Abstract
Objective
The primary aim of this study is to establish the validity of greyscale-based quantitative ultrasound (QUS) measures of the biceps and supraspinatus tendons.
Design
Nine QUS measures of the biceps and supraspinatus tendons were computed from ultrasound images collected from sixty-seven manual wheelchair users. Shoulder pathology was measured using questionnaires, physical examination maneuvers, and a clinical ultrasound grading scale.
Results
Increased age, duration of wheelchair use, and body mass correlated with a darker, more homogenous tendon appearance. Subjects with pain during physical examination tests for biceps tenderness and acromioclavicular joint tenderness exhibited significantly different supraspinatus QUS values. Even when controlling for tendon depth, QUS measures of the biceps tendon differed significantly between subjects with healthy tendons, mild tendinosis, and severe tendinosis. Clinical grading of supraspinatus tendon health was correlated with QUS measures of the supraspinatus tendon.
Conclusions
Quantitative ultrasound is valid method to quantify tendinopathy and may allow for early detection of tendinosis. Manual wheelchair users are at a high risk for developing shoulder tendon pathology and may benefit from quantitative ultrasound-based research that focuses on identifying interventions designed to reduce this risk.
Keywords: Ultrasound, Greyscale, Tendinopathy, Shoulder
Ultrasound enables dynamic real-time evaluation of musculoskeletal structures and has been widely applied to evaluate shoulder integrity1,2. Tendinopathy on ultrasound has been qualitatively described as an enlargement of the tendon and a disruption of the normal fibrillar pattern3. Often the diagnosis of tendinopathy is subjective and based on the experience of the examiner. We have recently described a grading scale of musculoskeletal shoulder pathology that includes a rating of tendon health ranging from normal to varying degrees of tendinopathy or tears4. While this scale allows researchers to quantify various pathologies at the shoulder, the validity of the ratings is still dependent on the operator's perception of the scan. Using image analysis and a unique localization method, we aim to derive objective, quantitative descriptors of tendon health which will facilitate ultrasound-based research.
Few attempts have been made to relate quantitative measures of tendon appearance to clinically documented pain or pathology. Subjects with chronic tendinopathy have been shown to have larger tendon cross sectional areas (CSA) compared to an asymptomatic control group3. An MRI study of chronic Achilles tendinopathy found that increased intratendinous signal (mean greyscale) correlated to severity of pain and functional impairment5. Quantitative analysis of tendon appearance has primarily been limited to these two simple measures (CSA and mean greyscale) which do not quantify the fibrillar pattern that becomes more disorganized with tendon degeneration. One recent study explored the spatial frequency content of Achilles tendon ultrasound images as a measure of the degree of collagen fiber organization within the tendon6. Using eight spatial frequency parameters derived from two-dimensional Fourier Analysis, the authors were able to discriminate between subjects with and without tendinopathy with approximately 80% accuracy. Image analysis of tendon structure using greyscale information appears to delineate between healthy and pathological tendons.
We have identified nine greyscale-based quantitative ultrasound (QUS) measures of biceps and supraspinatus tendon appearance including tendon width and mean echogenicity. The reliability of these QUS measures when using a standardized protocol and reference marker has been established7, but the content validity of these measures has not been determined. The greyscale values of all pixels in a region of interest within the tendon can be represented as a histogram that captures information about greyscale variation within the tendon. In this study we used first-order statistics (variance, skewness, kurtosis, and entropy) from the histogram to describe the global greyscale distribution within a region of interest. Additionally, co-occurrence matrix derived measures (contrast, energy, and homogeneity) will quantify the greyscale distribution in the expected direction of the fibrillar pattern within the tendon8,9. These measures account for the directional nature of collagen fiber organization by comparing the greyscale values of pixel pairs perpendicular to the long axis of the tendon. On ultrasound, healthy tendons exhibit alternating bands of light and dark due to the reflection from the well-organized collagen fibers. This strong directional pattern translates to increased contrast and lower energy and homogeneity. For clarification, homogeneity here refers specifically to the variations within the greyscale distribution. Clinical sonographers often use the same term qualitatively in an opposite manner and refer to healthy tendons as having a homogenous fibrillar pattern (see also the Discussion).
In addition to objectively measuring tendinopathy, quantitative ultrasound measures may facilitate a new line of research to identify risk factors for and to prevent musculoskeletal injuries. One group that could benefit from this type of research is manual wheelchair users. It is well-established that the majority of manual wheelchair users develop shoulder pain or pathology over time due to repetitive loading of the upper limb and that this can have a negative impact on independence and quality of life10–12. Since shoulder pain and pathology is more common with increasing age and duration of wheelchair use, it is important to intervene as early as possible13. Fortunately, research in the area of wheelchair biomechanics has shown that interventions related to wheelchair setup or propulsion biomechanics can reduce cadence and the amount of force required to push a wheelchair14. Reliable and objective measures of tendon health may allow researchers to monitor the response of the tendons to varying propulsion conditions which could lead to earlier identification of interventions that reduce the risk of injury.
The primary aim of this study is to establish the content validity of greyscale-based QUS measures by describing their relationship to established measures of pain and pathology including questionnaires, physical examinations, and clinical ultrasound examination findings in manual wheelchair users4,15. We expect that tendinosis will present as an enlarged tendon with a less organized fibrillar pattern3. Quantitatively, this will translate to increased width, skewness, kurtosis, energy and homogeneity and to decreased echogenicity, variance, entropy, and contrast. Further discussion of the theoretical basis for selecting the proposed QUS measures will be presented to establish the face validity of these measures.
METHODS
Subjects
Study participants were recruited through a research registry, local rehabilitation clinics, as well as at the 2007 and 2008 National Veterans Wheelchairs Games. Twenty-two individuals participated in this study at the Human Engineering Research Laboratories. All testing equipment was transported to the National Veterans Wheelchair Games, where an additional 48 subjects were tested. These subjects volunteered to participate in this study during the National Veterans Wheelchair Games which are attended by approximately 500 wheelchair athletes each year. Subjects were eligible if they used a manual wheelchair as their primary means of mobility, were 18–65 years of age, and were at least one year post in-patient rehabilitation. Subjects were excluded if they had a progressive or degenerative disability, a history of cardiopulmonary disease, or traumatic upper extremity injury to the non-dominant shoulder. Ultrasound examinations were only performed for the non-dominant side. All subjects provided informed consent prior to participation in this study which was approved by our local Institutional Review Board.
Questionnaires
Basic demographic information including age, height, mass, diagnosis, and date of diagnosis/wheelchair prescription was collected using self-report questionnaires. The Wheelchair User's Shoulder Pain Index (WUSPI), which has a test-retest reliability of 0.99, was used to quantify shoulder pain during activities of daily living15. The WUSPI score is calculated by summing the pain score (0–10 on a visual analog scale) for each of 15 activities and corrected based on individual activity level. Higher scores indicate more severe shoulder pain during an activity and have been correlated to a loss of shoulder range of motion15. Subjects were also asked to report whether they had experienced shoulder pain in the last month and whether it was specific to overhead activities or occurred during wheelchair propulsion.
Physical Examination
Each subject underwent a a physical examination focused on signs of shoulder injury. All physicians who performed this examination were trained by a physiatrist (Boninger) with 10 years of experience performing this type of examination for research purposes. Specifically, subjects were tested for pain or discomfort during 11 clinical tests and each was scored as: 0 = symptom/sign absent, 1 = equivocal finding, 2 = symptom/sign present. The clinical tests have been previous described4 and included bicipital tendon/groove tenderness, supraspinatus tendon/greater tuberosity tenderness, resisted external rotation, resisted internal rotation, acromioclavicular (AC) joint tenderness, supraspinatus test, painful arc test, Neer's sign, Hawkin's sign, O'Brien's sign for AC joint pathology, and O'Brien's sign for labrum pathology.
Clinical Ultrasound Examination
All participants underwent a clinical ultrasound examination by a physiatrist with musculoskeletal ultrasound training (Boninger or Fullerton) who assigned a numerical score for each of seven ultrasound signs. The total Ultrasound Shoulder Pathology Rating Scale (USPRS) score was calculated as the sum of the 7 individual examination scores and ranged from 0 to a possible maximum of 23. The USPRS has been previously described in detail4, although minor changes have been made including the addition of two new static examinations including: joint effusion scored as 0 (absent) or 1 (present) and bursal thickening scored as 0 (normal) or 1 (>2mm thick). The intra- and inter-rater reliability of this scale has not been evaluated, however it has been validated by correlating the USPRS scores to the presence of pain upon physical examination or risk factors for shoulder pathology4. Briefly, bicipital and supraspinatus tendinopathy were each scored on a scale from 0–6 where:
0=normal (hyperechoic, fibrillar echotexture),
1=mild tendinosis (heterogeneous echotexture with ill-defined hyperechoic regions),
2=severe tendinosis (diffuse abnormal hypoechogenicity, but without tendon volume loss),
3=intrasubstance abnormality (focal, well-defined, hypoechoic or anechoic area not extending to either the bursal or articular tendon surface),
4=partial-thickness tendon tear (focal, well-defined, hypoechoic or anechoic area extending to either the bursal or articular tendon surface),
5=focal full-thickness tendon tear (focal, well-defined, hypoechoic or anechoic area extending to both the bursal and articular tendon surfaces with tendon volume loss),, and
6=massive full-thickness tear (non-visualization of tendon with retraction).
Greater tuberosity cortical surface was graded as: 0=smooth hyperechoic surface, 1=mild, 2=moderate, 3=marked cortical irregularity. Finally, dynamic evaluation of supraspinatus and subscapularis tendon impingement resulted in a score ranging from 0–3 for each tendon where 0 = no impingement, 1= mild impingement, 2=moderate impingement, and 3 = marked impingement.
Quantitative Ultrasound Examination
A single examiner (Collinger) conducted a quantitative ultrasound examination of the biceps and supraspinatus tendons of the non-dominant shoulder using a Phillips HD11 1.0.6 ultrasound machine with a 5–12 MHz 50 mm linear array transducer (Phillips Medical Systems, Bothell, WA). The subject remained seated in his or her own wheelchair in a standardized posture7. A longitudinal image of the long head of the biceps tendon was obtained and a steel reference marker was taped to the skin, which produced an interference pattern in the ultrasound image. This reference marker has been shown to improve the reliability of QUS measures of tendon appearance7. A 2 cm wide region of interest (ROI) was defined 1.5 cm from the center of the interference pattern. A transverse view of the widest part of the supraspinatus tendon, with the rotator interval clearly in view, was saved for later analysis. An interference pattern from a second steel marker provided a landmark on the image to define a 1 cm wide ROI within the supraspinatus tendon. A longitudinal view of the biceps tendon was collected to optimize the viewing of the fibrillar pattern. For the supraspinatus tendon, however, a transverse view was chosen to minimize anisotropy that occurs in the longitudinal view allowing for more reliable imaging16.
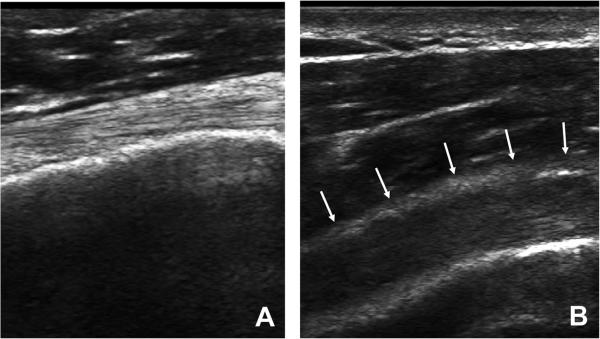
Saved ultrasound images were post-processed using Matlab (The Mathworks, Natick, MA). A detailed description of the greyscale-based QUS measures has been previously presented7. Tendon width was defined as the average distance between the top and bottom border within the ROI. Mean echogenicity, variance, skewness, kurtosis, and entropy were computed from a histogram that describes the greyscale distribution, or echotexture, within an ROI. Contrast, energy (smoothness), and homogeneity describe echotexture by comparing pixel pairs in the vertical direction since a horizontally oriented collagen fiber pattern exists within the tendons. Figure 1 illustrates the collagen fiber pattern in a healthy tendon (A) and for someone with severe tendinosis (B).
Figure 1.
Panel A illustrates a healthy biceps tendon with a strong fibrillar pattern. Panel B depicts a biceps tendon with severe tendinosis. The top border of the tendon is demarcated with arrows.
Statistical Analysis
Descriptive analysis of all data was performed first including mean and standard deviation for continuous variables (demographics and QUS variables) and frequency for discrete variables (pain, physical examination, clinical ultrasound scores). Content validity was determined by computing correlations between QUS variables, demographics, and clinical ultrasound tendon grades. Non-parametric (Spearman's) correlations were used for tests involving clinical ultrasound scores. Since fewer clinical scores were observed for the biceps tendon, an ANCOVA was used to compare QUS variables between subjects with healthy tendons, those with mild tendinosis, and those with severe tendinosis. Significant differences (p=.019) in tendon depth below the skin were noted between these groups. A larger distance between the skin and tendon could make it more difficult to obtain a clear image of the tendon due to attenuation of the ultrasound beams and therefore tendon depth was entered as a covariate. T-test comparisons of QUS descriptors of tendon appearance were made between subjects with and without pain and between subjects with and without symptoms upon physical examination. All statistical analysis was performed using SPSS (SPSS, Inc., Chicago, IL).
RESULTS
Subjects and Questionnaires
Seventy subjects were recruited for this study and data from 67 manual wheelchair users is presented. Two subjects did not return for testing after providing informed consent, and were withdrawn from the study. One subject's data was excluded because of poor image quality. Another subject had a completely ruptured biceps tendon so ultrasound examinations were only performed for the supraspinatus tendon. One subject had poor image quality for the supraspinatus, and therefore data was only analyzed for the biceps tendons. On average, subjects were 45.2 ± 11.0 years old, weighed 82.6 ± 19.9 kg, were 1.77 ± 0.09 m tall, and had been using a wheelchair for 13.8 ± 11.2 years.
The prevalence of shoulder pain, physical examination symptoms, and clinical ultrasound examination findings has been previously described for 49 manual wheelchair users with spinal cord injury4. Although only 5 subjects participated in both studies, we found a similar, although slightly lower, incidence of shoulder pain and pathology. A brief summary of findings among the current group is provided.
The average WUSPI score was 11.8±26.5, however the data was highly skewed as 30 subjects had WUSPI score of 0. Another thirty-one subjects had a score of 25 or less. One subject scored 40.9, while the remaining five participants had a WUSPI score greater than 82.
Thirty-three subjects (49.3%) reported experiencing shoulder pain within the last month. Specifically, 17 subjects (25.4%) reported pain during overhead activities, while 15 (22.4%) experienced shoulder pain during wheelchair propulsion.
Physical Examination
Thirty-five (52.2%) participants exhibited at least one sign of pain/discomfort for the non-dominant shoulder during the physical examination. 10–16% of subjects showed symptoms during the supraspinatus test (n=8), resisted external rotation (n=7), supraspinatus tenderness (n=11), the painful arc test (n=10), Neer's sign (n=9), and O'Brien's sign for labrum pathology (n=8). 20–25% of subjects exhibited pain during tests for biceps tenderness (n=17), acromioclavicular (AC) joint tenderness (n=13), Hawkin's sign (n=16), and O'Brien's sign for AC joint pathology (n=14). Only 6% of subjects experienced pain during resisted internal rotation (n=4).
Clinical Ultrasound Examination
All but 1 participant showed some sign of shoulder pathology during the clinical ultrasound examination. Recorded total USPRS scores ranged from 0 to 16 points with a mean score of 6.3 ± 3.6. Most subjects had a normal biceps tendon appearance (39%) or presented with mild tendinosis (47%). Only 12% of subjects exhibited a normal supraspinatus tendon appearance. The majority of subjects either presented with mild supraspinatus tendinosis (29%) or a partial tear (28%).
61.2% of participants showed signs of supraspinatus impingement ranging from mild (40.3%), to moderate (19.4%), to marked (1.5%). 29.9% of subjects exhibited subscapularis impingement classified as either mild (25.4%) or moderate (4.5%). The majority of subjects (85.0%) showed some degree of cortical irregularity. 41.8% presented with mild irregularity, while 31.3% had moderate irregularity, and 11.9% showed marked cortical irregularity or pitting. 23.9% of subjects presented with bursal fluid or thickening and 11.9% showed signs of joint effusion of the long head of the biceps tendon sheath.
Quantitative Ultrasound
Mean quantitative ultrasound (QUS) values derived from the biceps and supraspinatus tendon ROI are presented in Table 1.
Table 1.
Quantitative Ultrasound (QUS) Descriptors of Biceps and Supraspinatus Tendon Appearance
| QUS Measure | Biceps Tendon | Supraspinatus Tendon |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Width (mm) | 5.01 (1.16) | 5.32 (1.00) |
| Echogenicity | 108.96 (24.11) | 98.18 (29.26) |
| Variance | 1729.38 (745.28) | 1221.26 (692.93) |
| Skewness | 0.54 (0.47) | 0.27 (0.42) |
| Kurtosis | 3.91 (0.96) | 3.81 (0.85) |
| Entropy | 6.98 (0.36) | 6.72 (0.49) |
| Contrast | 5.08 (2.07) | 3.73 (1.80) |
| Energy | 0.69 (0.32) | 0.95 (0.62) |
| Homogeneity | 3.59 (0.27) | 3.78 (0.35) |
SD= standard deviation
Note: No statistical comparisons were made between tendons.
Quantitative Ultrasound and Demographics
Significant correlations were observed between demographic variables and QUS descriptors of biceps and supraspinatus tendon appearance. Increased age, duration of wheelchair use, and body mass correlated with a darker, more homogenous tendon appearance, consistent with tendinopathy. Specifically, older individuals tended to have a darker biceps tendon appearance (p = .044, r = −.249) and decreased greyscale variance (p = .011, r = −.312), entropy (p = .041, r = −.253), and contrast (p = .007, r = −.331). Biceps tendon homogeneity increased with age (p = .017, r = .292). Duration of wheelchair use was correlated with decreased supraspinatus tendon mean echogenicity (p = .014, r = −.300), variance (p = .049, r = −.248), and contrast (p = .001, r = −.393), and increased skewness (p = .003, r = .364) and homogeneity (p = .003, r = .372). Heavier individuals tended to have a larger biceps tendon (p = .010, r = .320) and a darker supraspinatus tendon (p = .004, r = −.357). They also exhibited less contrast (p = .009, r = −.324; p = .001, r = −.393) and increased homogeneity (p = .014, r = .304; p = .003, r = .372) for the biceps and supraspinatus tendons respectively. Increased body mass also correlated with less entropy of the biceps tendon (p = .047, r = −.249) and less greyscale variance in the supraspinatus tendon (p = .049, r = −.248). Overall, these relationships suggest that the biceps tendon degenerates with age, while the supraspinatus tendon appears to be more affected by the duration of wheelchair use. Increased body mass correlated with some indicators of tendon degeneration, while subject height showed no correlation with tendon health. Quantitative ultrasound features did not discriminate between people with and without shoulder pain as reported by the WUSPI and other questionnaires.
Quantitative Ultrasound and Physical Exam Scores
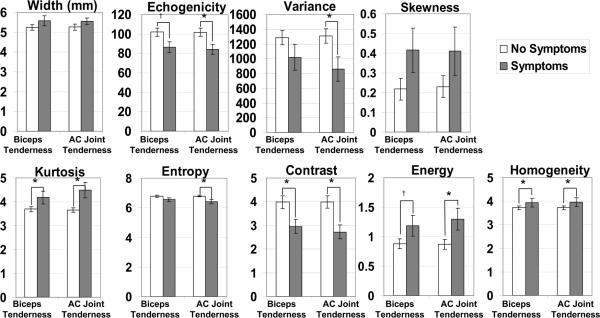
QUS descriptors of supraspinatus tendon health were significantly different between subjects with and without pain during tests for biceps tenderness and AC joint tenderness. In both cases, subjects with positive physical examination findings had significantly lower tendon echogenicity, variance, entropy, and contrast and significantly higher kurtosis, energy, and homogeneity (Figure 2). In general, QUS measures of the biceps tendon were not significantly different between those who experienced pain upon physical exam, and those who did not. The only exception was that those who had pain during the painful arc examination (n=9) had significantly lower homogeneity and energy in the biceps tendon.
Figure 2.
Quantitative ultrasound measures of the supraspinatus tendon for subjects with and without symptoms of biceps tendon tenderness and AC joint tenderness.
* indicates significant difference (p < .05) between subjects with and without symptoms
† indicates trend (0.05 ≤ p < .10) towards difference between subjects with and without symptoms
Quantitative Ultrasound and Clinical Ultrasound Examination (USPRS)
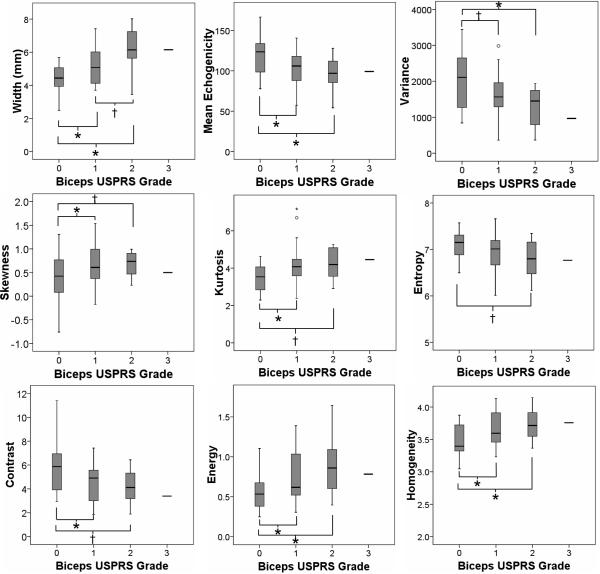
An ANOVA was used to test for differences in QUS measures of tendon appearance between subjects with different tendon grades upon clinical ultrasound examination. For the biceps tendon, three groups were compared: Biceps Grade = 0; Biceps Grade = 1; Biceps Grade = 2 or 3. ANOVA revealed that subjects with more severe pathology, or a higher biceps tendon grade, were older (p=.011) and weighed more (p=.011) than subjects with healthy tendons. When controlling for tendon depth below the skin, subjects with more tendon pathology upon clinical ultrasound examination showed the following tendon characteristics upon QUS analysis: larger tendon width (p<.001), darker echogenicity (p=.005), less greyscale variance (p=.017), increased skewness (p=.004), increased kurtosis (p=.011), less entropy (p=.057), less contrast (p=.012), increased energy (p=.006), and greater homogeneity (p=.004). Significant post-hoc differences are indicated in Figure 3.
Figure 3.
Box-plots showing the median and quartiles of raw quantitative ultrasound descriptors of biceps tendon appearance vs. biceps grade from the clinical ultrasound examination. Only 1 subject had a biceps grade of 3, and his data is represented as a single line for reference only. This subject's data was combined with the Biceps Grade = 2 group for statistical analyses. Post-hoc significant differences, when controlling for tendon depth below the skin, are noted as * (p < .05) or † (0.05 ≤ p < 0.10).
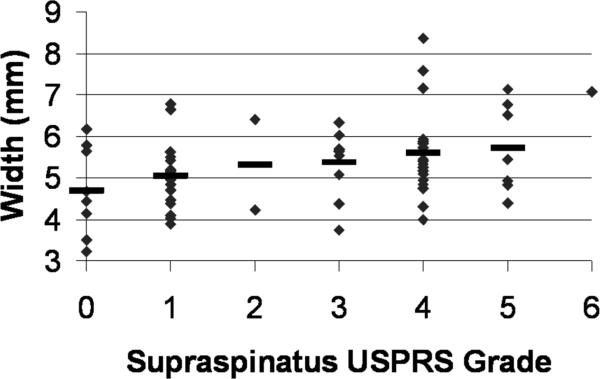
Increased supraspinatus tendon pathology upon clinical ultrasound examination correlated with a larger supraspinatus tendon width (p = .010, ρ = .317), darker tendon echogenicity (p = .013, ρ = −.304), and greater homogeneity (p = .029, ρ = .269). Other relationships trended towards being significant, including increased greyscale skewness (p = .062, ρ = .231) and energy (p = .064, ρ = .229) and decreased contrast (p = .064, ρ = −.230). A scatterplot of individual and mean values of tendon width for each USPRS supraspinatus tendon grade is shown in Figure 4.
Figure 4.
Scatter-plot of supraspinatus tendon width vs. supraspinatus grade from the clinical ultrasound examination. Mean values are denoted with a bold dash `-'. Only 1 subject had a supraspinatus grade of 6 so that subject's data is represented as a single dot.
Total USPRS, a measure of overall shoulder health, was significantly correlated to many QUS descriptors of biceps and supraspinatus tendon appearance. Spearman's correlation coefficients are presented in Table 2.
Table 2.
Correlations between Quantitative Ultrasound (QUS) measures and Ultrasound Shoulder Pathology Rating Scale (USPRS) score for the Biceps and Supraspinatus Tendons
| QUS measure | Biceps Tendon QUS and Total USPRS score | Supraspinatus Tendon QUS and Total USPRS score | ||
|---|---|---|---|---|
| p-value | Spearman's rho | p-value | Spearman's rho | |
| Width | .034 | .261 | .012 | .307 |
| Echogenicity | .098 | −.206 | .005 | −.339 |
| Variance | .069 | −.225 | .176 | −.168 |
| Skewness | .423 | .100 | .083 | .215 |
| Kurtosis | .188 | .164 | .986 | .002 |
| Entropy | .035 | −.260 | .120 | −.193 |
| Contrast | .037 | −.258 | .029 | −.270 |
| Energy | .023 | .279 | .040 | .254 |
| Homogeneity | .024 | .278 | .012 | .307 |
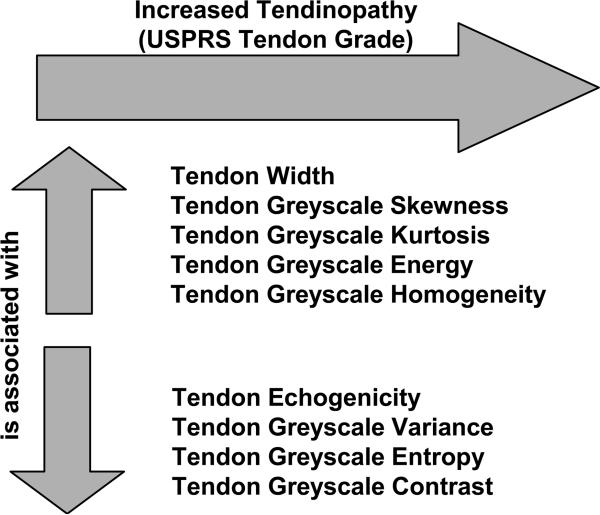
Figure 5 summarizes the relationship between increasing tendinopathy graded using the USPRS and greyscale-based QUS. Specific relationships, supported by statistical tests, have been described above.
Figure 5.
Summary of relationships between increasing tendinopathy and greyscale-based quantitative ultrasound.
DISCUSSION
This study is unique in that it is the first to describe the relationship between greyscale-based quantitative measures of tendon appearance and clinical measures of shoulder pain and pathology. In a previous study, using standardized positioning and a specially designed reference marker, we established a reliable quantitative ultrasound (QUS) examination protocol7. Here we have established the content validity of these QUS measures by confirming their relationship with demographic risk factors for shoulder pathology and established clinical examinations of shoulder integrity in a sample of manual wheelchair users.
In agreement with our hypothesis, as tendinosis became more severe, tendons appeared larger, less echogenic, and showed less greyscale variance, entropy, and contrast. Compared to a normal tendon, more severe tendinosis also presented as increased greyscale skewness, kurtosis, energy, and homogeneity. All of these changes indicate a more diffuse collagen fiber organization that has been described clinically as a sign of tendon degeneration. Total USPRS score is a measure of overall shoulder integrity, specifically as it relates to risk factors of rotator cuff disease4. Higher USPRS scores correlated with tendinopathy of the biceps and supraspinatus tendon measured using greyscale-based QUS.
Clinically, the grading of tendinosis is subjective so there is no gold standard for comparison, however the USPRS is the first scale to quantitatively describe musculoskeletal shoulder pathology. We are encouraged that even with this relatively small sample size, greyscale-based QUS features change with tendon degeneration. In the future, this may prove valuable in a clinical setting to identify early stages of tendinopathy that have previously been described subjectively.
We have confirmed that increased age, duration of wheelchair use, and body mass are risk factors for greater musculoskeletal shoulder pathology. Older individuals tended to have a more degenerated biceps tendon appearance, while duration of wheelchair use was more correlated to QUS descriptors of supraspinatus tendon appearance. Heavier individuals tended to have a tendon appearance consistent with degeneration of both their biceps and supraspinatus tendons. Since heavier individuals likely experience more loading during propulsion and thus may develop more pathology12, controlling for subject mass directly could obscure the relationship between clinical and QUS measures. Instead, we controlled for the distance from the skin to the top of the biceps tendon since ultrasound waves are attenuated as they pass through this tissue.
Physical examination findings, specifically biceps tenderness and AC tenderness, were accompanied by changes in QUS measures of supraspinatus tendon appearance. The direction of these changes was consistent with our hypothesis that persons with shoulder pain or pathology would have a larger tendon with a less organized collagen fiber structure. No causal relationship can be established since QUS changes in the supraspinatus tendon correlated to discomfort in surrounding musculoskeletal structures. However, previous studies have shown that biceps tendon pathology, which can present as bicipital groove tenderness, is often indicative of rotator cuff pathology17. It seems clear that chronic tendon pathology does not occur in isolation, but rather in combination with the degeneration of surrounding musculoskeletal structures. Contrary to our hypothesis, self-reported shoulder pain was not predictive of tendon health as described by QUS. Similarly, degenerative changes measured by QUS did not correlate to symptoms on physical examination of the same structure (i.e. biceps tenderness was not correlated to degeneration of the biceps tendon). However, as previously stated, QUS of the biceps and supraspinatus tendon did correlate well with subjective clinical gradings of tendon microstructure changes measured with the USPRS. QUS is based on analysis of tendon echotexture as a measure of tendon health and is not designed to diagnose specific tendon pathologies. The results indicate that some of the pathology identified in this study was still in the early stages of development and was asymptomatic4. Intervening before the development of pain is critical to preserving long term function of the upper limb.
Using clinical measures of shoulder pain and pathology including physical examinations and ultrasound-based grading scales, we have established the content validity of objective, QUS measures. It is also important to establish face validity by understanding the theoretical basis for the selection of these features. Tendinopathy results in tendon enlargement with reduced echogenicity and a loss of the normal fibrillar collagen pattern18. All of the greyscale-derived measures were chosen to quantify the presence or loss of a normal fibrillar pattern. A histogram describes the distribution of greyscale values, ranging from 0 (black) – 255 (white), within a region of interest. The mean value of this histogram, echogenicity, is often reduced with tendon degeneration because of a loss of the bright well-organized collagen structure.
Other first order statistics, including variance, skewness, kurtosis, and entropy, can be derived from the greyscale histogram. A healthy tendon would have a heterogeneous appearance because of alternating light and dark striations with a wide range of greyscale values, whereas a tendon with pathology would have a more homogeneous appearance since it is lacking the bright collagen pattern (Figure 1). Tendon degeneration translates to reduced greyscale variance and entropy, and increased skewness and kurtosis as observed in this study. To our knowledge, no one has applied first-order greyscale statistics to describe tendon appearance but researchers have employed these techniques to differentiate between muscles with varying amounts of contractile components9 and between muscle appearance of subjects with and without neuromuscular disease19,20.
Co-occurrence derived measures provide additional information about image greyscale texture in a specific orientation8,21. Contrast, energy, and homogeneity were computed in the vertical direction, perpendicular to the expected direction of collagen fiber alignment for a healthy tendon. Contrast quantifies the difference in greyscale level between adjacent pixels and is equal to 0 for an image with a constant greyscale, and increases for an image with sharp greyscale variations. Energy is equal to 1 for a constant image and decreases with non-uniformity. Homogeneity is equal to 1 for a completely uniform image and decreases when structural variations are present. Differences in image texture have been exploited to improve medical imaging segmentation21, to develop iris recognition systems22, and to differentiate between benign and malignant breast tumors using ultrasound23. To our knowledge, cooccurrence derived measures have not been used to quantify tendinosis, however theoretically a healthy tendon with a strong fibrillar organization should exhibit higher contrast and lower energy and homogeneity as was observed in this study. This is supported by Bashford et al.6 who applied 2-D Fourier analysis to quantify the loss of collagen fiber organization in subjects with tendinosis. A combination of eight spatial frequency parameters discriminated between a group with Achilles tendinopathy and a control group.
This study was limited because the degree of tendon pathology was not evenly distributed when scored using the USPRS. Specifically, no partial or full thickness tears of the biceps tendon were observed and only two subjects were graded as having severe supraspinatus tendinosis. However, we were still able to measure significant correlations between QUS measures of tendon health, demographic variables (age, body mass, duration of wheelchair use) and clinical measures of pathology using ultrasound and physical examination techniques. The combined results from the biceps and supraspinatus tendons provide strong evidence that greyscale-based QUS is a valid measurement of tendinosis. Previously, tendinosis has only been judged subjectively, even when quantitative scores are assigned4. The combination of clinical grading scales and objective quantitative measures will enhance research related to musculoskeletal pathology and injury prevention. Further, while the physical examination maneuvers performed in this study are commonly used to diagnose shoulder pathology, the scoring system has not been fully validated. This may have limited our ability to measure relationships between structure specific physical examination findings and QUS measures.
Ultrasound is known to be an operator-dependent modality and for that reason, a single investigator collected all of the images in this study. The reliability of these QUS measures has been shown to be acceptable particularly when using a standardized protocol and reference marker as described in the current study7. One limitation of this protocol is that capturing a single image will only identify global tendinopathy and may miss partial tears or other localized abnormalities. However, for research purposes, it is important to capture the same area anatomically in each subject to derive reliable and objective measures of tendon appearance. We believe that subjects with more severe pathology will experience larger changes in these QUS measures globally throughout the tendon when subjected to upper limb loading. This remains to be tested.
Manual wheelchair users are a unique group of individuals who experience chronic upper limb loading. We chose to focus our analysis on manual wheelchair users since it is important to prevent the development of upper limb pathology in this group, however this must be considered when generalizing the results to other populations. We believe that the relationship between QUS and tendinosis severity will translate to other populations with a high prevalence of tendon overuse injuries. Future work will need to establish which QUS measures are sensitive to change in order to identify risk factors and test interventions to reduce the risk of developing upper limb pathology. Currently, QUS is likely to be most useful in a research setting since it requires operator-guided image analysis. The region of interest was outlined manually in this study and the QUS variables were calculated using Matlab. However, the QUS variables themselves are computationally simple to calculate. It is possible that the image analysis could be automated and integrated with the ultrasound machine software so that objective, quantitative analysis of tendon healthy could be performed during a clinical ultrasound examination.
CONCLUSIONS
In this work we have established the face and content validity of greyscale-based QUS measures. These measures correlate with known risk factors of shoulder pain and pathology including increased age, duration of wheelchair use, and body mass. Quantitative ultrasound descriptors of tendon appearance also correlated with clinically graded shoulder pathology (USPRS). We believe that QUS will provide a unique opportunity to evaluate risk factors for the development of shoulder pathology and the effectiveness of interventions to reduce this risk. In particular, manual wheelchair users have a high risk for developing shoulder pain and pathology that can negatively impact their quality of life. Fortunately, numerous interventions related to wheelchair setup and propulsion biomechanics can be tested to reduce this risk and to preserve independent mobility.
Acknowledgments
This material is based upon work supported by the Office of Research and Development, Rehabilitation Research & Development Service, Department of Veterans Affairs, Grant# B3142C, the National Institute of Health, Grant# R21HD054529, the National Institute on Disability and Rehabilitation Research (NIDRR) Rehabilitation Engineering Research Center on Spinal Cord Injury, Grant# H133E070024, and an NSF graduate research fellowship.
Footnotes
Disclosures: Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Teefey S, Middleton W, Yamaguchi K. Musculoskeletal Ultrasound: Shoulder Sonography: State of the Art. Radiol Clin North Am. 1999;37:767–85. doi: 10.1016/s0033-8389(05)70128-7. [DOI] [PubMed] [Google Scholar]
- 2.Churchill RS, Fehringer EV, Dubinsky TJ, Matsen FA., III Rotator cuff ultrasonography: diagnostic capabilities. J Am Acad Orthop Surg. 2004;12:6–11. doi: 10.5435/00124635-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Leung JLY, Griffith JF. Sonography of Chronic Achilles Tendinopathy: A Case-Control Study. J Clin Ultrasound. 2008;36:27–32. doi: 10.1002/jcu.20388. [DOI] [PubMed] [Google Scholar]
- 4.Brose SW, Boninger ML, Fullerton B, McCann T, Collinger JL, Impink BG, et al. Shoulder Ultrasound Abnormalities, Physical Examination Findings, and Pain in Manual Wheelchair Users with Spinal Cord Injury. Arch Phys Med Rehabil. 2008;89:2086–93. doi: 10.1016/j.apmr.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Gardin A, Bruno J, Movin T, Kristoffersen-Wiberg M, Shalabi A. Magnetic Resonance Signal, Rather Than Tendon Volume, Correlates to Pain and Functional Impairment in Chronic Achilles Tendinopathy. Acta Radiol. 2006;7:718–24. doi: 10.1080/02841850600774035. [DOI] [PubMed] [Google Scholar]
- 6.Bashford GR, Tomsen N, Arya S, Burnfield JM, Kulig K. Tendinopathy Discrimination by Use of Spatial Frequency Parameters in Ultrasound B-Mode Images. IEEE Trans Med Imag. 2008;27:608–15. doi: 10.1109/TMI.2007.912389. [DOI] [PubMed] [Google Scholar]
- 7.Collinger JL, Gagnon D, Jacobson J, Impink BG, Boninger ML. Reliability of Quantitative Ultrasound Measures of the Biceps and Supraspinatus Tendons. Academic Radiology. doi: 10.1016/j.acra.2009.05.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haralick RM, Shanmugam K, Dinstein I. Textural Features for Image Classification. IEEE Trans Sys Man Cybern. 1973;SMC-3(6):610–21. [Google Scholar]
- 9.Nielsen PK, Jensen BR, Darvann T, Jorgensen K, Bakke M. Quantitative ultrasound tissue characterization in shoulder and thigh muscles- a new approach. BMC Musculoskelet Disord. 2006;7:2. doi: 10.1186/1471-2474-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundqvist C, Siosteen A, Blomstrand C, Lind B, Sullivan M. Spinal cord injuries. Clinical, functional, and emotional status. Spine. 1991;16:78–83. [PubMed] [Google Scholar]
- 11.Subbarao JV, Klopfstein J, Turpin R. Prevalence and impact of wrist and shoulder pain in patients with spinal cord injury. J Spinal Cord Med. 1994;18:9–13. doi: 10.1080/10790268.1995.11719374. [DOI] [PubMed] [Google Scholar]
- 12.Mercer JL, Boninger ML, Koontz AM, Ren D, Dyson-Hudson TA, Cooper RA. Shoulder Joint Pathology and Kinetics in Manual Wheelchair Users. Clin Biomech. 2006;21:781–9. doi: 10.1016/j.clinbiomech.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Dyson-Hudson T, Kirshblum S. Shoulder Pain in Chronic Spinal Cord Injury, Part I: Epidemiology, Etiology, and Pathomechanics. J Spinal Cord Med. 2004;27(1):4–17. doi: 10.1080/10790268.2004.11753724. [DOI] [PubMed] [Google Scholar]
- 14.Boninger ML, Koontz AM, Sisto SA, Dyson-Hudson TA, Chang M, Price R, et al. Pushrim Biomechanics and Injury Prevention in Spinal Cord Injury: Recommendations Based on CULP-SCI Investigations. J Rehab Res Dev. 2005;42(3, Supp. 1):9–20. doi: 10.1682/jrrd.2004.08.0103. [DOI] [PubMed] [Google Scholar]
- 15.Curtis KA, Roach KE, Applegate EB, Amar T, Benbow CS, Genecco TD, et al. Reliability and validity of the wheelchair user's shoulder pain index (WUSPI) Paraplegia. 1995;33:595–601. doi: 10.1038/sc.1995.126. [DOI] [PubMed] [Google Scholar]
- 16.Crass JR, van de Vegte GL, Harkavy LA. Tendon Echogenicity: Ex Vivo Study. Radiology. 1988;167:499–501. doi: 10.1148/radiology.167.2.3282264. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto B, Kramer D, Wiitala L. Applications of musculoskeletal sonography. J Clin Ultrasound. 1999;27(6):293–318. doi: 10.1002/(sici)1097-0096(199907/08)27:6<293::aid-jcu1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Allen GM. Shoulder ultrasound imaging-integrating anatomy, biomechanics and disease process. Eur J Radiol. 2008;68:137–46. doi: 10.1016/j.ejrad.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Pillen S, Scholten RR, Zwarts MJ, Verrips A. Quantitative skeletal muscle ultrasonography in children with suspected neuromuscular disease. Muscle Nerve. 2003 Jun;27:699–705. doi: 10.1002/mus.10385. [DOI] [PubMed] [Google Scholar]
- 20.Arts IMP, van Rooij FG, Overeem S, Pillen S, Janssen H, Schelhaas H, et al. Quantitative muscle ultrasonography in Amyotrophic Lateral Sclerosis. Ultrasound Med Biol. 2008;34:354–61. doi: 10.1016/j.ultrasmedbio.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Sharma N, Ray AK, Sharma S, Shukla KK, Pradhan S, Aggarwal LM. Segmentation and classification of medical images using texture-primative features: Application of BAM-type artificial neural network. J Med Phys. 2008;33(3):119–26. doi: 10.4103/0971-6203.42763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X, An S, Shi P. Statistical Texture Analysis-Based Approach for Fake Iris Detection Using Support Vector Machines. ICB07; Seoul, Korea. 2007. pp. 540–6. [Google Scholar]
- 23.Alvarenga A, Pereira WCA, Infantosi AFC. Complexity curve and grey level cooccurrence matrix in the texture evaluation of breast tumor on ultrasound images. Med Phys. 2007 Feb;34(2):379–387. doi: 10.1118/1.2401039. [DOI] [PubMed] [Google Scholar]