. 2003a). These proteins bind 1 (DC3) or 1-2 (LC4) Ca2+ ions with affinities of 1×10-5 and ∼3×10-5 M, respectively; for LC4 binding is Ca2+-specific whereas DC3 will also bind Mg2+ although with much lower affinity (Casey et al. 2003b; King and Patel-King 1995). A third Ca2+ binding protein, centrin, is present in monomeric inner arm species b, e and g (Kagami and Kamiya 1992; Piperno et al. 1990), and binds two Ca2+ ions with high affinity (1.2×10-6 M) and two with significantly lower affinity (1.6×10-4 M) (Weber et al. 1994).
In Chlamydomonas, the cis and trans flagella respond differently to submicromolar Ca2+ levels which allows for phototactic steering (Kamiya and Witman 1984). Although this response does not require the outer arms, when present, those motors must still undergo a power stroke at the appropriate time. Waveform conversion from an asymmetric to a symmetric beat occurs as Ca2+ increase above 10-6 M (Bessen et al. 1980). This response consists of two distinct phases: flagellar quiescence at 10-5 M Ca2+ and backward swimming driven by the symmetric beat at 10-4 M. Strains which lack outer arms exhibit either an aberrant response or are completely incapable of switching (Kamiya and Okamoto 1985; Mitchell and Rosenbaum 1985). A third Ca2+-controlled pathway that leads to a transient increase in beat frequency and swimming velocity following the mechanical activation of flagellar Ca2+ channels has recently been described (Wakabayashi et al. 2009). Intriguingly, this response occurs at submicromolar levels of Ca2+ but still requires the outer arm. Neither of the known outer arm Ca2+-binding proteins has an appropriate affinity to signal this response, and indeed it is present in a DC3-null strain that has been rescued with a mutant form of the protein that is unable to bind Ca2+ (S.M. King and G.B. Witman, unpublished results). Thus, this observation suggests that an additional high affinity Ca2+-binding protein or pathway associated with or impinging on the outer arms remains to be identified.
Although Ca2+ binding to DC3 is of high affinity and redox-sensitive, the Ca2+-dependent role played by this protein remains unknown as rescue of a DC3-null strain with a mutant form unable to bind Ca2+ rescued all observed motility phenotypes (Casey et al. 2003b). In contrast, biochemical studies of LC4 in isolated dynein particles revealed that Ca2+ binding to this protein drives conformational changes and indeed leads to altered intra-dynein interactions (Sakato et al. 2007). Specifically, LC4 associates with the γ HC N-terminal domain through two binding sites, only one of which is Ca2+-dependent. Thus, upon ligand binding, one site releases the γ HC and instead contacts the IC1 intermediate chain that is located at the base of the dynein particle and associates directly with tubulin (King et al. 1991). This transition is associated with an alteration in the conformation of the N-terminal domain that in isolated particles is evidenced by enhanced flexibility around a nodal point ∼2/3 the way along the N-terminal region. Intriguingly, the Ca2+-binding protein calaxin was recently shown to associate with the β HC2 of outer dynein arm from Ciona sperm in a Ca2+-dependent manner and also to interact with tubulin (Mizuno et al. 2009). Thus, alterations in how dynein interacts with the A-tubule may represent a general mechanism by which Ca2+ signals are propagated.
The γ Heavy Chain as a Ca2+-activated Tethered Winch
The phenotype of the oda2-t mutant suggests that there is an intimate connection between Ca2+ signaling through LC4 and the mechanosensory pathway that includes LC1. Indeed, as the LC3 thioredoxin also interacts with this HC (Harrison et al. 2002), it appears that the γ HC may be a key regulatory node for control of outer arm function in response to a variety of signaling ligands. These biochemical and physiological data combined with our current understanding of the dynein force generating mechanism (Burgess et al. 2003; Carter et al. 2008; Roberts et al. 2009; Ueno et al. 2008) now allow us to propose a testable model for how these pathways are integrated at the molecular level to yield distinct motor outputs that ultimately lead to waveform conversion in response to altered ligand levels (Fig. 2). The model described below is based on the proposed winch mechanism (Ueno et al. 2008) by which the dynein AAA motor unit does not rotate, but rather acts on a linker that spans the AAA ring to pull the anchored N-terminal region towards the microtubule minus end. This mechanism necessitates that the orientation of the microtubule binding stalk does not shift with respect to the B-tubule but rather undergoes cycles of strong and weak binding dictated by the nucleotide state of the AAA ring.
Fig. 2. Models for Mechano-sensing and Ca2+ Signaling by Outer Arm Dynein.
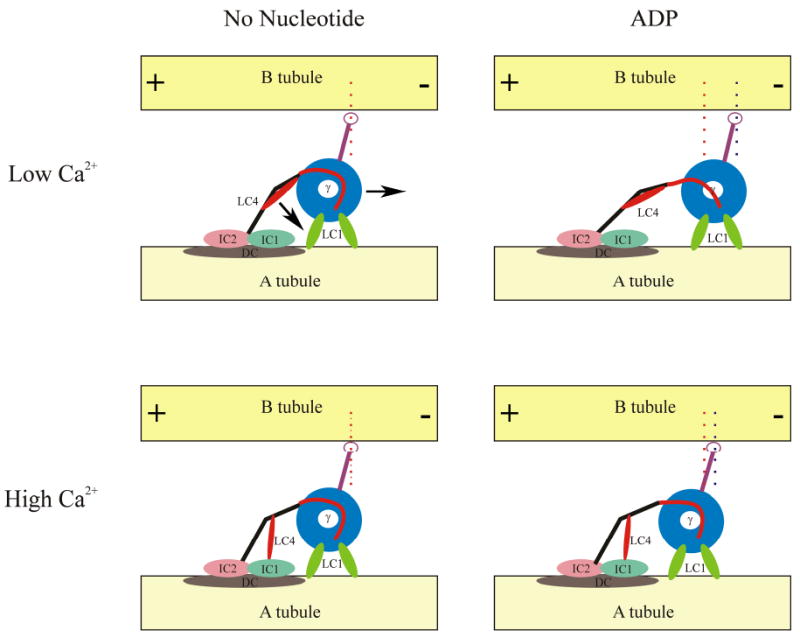
This model depicts the γ HC of outer arm dynein and one possible scenario for its response to nucleotide hydrolysis under low and high Ca2+ conditions. The diagrams are oriented with the distal or + end of the microtubules to the left. The HC regions are color-coded as follows: blue, motor unit; red, linker, black, N-terminal domain; purple, microtubule-binding domain and coiled coil domain. The γ HC-associated LC1 and LC4 proteins and the ICs and docking complex (DC) are also illustrated; the view is from the outside of the axoneme looking inwards. In this version, we have assumed that both copies of LC1 are bound simultaneously to the A-tubule and act as a loose tether. Thus, during a cycle of ATP binding and hydrolysis, the γ HC motor unit moves with respect to the A-tubule. Note that the N-terminal domain alters its orientation during this process. In contrast, under high Ca2+ conditions, LC4 releases one site on the γ HC N-terminal region and instead binds IC1 leading to a triple-tethered state. We suggest that this third interaction with the A-tubule disallows motion of the N-terminal region and consequently inhibits this HC from acting as a motor. Indeed under these conditions, the γ HC may act as an ATP-dependent brake to inhibit sliding.
Features of the model
The γ HC motor unit is bound to the A-tubule by one or both copies of LC1. Several scenarios for the interactions are possible, including: a) either one or both copies bind the A-tubule and never release; b) one copy of LC1 is bound in the absence of nucleotide and this is shifted to the other copy during the power stroke; c) LC1 acts as a “loose tether” and exhibits low affinity binding to the A-tubule that can be dragged from one tubulin monomer to the next by the dynein power stroke; d) LC1 does release and rebind during the power stroke but this transient behavior cannot be detected by currently available assays.
The γ HC N-terminal domain alters conformation following Ca2+ binding to LC4 and there is a consequent interaction with IC1 which is located near the A-tubule. Note that in this high Ca2+ state, the γ HC is now tethered to the A-tubule via three different regions (i. through interaction of the N-terminal domain with the IC/LC complex and docking complex; ii. via association of LC4 with IC1 which in turn binds α-tubulin directly; and, iii. by binding of the motor unit via one or both copies of LC1). If the orientation of the N-terminal domain with respect to the A-tubule changes during the mechanochemical cycle, this would be inhibited by the third tether; an apparent structural change in this region between the rigor and ADP states can be seen in cryo-positive stained images (Ueno et al. 2008). It also seems reasonable that this conformational alteration would be transmitted to the adjacent linker region that is key to the power stroke. One possible result of this would be to stop the motor domain moving to its next binding site along the B-tubule
An ATPase cycle of the γ HC will cause a transient tight binding to the B-tubule of the adjacent doublet. However, if the LC1 tether to the A-tubule is tight so that the AAA ring cannot move or the N-terminal domain is fixed by an additional tether as occurs in the presence of high Ca2+, the microtubule-binding domain consequently would be unable to find its next interaction site along the B-tubule. This would not lead to a power stroke but rather merely ATP-dependent binding, i.e. the γ HC would act as an ATP-dependent brake to limit the degree of sliding.
Lack of the γ HC motor/LC1 in the oda2-t mutant results in significantly reduced beat frequency and an ineffective symmetric waveform (Liu et al. 2008), whereas lack of the β HC motor unit leads to an even more severe motility phenotype (Sakakibara et al. 1993). The current data suggest that these motors are essential for very different reasons: the β HC provides the motive force, whereas the γ HC controls the degree of sliding and thus the waveform. This is consistent with in vitro experiments using fractionated sea urchin outer arm dynein components which found that the α HC2 in solution functioned to bundle microtubules in an ATP-dependent manner (Moss et al. 1992b), whereas the β HC did not (Moss et al. 1992a); the latter motor was also capable of inducing microtubule gliding when adsorbed to a glass surface. Under similar conditions, the Chlamydomonas γ HC can indeed also act to translocate microtubules (Sakakibara and Nakayama 1998). However, it is important to recognize that in this scenario the geometry of the system is different to that seen in situ, as LC1 is no longer acting to tether the motor unit to the A tubule.
In conclusion, recent data have allowed us to propose a model for how the outer arm dynein motor is controlled such that a wave of activity can be propagated along the axoneme and provides a potential molecular mechanism for how this can be combined with Ca2+ signaling pathways to result in alterations in motor function that ultimately lead to waveform conversion.
The question of what precise signal the γ HC-LC1-LC4-microtubule complex actually responds to remains open. For example, one might envisage that microtubule bending generates tension in this dual/triple-tether system that leads to a change in motor output once some critical value has been reached. Alternatively, it is possible that LC1 may act in the direct readout of changes in the microtubule lattice structure that occur upon bending. A third possibility is that this complex functions as one of the springs (in this case one whose compliance is tunable in a Ca2+-dependent manner) that have been hypothesized recently to be necessary for “self-organized oscillation” (Mitchison and Mitchison 2010). Experimental tests of these concepts will be challenging but potentially highly informative. In addition, it will be fascinating to determine how other regulatory pathways (such as the response to alterations in redox poise mediated through HC-associated thioredoxins) are integrated to provide coordinated responses to multiple signals.
Acknowledgments
I thank the reviewers for their highly constructive comments. My laboratory is supported by grant GM51293 from the National Institutes of Health.
Abbreviations used
- HC
heavy chain
- IC
intermediate chain
- LC
light chain
Footnotes
Note that dynein heavy chain nomenclature can be confusing. The heavy chain designated “γ” within the Chlamydomonas outer dynein arm is equivalent to the α and β heavy chains from sea urchin and Ciona sperm outer arm dyneins, respectively (Wilkes et al 2008; Mizuno et al. 2009)
References
- Baron DM, Kabututu ZP, Hill KL. Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement. J Cell Sci. 2007;120:1513–1520. doi: 10.1242/jcs.004846. [DOI] [PubMed] [Google Scholar]
- Benashski SE, Patel-King RS, King SM. Light chain 1 from the Chlamydomonas outer dynein arm is a leucine-rich repeat protein associated with the motor domain of the γ heavy chain. Biochemistry. 1999;38:7253–7264. doi: 10.1021/bi990466y. [DOI] [PubMed] [Google Scholar]
- Bessen M, Fay RB, Witman GB. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J Cell Biol. 1980;86:446–455. doi: 10.1083/jcb.86.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw C. Thinking about flagellar oscillation. Cell Motil Cytoskeleton. 2009;66:425–436. doi: 10.1002/cm.20313. [DOI] [PubMed] [Google Scholar]
- Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–718. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- Carter AP, Garbarino JE, Wilson-Kubalek EM, Shipley WE, Cho C, Milligan RA, Vale RD, Gibbons IR. Structure and functional role of dynein's microtubule-binding domain. Science. 2008;322:1691–1695. doi: 10.1126/science.1164424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey D, Inaba K, Pazour G, Takada S, Wakabayashi K, Wilkerson C, Kamiya R, Witman G. DC3, the 21-kD subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol Biol Cell. 2003a;14:3650–3663. doi: 10.1091/mbc.E03-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey D, Yagi T, Kamiya R, Witman G. DC3, the smallest subunit of the Chlamydomonas flagellar outer dynein arm-docking complex, is a redox-sensitive calcium-binding protein. J Biol Chem. 2003b;278:42652–42659. doi: 10.1074/jbc.M303064200. [DOI] [PubMed] [Google Scholar]
- DiPetrillo C, Smith E. Calcium regulation of ciliary motility: analysis of axonemal calcium-binding proteins. Methods Cell Biol. 2009;92:163–180. doi: 10.1016/S0091-679X(08)92011-2. [DOI] [PubMed] [Google Scholar]
- Furuta A, Yagi T, Yanagisawa HA, Higuchi H, Kamiya R. Systematic comparison of in vitro motile properties of Chlamydomonas wild-type and mutant outer arm dyneins each lacking one of the three heavy chains. J Biol Chem. 2009;284:5927–5935. doi: 10.1074/jbc.M807830200. [DOI] [PubMed] [Google Scholar]
- Gibbons BH, Gibbons IR. Flagellar movement and adenosine triphosphatease activity in sea urchin sperm extracted with Triton X-100. J Cell Biol. 1972;54:75–97. doi: 10.1083/jcb.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Sakato M, Tedford HW, Benashski SE, Patel-King RS, King SM. Redox-based control of the γ heavy chain ATPase from Chlamydomonas outer arm dynein. Cell Motil Cytoskeleton. 2002;52:131–43. doi: 10.1002/cm.10044. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Shingyogi C. Mechanism of flagellar oscillation - bending-induced switching of dynein activity in elastase-treated axonemes of sea urchin sperm. J Cell Sci. 2008;121:2833–2843. doi: 10.1242/jcs.031195. [DOI] [PubMed] [Google Scholar]
- Hayashibe K, Shingyoji C, Kamiya R. Induction of temporary beating in paralyzed flagella of Chlamydomonas mutants by application of external force. Cell Motil Cytoskeleton. 1997;37:232–239. doi: 10.1002/(SICI)1097-0169(1997)37:3<232::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kagami O, Kamiya R. Translocation and rotation of microtubules caused by multiple species of Chlamydomonas inner-arm dynein. J Cell Sci. 1992;103:653–664. [Google Scholar]
- Kamiya R, Okamoto M. A mutant of Chlamydomonas reinhardtii that lacks the flagellar outer dynein arm but can swim. J Cell Sci. 1985;74:181–191. doi: 10.1242/jcs.74.1.181. [DOI] [PubMed] [Google Scholar]
- Kamiya R, Witman GB. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984;98:97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Kamiya R. Axonemal dyneins: assembly, structure and force generation. In: Witman GB, editor. The Chlamydomonas Source Book, 2nd Edition. Volume 3: Cell Motility and Behavior. San Diego: Elsevier; 2008. pp. 131–208. [Google Scholar]
- King SM, Patel-King RS. Identification of a Ca(2+)-binding light chain within Chlamydomonas outer arm dynein. J Cell Sci. 1995;108:3757–3764. doi: 10.1242/jcs.108.12.3757. [DOI] [PubMed] [Google Scholar]
- King SM, Wilkerson CG, Witman GB. The Mr78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with α tubulin in situ. J Biol Chem. 1991;266:8401–8407. [PubMed] [Google Scholar]
- Lechtreck K, Witman G. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J Cell Biol. 2007;176:473–482. doi: 10.1083/jcb.200611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Delmotte P, Robinson ML, Sanderson MJ, Witman GB. Mutations in hydin impair ciliary motility in mice. J Cell Biol. 2008;180:633–643. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB. Geometric Clutch model version 3: The role of the inner and outer arm dyneins in the ciliary beat. Cell Motil Cytoskeleton. 2002;52(4):242–54. doi: 10.1002/cm.10049. [DOI] [PubMed] [Google Scholar]
- Liu Z, Takazaki H, Nakazawa Y, Sakato M, Yagi T, Yasunaga T, King SM, Kamiya R. Partially functional outer arm dynein in a novel Chlamydomonas mutant expressing a truncated γ heavy chain. Eukaryotic Cell. 2008;7:1136–1145. doi: 10.1128/EC.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Rosenbaum JL. A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J Cell Biol. 1985;100:1228–1234. doi: 10.1083/jcb.100.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Mitchison HM. Cell biology: how cilia beat. Nature. 2010;463:308–309. doi: 10.1038/463308a. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Padma P, Konno A, Satouh Y, Ogawa K, Inaba K. A novel neuronal calcium sensor family protein, calaxin, is a potential Ca2+-dependent regulator for the outer arm dynein of metazoan cilia and flagella. Biol Cell. 2009;101:91–103. doi: 10.1042/BC20080032. [DOI] [PubMed] [Google Scholar]
- Moss AG, Gatti JL, Witman GB. The motile β/IC1 subunit of sea urchin sperm outer arm dynein does not form a rigor bond. J Cell Biol. 1992a;118:1177–1188. doi: 10.1083/jcb.118.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss AG, Sale WS, Fox LA, Witman GB. The α subunit of sea urchin sperm outer arm dynein mediates structural and rigor binding to microtubules. J Cell Biol. 1992b;118:1189–200. doi: 10.1083/jcb.118.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano I, Kobayashi T, Yoshimura M, Shingyoji C. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J Cell Sci. 2003;116:1627–1636. doi: 10.1242/jcs.00336. [DOI] [PubMed] [Google Scholar]
- Oda T, Hirokawa N, Kikkawa M. Three-dimensional structures of the flagellar dynein-microtubule complex by cryoelectron microscopy. J Cell Biol. 2007;177:243–252. doi: 10.1083/jcb.200609038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-King R, Gorbatyuk O, Takebe S, King S. Flagellar radial spokes contain a Ca2+-sensitive nuceloside diphosphate kinase. Mol Biol Cell. 2004;15:3891–3902. doi: 10.1091/mbc.E04-04-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-King RS, Benashki SE, Harrison A, King SM. Two functional thioredoxins containing redox-sensitive vicinal dithiols from the Chlamydomonas outer dynein arm. J Biol Chem. 1996;271:6283–6291. doi: 10.1074/jbc.271.11.6283. [DOI] [PubMed] [Google Scholar]
- Patel-King RS, King SM. An outer arm dynein light chain acts in a conformational switch for flagellar motility. J Cell Biol. 2009;186:283–295. doi: 10.1083/jcb.200905083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Ramanis Z, Smith EF, Sale WS. Three distinct inner dynein arms in Chlamydomonas flagella: molecular composition and location in the axoneme. J Cell Biol. 1990;110:379–89. doi: 10.1083/jcb.110.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Numata N, Walker ML, Kato YS, Malkova B, Kon T, Ohkura R, Arisaka F, Knight PJ, Sutoh K, et al. AAA+ ring and linker swing mechanism in the dynein motor. Cell. 2009;136(3):485–495. doi: 10.1016/j.cell.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Patel-King RS, King SM. Outer arm dynein is required for gliding motility in planaria. Am Soc Cell Biol abstract 2009 [Google Scholar]
- Sakakibara H, Nakayama H. Translocation of microtubules caused by the αβ, β and γ outer arm dynein subparticles of Chlamydomonas. J Cell Sci. 1998;111:1155–1164. doi: 10.1242/jcs.111.9.1155. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Takada S, King SM, Witman GB, Kamiya R. A Chlamydomonas outer arm dynein mutant with a truncated β heavy chain. J Cell Biol. 1993;122:653–661. doi: 10.1083/jcb.122.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakato M, Sakakibara H, King SM. Chlamydomonas outer arm dynein alters conformation in response to Ca2+ Mol Biol Cell. 2007;18:3620–3634. doi: 10.1091/mbc.E06-10-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Matsuoka T. Splitting the ciliary axoneme: implications for a “switch-point” model of dynein arm activity in ciliary motion. Cell Motil Cytoskeleton. 1989;14:345–358. doi: 10.1002/cm.970140305. [DOI] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga N, Wang J, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Naitoh Y. Simulated cross-bridge patterns corresonding to ciliary beating in Paramecium. Nature. 1982;295:609–611. [Google Scholar]
- Ueno H, Yasunaga T, Shingyoji C, Hirose K. Dynein pulls microtubules without rotating its stalk. Proc Natl Acad Sci USA. 2008;105:19702–19707. doi: 10.1073/pnas.0808194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, King SM. Modulation of Chlamydomonas reinhardtii flagellar motility by redox poise. J Cell Biol. 2006;173:743–754. doi: 10.1083/jcb.200603019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K-i, Ide T, Kamiya R. Calcium-dependent flagellar motility activation in Chlamydomonas reinhardtii in response to mechanical agitation. Cell Motil Cytoskeleton. 2009;66:736–742. doi: 10.1002/cm.20402. [DOI] [PubMed] [Google Scholar]
- Wargo M, Smith E. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc Natl Acad Sci USA. 2003;100:137–142. doi: 10.1073/pnas.0135800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo MJ, Dymek E, Smith E. Calmodulin and PF6 are components of a complex that localizes to the C1 microtubule of the flagellar central apparatus. J Cell Sci. 2005;118:4655–4665. doi: 10.1242/jcs.02585. [DOI] [PubMed] [Google Scholar]
- Wargo MJ, McPeek MA, Smith EF. Analysis of microtubule sliding patterns in Chlamydomonas flagellar axonemes reveals dynein activity on specific doublet microtubules. J Cell Sci. 2004;117:2533–2544. doi: 10.1242/jcs.01082. [DOI] [PubMed] [Google Scholar]
- Weber C, Lee VD, Chazin WJ, Huang B. High level expression in Escherichia coli and characterization of the EF-hand calcium-binding protein caltractin. J Biol Chem. 1994;269:15795–15802. [PubMed] [Google Scholar]
- Wilkes DE, Watson HE, Mitchell DR, Asai DJ. Twenty-five dyneins in Tetrahymena: a re-examination of the multidynein hypothesis. Cell Motil Cytoskeleton. 2008;65:342–351. doi: 10.1002/cm.20264. [DOI] [PubMed] [Google Scholar]
- Wu H, Blackledge M, Maciejewski MW, Mullen GP, King SM. Relaxation-based structure refinement and backbone molecular dynamics of the dynein motor domain-associated light chain. Biochemistry. 2003;42:57–71. doi: 10.1021/bi026762j. [DOI] [PubMed] [Google Scholar]
- Wu H, Maciejewski MW, Marintchev A, Benashski SE, Mullen GP, King SM. Solution structure of a dynein motor domain associated light chain. Nature Struct Biol. 2000;7:575–579. doi: 10.1038/76804. [DOI] [PubMed] [Google Scholar]
- Yagi T, Minoura I, Fujiwara A, Saito R, Yasunaga T, Hirono M, Kamiya R. An axonemal dynein particularly important for flagellar movement at high viscosity: Implications from a new Chlamydomonas mutant deficient in the dynein heavy chain gene DHC9. J Biol Chem. 2005;280:41412–41420. doi: 10.1074/jbc.M509072200. [DOI] [PubMed] [Google Scholar]
- Yang P, Diener DR, Rosenbaum JL, Sale WS. Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J Cell Biol. 2001;153:1315–1326. doi: 10.1083/jcb.153.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Diener DR, Yang C, Kohno T, Pazour GJ, Dienes JM, Agrin NS, King SM, Sale WS, Kamiya R, Rosenbaum JL, Witman GB. Radial spoke proteins of Chlamydomonas flagella. J Cell Sci. 2006;119:1165–1174. doi: 10.1242/jcs.02811. [DOI] [PMC free article] [PubMed] [Google Scholar]