Abstract
Immune dysregulation and inflammation play a major role in the pathology of age-related disorders. In an earlier study, the microarray data from our laboratory indicated an increase in inflammation-related gene expression in the liver with age. We further investigated immune-related changes in the aged liver and found that the levels of inflammatory cytokines, chemokines, and inflammatory genes were higher in aged animals. Immunohistochemical studies showed that immune cells formed clusters or foci in the livers of old mice, preferentially near the perivascular regions. Further analysis revealed an enrichment of macrophages, T cells, B cells, natural killer cells, and neutrophils in old liver. Characterization of the immune clusters showed the presence of shared markers of tertiary lymphoid neogenesis. Levels of lymph node homing cytokines were elevated. Expression of immunoglobulin and recombinase gene transcripts was also higher, indicating the presence of ectopic lymphoid structures in the aged liver. Conclusion Aged liver exhibits a marked inflammatory status accompanied by increased immune cell infiltration. Inflammation and ectopic lymphoid structures have previously been shown to be associated with carcinogenesis, a condition that becomes more prevalent with age. Thus, further study of inflammation-related changes in the microenvironment of the aged liver could provide insights into these disorders.
The aging process has been shown to be associated with an increase in the proinflammatory status of the organism.1 Age-associated inflammation has been described to occur in lower organisms such as C. elegans and Drosophila, which showed marked up-regulation of immune defense genes.2, 3 In mice and humans, an increase in the circulating levels of proinflammatory cytokines such as IL -6 , TNF-α, and IL-1 with age has been reported.4–6 Global mRNA expression studies done in various tissues including liver tissue have shown that one of the major categories of genes up-regulated with age is that involved in the inflammatory and stress response.7–9
Gene expression profiling data published from our laboratory has also reported similar results. Regression analysis carried out with gene expression data from 3-, 6-, 12-, and 24-month-old C57BL/6 mice showed that many of the genes exhibiting the highest elevation in expression belonged to the functional category of inflammation-related genes. These included hepatospecific acute-phase genes such as lipopolysaccharide-binding protein and haptoglobin.10 Also, flow cytometry studies have shown an increase in the total numbers of immune cells in the livers of aged animals.11
In this article we report a comprehensive study of inflammatory signaling and localization of various immune cell types in aged liver tissue. We show that the message levels of inflammation-related genes, that is, inflammatory cytokines and chemokines, are higher in old mice. Using cell surface markers, we also show that various immune cells are more prevalent in the livers of aged animals and are localized predominantly in focal clusters as opposed to being distributed throughout the parenchyma. We quantify the differences in numbers of these immune cell clusters as well as characterize their size and tissue distribution in 3-month-old and 24-month-old mice. It has been shown in several studies that the presence of chronic inflammation leads to the development of ectopic lymphoid structures in the tissue.12 Further characterization of immune clusters in aged liver revealed the shared presence of markers of tertiary lymphoid neogenesis. Thus, our study has shown higher levels of proinflammatory cytokines and increased immune cell infiltration of liver tissue with age. Chronic inflammation has been linked to carcinogenesis and autoimmune disorders.12,13 The presence of these conditions in aged liver also may potentially impair recovery from hepatic injury and diminish the metabolic output of the liver, thus identifying an important area of research in liver gerontology.
Materials and Methods
Materials
Mice
C57BL/6 mice were purchased from Jackson Laboratories. The animals were housed in groups of three to five animals of the same sex in a room with a controlled photoperiod of 12 hours light/12 hours dark (lights on from 7:00 &rtf-scaps-start;am&rtf-scaps-end; to 7:00 &rtf-scaps-start;pm&rtf-scaps-end;) and a temperature of 22°C ± 2°C. Animals were given free access to water and a pellet diet (5010 rodent diet, LabDiet; PMI Nutrition International, Brentwood, MO). For tissue harvesting, the animals were anesthetized with isofluorane followed by cervical dislocation; livers were collected, flash-frozen in liquid nitrogen, and stored at −70°C. All procedures with mice were carried out in accordance with the guidelines for laboratory animal welfare provided by the Association of Assessment and Accreditation of Laboratory Animal Care–approved Center of Comparative Medicine at Baylor College of Medicine, Houston, TX, under specific pathogen–free conditions in microisolator cages.
Liver Perfusion and Collection of Nonparenchymal Cells
The mice were anesthetized using isofluorane. The peritoneal cavity was opened, and the inferior vena cava was cannulated using a catheter. The liver was perfused with EGTA solution (0.2 mg/mL) for 5 minutes followed by collagenase IV solution (0.3 mg/mL) until the liver began to dissociate under Glisson’s capsule. Dissociated liver cells were separated from the tissue debris using a coarse mesh. The liver cell suspension was centrifuged at 50g for 2 minutes and supernatant collected. Centrifugation was repeated twice at 50g to separate the hepatocytes from the nonparenchymal cell (NPC) population. The supernatant was finally spun at 1,000g, and the NPC fraction was collected, frozen in liquid nitrogen, and stored at −80°C
RT-PCR
Total RNA was extracted from frozen livers using an RNeasy purification kit (Qiagen) in accordance with the manufacturer’s protocol. DNase-treated total liver RNA was reverse-transcribed using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). Realtime polymerase chain reaction (RT-PCR) was performed using SYBR Green Master Mix and an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). γ-Tubulin and β-actin were used as concentration control genes. Thermal cycling conditions consisted of an initial step at 95°C for 10 minutes to activate the Taq DNA polymerase and 40 cycles of sequential denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 60 seconds. Data analysis was performed using the ABI Prism 7000 SDS Software (Applied Biosystems). The primers used are listed in Supplementary Table 1. Real-time PCR analysis was performed according to the comparative CT method.10 The P values reported for these changes refer to a 2-tailed t test comparing normalized CT values in old and young mice.
Immunohistochemistry
For immunohistochemistry, tissue was either fixed in 10% formalin in PBS for 8 hours at room temperature and stored in 70% ethanol for paraffin sections or embedded directly in Cryomatrix (Shandon, Waltham, MA) on dry ice for frozen sections which were stored at −80°C. Paraffin-embedded tissue blocks were cut into 4-µm sections, and hematoxylin and eosin (H&E) staining was carried on them for histological studies. The paraffin-embedded sections were also used to stain the liver samples for macrophages using rat antimouse F4/80 antigen (MCA497GA; Serotec, Raleigh, NC). Frozen tissue was also sectioned to a thickness of 4 µm and fixed in 4% paraformaldehyde for immunofluorescence staining. To ascertain the types of immune cells present in the immune clusters, the frozen sections were incubated overnight at 4°C with the following primary antibodies: pan leukocyte marker—CD45 (550539 and 555480; BD Biosciences, San Jose, CA); for macrophages—F4/80 (MF48000; Caltag, Carlsbad, CA); T cells (total)—CD3 (153501; Southern Biotech, Birmingham, AL); cytotoxic T cells—CD8 (550281; BD); helper T cells (550278; BD); B cells—B220 (557390; BD); dendritic cells—CD11c (550283; BD); neutrophils—GR1 (550291; BD); proliferating cell nuclear antigen (PCNA; sc-7907; Santa Cruz, CA); and PNA-FITC (FL-1071; Vector, Burlingame, CA). Sections were incubated with anti-HRP-labeled rat, rabbit, and hamster secondary antibodies (1:500; The Jackson Laboratory, West Grove, PA), and subsequently an HRP–fluorophore reaction was carried out with Alexa Fluor 568 substrate dye (T20924; Molecular Probes–Invitrogen, Carlsbad, CA).
Imaging and Statistical Analysis
Immunohistochemistry imaging was carried out using a Zeiss Axioskop 2 plus microscope. Image capture and calculation of cluster size were done using AxioVision 3.1.2.1 image analysis software. Statistical analysis including calculation of mean distribution and standard deviation for the cluster size study was carried out using Microsoft Excel software.
Results
Increase in Inflammation in Liver Tissue with Age
Microarray data analysis comparing changes in the liver tissue with age suggested the presence of a proinflammatory environment in the aged animals, with induction of inflammatory genes such as haptoglobin, serum amyloid, lysozyme, and lymphoid cell–specific genes such as heavy-chain and light-chain immunoglobulin. As a more sensitive measure of the magnitude of changes, message levels of inflammation-related genes were determined by real-time PCR from liver cDNA samples of five young (3-month-old) and five old (24-month-old) mice (Table 1). There was a general increase in the expression of proinflammatory cytokines IFN-γ, IL-β, IL-6, TNF-α, and IL-2 as well as anti-inflammatory cytokines TGF-β and IL-10, which are up-regulated in response to inflammation and also participate in its regulation.14,15 Inflammation-inducible genes—fibrinogen, haptoglobin, intercellular adhesion molecule 1, lipo-polysaccharide binding protein (LBP), promyelocytic leukemia protein, and serum amyloid-A (SAA) were more highly expressed in aged liver, and all but fibrinogen showed a significant difference (P<0.05). Fibrinogen, haptoglobin, LBP, and SAA are acute-phase genes produced solely by hepatocytes and are elevated during systemic inflammation.16 Increased expression of these genes strongly suggested that the hepatocytes in the older animals were responding to inflammatory signals. Tissue inflammation and induction of the inflammatory genes are a result of signaling via proinflammatory cytokines and chemokines. We also examined the message levels of the chemokines RANTES, MIP1-α, IP-10, and GRO1, which recruit various immune cells in inflamed tissue (Table 1c). These chemokines are potent chemoattractants for T cells, monocytes, natural killer (NK) cells, macrophages, and neutrophils, the immune cells known to produce inflammation-mediating proteins and proinflammatory cytokines. All these chemokines had higher message levels in aged tissue, especially RANTES and MIP1α which showed statistically significant increases. Thus, aged liver shows an increased inflammatory signaling compared with that in the young.
Table 1.
Changes in Expression of Inflammation-Related Genes with Age in Mice
| Gene name | Symbol | Fold Change (old/young) |
P value | Role in inflammation | Reference | Source |
|---|---|---|---|---|---|---|
| A–Inflammatory cytokines | ||||||
| Tumor necrosis factor alpha |
TNFα | 6.39 | 0.0118 | Major proinflammatory cytokine involved in induction of fever, inflammatory genes, and IL-6 and IL-1 cascade. |
17, 18 | Macrophages, mast cells, NK cells, B cells, T cells, fibroblasts, dendritic cells |
| Interleukin-6 | IL-6 | 2.51 | 0.0904 | Proinflammatory cytokine induces acute-phase response in liver, differentiation of B cells into Ig-secreting cells, activation and induction of IL-2 in T-cells. |
18, 19 | Monocytes, macrophages, Th2 cells, endothelial cells, fibroblasts, dendritic cells |
| Interleukin-1 beta | L-β | 1.92 | 0.3077 | Proinflammatory cytokine. Causes fever induction, leads to prostaglandin synthesis and induction of inflammatory genes. |
18, 20 | Monocytes, macrophages, B cells, dendritic cells |
| Interferon gamma | FNγ | 2.72 | 0.0008 | Proinflammatory cytokine, activates macrophages and neutrophils, induces NOS and MMP production, chemokine production and cases lymphocytic tissue infiltration. |
21 | Th1 cells, Tc cells, NK cells, macrophages |
| Transforming growth factor beta |
TGFβ | 1.55 | 0.0166 | Anti-inflammatory cytokine induces tissue inhibitors of metalloproteinases, inhibits nitric oxide and superoxide production, and induces interleukin-1 receptor antagonist expression. |
15 | T cells, monocytes |
| Interleukin-2 | IL-2 | 2.74 | 0.2080 | T-cell proliferation, regulation of immune response. B-cell, monocyte, NK cell activation. |
22 | Th1 cells |
| Interleukin-10 | IL-10 | 4.31 | 0.0072 | Anti-inflammatory action. Inhibits synthesis of several cytokines, including IFNγ, IL-2, IL-3, and TNF |
22 | Th2 and Th0 T cells, B cells, dendritic cells, monocytes |
| B—Inflammation-induced genes | ||||||
| Haptoglobin | Hp | 3.02 | 0.0075 | Inflammation-inducible acute-phase protein. Binds hemoglobin and helps to prevent iron loss and protects kidney from damage. |
23 | Hepatocytes |
| Fibrinogen | Fba | 1.49 | 0.1631 | Inflammation-inducible acute-phase protein. Involved in blood clot formation and wound healing. |
23 | Hepatocytes |
| Lipopolysaccharide- binding protein |
Lbp | 2.67 | 0.0012 | Inflammation-inducible acute-phase protein. Binds to bacterial LPS and facilitates antigen presentation and immune activation. |
24 | Hepatocytes |
| Serum amyloid A1 | Saa1 | 7.05 | 0.0339 | Inflammation-inducible acute-phase protein. An apolipoprotein of the HDL complex. |
23 | Hepatocytes |
| Intercellular adhesion molecule 1 |
Icam1 | 3.03 | 0.0009 | Involved in leukocyte recruitment into tissue and inhibition of IL-4 production by naive T-cells. |
25 | Endothelial cells, lymphocytes |
| Promyelocytic leukemia |
Pml | 2.08 | 0.0006 | Transcriptional regulation and scaffolding. Nuclear levels are up-regulated during inflammation. |
26 | Various cell types |
| C—Chemokines | ||||||
| Chemokine (C-X-C motif) ligand 10 |
CXCL10/IP-10 | 3.74 | 0.0602 | FN-γ-inducible proinflammatory chemokine Chemotactic for CD4+ve T cells and monocytes. |
27 | Neutrophils monocytes, dendritic cells, hepatocytes, endothelial cells |
| Macrophage inflammatory protein 1 alpha |
CCL3/MIP1α | 11.54 | 0.0135 | Involved in acute inflammation by recruitment and activation of polymorphonuclear leukocytes. |
27 | T cells, B cells, macrophages, dendritic cells |
| Chemokine (C-X-C motif) ligand 1 |
CXCL1/Gro1 | 1.31 | 0.79208 | Chemotactic activity for neutrophils and their activation by stimulating exocytosis and the respiratory burst. |
28 | Monocytes, fibroblasts, epithelial cells |
| Chemokine (C-C motif) ligand 5 |
CCL5/RANTES | 13.97 | 0.0043 | Chemoattractant for monocytes, memory T-helper cells, and eosinophils. Causes the release of histamine from basophils and activates eosinophils. |
27 | T cells, platelets |
The table shows fold changes in the expression of mRNA of genes involved in the inflammatory process in the liver. Gene expression was assessed using real-time PCR with cDNA from total liver mRNA of 3-month-old and 24-month-old C57BL/6 mice (n = 5). To determine the significance confidence limit, the t test of sample groups was carried out.
Increased Immune Cell Infiltration of Hepatic Tissue with Age
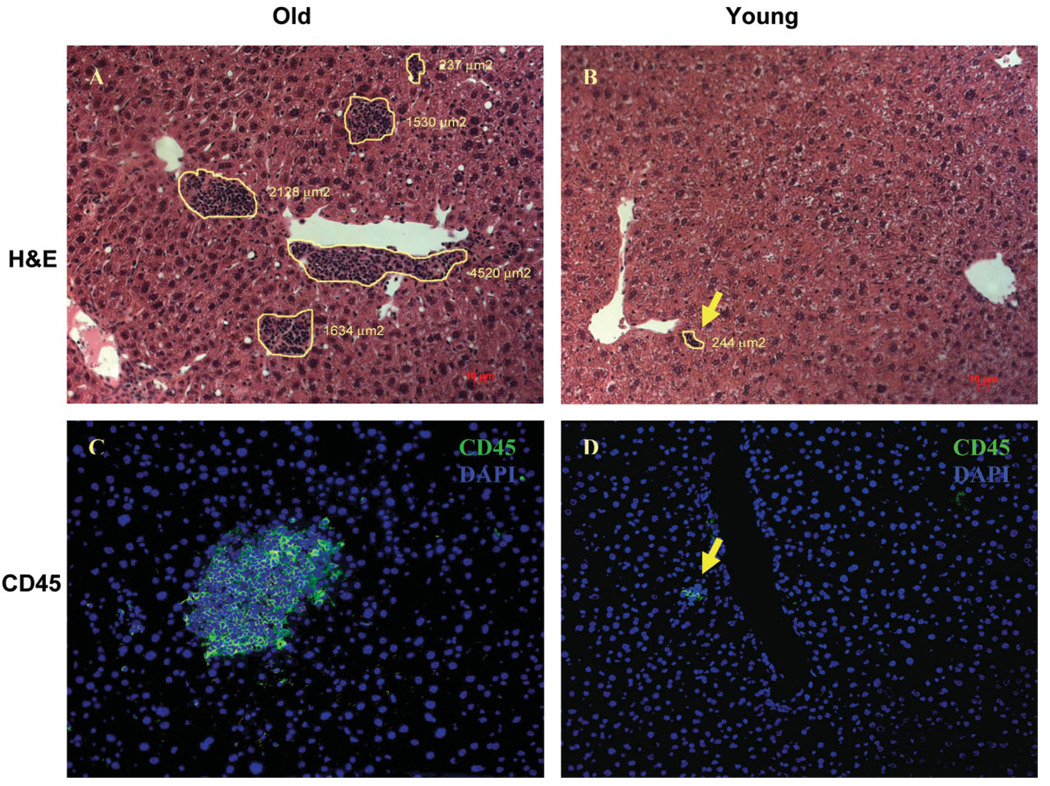
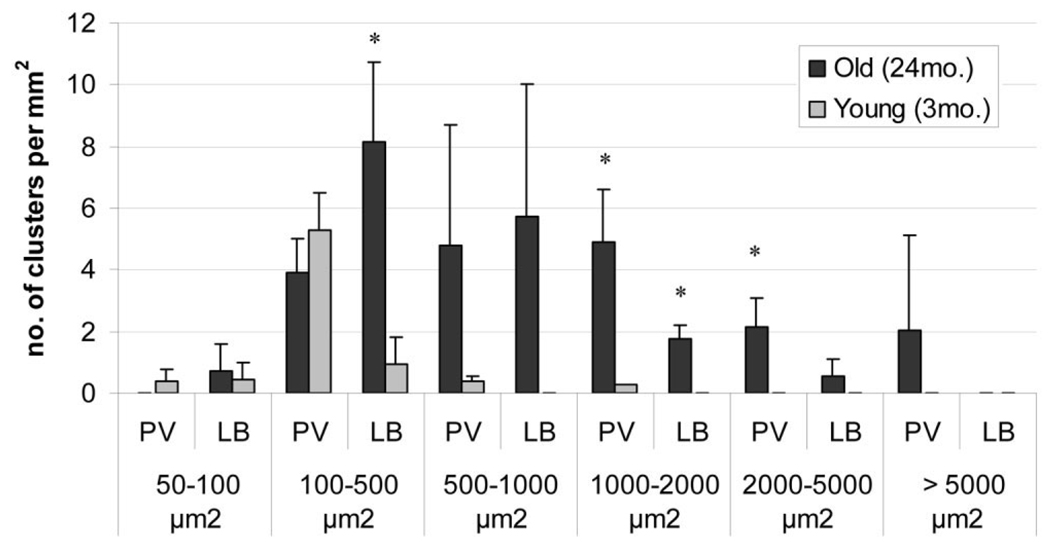
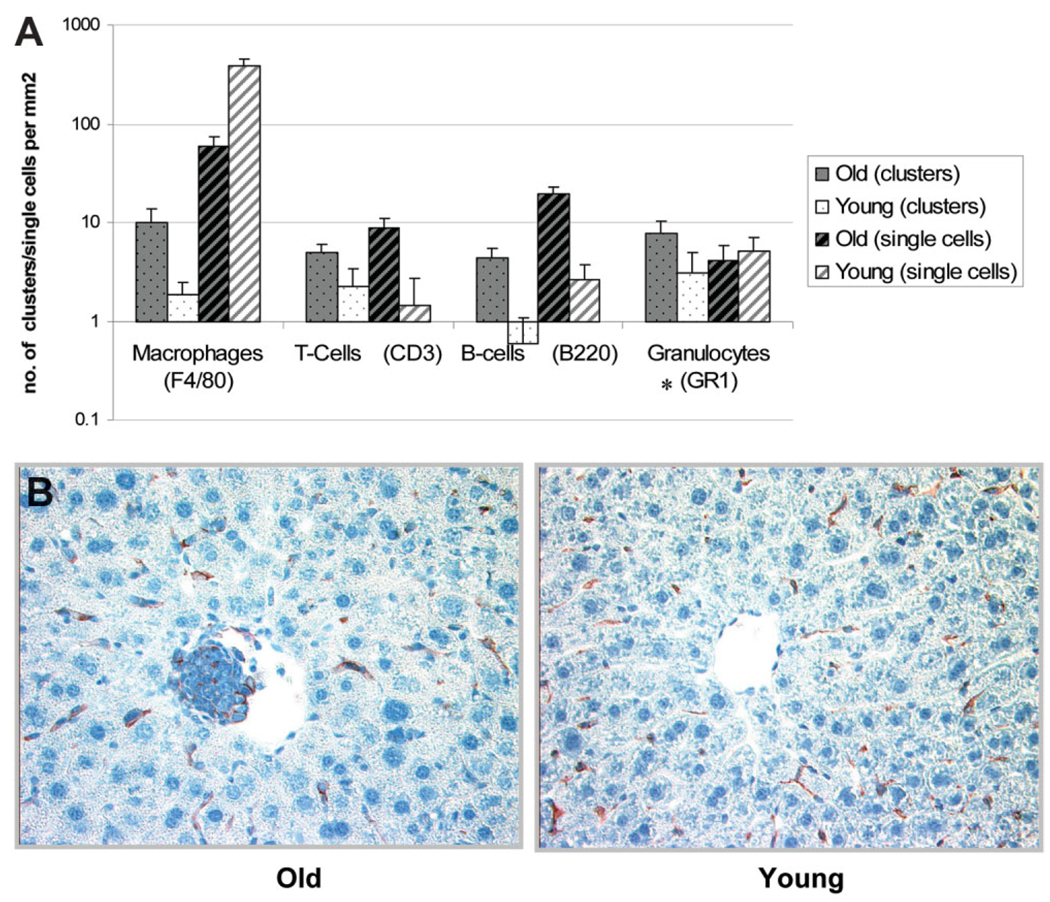
The liver consists mainly of hepatocytes but also has a sizable pool of immune cells, which protect the organism by responding to antigenic threats in portal circulation. Because our gene expression data showed an increase in inflammatory gene expression in the livers from old mice, we determined whether there were changes in the immune cell population of the liver, as these cells play a major regulatory role in inducing and sustaining inflammatory conditions. Liver sections were visualized using H&E staining on formalin-fixed tissue of old and young mice. The hepatocytes showed a characteristic morphology of large cells with pink cytoplasm and round nuclei. Interestingly, foci of nonhepatocytic cells that were much smaller in size with little cytoplasm were also observed. These foci were much more abundant and larger in size in the old livers (Fig. 1). To determine the composition of these cell clusters, we stained the liver sections with the panleukocytic marker CD45, which is expressed in all cells of hematopoietic lineage except red blood cells (Fig. 1). Ninety-six percent of the foci analyzed from both young and old animals (n = 50 clusters) showed staining with the CD45 marker, indicating that these foci are primarily composed of leukocytic cells. We characterized the sizes as well as the position of these foci in liver lobules from both young and old animals. These results are summarized in Table 2 and Fig. 2. The number of clusters was nearly 5 times greater in old liver than in young liver. The clusters in the older animals were larger in size, as assessed by area, with almost 50% exceeding a size of 1,000 µm2, whereas the cluster size in young tissue rarely exceeded 500 µm2. In the old liver samples, the immune cells formed foci in the central lobular region as well, but most of the larger clusters were localized near the perivascular regions. This observation along with the high expression of proinflammatory cytokines and chemokines indicated increased extravasation of immune cells into the liver tissue of old mice. Preliminary data from healthy young and old human subjects aged 25–35 and 60–68 years, respectively, indicated a trend toward elevated levels of inflammatory genes and more numerous immune foci in the aged livers as well (Supplementary Table 4 and Supplementary Fig. 1).
Fig. 1.
Changes in the immune cell population in liver with age. (A, B) Liver tissue from young (3-month-old) and old (24-month-old) mice was stained with H&E to ascertain tissue morphology and cell distribution. The immune clusters have been outlined and their respective size per square micrometer reported. (C, D) Frozen liver sections were stained for pan leukocytic marker CD45 (green) along with nuclear 4′,6-diamidino-2-phenylindole (DAPI)-stained (blue) nuclei. Hepatocytes do not show any staining with CD45, whereas the smaller nuclei in the clusters show strong peripheral CD45 staining, indicating the presence of immune cells. (magnification ×20).
Table 2.
Difference in Numbers and Distribution of Immune Cell Clusters in liver Tissue of Aged and Young Mice
| Average number of clusters/mm2 |
|||
|---|---|---|---|
| Age | Total | PV | LB |
| Aged (24 months old) | 66.67 | 44.44 | 22.22 |
| Young (3 months old) | 10.67 | 2.22 | 12.89 |
The table shows the total number of immune clusters present per square millimeter in livers of old and young mice. It also shows the differences in the distribution of these clusters between the perivascular (PV) regions and the lobular (LB) regions. A total of 150 clusters in old livers and 29 clusters in young livers were analyzed corresponding to the total number of clusters present in an 2.25-mm2 area (corresponding to 5 fields per mouse at 20× magnification; nice= 3).
Fig. 2.
Distribution of the immune clusters between the perivascular (PV) and the lobular (LB) regions of the liver in young (3-month-old) and aged (24-month-old) mice in different size brackets. Six fields per individual animal at magnification ×10 (total area corresponding to 3.63 mm2) were analyzed for each sample (n = 3 young and 3 old; *P < 0.05.
Analysis of Changes in Immune Cell Composition of Hepatic Tissue with Age
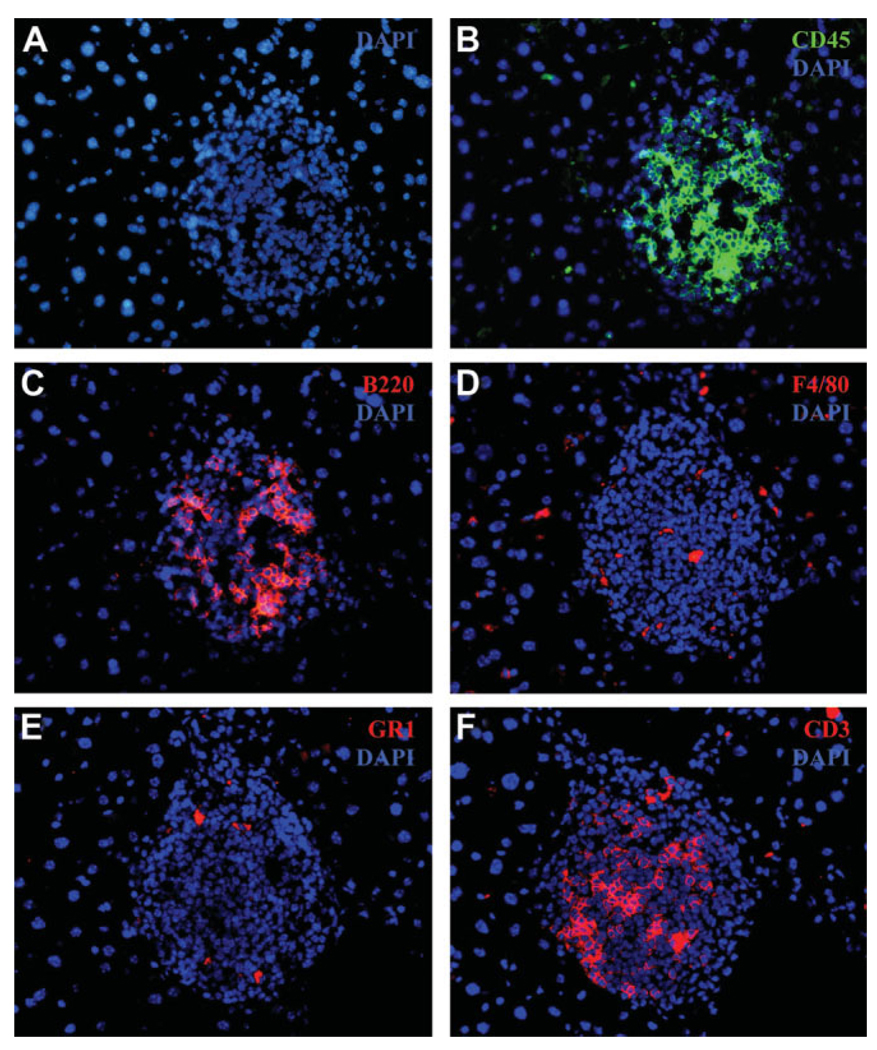
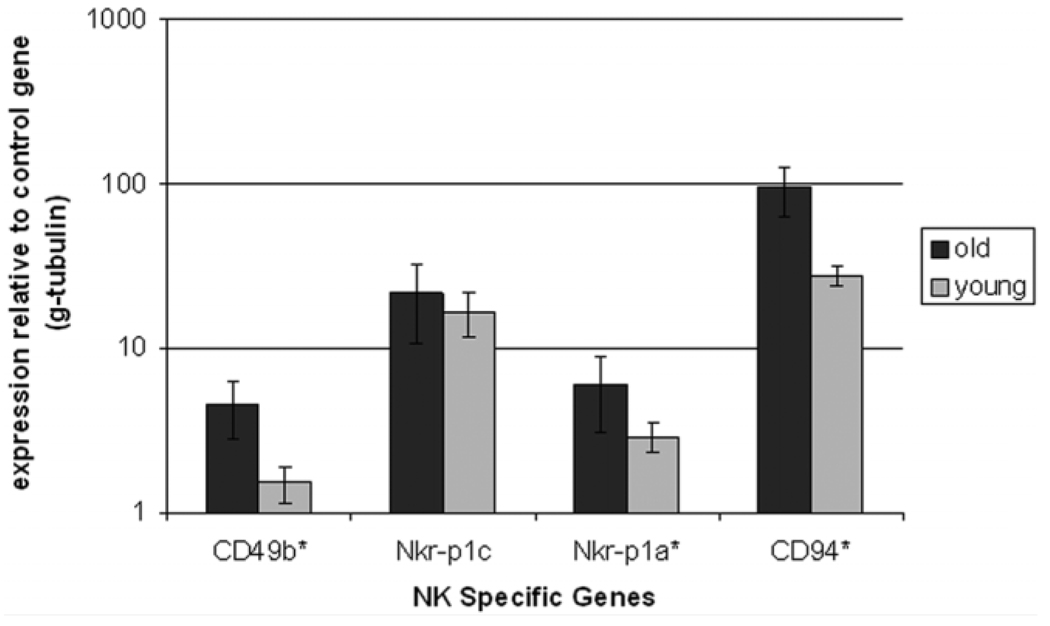
It is well known that the liver has a large resident macrophage (Kupffer cells) and NK cell (pit cells) population. These cells are major players in the inflammatory cascade. Several flow-cytometry studies have shown an increase in the total numbers of immune cells in the liver with age,11 but comprehensive studies on the localization of various cell types in the aged liver have not been done. The presence of an increased number of immune-cell foci in the older animals raises questions about which cell types are predominantly expressed in aged liver and whether these immune cells are scattered throughout the parenchyma or are localized in the foci we observed in the older animals. To address these questions, we stained the liver tissue from aged animals for various immune markers, that is, F4/80 (macrophages), NK1.1 (NK cells), CD3 (T cells), B220 (B cells), and GR1 (neutrophils), as these cell types play a key role in inflammation in tissues. Figure 3 illustrates the staining of consecutive sections of an immune cluster from old liver for various immune cell markers. The staining for NK cells was not successful when we tried different anti-bodies against NK1.1 as well CD49b markers for NK cells. We carried out RT-PCR quantification using NK cell–specific markers—CD49b, NKR-P1C, NKR-P1A, and CD94—in order to discern the relative numbers of NK cells in the liver. An increase in the number of NK cells in hepatic tissue was observed similar to the age-dependent increase in the NK markers seen in humans and in mice circulation (Fig. 4).11,17 The total numbers of single nonclustering cells and the number of immune cell marker–positive foci was assessed in young and old liver tissue, and the results are summarized in Fig. 5A. In general, the number of clusters staining positive for an immune marker was much higher in the old liver samples, as was the number of single cells scattered throughout the parenchyma except macrophages, which were more prevalent as single cells in the younger animals (Fig. 5B). Because the number of macrophage (F4/80)–staining clusters was higher in old livers, this could suggest aggregation of single macrophages from the parenchyma into large foci, as is seen in cases of chronic inflammatory conditions in various tissues.18
Fig. 3.
Frozen sections from old liver tissue (24 months) were paraformaldehyde-fixed and stained with (A) nuclear DAPI stain (blue) and the following immune cell markers: (B) CD45 for leukocytes, (C) B220 for B cells, (D) F4/80 for macrophages, (E) GR1 for granulocytes (neutrophils), and (F) CD3 for T cells (total). Hepatocytes can be visualized with DAPI as larger, round, isolated nuclei, whereas the immune cell cluster can be seen as a region of denser concentration of DAPI staining smaller nuclei (magnification ×40).
Fig. 4.
Bar graph compares the mRNA levels of NK cell-specific genes in old and young mice. Gene expression was assessed by real-time PCR using cDNA from young (3-month-old) and old (24-month-old) mice (*P value < 0.05).
Fig. 5.
Distribution of various immune markers in the hepatic tissue of old and young mice. (A) Number of immune clusters staining positive for a given marker per square millimeter of the tissue as well as the number of single isolated immune cells present per square millimeter of the tissue in the old and young mice (*All comparisons between young and old mice showed a significant difference [P < 0.05} except granulocytes, which showed no significant difference in single-cell distribution with age. (B) Resident macrophages of liver were visualized by immunohistochemistry for the F4/80 antigen. F4/80 staining was seen in the immune foci observed in the old mice, indicating the presence of macrophages in these foci. In both young and old liver samples, Kupffer cells were present in the sinusoids. Nuclei have been counterstained with hematoxylin (mgnification ×20).
Chronic Inflammation in Old Tissue Leads to Tertiary Lymphoid Neogenesis
Tertiary lymphoid organs are ectopic accumulations of lymphoid cells that arise in chronically inflamed tissue, a process called lymphoid neogenesis.12 These structures are induced in the presence of chronic inflammatory conditions, which lead to the production of homing chemokines by both lymphocytes and nonparenchymal cells.19 In older mice the levels of proinflammatory cytokines and chemokines were up-regulated compared with those in younger animals along with increased expression of proinflammatory genes. Also present were clusters of immune cells, which were enriched in macrophages, T cells, B cells, and neutrophils. These conditions recapitulate the proinflammatory conditions required for induction of tertiary lymphoid organs. Thus, we further tested the old liver samples for the shared presence of markers observed in tertiary lymphoid structures, as seen in cases of chronic inflammation.
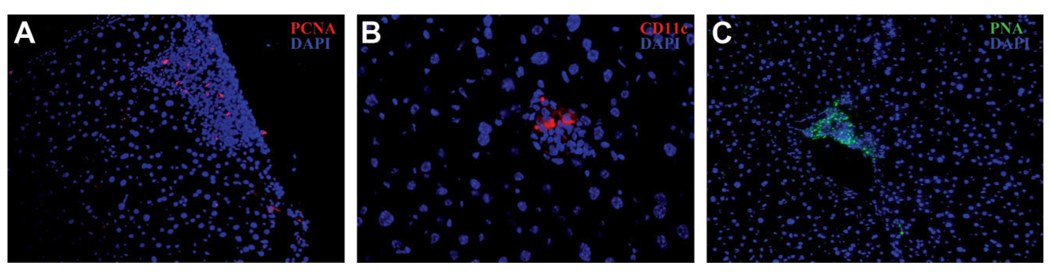
One of the hallmarks of cells in tertiary lymphoid structures is that they proliferate in the host organ.12 Old and young liver tissue was stained with the proliferative marker PCNA (Fig. 6). The percentage of clusters showing PCNA-positive cells in the old tissue was significantly higher compared with tissue from young animals. Seventy-five percent of the clusters (n = 108) in the liver tissue from aged mice contained at least 1 PCNA-positive cell, whereas in young mice only 11% of the clusters (n = 46) contained PCNA-positive cells for the same area analyzed (3mm2 per animal; nmice = 2). Earlier studies have shown that tertiary lymphoid structures contain B cells, T cells, and dendritic cells and have increased expression of immunoglobulin transcripts and various chemokines.19,20 Figures 3 and 5 demonstrate the presence of B cells and T cells in the immune clusters in old animals. Further analysis of the old tissue for the presence of dendritic cells (CD11c marker) and a germinal center marker (peanut agglutinin) was carried out by immunohistochemistry. A high proportion of the larger clusters (exceeding 1,000 µm2) showed the presence of these markers (Fig. 6).
Fig. 6.
Frozen sections from liver tissue of old mice were paraformaldehyde- fixed and stained with the following markers associated with lymphoid neogenesis and nuclear DAPI stain (blue): (A) proliferation marker PCNA (magnification ×20); (B) dendritic cell marker CD11c (magnification ×40); and (C) germinal center marker peanut agglutinin (magnification ×20).
Lymphotoxin alpha production has been shown to lead to the formation of ectopic tertiary lymphoid structures in chronically inflamed tissues. It also induces lymph node homing cytokines CXCL13, CCL20, CCL21, and CCL19 and leads to up-regulation of cell adhesion proteins PNAd and Madcam1.21,22 We predicted that expression of these genes would be increased in the livers of the old mice. Real-time PCR was carried out on whole liver tissue from both young and old animals to determine the levels of these genes. The levels of lymphotoxin alpha were 10 times higher in old tissue along with an up-regulation of CXCL13, CCL19, CCL20, and PNAd (Table 3). Finally, to assess B-cell clonal expansion, we looked at the levels of B-cell transcripts as wells as levels of recombination activating genes (rag1/rag2), which are involved in the rearrangement of V(D)J gene segments in primary lymphoid organs, in the nonparenchymal fraction of the liver, which is highly enriched for immune cells (Supplementary Table 2). The levels of both rag1 and rag2 genes were up-regulated along with increased expression of various immunoglobulin transcripts similar to the tertiary lymph node–like structures seen in inflamed tissues, indicating that the old liver was undergoing lymphoid neogenesis (Table 4).
Table 3.
Changes in Expression of Genes Associated with Development of Ectopic Germinal Centers in Chronically Inflamed Tissue
| Gene name | Symbol | Fold change (old/young) |
P value | Role in lymphoid neogenesis | Reference | Tissue fraction |
|---|---|---|---|---|---|---|
| Peripheral node addressin | PNAd | 1.36 | 0.013 | Homing of lymphocytes to peripheral lymph nodes. Interaction between L-selectin on lymphocytes and PNAd. |
37 | Whole liver |
| Lymphotoxin-alpha | LTα | 10.53 | 0.028 | Induces expression of adhesion molecules VCAM, ICAM, and MAdCAM-1. Induces expression of leukocyte homing chemokines. |
37 | Whole liver |
| Mucosal addressin cell adhesion molecule |
MAdCAM-1 | 0.69 | 0.043 | Endothelial cell adhesion molecule that directs leukocytes into lymph nodes and inflamed tissues. |
37 | Whole liver |
| Chemokine (C-C motif) ligand 19 |
CCL19 | 3.25 | 0.023 | Trafficking of T cells in thymus. T-cell and B-cell migration to lymphoid organs. |
38 | Whole liver |
| Chemokine (C-X-C motif) ligand 13 |
CXCL13 | 17.03 | 0.203 | Chemotactic for B cells. | 34, 38 | Whole liver |
| Chemokine (C-C motif) ligand 20 |
CCL20 | 1.76 | 0.097 | Involved in formation and function of mucosal lymphoid tissues by attracting lymphocytes and dendritic cells toward epithelial cells. |
39 | Whole liver |
| Chemokine (C-C motif) ligand 21 |
CCL21 | 0.95 | 0.936 | Chemotactic for thymocytes and activated T cells. | 34, 38 | Whole liver |
Fold changes in expression of mRNA of genes involved in the development of ectopic germinal centers in chronically inflamed tissues. Gene expression was assessed using real-time PCR with cDNA from total liver mRNA of 3-month-old and 24-month-old C57BL/6 mice (N = 5). To determine the significance confidence limit the t test of sample groups was carried out
Table 4.
Changes in Message Levels of Recombinase and Immunoglobulin Transcripts with Age in Immune Cell Pool of Mouse Liver
| Gene name | Symbol | Fold change (old/young) |
P value | Tissue fraction |
|---|---|---|---|---|
| Recombination activating gene 1 | rag1 | 9.52 | 0.026 | Nonparenchymal cell fraction |
| Recombination activating gene 1 | rag2 | 7.06 | 0.040 | Nonparenchymal cell fraction |
| Immunoglobulin J chain | IgJ | 18.79 | 0.041 | Nonparenchymal cell fraction |
| Immunoglobulin light chain kappa variable region 1 | IgkV1 | 12.42 | 0.141 | Nonparenchymal cell fraction |
| Immunoglobulin light chain kappa variable region 8 | IgKV8 | 29.18 | 0.260 | Nonparenchymal cell fraction |
| Immunoglobulin heavy chain 6 | Ig heavy 6 | 8.97 | 0.163 | Nonparenchymal cell fraction |
| Immunoglobulin heavy chain 4 | Ig heavy 4 | 19.39 | 0.066 | Nonparenchymal cell fraction |
Fold changes in RAG 1/2 and immunoglobulin gene expression in the nonparenchymal fraction (enriched in immune cells) in 3-month-old and 24-month-old livers. Gene expression was assessed using real-time PCR (Nmice= 4). To determine the significance confidence limit, the t test of the sample groups was carried out.
Discussion
Various studies have shown that the innate immune system becomes relatively more active with advancing age.23 In previous studies from our laboratory, gene expression profiling of livers in mice grouped by age into 3-, 6-, 12-, and 24-month-old groups showed distinct immunological changes. The genes showing maximal changes with age in the data set predominantly belonged to the “immune response” category, especially the inflammatory branch of the immune system, indicating the presence of inflammation in the livers of aged mice. 13
To substantiate our findings, we measured mRNA expression of genes involved in inflammation in older and younger mouse livers and found the expression of inflammatory genes to be higher in the older animals. The levels of proinflammatory cytokines that initiate and sustain the process of inflammation were consistently higher in the livers of aged mice, indicating that the immune cells or other resident cells of the liver were releasing signals into hepatic tissue, inducing expression of inflammatory genes in hepatocytes. Further, elevated message levels of the chemoattractants, for example, rantes, mip-1 α, and cxcl1, in the aged liver, which attract the B cells, T cells, monocytes, and neutrophils to the liver tissue, were documented.
Histological analysis together with immunohisto-chemistry of the liver tissue revealed that immune cells formed foci in the perivascular and the lobular regions of the old livers. The immune cell foci were concentrated predominantly in the perivascular region, suggesting increased extravasation of the immune cells in the liver with age. Immunohistochemistry for cell surface markers of macrophages, T cells, B cells, and neutrophils showed that all these cell types were congregating in immune cell clusters in the older tissue. Higher expression of the NK-specific markers was also observed in the liver tissue of the aged mice.
The increase in inflammatory conditions in the aged animal livers may be explained by several factors. It has been observed that with time the epithelial lining of the gut becomes compromised in older animals and that there is a greater influx of foreign antigens into the portal circulation from the intestine into the liver.24 The increased antigenic load on the liver can lead to the chronic inflammatory conditions seen in the liver. Interestingly, there is a genetic component also involved in the increased functioning of the innate immune system, as it is known to be associated with the canonical insulin insulin-like growth factor (IGF) aging pathway. Long-lived DAF-2 mutants in C. elegans show an increased resistance to bacterial infection with age compared with wild-type worms, indicating that regulation of longevity and immunity is tied to the insulin–IGF pathway.25 Similar results were seen in growth hormone–deficient long-lived rats, which showed a smaller population and lower activity of NK cells than wild-type rats, indicating a decreased rate of up-regulation of innate immunity with age.26 Aging is usually accompanied by increased accumulation of fat in the liver.27 Increased fatty acid levels in the liver are associated with elevated levels of reactive oxygen species and apoptosis, which can lead to hepatocellular injury and development of inflammatory conditions in the liver.28,29 The analysis of microarray data in our laboratory also indicated significant differential expression of genes involved in lipid metabolism, ROS, and apoptosis (Supplementary Table 3). Thus, these physiological and genetic factors might have been acting in concert to cause the immune up-regulation observed in the older animals.
Tertiary lymphoid neogenesis (TLN) is the phenomenon entailing formation of ectopic lymphoid structures frequently observed in chronically inflamed tissues.12,30 These structures are seen in a transgenic model of chronic hepatic inflammation and helicobacter infection in mice liver as well as in humans with liver inflammation because of infectious agents such as hepatitis C.20,31,32 The immune clusters in the aged livers exhibited various features of tertiary lymphoid structures—proliferation, aggregation of T cells, B cells, and dendritic cells,33,34 and elevated levels of chemokine messages.35–37 TLN can play a positive role in the induction of tumors, and tertiary lymphoid organs have previously been shown to be associated with lymphomas.38 In a murine model of chronic liver inflammation, formation of tertiary lymphoid tissue was associated with the development of hepatocellular carcinoma.13 In cases of long-term allograft failure, ectopic germinal centers have been shown to develop in the chronically rejected transplanted tissue, where they provide a resident source of alloimmune lymphocytes that are likely to contribute to tissue rejection.39 Our study also showed increased expression of RAG genes and antibody transcripts in the immune cell compartment of the liver. Immunoglobulin gene rearrangement occurs in ectopic germinal centers, where B cells undergo antigen-driven clonal expansion and somatic hypermutation that can lead to production of autoimmune antibodies.40,41 Thus, hepatic inflammation can potentially contribute to the development of various pathologies. In case of invasive liver procedures such as liver transplant and resection, the presence of inflammatory conditions could impede recovery, as some of the inflammatory cytokines can have an inhibitory effect on the hepatic regeneration process. Further studies on the impact of the development of inflammatory conditions in the aging liver, especially on liver disease treatment and surgical procedures, are warranted to facilitate better recovery in the aged population.
Supplementary Material
Acknowledgment
We thank the Digestive Disease Center, Baylor College of Medicine, for help with immunohistochemical analysis. We also thank Dr. Milton Finegold for his expertise in the histological analysis of the liver samples.
Supported by the NIH (PO1 AG20752), the Texas Medical Center Digestive Disease Center (DK56338, DK53045), and the Ellison Medical Foundation.
Abbreviations
- NK
natural killer
- PCNA
proliferating cell nuclear antigen
Footnotes
Potential conflict of interest: Nothing to report.
Supplementary material for this article can be found on the HEPATOLOGY website (http://interscience.wiley.com/jpages/0270-9139/suppmat/index.html).
References
- 1.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflammaging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 2.Golden TR, Melov S. Microarray analysis of gene expression with age in individual nematodes. Aging Cell. 2004;3:111–124. doi: 10.1111/j.1474-9728.2004.00095.x. [DOI] [PubMed] [Google Scholar]
- 3.Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- 4.Baggio G, Donazzan S, Monti D, Mari D, Martini S, Gabelli C, et al. Lipoprotein(a) and lipoprotein profile in healthy centenarians: a reappraisal of vascular risk factors. FASEB J. 1998;12:433–437. doi: 10.1096/fasebj.12.6.433. [DOI] [PubMed] [Google Scholar]
- 5.Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230. [PubMed] [Google Scholar]
- 6.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 7.Prolla TA. DNA microarray analysis of the aging brain. Chem Senses. 2002;27:299–306. doi: 10.1093/chemse/27.3.299. [DOI] [PubMed] [Google Scholar]
- 8.Ida H, Boylan SA, Weigel AL, Hjelmeland LM. Age-related changes in the transcriptional profile of mouse RPE/choroid. Physiol Genomics. 2003;15:258–262. doi: 10.1152/physiolgenomics.00126.2003. [DOI] [PubMed] [Google Scholar]
- 9.Reyes I, Reyes N, Iatropoulos M, Mittelman A, Geliebter J. Aging-associated changes in gene expression in the ACI rat prostate: implications for carcinogenesis. Prostate. 2005;63:169–186. doi: 10.1002/pros.20164. [DOI] [PubMed] [Google Scholar]
- 10.Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3:423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsukahara A, Seki S, Iiai T, Moroda T, Watanabe H, Suzuki S, et al. Mouse liver T cells: their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301–309. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 12.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 13.Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341–350. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Salder H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597–1606. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- 15.Williams AO, Knapton AD, Geiser A, Letterio JJ, Roberts AB. The liver in transforming growth factor-Beta-1 (TGF-beta 1) null mutant mice. Ultrastruct Pathol. 1996;20:477–490. doi: 10.3109/01913129609016352. [DOI] [PubMed] [Google Scholar]
- 16.Ruminy P, Gangneux C, Claeyssens S, Scotte M, Daveau M, Salier JP. Gene transcription in hepatocytes during the acute phase of a systemic inflammation: from transcription factors to target genes. Inflamm Res. 2001;50:383–390. doi: 10.1007/PL00000260. [DOI] [PubMed] [Google Scholar]
- 17.Miyaji C, Watanabe H, Toma H, Akisaka M, Tomiyama K, Sato Y, et al. Functional alteration of granulocytes, NK cells, and natural killer T cells in centenarians. Hum Immunol. 2000;61:908–916. doi: 10.1016/s0198-8859(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 18.Williams GT, Williams WJ. Granulomatous inflammation—a review. J Clin Pathol. 1983;36:723–733. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hjelmströom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikenwalder M, Seeger H, Prinz M, Klöhn PC, Schwarz P, Ruddle NH, et al. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science. 2005;307:1107–1110. doi: 10.1126/science.1106460. [DOI] [PubMed] [Google Scholar]
- 21.Cuff CA, Schwartz J, Benjamin CM, Russell KS, Bender JR, Ruddle NH, et al. Lymphotoxin alpha3 induces chemokines and adhesion molecules: insight into the role of LT alpha in inflammation and lymphoid organ development. J Immunol. 1998;161:6853–6860. [PubMed] [Google Scholar]
- 22.Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 24.Ma TY, Hollander D, Dadufalza V, Krugliak P. Effect of aging and caloric restriction on intestinal permeability. Exp Gerontol. 1992;27:321–333. doi: 10.1016/0531-5565(92)90059-9. [DOI] [PubMed] [Google Scholar]
- 25.Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 26.Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, et al. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am J Pathol. 2002;160:2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engler MM, Engler MB, Nguyen H. Age-related changes in plasma and tissue fatty acid composition in Fischer 344 rats. Biochem Mol Biol Int. 1998;46:1117–1126. doi: 10.1080/15216549800204672. [DOI] [PubMed] [Google Scholar]
- 28.Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- 29.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 31.Freni MA, Artuso D, Gerken G, Spanti C, Marafioti T, Alessi N, et al. Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. HEPATOLOGY. 1995;22:389–394. [PubMed] [Google Scholar]
- 32.Shomer NH, Fox JG, Juedes AE, Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect Immun. 2003;71:3572–3577. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 34.Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, et al. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195:1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res. 1999;19:119–125. doi: 10.1007/BF02786481. [DOI] [PubMed] [Google Scholar]
- 36.Salomonsson S, Larsson P, Tengnér P, Mellquist E, Hjelström P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren’s syndrome. Scand J Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 37.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P, et al. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–3027. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 38.Isaacson PG, Spencer J. The biology of low grade MALT lymphoma. J Clin Pathol. 1995;48:395–397. doi: 10.1136/jcp.48.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaunat O, Patey N, Morelon E, Michel JB, Nicoletti A. Lymphoid neogenesis in chronic rejection: the murderer is in the house. Curr Opin Immunol. 2006;18:576–579. doi: 10.1016/j.coi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Stott DI, et al. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren’s syndrome. J Clin Invest. 1998;102:938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magalhaes R, Stiehl P, Morawietz L, Berek C, Krenn V. Morphological and molecular pathology of the B cell response in synovitis of rheumatoid arthritis. Virchows Arch. 2002;441:415–427. doi: 10.1007/s00428-002-0702-1. [DOI] [PubMed] [Google Scholar]
- 42.Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, et al. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- 43.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 44.Choi I, Kang HS, Yang Y, Pyun KH. IL-6 induces hepatic inflammation and collagen synthesis in vivo. Clin Exp Immunol. 1994;95:530–535. doi: 10.1111/j.1365-2249.1994.tb07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 46.Jaruga B, Hong F, Kim WH, Gao B. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1044–G1052. doi: 10.1152/ajpgi.00184.2004. [DOI] [PubMed] [Google Scholar]
- 47.Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–765. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 48.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 49.Kirschning C, Unbehaun A, Lamping N, Pfeil D, Herrmann F, Schumann RR. Control of transcriptional activation of the lipopolysaccharide binding protein (LBP) gene by proinflammatory cytokines. Cytokines Cell Mol Ther. 1997;3:59–62. [PubMed] [Google Scholar]
- 50.Volpes R, van den Oord JJ, Desmet VJ. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology. 1990;12:59–65. doi: 10.1002/hep.1840120110. [DOI] [PubMed] [Google Scholar]
- 51.Terris B, Baldin V, Dubois S, Degott C, Flejou JF, He´nin D, et al. PML nuclear bodies are general targets for inflammation and cell proliferation. Cancer Res. 1995;55:1590–1597. [PubMed] [Google Scholar]
- 52.Lalor PF, Shields P, Grant A, Adams AH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 53.Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J Biol Chem. 1993;268:15419–15424. [PubMed] [Google Scholar]
- 54.Cupedo T, Mebius RE. Role of chemokines in the development of secondary and tertiary lymphoid tissues. Semin Immunol. 2003;15:243–248. doi: 10.1016/j.smim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Caux C, Vanbervliet B, Massacrier C, Ait-Yahia S, Vaure C, Chemin K, et al. Regulation of dendritic cell recruitment by chemokines. Transplantation. 2002;73:S7–S11. doi: 10.1097/00007890-200201151-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.