Abstract
Mosquito coils are used to prevent mosquito exposures indoors by ~ 2 billion people worldwide. However, the smoldering of organic matters used as base materials of mosquito coils emits particulate and gaseous toxic compounds. A previous study indicates that emission rates of toxic compounds depend on types of base materials and can be high enough to generate room concentrations markedly higher than health based standards or references. The objective of the present study is to evaluate a new type of mosquito coil that uses charcoal powder as base material and to compare its emission rates with those of several current-market brands and several brands tested in the previous study. Results show that the charcoal-based coil had emission rates of PM2.5 mass, total particle number, PAHs, and aldehydes, substantially (up to 10 times) lower than other tested conventional mosquito coils. Results also show that particles freshly generated from burning mosquito coils were all fine and mostly ultrafine in size. This paper presents emission rates for PM2.5 mass, total particle number, gas-phase and particle-phase PAHs, 14 aldehydes and acetone, and 10 volatile hydrocarbons. These data, along with emission rates presented in the previous study are useful for estimating indoor concentrations of toxic compounds generated from mosquito coil uses.
Keywords: mosquito control, product of incomplete combustion, indoor air pollutants, biomass combustion
Introduction
The use of mosquito repellents helps reduce or prevent mosquito bites that may lead to not only discomfort but also mosquito-borne diseases such as malaria and West Nile virus illness. Mosquito coils have been and are still the most commonly used mosquito repellents worldwide. Based on our personal communications with a mosquito coil manufacturer, it is estimated that 45 to 50 billion mosquito coils are used annually by approximately 2 billion people worldwide. To be effective, mosquito coils are typically used indoors, primarily at night during sleeping hours.
Mosquito coils are made of biomass base materials impregnated with insecticides. Through slow and steady combustion (smoldering), a mosquito coil evaporates insecticides that prevent mosquitoes from entering indoor environments. Although insecticides impregnated in mosquito coils, most likely pyrethrins, are not particularly harmful to humans, the smoldering combustion condition is ideal for generating products of incomplete combustion (PICs) (Lukwa and Chandiwana 1998; Zhang et al 1991; Abubakar and Hassan 2007; Morello-Frosch et al 2006). These PICs are no different from those emitted from other types of biomass combustion (e.g., wood burning and tobacco smoking), including fine particles, carbon monoxide, and numerous volatile organic compounds (VOCs) (Liu et al 2003; Zhang et al 2000; Lee and Wang 2006; Chen et al 2008). Some of biomass-emitted PICs are carcinogens (e.g, polycyclic aromatic hydrocarbons, formaldehydes, benzene) and some are respiratory and eye irritants (e.g., aldehydes). Particulate matter, especially fine particles (i.e., particles with an aerodynamic diameter ≤ 2.5 µm, PM2.5), has been most commonly used as a “single” air pollution predictor of mortality and morbidity in numerous air pollution epidemiologic studies.
Until the publication of a seminal paper in 2003 (Liu et al 2003), however, PICs emitted from mosquito coils have not been characterized. Using a well controlled emission testing protocol, Liu et al measured particle size distribution and emission rates of PM2.5, particulate-phase polycyclic aromatic hydrocarbons (PAHs), carbonyls (aldehydes and ketones) from several popular brands of mosquito coils that were purchased from the Chinese and Malaysian markets in 2001 –2002. The study reported that burning one mosquito coil releases the same amount of fine particles as burning 75 – 135 cigarettes, largely depending on what biomass (saw dust or coconut husks) was used as base materials (Liu et al 2003). The emission of formaldehyde per coil, as high as a 51 cigarette emission equivalent, also largely depended on coil base materials.
The publication of the above mentioned paper generated tremendous amount of responses from governmental agencies, the public, the mosquito coil industry, in China and Southeastern Asia, catalyzed by the media coverage of the topic. Inspired by the findings of Liu et al, some mosquito coils manufacturers started to seek new base materials with a goal to reduce PIC emissions. In 2008, we have noticed smokeless mosquito coils existing in the Chinese market. This observation stimulated our interest in conducting a follow-up study to evaluate this new type of mosquito coils in particular and to evaluate other common mosquito coils in the current global market. In this follow-up study, we used the same emission testing protocol and the same laboratory as reported in the Liu et al paper to measure emission rates of PICs of most health concerns (Liu et al 2003). We compared the results from the current tests with those reported previously to see whether there have been any product improvements since the original study. (It should be noted that neither study employed a thorough market analysis in selecting test coils.)
Materials and Methods
Mosquito Coils Tested
The main motivation for this study is to examine whether smokeless mosquito coils emit a lesser amount of toxic PICs than conventional mosquito coils. We acquired one brand of smokeless mosquito coil (Lanju) from China. The “smokeless” status was granted officially by the China Environmental Labeling. For comparison purposes, we acquired four additional brands of mosquito coils (Bison, Godzilla, Jambo, and Raid) and tested them in the current study. Product information, derived mainly from the product labels and physical appearance, are summarized in Table 1 for the 5 mosquito coil brands. It is clear from the product information listed in the table (e.g., manufacture year, etc) that the present study is not a systematic evaluation of mosquito coils.
Table 1.
Product information about the mosquito coils tested in the present study
| Brand Name |
Description | Coil Color |
Manufacturer | Manufacture Year |
Net Weight (g/coil) |
|---|---|---|---|---|---|
| Lanju | Smokeless (G Series). Active Ingredient: dimefluthrin 0.02%, Inert Ingredient: Charcoal powder | Black | Zhongshan Lanju Daily Chemical Inc., Guangdong, China | 2008 | 19.8 |
| Bison | Big size. Active Ingredient: Allethrin 0.20% | Green | Bison (Group) of Pingle Kwangsi Inc., Guangxi, China | 2007 | 17.7 |
| Godzilla | 12-Anti Mosquito Coils. Active Ingredient: d-allethrin 0.30% w/w | Red | Godzilla Inc., Thailand | N/A* | 14.2 |
| Jumbo | 12 coils. Active Ingredient: d-allethrin 0.30% w/w | Green | Technopia SDN. BHD., Malaysia | N/A* | 12.4 |
| Raid | 10 Antimosquitos. Active Ingredient: d-allethrin 0.19% | Yellow | SC Johnson Wax Inc., Spain | N/A* | 11.9 |
Information not available, but purchased in 2008
Experimental Apparatus
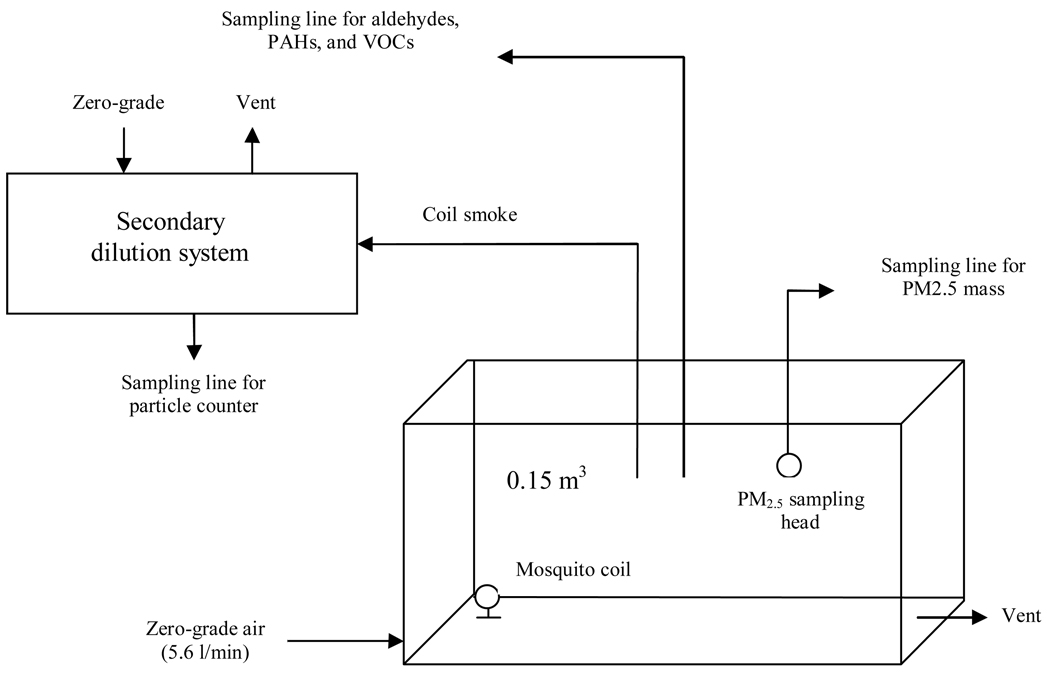
The core of the experimental apparatus is a 0.15 m3 test chamber, as described in detail previously (Liu et al 2003) and shown in Figure 2. The incoming air, after passing through a series of activated carbon filters, was introduced into the chamber through the inlet valve at a constant flow rate of 5.6 l/min. The chamber was under a slightly positive pressure to prevent the infiltration of air from outside by controlling the flow rate of exiting air via the sampling ports and the outlet valve. During each experimental run, a lit mosquito coil, on the metal stand provided within the coil packet, was placed inside the chamber. Air samples were drawn out of the chamber at 10 ml/min through Teflon tubing and diluted with clean air 80 times in a flask to match the measurement range of an eight-channel optical particle counter and a condensation particle counter. Additional sampling ports on the chamber were used to collect samples for measuring particulate and gaseous phase PICs (see below).
Figure 2.
Schematic diagram of the mosquito coil testing system
Measurement of Particle Number and Mass Concentrations
We used a real-time SidePak particle mass concentration monitor (AM510 Aerosol Monitor, TSI Inc., Shoreview, MN, USA), a condensation particle counter (CPC Model 3007, TSI Inc.), and an eight-channel optical particle counter (LASAIR Model 1002, Particle Measuring Systems, Inc., Boulder, CO, USA) to measure PM2.5 mass concentration, number concentrations of all particles larger than 0.01 µm, and number concentrations of particles from 0.1 to 10 µm in diameter with eight size ranges: 0.1–0.2 µm, 0.2–0.3µm, 0.3–0.4 µm, 0.4–0.5 µm, 0.5–0.7 µm, 0.7– 1.0 µm, 1.0–2.0 µm, and > 2 µm, respectively.
The SidePak particle mass monitor was used to calculate the particle removal rate in the chamber. It was operated for 5-hrs, including a 1-hr coil burning period, and 4-hrs post-burning period after coils were removed from the chamber. The LASAIR eight-channel particle counter was used to test particle distribution and operated for 2 hours for each coil test, which included a 1-hr equilibration period before initiating the coil smoke and a 1-hr coil burning period. The condensation particle counter was used in addition to the eight-channel optical particle counter to monitor particles with diameters as small as 0.01 µm.
A sampling pump, coupled with a PM2.5 sampling impactor (PEM Model 200 by MSP Co., Minneapolis, MN, USA) and a 25-mm Teflon filter (Pall Co., Ann Arbor, MI, USA), were used to collect PM2.5 mass. Each of these PM2.5 samples were collected after coils had been steadily burned for 1-hr. The particle mass on the filters was determined gravimetrically.
Measurement of Gas-phase and Particle-phase PAHs
A sampling system, consisting of a PM2.5 sampling inlet with a 25-mm pre-baked (at 500°C for 12 hours) quartz fiber filter (Pall Co., Ann Arbor, MI, USA) followed by a tube filled with polyurethane foam (PUF, medium density, diameter: 17mm, length: 100 mm), was used at a flow rate of ~ 4 l/min for 2 minutes to collect particle-phase and gas-phase PAHs, respectively. Given the short sampling duration, the sampling pumps were turned on at least 15 minutes before sampling to get a stable sampling rate. Both the quartz fiber filters and PUF samples were extracted with 150 ml dichloromethane individually for 16 hr at 75°C using a Soxhlet apparatus (Han et al 2004; Zhang et al 2008). The extracts were evaporated to near dryness and re-dissolved in 1 ml acetonitrile (ACN). The extracts were analyzed using an HPLC system with a fluorescence detector (Waters 2695 Alliance HPLC system with 2475 Multichannel Fluorescence Detector). The HPLC column used was a Supelcosil LC-PAH column (4.6 × 250 mm) (Supelco, Inc., Bellefonte, PA, USA) under controlled temperature at 30°C. The mobile phase used was as follows: solution A = 50% ACN and 50% water; solution B = 100% ACN. The gradient program was 100% A for 20 min, linear gradient from 100% A to 100% B in 20 min, 100% B for 15 min, then from 100% B back to 100% A in 10 min, and held at 100% A for 10 min. The mobile phase flow rate was 1 ml/min. The injection volume was 20 µl. The fluorescence detector program was started at an excitation wavelength of 270 nm and an emission wavelength of 350 nm; at 34 min, the wavelength was changed to excitation at 250 nm and emission at 400 nm and held for 13.5 min; then it was changed to excitation at 280 nm and emission at 425 nm for another 22.5 min. PAHs concentrations were determined through calibration curves prepared using certified standard solutions of PAHs purchased commercially (Supelco, Inc.). Using this method, we only needed 2 minutes to collect enough material (and not overloading the sampling media) for subsequent PAH analyses. We found that PAH concentrations in the coil smoke were all far above the method detection limits of 1.2 ng/m3 to 140 ng/m3 for the following 16 target PAHs: naphthalene, acenaphthene, acenaphthrene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, benzo[g,h,i]perylene, and indeno(1,2,3-cd)pyrene. The precision of the analytical method, measured as coefficient of variation (CV) from 6 repeated analyses, ranged from 2.5% to 21% depending on PAH species. The method accuracy, measured as recovery of spiked PAH standards, ranged from 73% to 136%.
Measurement of Carbonyl Compounds
Carbonyl compounds (aldehydes and ketones) were measured using the U.S. Environmental Protection Agency (EPA) Method TO-11A (U.S. EPA 1999a). Coil emission samples were collected onto a 2,4-dinitrophenyl hydrazine (DNPH)-coated C18 cartridge using a sampling pump at ~0.6 l/min for 5 minutes during the steady burning stage. The samples were slowly eluted with 4 ml acetonitrile (ACN) immediately after the sampling; the extracts were analyzed using an HPLC system with a reverse-phase Nova-Pak C18 column (3.9 × 150 mm; Waters Corp.). The mobile phase gradient program used was as follows: 100% of solvent A (water/ACN/tetrahydrofuran 60/30/10), hold for 5 min, then program to 100% solvent B (ACN/water 60/40) in 28 min, and hold at 100% B for 10 min, and then program back to 100% A in 5 min. The flow rate of the mobile phase was kept constant at 1 ml/min. The sample injection volume was 20 µl. The ultraviolet detector was set at 365 nm. Carbonyl compound concentrations were determined through calibration curves prepared using certified standard solutions of DNPH-carbonyl derivatives purchased commercially (Supelco, Inc., and Accustandards Inc., New Haven, CT, USA). Using this method with a 5-minute sampling duration, we could detect 15 target carbonyl compounds (formaldehyde, acetaldehyde, acrolein, acetone, propionaldehyde, crontonaldehyde, butyraldehyde, benzaldehyde, isovaleraldehyde, valeraldehyde, o-tolualdehyde, m-tolualdehyde, hexaldehyde, and 2,5-dimethylbenzaldehyde) with detection limits ranging from 0.19 to 0.83 µg/m3 (depending on carbonyl species). The concentrations we measured were all far above the detection limits. The precision of the method, measured as coefficient of variation (CV) from 8 repeated analyses, ranged from 8.5% to 19.2%. The analytical method accuracy, measured as recovery of spiked carbonyl standards, ranged from 80% to 109%.
Measurement of Volatile Hydrocarbons
Gas-phase hydrocarbons (often referred to as VOCs) were collected using ORBO-32 Standard Charcoal Tubes (Supelco Inc., Bellefonte, PA) at a flow rate of ~0.6 l/min for 16 minutes during the steady burning stage for each test. A glass fiber filter was placed in front of the charcoal tube to remove particles. After collection, the samples were extracted with 1.0 ml of carbon disulfide (Reagent A.C.S. grade, Fisher Scientific, Fair Lawn, NJ). A 200 µl of aliquot was pipetted into an autosampler vial with a 250 µl glass insert containing 10 μl of internal standard solution (1,000 ng of bromochloromethane, chlorobenzene-d5 and 1,4-difluorobenzene, respectively). These extract vials were placed in the autosampler tray and a 1 µl aliquot was injected for VOC analysis. A split ratio of 11:1 was used and the flow rate was set at 1.5 ml/min. The analysis was performed using a Varian Star 3400GC/Saturn 2000 ion trap mass spectrometer. The column used was a Restek RTX 624 60m × 0.25 mm with 1.4 µm thickness. The GC oven temperature was programmed as follows: 35 °C for 4 min, a 4 °C /min ramp to 200 °C, and a 5 min hold at 200 °C for a total analysis time of 50 min. The injector temperature was 180 °C and the ion trap temperature was 220 °C.
Determination of Emission Rates
A single-compartment mass balance model (box model) was used to determine emission rates. Basic assumptions for this model were as follows: a) background concentration was zero; b) pollutant concentrations were homogeneous within the chamber; and c) emission rate and decay rate of the pollutants remained constant throughout the entire period of concern. The details of box model can be found in Lin et al (2003). Briefly, at the steady-state, i.e, during the steady-combustion stage, emission rate (P) can be calculated using the following equation:
| (Equation 1) |
where C is steady-state concentration, V is the volume of the chamber, and k is pollutant removal rate in the chamber. We used real-time PM2.5 monitoring data (from the SidePak) to calculate k for particles, i.e., k is the slope of the linear regression of ln(C) against time t for the post-combustion period measurements. For gaseous pollutants, we used air exchange rate as the total removal rate (k). The air exchange rate was the volumetric flow rate of the incoming air divided by the chamber volume (V). The air exchange rate for all coil tests in this study was set constant at a nominal value of 2.0 hr−1.
Results
Based on our visual examination of coil burning, we observed smokes from all the tested conventional brands using raw biomass as base materials (e.g., Bison, Godzilla, Jumbo, and Raid). However, no smoke was seen during the steady combustion stage of the Lanju smokeless coil. A comparison of visible smoke between the Lanju coil and the Bison coil is shown in Figure 1. Determined in the test chamber at an air exchange rate of 2.0 hr−1, the burn rates for the test coils were 2.5, 1.8, 1.9, 1.7, and 2.1 g/hr for Lanju, Bison, Godzilla, Jumbo, and Raid, with corresponding burn durations of 7.8, 10.0, 7.6, 7.5, and 5.8 hours, respectively.
Figure 1.
Comparison of visible smoke between a charcoal-based smokeless mosquito coil (Lanju Smokeless coil appearing in black) and a sawdust-based conventional mosquito coil (Bison coil appearing in gray)
Particle Size Distribution
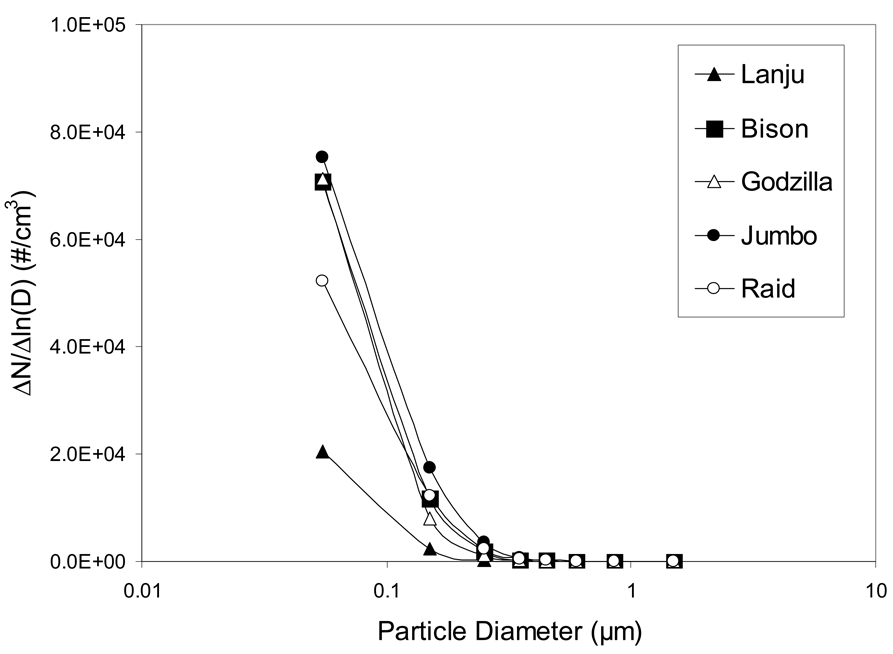
Particle number concentrations measured during the steady burning stage of the tested mosquito coils in nine different size ranges are shown in Figure 3. The plots present the normalized distribution with ΔN/ΔlnD versus particle diameter D in logarithmic scale, where ΔN is the number of particles within a specified size interval and ΔlnD is the difference in the logarithms of the largest and smallest particle sizes of that interval. The LASAIR optical counter measured particles with the following eight size ranges: 0.1–0.2 µm, 0.2–0.3µm, 0.3–0.4 µm, 0.4–0.5 µm, 0.5–0.7 µm, 0.7–1.0 µm, 1.0–2.0 µm, and > 2 µm. The condensation particle counter measured all particles larger than 0.01 µm. Number concentration of particles in the size range of 0.01–0.1 µm was obtained by subtracting the total number concentration measured with the LASAIR from the total number concentration measured with the condensation particle counter. Since the LASAIR and the condensation particle counter use somewhat different principles to measure particles, this subtraction can only provide rough concentration estimates for particles in the range of 0.01–0.1 µm. As shown in Figure 3, highest normalized concentration fell in the size range of 0.01–0.1 µm. Nearly all particles were smaller than 0.35 µm; no particles larger than 1 µm were measured during the steady combustion stage.
Figure 3.
Normalized number concentrations of particles emitted from 5 tested mosquito coils as a function of particle diameter
Emission Rates
Emission rates of particle mass and number are summarized in Table 2. Particle mass emission rate was determined from gravimetric analysis of PM2.5 and particle number emission rate was determined from all particles larger than 0.01 µm meaured by the condensate particle counter (CPC). Results were based on an average of 3 experiments for each coil tested. As shown in Table 2, the Lanju smokeless coil had substantially lower emission rates of both PM2.5 mass and total number of particles. PM2.5 emission rates varied largely across the tested coils, ranging from 12.6 ± 0.4 to 127 ± 2 mg/hr. PM2.5 emission rates for Bison, Godzilla, Jumbo, and Raid were higher than the emission rate for the Lanju smokeless coil by a factor of 5.0, 6.5, 10.0, and 7.8, respectively. Particle number emission rates ranged from (14.4 ± 3.0) × 109 to (69.0 ± 7.3) × 109 particles/hr. Compared to Lanju with the lowest particle number emission rate, Bison, Godzilla, Jumbo, and Raid had higher emission rates by a factor of 3.8, 4.2, 4.8, and 3.0, respectively.
Table 2.
Emission Rates of PM2.5 mass and total particle number for the five tested mosquito coils
| Lanju | Bison | Godzilla | Jumbo | Raid | |
|---|---|---|---|---|---|
| PM2.5 mass (mg/hr) | 12.6 ± 0.4 | 62.8 ± 1.8 | 82.1 ± 0.5 | 127 ± 2 | 98.7 ± 1.9 |
| Total particle number (109 particle/hr) |
14.4 ± 3.0 | 54.2 ± 3.7 | 59.9 ± 5.4 | 69.0 ± 7.3 | 43.4 ± 4.1 |
Note: Data are expressed as mean ± SD; number of repeated tests n=3 for each coil brand; emission rates were determined at air exchange rate of 2.0 hr−1.
As shown in Table 3, emission rates for the following PAHs were determined based on 3 experiments for each test coil: naphthalene, acenaphthene, acenaphthrene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, benzo[g,h,i]perylene, and indeno(1,2,3-cd)pyrene. Table 3 shows that the emission rates for low-molecular-weight PAHs (e.g. 2–4 ring compounds) were higher than those for heavier ones (5–6 ring compounds). In the gas phase, 2-ring PAHs (naphthalene) and all the 3-ring PAHs including acenaphthylene, fluorene, phenanthrene, anthracene, and most of the 4-ring PAHs including fluoranthene, pyrene, benzo[a]anthracene, were all detected in the gas phase, except chrysene which was detected only in 3 out of 6 samples. As expected based on their low volatility, heavier PAHs such benz[a]anthracene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[k]fluoranthene, dibenz[a,h]anthracene, and indeno[1,2,3-cd]pyrene were not detected in the gas phase. However, these PAHs are of greater concern as they are classified as probable human carcinogens (U.S. EPA 1994). Nearly all of the 16 PAHs were detected in the particulate phase of mosquito coil emission samples. Among 5 brands of mosquito coils tested in this study, the Lanju smokeless coil, had the lowest emission rate for PAHs. The emission rate of total gasphase PAHs (sum of the 16 PAHs) for Lanju was 2.5, 2.8, 2.3, and 1.9 times lower than for Bison, Godzilla, Jumbo, and Raid, respectively. The emission rates of total particle-phase PAHs for Lanju was 25, 13, 13, and 9 times lower than for Bison, Godzilla, Jumbo, and Raid, respectively.
Table 3.
Emission rates (µg/hr) of PAHs in the particle phase and in the gas phase, respectively
| Coils | Lanju | Bison | Godzilla | Jumbo | Raid | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Gas phase | Particle phase | Gas phase | Particle phase | Gas phase | Particle phase | Gas phase | Particle phase | Gas phase | Particle phase |
| Naphthalene | 4.81 ± 0.73 | 0.566 ± 0.085 | 19.3 ± 2.9 | 17.6 ± 2.7 | 19.5 ± 2.9 | 7.58 ± 1.14 | 13.3 ± 2.0 | 5.31 ± 0.80 | 11.4 ± 1.7 | 6.14 ± 0.93 |
| Acenaphthylene | 0.41 ± 0.04 | 0.124 ± 0.013 | 1.24 ± 0.13 | 3.68 ± 0.38 | 2.70 ± 0.28 | 1.78 ± 0.19 | 0.89 ± 0.09 | 4.14 ± 0.42 | 1.83 ± 0.19 | 0.911 ± 0.090 |
| Acenaphthrene | 0.991 ± 0.078 | 0.357 ± 0.028 | 0.827 ± 0.065 | 12.2 ± 1.0 | 0.941 ± 0.074 | 7.64 ± 0.60 | 0.462 ± 0.036 | 13.3 ± 1.0 | 0.720 ± 0.057 | 3.29 ± 0.26 |
| Fluorene | 0.679 ± 0.141 | 0.291 ± 0.060 | 3.26 ± 0.68 | 13.9 ± 2.9 | 3.08 ± 0.64 | 4.73 ± 0.98 | 1.62 ± 0.34 | 6.47 ± 1.34 | 2.31 ± 0.48 | 3.05 ± 0.63 |
| Phenanthrene | 1.20 ± 0.05 | 0.541 ± 0.022 | 1.34 ± 0.06 | 5.96 ± 0.25 | 1.52 ± 0.06 | 3.52 ± 0.15 | 2.65 ± 0.11 | 2.95 ± 0.12 | 1.21 ± 0.05 | 2.70 ± 0.11 |
| Anthracene | 0.048 ± 0.007 | 0.049 ± 0.007 | 0.044 ± 0.006 | 0.776 ± 0.109 | 0.061 ± 0.009 | 0.997 ± 0.140 | 0.057 ± 0.008 | 0.430 ± 0.061 | 0.042 ± 0.006 | 0.344 ± 0.048 |
| Fluoranthene | 2.76 ± 0.35 | 0.543 ± 0.069 | 2.38 ± 0.30 | 20.5 ± 2.6 | 3.52 ± 0.45 | 11.0 ± 1.4 | 5.87 ± 0.74 | 6.72 ± 0.85 | 3.23 ± 0.41 | 8.84 ± 1.12 |
| Pyrene | 0.444 ± 0.058 | 0.287 ± 0.037 | 0.263 ± 0.034 | 1.35 ± 0.18 | 0.544 ± 0.071 | 0.729 ± 0.095 | 0.919 ± 0.120 | 0.580 ± 0.075 | 0.540 ± 0.070 | 0.565 ± 0.074 |
| Benzo(a)anthracene | 0.011 ± 0.001 | 0.118 ± 0.006 | 0.013 ± 0.001 | 0.672 ± 0.033 | 0.013 ± 0.001 | 0.562 ± 0.028 | 0.022 ± 0.001 | 0.202 ± 0.010 | 0.014 ± 0.001 | 0.869 ± 0.043 |
| Chrysene | < 0.003 | 0.144 ± 0.018 | < 0.003 | 1.63 ± 0.20 | 0.005 ± 0.001 | 1.82 ± 0.22 | < 0.003 | 0.11 ± 0.01 | < 0.003 | 1.04 ± 0.13 |
| Benzo(b)fluoranthene | < 0.001 | 0.064 ± 0.002 | < 0.001 | 0.200 ± 0.005 | < 0.001 | 0.131 ± 0.003 | < 0.001 | 0.045 ± 0.001 | < 0.001 | 0.068 ± 0.002 |
| Benzo(k)fluoranthene | < 0.001 | 0.015 ± 0.001 | < 0.001 | 0.092 ± 0.006 | < 0.001 | 0.046 ± 0.003 | < 0.001 | 0.007 ± 0.000 | < 0.001 | 0.018 ± 0.001 |
| Benzo(a)pyrene | < 0.001 | 0.053 ± 0.008 | < 0.001 | 0.240 ± 0.038 | < 0.001 | 0.230 ± 0.037 | < 0.001 | 0.052 ± 0.008 | < 0.001 | 0.095 ± 0.015 |
| Cdibenzo(a,h)anthracene | < 0.001 | 0.009 ± 0.001 | < 0.001 | 0.022 ± 0.001 | < 0.001 | 0.027 ± 0.001 | < 0.001 | 0.003 ± 0.001 | < 0.001 | < 0.001 |
| Benzo(ghi)perylene | < 0.001 | < 0.001 | < 0.001 | 0.119 ± 0.005 | < 0.001 | 0.042 ± 0.002 | < 0.001 | 0.030 ± 0.001 | < 0.001 | < 0.001 |
| Indeno(1,2,3-cd)pyrene | < 0.001 | < 0.001 | < 0.001 | 0.012 ± 0.001 | < 0.001 | 0.003 ± 0.001 | < 0.001 | 0.004 ± 0.001 | < 0.001 | < 0.001 |
| Sum of PAHs* | 11.4 ± 1.5 | 3.16 ± 0.36 | 28.7 ± 4.2 | 79.0 ± 10.3 | 31.9 ± 4.5 | 40.8 ± 5.0 | 25.8 ± 3.5 | 40.3 ± 4.8 | 21.3 ± 3.0 | 27.9 ± 3.5 |
Note: Data are expressed as mean ± SD, or < detection limit if the value is below detection limit; number of repeated tests n=3 for each coil brand; Emission rates were determined at air exchange rate of 2.0 hr−.
In computing the sum of PAHs, all values below detection limits were set at zero.
Table 4 shows the emission rates of carbonyl compounds identified in the mosquito coil emission samples (n=3 for each test coil). Among all the carbonyls detected, formaldehyde and acetaldehyde had the highest emission rates and together represented as much as 55% of the total carbonyl compounds emitted from the coil combustion. Acrolein, glyoxal, and methylglyoxal, known for their high reactivity, strong irritation effects, and suspected carcinogenic effects, were also detected in the coil smoke in relatively high concentrations. Among the five tested brands, Bison, Godzilla, Jumbo, and Raid all had substantially higher emission rates of aldehydes than Lanju. Compared to Lanju, for instance, the other four brands had formaldehyde emission rates 2.0 – 9.2 times higher and emission rates of total carbonyls ~ 2 to 4 times higher.
Table 4.
Emission rates (mg/hr) of gas-phase carbonyl compounds
| Compound | Lanju | Bison | Godzilla | Jumbo | Raid |
|---|---|---|---|---|---|
| Fomaldehyde | 0.435 ± 0.035 | 0.866 ± 0.069 | 2.69 ± 0.22 | 4.02 ± 0.32 | 1.37 ± 0.11 |
| Acetaldehyde | 0.657 ± 0.089 | 1.21 ± 0.16 | 2.29 ± 0.31 | 1.81 ± 0.25 | 2.88 ± 0.39 |
| Acrolein | 0.255 ± 0.035 | 0.521 ± 0.072 | 0.303 ± 0.042 | 0.078 ± 0.011 | 0.481 ± 0.066 |
| Acetone | 0.200 ± 0.036 | 0.531 ± 0.095 | 0.794 ± 0.142 | 0.450 ± 0.081 | 1.01 ± 0.18 |
| Propionaldehyde | 0.136 ± 0.026 | 0.189 ± 0.036 | 0.316 ± 0.060 | 0.211 ± 0.040 | 0.404 ± 0.077 |
| Crotonaldehyde | 0.141 ± 0.021 | 0.271 ± 0.040 | 0.137 ± 0.020 | 0.077 ± 0.011 | 0.209 ± 0.031 |
| Butyraldehyde | 0.023 ± 0.002 | 0.064 ± 0.006 | 0.080 ± 0.008 | 0.046 ± 0.004 | 0.096 ± 0.009 |
| Benzaldehyde | 0.042 ± 0.004 | 0.058 ± 0.006 | 0.057 ± 0.006 | 0.018 ± 0.002 | 0.056 ± 0.005 |
| Isovaleraldehyde | 0.038 ± 0.005 | 0.108 ± 0.015 | 0.313 ± 0.044 | 0.573 ± 0.080 | 0.322 ± 0.045 |
| Valeraldehyde | 0.084 ± 0.012 | 0.128 ± 0.018 | 0.243 ± 0.034 | 0.292 ± 0.041 | 0.245 ± 0.034 |
| o-Tolualdehyde | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| m-Yolualdehyde | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| p-Tolualdehyde | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Hexaldehyde | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| 2,5-Dimethylbenzaldehyde | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Sum of carbonyl | |||||
| compounds* | 2.01 ± 0.27 | 3.94 ± 0.52 | 7.22 ± 0.88 | 7.58 ± 0.84 | 7.07 ± 0.95 |
Note: Data are expressed as mean ± SD, or < detection limit if the value is below detection limit; number of repeated tests n=3 for each coil brand; Emission rates were determined at air exchange rate of 2.0 hr−.
In computing sum of gas-phase carbonyl compounds, all values below detection limits were set at zero.
More than 30 hydrocarbons (VOCs) were tentatively identified, using a GC/MS technique, to be present in coil emission samples; and 10 target VOCs were quantified and their emission rates, based on 2 experiments for each test coil, are shown in Table 5. Results show that benzene and toluene had the highest emission rates and together represented as much as 67% of the sum of the 10 reported VOCs. Although the Lanju smokeless coil had a slightly higher emission rate for benzene, the other brands had higher emission rates for the other 9 VOCs individually and for the sum of 10 VOCs. The emission rate of Lanju for the total of 10 VOCs was similar to the VOC emission rates for the other 4 tested brands.
Table 5.
Emission rates (mg/hr) of gas-phase hydrocarbons
| Compound | Lanju | Bison | Godzilla | Jumbo | Raid |
|---|---|---|---|---|---|
| Benzene | 0.396 ± 0.007 | 0.333 ± 0.005 | 0.354 ± 0.006 | 0.253 ± 0.008 | 0.318 ± 0.006 |
| Toluene | 0.113 ± 0.001 | 0.243 ± 0.010 | 0.233 ± 0.002 | 0.145 ± 0.004 | 0.222 ± 0.013 |
| Ethylbenzene | 0.008 ± 0.001 | 0.027 ± 0.003 | 0.022 ± 0.001 | 0.012 ± 0.001 | 0.026 ± 0.002 |
| m,p-Xylene | 0.013 ± 0.001 | 0.035 ± 0.004 | 0.050 ± 0.001 | 0.031 ± 0.001 | 0.026 ± 0.001 |
| o-Xylene | 0.011 ± 0.001 | 0.022 ± 0.001 | 0.018 ± 0.001 | 0.012 ± 0.001 | 0.014 ± 0.002 |
| Styrene | 0.028 ± 0.008 | 0.065 ± 0.028 | 0.081 ± 0.008 | 0.062 ± 0.007 | 0.089 ± 0.006 |
| 1,2,4-Trimethylbenzene | < 0.001 | 0.002 ± 0.001 | 0.011 ± 0.001 | 0.008 ± 0.001 | < 0.001 |
| 1,3,5-Trimethylbenzene | 0.007 ± 0.001 | 0.014 ± 0.002 | 0.011 ± 0.001 | 0.006 ± 0.001 | 0.008 ± 0.001 |
| Trichloroethene | 0.024 ± 0.001 | 0.047 ± 0.001 | 0.063 ± 0.004 | 0.059 ± 0.001 | 0.077 ± 0.001 |
| Sum of hydrocarbons* | 0.599 ± 0.020 | 0.790 ± 0.053 | 0.843 ± 0.022 | 0.586 ± 0.016 | 0.780 ± 0.031 |
Note: Data are expressed as mean ± SD, or < detection limit if the value is below detection limit; number of repeated tests n=2 for each coil brand; Emission rates were determined at air exchange rate of 2.0 hr−.
In computing sum of hydrocarbons, all values below detection limits were set at zero.
Discussion
This paper reports emission rates of particle mass and number, 16 PAHs present in the gas phase and/or the particle phase of mosquito coil emissions, 14 aldehydes and acetone (carbonyls), and 10 gas-phase hydrocarbons (VOCs). These emission rates data expand the data reported in the previous paper of Liu et al (2003) not only by testing five additional current-market brands of mosquito coils but also by measuring additional pollutants (particle number, gas-phase PAHs, and VOCs). The 6 brands tested in the previous study did not include a smokeless mosquito coil and were acquired from China and Malaysia. The previously tested brands had PM2.5 emission rates ranging from 51±7 to 117±14 mg/hr, comparable to the range from 63 ± 2 to 127 ± 2 mg/hr for the 4 non-smokeless brands tested in the present study. However, the smokeless brand (Lanju) had a substantially lower PM2.5 emission rate of 12.6 ± 0.4 mg/hr. The 6 brands tested in the previous study had emission rates of particle-phase total PAHs ranging from 3.5 to 176 µg/hr (Liu et al 2003). In the present study, Lanju had 3.2 µg/hr; but the other four brands had particle-phase total PAHs emission rates ranging from 28 to 79 µg/hr (see Table 3). The previously tested 6 brands had emission rates of total carbonyls ranging from 5.0 to 19 mg/hr, overlapping the range (7.2 to 7.6 mg/hr) for Godzilla, Jumbo, and Raid but higher than the emission rates for Lanju (2.0 mg/hr) and Bison (4.0 mg/hr).
Among the 6 previous tested brands, emissions rates for the 2 Malaysian brands were substantially higher than those of the 4 Chinese brands. Liu et al concluded that this difference was due to the difference in contents of organic fillers (base materials) used for coil smoldering. The Malaysian coils were likely to be made of coconut shells and husks while the Chinese coils were made of sawdust (Liu et al 2003). The difference in emission rates measured for the 5 brands tested in the present study also reflects the difference in base coil materials. The Lanju smokeless coil was clearly labeled as being made of charcoal powder. No base material information was available from the product labeling for the other 4 brands. However, we found the burn rates for the 4 tested coils (1.66 to 2.07 g/hr) in this study were comparable to the previously tested coils (1.48 to 2.05 g/hr). In contrast, the charcoal-based coil (Lanju) had a higher burn rate (2.56 g/hr), perhaps due to base-material’s uniformity that promotes more constant uptake of oxygen, meaning improved combustion efficiency. Therefore, we think base materials for the 4 brands tested in the present study were sawdust or similar biomass materials.
These emission rates data (reported in both the previous and present study) are useful in estimating (modeling) indoor concentrations while a mosquito coil is being used, as done in the previous paper by Liu et al (2003). Using the emission rates reported in the previous study, Liu et al (2003) presented a range of estimates of 24-hr average concentrations in a room where a mosquito coil was used for 8 hours, based on lower- and upper-bound estimates of room volume 16 and air exchange rate. Their upper-bound estimates of room concentrations for PM2.5, formaldehyde, and acrolein all substantially exceeded health-based reference exposure values. However, given that PM2.5 emission rate for the Lanju smokeless coil was 5 fold lower than that of the lowest PM2.5 emitting coil (Coil coded C3) tested by Liu et al (2003), the upper-bound estimate of PM2.5 24-hour average concentration would be approximately 1/5 of the estimated concentration for C3, i.e., 34 µg/m3, lower than the US National Ambient Air Quality Standard for 24-hr average PM2.5. Similarly, a 2 to 3 fold reduction in emissions of formaldehyde and other aldehydes, by using charcoal-based mosquito coils, would most likely bring the indoor average concentrations below health-based references.
Due to instrumentation constraints, the previous study was unable to measure particles with a diameter < 0.1 µm in the test chamber. The present study measured particles as small as 0.01 µm and confirmed that particles freshly emitted from mosquito coils were mostly ultrafine in size (≤ 0.1 µm) and all < 0.35 µm. Ultrafine particles have a large potential to deposit into the deep lung and to even directly enter the blood stream (Oberdorster et al. 2005). Therefore, it is very important to reduce exposure to ultrafine particles. Substituting the use of conventional mosquito coils (using sawdust, coconut shells, etc, as base materials) with that of charcoal-based ‘smokeless’ mosquito coils can reduce ultrafine particle emissions (and thus exposures) substantially (at least by a factor of 3 based on the present study, see Table 2). Although we only tested one brand of charcoal-based mosquito coils, the findings from this study should be considered generic to charcoal-based coils. This statement can be supported by previous measurements of PIC emission factors for charcoal and other “raw” biomass (wood, crop residue, animal dung) as household cooking fuels (Smith et al. 2000). During the manufacturing process of converting wood to charcoal, lots of “impurities” have already been released and consequently, charcoal has much higher carbon content than wood. Charcoal can be burned more steadily with more constant oxygen uptake, meaning improved combustion efficiency and lower PIC emissions.
Conclusions
Organic fillers, or base materials, of mosquito coils are used to produce smoldering through which pesticides impringnated in the base materials are slowly and steadily emitted to the room for mosquito repelling. Such smoldering of organic matters emits product of incomplete combustion (PIC) including fine and ultrafine particles, aldehydes and hydrocarbons, as well as gas-phase and particle-phase PAHs. Emission rates of PIC depend on the types of base materials and can be high enough to generate room concentrations markedly higher than health based standards or references. However, using charcoal powder as base material not only reduces visible smoke, as officially labeled as smokeless coils, but also reduces emissions of PM2.5 mass, total particle number, PAHs, and aldehydes substantially. Based on emission rates determined in the present study and those reported in a previous study, substituting the use of conventional mosquito coils (using sawdust, coconut shells, etc, as base materials) with that of charcoal-based ‘smokeless’ mosquito coils may bring average room concentrations of PM2.5 and several aldehydes below health-based references or standards.
Practical Implications.
Mosquito coils are widely used indoors to prevent mosquitoes from entering indoor environments. This is achieved through the release of insecticides impregnated in biomass base materials of mosquito coils during coil combustion. A previous study reported that burning one mosquito coil releases the same amount of fine particles as burning 75 – 135 cigarettes, largely depending on what biomass (saw dust or coconut husk) is used as base material. This “follow-up” study measured several current-market brands of mosquito coils, including a new charcoal-based coil labeled as smokeless coil by China Environmental Labeling, for their emissions of particulate and gaseous pollutants. Results show that using charcoal powder as base material reduces fine particle emissions by a factor of 5 to 10 and also reduces emissions of pollutants such as formaldehyde and PAHs substantially.
Acknowledgement
The authors wish to thank Dr. Yizhong You of (Chinese) Journal of Aerosol Communication for initiating the idea of conducting this study. JZ is in part supported by an NIEHS Center of Excellence grant (#P30 ES05022) awarded to EOHSI at Rutgers and UMDNJ.
References
- Abubakar MG, Hassan LG. Toxicological Effects Of Some Mosquito Coils Brands In Experimental Rats. The Internet J. Toxic. 2007;4(1):1–6. [Google Scholar]
- Chen SC, Wong RH, Shiu LJ, Chiou MC, Lee H. Exposure to mosquito coil smoke may be a risk factor for lung cancer in Taiwan. J. Epidemiology. 2008;18(1):19–25. doi: 10.2188/jea.18.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Kammen DM. Quantifying the Effects of Exposure to indoor air pollution from biomass combustion on acute respiratory infections in developing countries. Environ. Health Perspect. 2001;109:481–488. doi: 10.1289/ehp.01109481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han IK, Zhang J, Zhang L, Liu W, Rhoads G. ISEA 2004. Pennsylvania, USA: International Society of Exposure Analysis; 2004. Relationships between hydroxylated Metabolites in urine and pyrene/benzo(a)pyrene exposure; p. 256. [Google Scholar]
- Lee SC, Wang B. Characteristics of emissions of air pollutants from mosquito coils and candles burning in a large environmental chamber. Atmos. Environ. 2006;40(12):2128–2138. [Google Scholar]
- Liu WL, Zhang JF, Hashim JH, Jalaludin J, Hashim Z, Bernard D, Goldstein BD. Mosquito Coil Emissions and Health Implications. Environ. Health Perspec. 2003;111(12):1454–1460. doi: 10.1289/ehp.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukwa N, Chandiwana SK. Efficiency of mosquito coils containing 0.3% and 0.4% pyrethrins against An. Gambiae sensulato mosquitoes. Cent. Afr. J. Med. 1998;44(4):104–107. [PubMed] [Google Scholar]
- Morello-Frosch RA, Woodruff RJ, Axelrad DA, Caldwell JC. Air toxics and health risks in California: The public health implications of outdoor concentrations. Risk Anal. 2006;20:273–290. doi: 10.1111/0272-4332.202026. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspec. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Uma R, Kishore VVN, Zhang J, Joshi V, Khalil MAK. Greenhouse implications of household stoves. Annual Review of Energy and Environment. 2000;25:741–763. [Google Scholar]
- U.S. EPA. Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge followed by High Performance Liquid Chromatography (HPLC) Washington, DC: U.S. Environmental Protection Agency; 1999. (U.S. EPA 1999a, Method TO-11A) [Google Scholar]
- Zhang J, Smith KR, Ma Y, Ye S, Jiang F, Qi W, Liu P, Khalil MAK, Rasmussen RA, Thorneloe SA. Greenhouse gases and other pollutants from household stoves in China: A database for emission factors. Atmos. Environ. 2000;34(26):4537–4549. [Google Scholar]
- Zhang J, Han IK, Zhang L, Crain W. Hazardous chemicals in synthetic turf materials and their bioaccessibility in digestive fluids. J. Expo. Sci. Environ. Epi. 2008;18:600–607. doi: 10.1038/jes.2008.55. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sun J, Chen S, Wu Y, He F. Levels of exposure and biological monitoring of pyrethroids in spray men. Br. J. Ind. Med. 1991;48:82–86. doi: 10.1136/oem.48.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]