Abstract
The ability to regulate wound contraction is critical for wound healing as well as for pathological contractures. Matrix metalloproteinases (MMPs) have been demonstrated to be obligatory for normal wound healing. This study examined the effect the broad-spectrum MMP inhibitor BB-94 has when applied topically to full-thickness skin excisional wounds in rats and its ability to inhibit the promotion of myofibroblast formation and function by latent transforming-growth factor-β1 (TGF-β1). BB-94 delayed wound contraction, as well as all other associated aspects of wound healing examined, including myofibroblast formation, stromal cell proliferation, blood vessel formation, and epithelial wound coverage. Interestingly, BB-94 dramatically increased the level of latent and active MMP-9. The increased levels of active MMP-9 may eventually overcome the ability of BB-94 to inhibit this MMP and may explain why wound contraction and other associated events of wound healing were only delayed and not completely inhibited. BB-94 was also found to inhibit the ability of latent TGF-β1 to promote the formation and function of myofibroblasts. These results suggest that BB-94 could delay wound closure through a two-fold mechanism; by blocking keratinocyte migration and thereby blocking necessary keratinocyte-fibroblast interactions needed for myofibroblast formation and by inhibiting the activation of latent TGF-β1.
Introduction
Skin wound healing is a complex process involving inflammation, granulation tissue formation, epithelialization, wound contraction, and scar formation.1 Matrix metalloproteinases (MMPs), also called matrixins, comprise 23 different human zinc-dependent endopeptidases that belong to the metzincin superfamily.2-4 MMPs have broad substrate specificities.4 Apart from the traditional role in degradation of ECM molecules MMPs also regulate cellular processes through activation and release of signaling molecules and receptors.2, 4 MMPs are thus believed to be involved in all phases of wound healing.5, 6 Following tissue injury, MMPs contribute to removal of cellular debris and initial detachment of keratinocytes from the basement membrane. Later in the wound healing cascade, MMPs remodel the deposited provisional matrix as wound contraction occurs and facilitate scar formation.5, 6
MMP involvement in wound healing has been substantiated by the observation that MMP-3,7 MMP-98, 9 and MMP-139 depleted mice have reduced healing ability of excisional wounds. Interestingly, the healing impairment was more severe in double MMP-9/MMP-13 knockout mice than in the single MMP-9 and MMP-13 null mice.9 Similarly, the broad spectrum hydroxamate MMP inhibitor (MMPI) GM6001 dramatically delays epithelialization of excisional wounds.10, 11 We have recently demonstrated that systemic application of GM6001, in addition to delaying epithelialization, delays wound closure and correlated with this is a delay in the formation of myofibroblasts.12 Myofibroblasts are specialized fibroblasts that express smooth muscle α-actin (SMAA) and are believed to be the key effectors of long-term wound contraction during tissue remodeling and scar formation.13, 14 The correlation observed between delayed myofibroblast formation and wound contraction is consistent with the force generating role for myofibroblasts during wound contraction. The underlying mechanism by which this MMPI inhibits myofibroblast formation and thereby wound contraction is currently unclear. It should be emphasized that this class of peptidomimetic MMPI are also capable of chelating the active-site zinc ion of other metzincin members such as ADAMs and ADAMTSs.3
The growth factor transforming growth factor-β1 (TGF-β1) has been demonstrated to promote myofibroblast formation and function, including increased expression of SMAA. In our previous study we found that while GM6001 could reduce myofibroblast formation and function in granulation tissue in vivo, it had no effect upon the ability of active TGF-β1 to promote myofibroblast formation and function in in vitro models, demonstrating that GM6001 inhibition must be indirect.12 Recent studies have demonstrated that in various cell types MMPs are capable of activating latent TGF-β1 resulting in TGF-β1-mediated functional consequences that can be inhibited by GM6001.15-18 Fibroblasts in quiescent skin predominantly express MMP-1419 but wounding induces additional MMPs.20, 21 These results suggest that fibroblasts could activate latent TGF-β1 through a MMP-mediated mechanism that would promote myofibroblast formation and function and would be inhibited by the broad spectrum hydroxamate MMPI BB-94 providing an indirect mechanism by the MMPIs could inhibit myofibroblast formation and function.
To elucidate the role of MMPs in wound contraction we have investigated the effect topical treatment with the hydroxamate MMPI BB-94 has on in vivo wound contraction to exclude possible systemic influences of the substance and to expand the temporal findings of our previous study. In addition, in vitro studies were performed on interactions between MMPs, TGF-β1 activation and myofibroblast formation to test the hypothesis that the MMPI BB-94 can delay myofibroblast formation and function by inhibiting the activation of latent TGF-β1.
Materials and Methods
BB-94 (batimastat) and vehicle formulation
BB-94 was donated by British Biotech Pharmaceuticals Ltd. (Oxford, UK). BB-94 inhibits MMP with IC50 values of 3 nM for MMP-1, 4 nM for MMP-2, 20 nM for MMP-3, 10 nM for MMP-8 and 10 nM for MMP-9. BB-94 (10 mg/ml, final concentration) was suspended manually into a hydrogel (Dr August Wolff GmbH and Co., Bielefeld, Germany) which is a transparent liquid at 4°C but solidifies at body temperature. Gentamycin (100 μg/ml) was added to prevent wound infections.
Animal experiments
The study was approved by the ethics committee for animal studies at Landesverwaltungsamt Sachsen-Anhalt (2-682 Uni MD). Seventy-two female SPF Sprague-Dawley rats weighing 180-220 g were allocated to topical treatment with the inhibitor BB-94 in hydrogel or to hydrogel alone (control). BB-94 (n=12) and control animals (n=12) were analyzed postoperatively at three time points: 4, 8 and 14 days (n=24 for each day).
Rats were anesthetized with an intraperitoneal injection of a mixture of ketamine hydrochloride (100 mg/kg body weight)/xylazine hydrochloride (5 mg/kg). Two sets of two circular (8 mm) full-thickness skin wounds were made on each side of the spine as described elsewhere.12 After hemostasis, 100 μl (50 μl from day 2 onward) of the BB-94 hydrogel or the hydrogel alone were applied to each wound. A non-occlusive dressing (Gothaplast®, Gotha, Germany) covered the hydrogels and was fixed with Peha-Haft® (Hartmann, Heidenheim, Germany) and a rat jacket (Lomir®; Lomir Biomedical, Ontario, Canada). On days 2, 4, 6, 8, 10 and 12, dressings and hydrogels were removed, wounds cleaned with saline-moistened swabs and new treatments applied.
Wound healing and contraction were evaluated every other day from images taken together with a mm-scale.12 From the digitized images the visible wound margins were traced and the area in mm2 calculated by an image analysis software (AxioVision release 4.5; Carl Zeiss, Göttingen, Germany). The mean of the measurements of the 4 wounds was used in the statistical analyses. Wounds were judged completely closed when wound surfaces had turned from glossy into dry.
At 4, 8 or 14 days, 5-bromo-2′-deoxyuridine (BrdU, B9285; Sigma-Aldrich, St. Louis, MO) was injected intramuscularly at 50 mg/kg 1 hour before animals were killed by carbon dioxide asphyxiation. Upper left and lower right wounds were fixed in buffered 4% formaldehyde and subjected to histological and immunohistochemical analyses.12 At the largest wound diameter, 5 μm sections were cut vertically to the wound surface and used for analysis. The length of neoepithelium was measured histomorphometrically in hematoxylin-eosin-stained sections using image analysis software (AxioVision release 4.5) and expressed as percent of wound coverage. Incorporation of BrdU into DNA was immunodetected using a mouse monoclonal anti-BrdU antibody diluted 1:100 (M744; Dako, Glostrup, Denmark) and a visualization system with 3,3′-diaminobenzidine-HCl (DAB; Sigma-Aldrich) as chromogen. Control sections treated identically but without the primary antibody did not show any positive staining. In each tissue specimen, BrdU-positive cells were counted in the epithelium of 0.9 mm segment of normal skin 2 mm from the wound margins, in a 0.5 mm segment of epidermis adjacent to the left and right wound margin, in the new epithelium covering the wound, in the dermis of the wound margin (0.5 mm2 area) and in the entire thickness of central granulation tissue (0.16 mm2 area).
SMAA was stained by immunohistochemistry using a mouse monoclonal antibody diluted 1:300 (CBL171; Chemicon, Temecula, CA) and DAB as chromogen.12 Control sections treated identically but without the primary antibody did not show any positive staining. The extent of SMAA immunostaining was assessed on a four-graded scale: 0 = no positive staining, 1 = weak, 2 = moderate and 3 = abundant. SMAA immunostaining was investigated in the superficial and the deep dermis of the wound margins and in the granulation tissue of the wound centers. Blood vessels were counted in an area of 0.25 mm2 of wound margins and in a granulation tissue area of 0.49 mm2 per section. Blood vessels were determined by SMAA immunostaining of perivascular cells surrounding the blood vessel. Histological and immunohistochemical analyses were performed in a blinded manner by an investigator unaware of group affiliation. Each morphometric analysis was done on 3-6 sections per wound and animal. The mean values of the sections from each animal were used for statistical analysis.
Gelatin zymography was carried out on the upper right and lower left wounds. Wound margin biopsies including 1 mm of adjacent uninjured skin were obtained using 2-mm trephine and wound center biopsies using 4-mm trephine. MMPs were extracted at 4°C for 18 hours as described in detail elsewhere.22 Aliquots of 25 μl containing 5 μg of total protein were loaded into each lane.22 A human MMP-2/MMP-9 standard (CC073; Chemicon) was run in parallel lane. Densitometry was performed using the software ImageJ 1.37v (National Institutes of Health, Bethesda, MD). For quantification of the enzymes, profile plots of the gelatinolytic bands (grey values 0-255) were generated and the amount of latent and active forms of MMP-2 and MMP-9 was estimated by the area under the curve (AUC) for the respective lysis band. AUC of gelatinase standards run in parallel on each gel were used as reference. Gelatinase levels in wounds were normalized to those of adjacent normal skin. The calculated multiples of normal skin were used for statistical analyses.
In vitro experiments
Human fibroblasts were obtained as explant cultures of palmar aponeurosis from patients undergoing carpel tunnel release as previously described.23 The use of the human tissue for this study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board. Fibroblasts were cultured after standard protocol23 passaged by trypsinization and used in experiments between passages 4-6. Fibroblasts were trypsinized and cultured on glass coverslips for 4 days in DMEM (Invitrogen/GIBCO, Carlsbad, CA) containing 1% FBS (Atlanta Biologicals, Norcross, GA) and supplemented with either active human recombinant TGF-β1 (1 ng per ml; T7039; Sigma-Aldrich) or varying concentrations of latent human recombinant TGF-β1 (299-LT; R&D Systems, Minneapolis, MN). BB-94 was made up as a 20 mM stock solution in dimethyl sulfoxide and added to cultures at 0.25, 0.5, 1.0, 2.5 or 5 μM; controls contained appropriate concentrations of dimethyl sulfoxide. The soluble TGF-β receptor antagonist, TGF-β Receptor II/Fc Chimera (T3698; Sigma-Aldrich), was added at 100 ng/ml. Media without or with supplements were changed after 2 days. After 4 days in culture fibroblasts were fixed in 4% formaldehyde in 0.1 M sodium phosphate buffer pH 7.4, immunostained for SMAA and stained with 4′,6-diamidino-2-phenylindole HCl (DAPI) as previously described.23 Briefly, cultures were immunostained with a mouse anti-SMAA monoclonal antibody (1:1000 dilution, F3777 clone 1A4; Sigma-Aldrich) and stained with DAPI (1μg/ml; Invitrogen/Molecular Probes). The percent of cells expressing SMAA was determined as previously described.23 Images were captured using a Q-capture imaging program (Surrey, BC, Canada) maintaining a constant exposure threshold for determining the number of positive immunostained cells with the anti-SMAA antibody, while the number of cells in the field was determined based on DAPI-positive nuclei. The percentage of cells expressing SMAA was determined by counting at least 100 cells per treatment group from three treatment groups. Each experiment was repeated at least three times.
Fibroblasts were cultured within stabilized type I collagen lattices as previously described23 such that the final collagen concentration was 0.65 mg/ml and the cell concentration was 1.25 × 105 cells/ml using the same culture media described above. Lattices were cultured for 5 days with change of media at 2.5 days. After 5 days in culture, generation of contractile force was measured. Rapid contraction was analyzed by measuring the lattice diameter before release and at specific times after release using a Nikon SMZ-1 stereoscope. The initial collagen lattice diameters ranged from 14 to 16 mm. The fraction of the original collagen lattice diameter was obtained by dividing the diameter at each time point by the initial diameter of the lattice. All contraction assays were carried out in triplicate, and every experiment was repeated three or more times.
Statistical analyses
Groups were compared using the software SPSS 13.0 (SPSS Inc., Chicago, Illinois, USA). Significant differences between the control group and BB-94 treated group were determined with 2-way analysis of variance (ANOVA) followed by Mann-Whitney U test or by Wilcoxon test. Student's t-test was applied to the in vitro data. p<0.05 was considered statistically significant. Data are given as mean ± standard error of the mean (SEM).
Results
Wound contraction and myofibroblast formation are delayed by MMPI BB-94 treatment of wounds
Full-thickness skin wounds in rats were treated with a hydrogel supplemented with or without (control) the synthetic MMPI BB-94 and covered with a non-occlusive dressing for 14 days with re-application of new topical treatments every other day. Both BB-94 and control wounded rats, after an initial body weight drop of about 10%, gained body weight starting at day 4 at a similar rate. No local adverse effects of BB-94 treatment were observed on the surrounding skin or on hair growth.
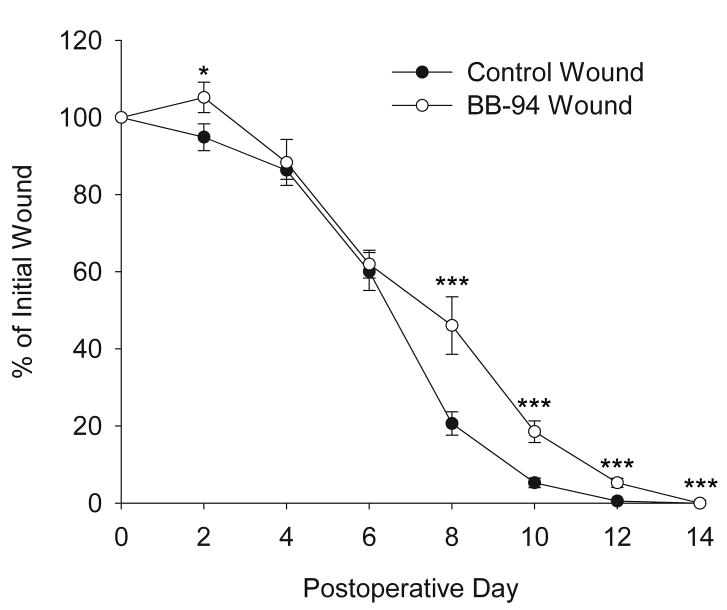
Wound closure and contraction were assessed by measuring the outer wound margins from digital images. Minimal scabs formed on the wounds due to the non-occlusive dressing, in contrast to the large scabs that formed on air-exposed wounds in a previous study.12 Two days after wounding, BB-94-treated wounds had expanded to 105±3.9% of their initial size, compared to control wounds that had reduced to 95±3.5%; however by day 4 wounds on both BB-94 and control rats were closed to a similar size (Figure 1). From day 6 to 8 control wounds decreased dramatically in size compared to BB-94-treated wounds (Figure 1). On postoperative day 8, wound areas of BB-94-treated animals decreased to 46% ± 7.5% of the original size compared to 21% ± 3% in controls. From postoperative day 8 onward BB-94-treated wounds were significantly (p<0.005) larger than control wounds (Figure 1). On day 14, all 4 wounds of each control animal were macroscopically closed; in contrast, only one BB-94-treated animal had all 4 wounds healed. These results are similar to those obtained with systemic administration of GM6001 except that the inhibition of wound closure by topical treatment appears to be slightly delayed to that of systemic treatment.12
Figure 1.
Effect of topical BB-94 treatment on wound closure of full-thickness skin wounds. Wound healing was expressed as percent change of initial wound area. Control, open diamonds; BB-94, filled squares. *p<0.05. ***p<0.005. Mean ± SEM. n=12 per time point.
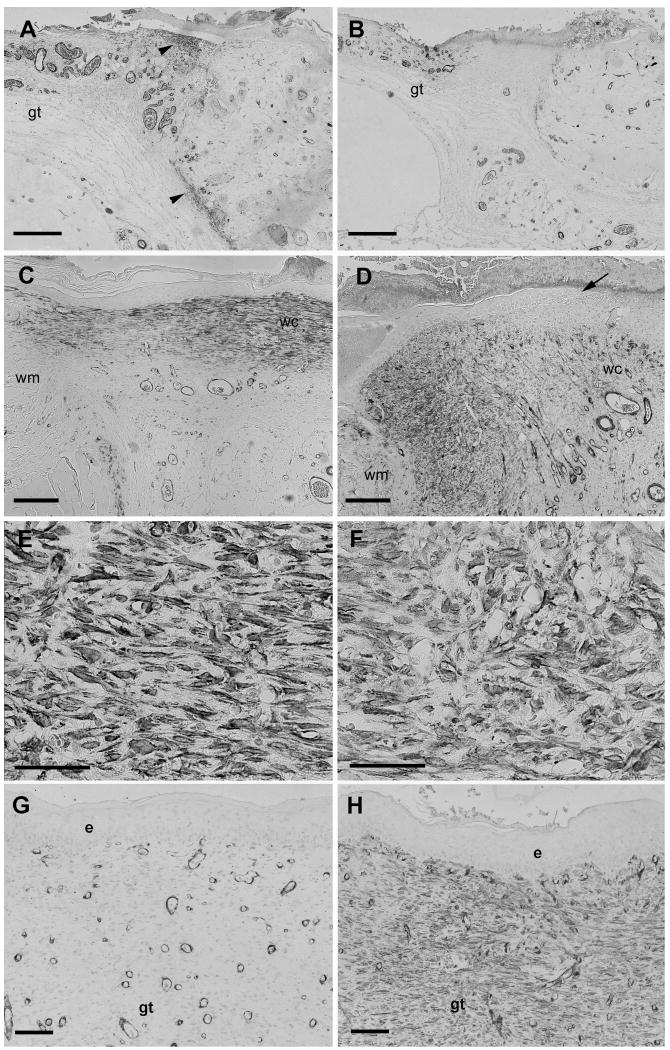
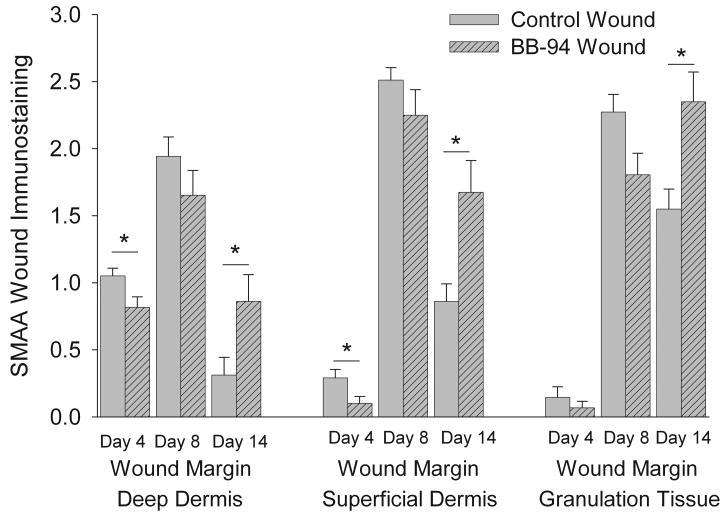
The presence of myofibroblasts was in granulation tissue determined by SMAA immunohistochemistry. Four days after wounding, control wounds had moderate positive staining for SMAA in dermis adjacent to the granulation tissue while the staining was weaker in central granulation tissue; in contrast, BB-94 wounds had a reduced positive staining for SMAA in all areas (compare Figures 2A and B). Quantitative analysis of staining demonstrated that at day 4 deep and superficial dermal SMAA staining was significantly more extensive in controls compared to BB-94 treated wounds (Figure 3). Immunoreactivity for SMAA increased strikingly in wounds of both groups on day 8 compared to day 4 (Figures 2C-F). In day 8 BB-94 treated wounds SMAA positive myofibroblasts were observed predominantly in granulation tissue under healing epidermis and not in granulation tissue that formed beneath the non-epithelialized central portion of the wound (Figure 2D). SMAA positive myofibroblasts had differing shape and orientation in control and BB-94-treated wounds; in controls, SMAA positive cells were spindle-shaped and oriented in parallel to the epithelial surface of the wound (Figure 2E); whereas, with BB-94 treatment numerous labeled cells were roundish and irregularly distributed in the granulation tissue (Figure 2F). The level of SMAA staining did not differ significantly between BB-94-treated and control groups in any of the analyzed regions on day 8, although the level of immunostaining for SMAA was consistently reduced in BB-94-treated compared with control groups in all three wound compartments (Figure 3). On postoperative day 14, SMAA immunostaining in the dermal compartments decreased in both groups (Figures 3); however, the reduction in immunostaining was significantly greater in control than BB-94 groups resulting in a significantly higher level of SMAA immunostaining in the BB-94-treated than in the control wounds. In the central granulation tissue SMAA immunostaining was reduced in the control group while it was increased in the BB-94 group. This resulted in a significant increase in the level of SMAA immunostaining in the central granulation tissue of BB-94 treated wounds compared to control wounds (Figures 2E and F; Figure 3). In summary, more SMAA positive myofibroblasts were present in controls on day 4. There was a significant increase in SMAA positive myofibroblasts in both groups on day 8; however, control groups tended to have a greater number of myofibroblasts than BB-94 treated wounds with myofibroblasts predominantly located only under healing epidermis. On day 14, more SMAA immunostained cells were consistently observed in the BB-94-treated group than in the control group. These results are consistent with a delay in both wound contraction and myofibroblast formation in BB-94 treated wounds.
Figure 2.
SMAA immunostaining of wounds. Immunostaining for SMAA on postoperative days 4 (A, B), 8 (C-F) or 14 (G, H) showing tissue sections of full-thickness skin wounds untreated (A, C, E, G) or treated with the MMPI BB-94 (B, D, F, H). (A) SMAA-stained myofibroblasts are present in the superficial and deep dermis (arrowheads) close to the edge of control wound on day 4. Myofibroblasts are absent from the adjacent granulation tissue (gt). (B) Few SMAA-stained myofibroblasts are present in the BB-94-treated wound on day 4. (C) Strongly SMAA-stained myofibroblasts are present in granulation tissue that has been re-epithelialized from wound margin (wm) to wound center (wc) of control wound on day 8. (D) SMAA stained myofibroblasts are mainly located in granulation tissue near wound margin (wm) with few in wound center (wc). Newly formed epidermis terminates prior to wound center (arrow); SMAA-stained myofibroblasts are present primarily beneath new epidermis. (E, F) Higher magnification images of figures C and D. SMAA-stained myofibroblasts in granulation tissue in day 8 control wounds are aligned parallel to the wound surface (E); in contrast, SMAA-stained myofibroblasts in BB-94 treated wounds are more rounded and have an irregular organization (F). (G) Few SMAA positive myofibroblasts are present in wound center granulation tissue (gt) of control wound on day 14. Blood vessels with rounded lumina surrounded by darkly stained cells are oriented in parallel to the wound bed. (H) Numerous SMAA-stained myofibroblasts are present in wound center granulation tissue (gt) beneath epidermis (e) of BB-94 treated wound on day 14. Blood vessels are are predominantly oriented perpendicular to the wound bed. Scale bars: A-B, 600 μm; C-D, 500 μm, E-F, 20 μm, G-H, 100 μm.
Figure 3.
Semiquantitative analysis of the SMAA immunostaining in superficial and deep dermis of wound margins and in granulation tissue of control and BB-94 treated wounds. SMAA immunostaining in superficial and deep dermis of wound margins and in granulation tissue was assessed semiquantitatively on a four-graded scale: 0 = no positive staining, 1 = weak, 2 = moderate and 3 = abundant. BB-94 treatment of wounds resulted in reduced SMAA immunostaining on day 4 and 8 and increased immunostaining on day 14, compared to control wounds. Control, open bars; BB-94, filled bars. *p<0.05. Mean ± SEM. n=12 on days 4 and 14, n=9 on day 8.
Blood vessel development and stromal cell proliferation are delayed by MMPI BB-94 treatment of wounds
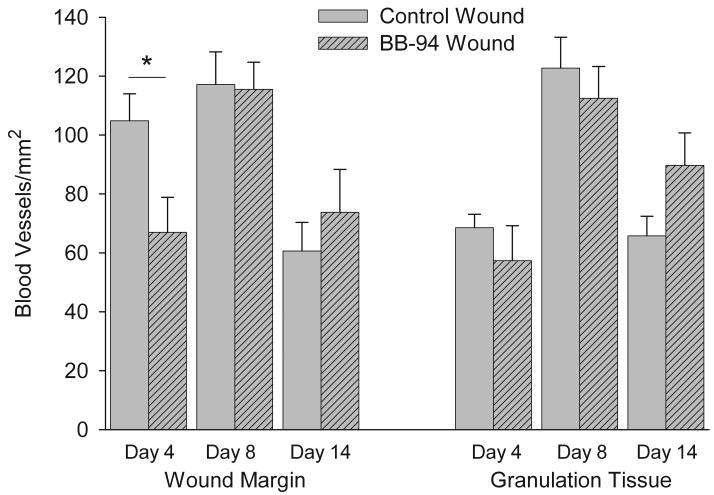
Angiogenesis in response to wounding was quantitatively assessed by determining the number of blood vessels per unit area examined. BB-94 treatment significantly reduced the number of blood vessels formed in wound margin granulation tissue at day 4 post-wounding compared with control wounds (Figure 4). Maximal blood vessel density was observed on day 8 with no significant difference between control and BB-94 groups (Figure 4). Blood vessel formation was reduced by day 14 in both control and BB-94 groups; however, more blood vessels were present in BB-94 treated wounds compared with control wounds (Figure 4). While these differences at day 14 are not statistically significant they are consistent with a delay in wound healing.
Figure 4.
Quantitative analysis of number of blood vessels in superficial and deep dermis of wound margins and in granulation tissue of wounds. Blood vessels were identified by SMAA immunostained perivascular cells surrounding a lumen and counted as described in Materials and Methods. BB-94 treated wounds were delayed in initial blood vessel formation and in the later reduction in blood vessel numbers. Control, open bars; BB-94, filled bars. *p<0.05. Mean ± SEM. n=12 on days 4 and 14, n=9 on day 8.
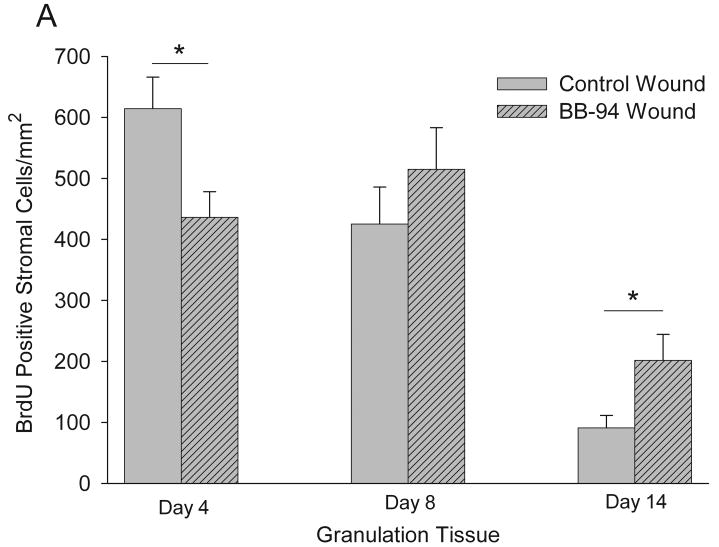
Cellular proliferation of stromal cells in granulation tissue was assessed by analysis of BrdU incorporation and quantified by counting the number of positive cells per unit area examined (Figure 5). In control wound granulation tissue the greatest proliferation of stromal cells was observed at day 4 with a reduction at day 8 and a further reduction at day 14 (Figure 6A). In contrast, in BB-94 treated wounds stromal cell proliferation had a different pattern with increased proliferation occurring from day 4 to 8 and a reduction at day 14 (Figure 6A). This resulted in stromal cell proliferation significantly greater in control wounds at day 4 and in BB-94 treated wounds at day 14 (Figure 6A). In the wound margins only mild changes in BrdU-positive stromal cells were noticed with similar numbers in both groups at all time points (data not shown).
Figure 5.
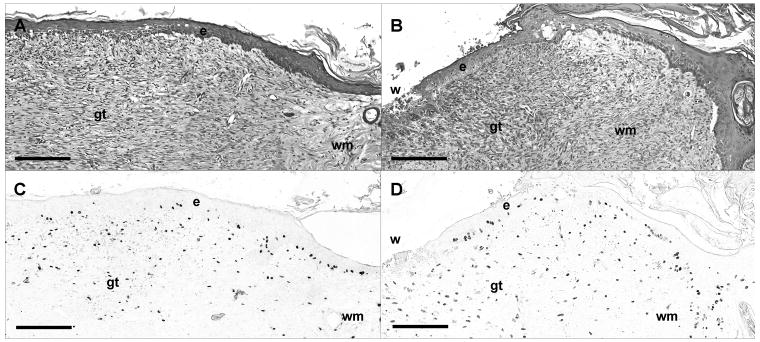
Hematoxylin-eosin staining (A, B) and BrdU immunohistochemistry (C, D) of control (A, C) and BB-94 (B, D) treated wounds on postoperative day 8. Animals were injected with BrdU for analysis of cell proliferation as described in Materials and Methods. (A) Multilayered neoepithelium (e) has formed over wound; stromal cells in granulation tissue (gt) are aligned parallel to the wound bed. (B) BB-94 treatment resulted in reepithelialization (e) of only edge of wound and not of wound center (w). (C, D): Adjacent sections to (A) and (B). BrdU-labeling depicts proliferating epithelial and stromal cells that have incorporated BrdU during the 1-hour pulse. Scale bar (A-D): 100 μm. Labels: epithelium (e); granulation tissue (gt); wound margin (wm); wound center (w).
Figure 6.
Quantification of BrdU incorporation in stromal cells in granulation tissue (A) and epithelium (B). (A): BB-94 treated wounds were delayed in initial BrdU incorporation and in the later reduction in incorporation. (B): In the epithelium, proliferation was higher with BB-94 compared to controls; particularly in the epithelial tongue on day 8. Control, open bars; BB-94, filled bars. *p<0.05. Mean ± SEM. n=12 on days 4 and 14, n=9 on day 8.
Epithelialization is delayed while keratinocyte proliferation is increased by MMPI BB-94 treatment of wounds
Neoepithelial coverage of wounds was analyzed microscopically. On day 4 neoepithelial coverage was significantly reduced in BB-94 treated wounds (0.5 ± 0.1 mm) compared to control wounds (1.2 ± 0.1 mm) (p≤0.01; not illustrated). This corresponds to 28 ± 3% reepithelialization of the control wounds and 11 ± 2% of the BB-94-treated wounds. On day 8, wound coverage for control and BB-94 treated wounds was significantly different with 96 ± 4% reepithelialization (4.1 ± 0.2 mm) for control-treated wounds compared with 52 ± 9% (2.1 ± 0.2 mm) for the BB-94-treated wounds (p≤0.05; compare Figures 2 C and D, and Figures 5A and B). Hyperplastic wound edges with few migratory keratinocytes were observed in BB-94-treated wounds in contrast to the thin epithelial tongues of control wounds on day 8 (compare Figures 2C and D). On postoperative day 14 all wounds had complete neoepithelial coverage by microscopic assessment. In contrast, by macroscopic examination the majority of the BB-94 wounds did not appear healed. The discrepancy between microscopic and macroscopic assessments could be explained by the fact that the thin layer of epithelium detectable by light microscopy is not visible by macroscopic observation. Furthermore, these results suggest that wound epithelialization precedes macroscopic wound contraction by several days and are consistent with our previous observations using systemic GM6001 treatment.12
BB-94 treatment also altered epithelial cell proliferation. Epithelial proliferation was significantly increased in hyperplastic wound margins in the BB-94 group on days 4, 8 and 14 (Figure 6B), and in the epithelial tongue on day 8 compared with control tissue (Figure 6B; compare Figures 5 C and D). These results suggest that BB-94 treatment delayed keratinocyte migration, while at the same time increasing keratinocyte proliferation.
MMP-9 levels in wounds are altered by MMPI BB-94
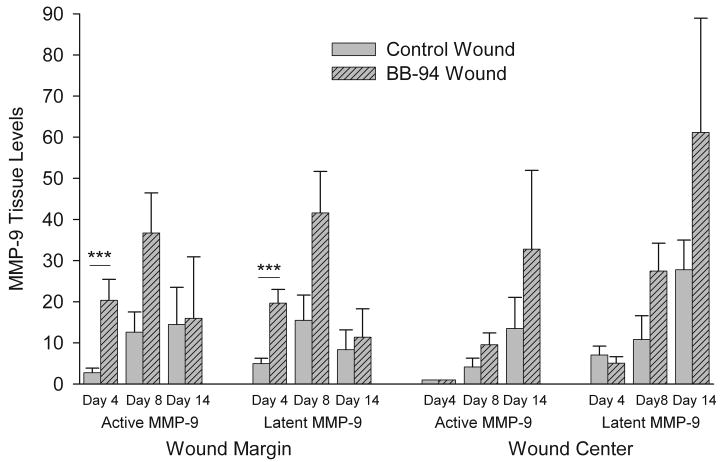
The levels of active and latent MMP-2 and MMP-9 in wounds were analyzed by gelatin zymography. The maximum increase in MMP-2 levels in response to wounding were 2-fold over skin in day 8 wound margin tissue and no differences in latent or active MMP-2 were observed between control and BB-94 treatment at any wound region or time measured (not illustrated). In contrast, wounding resulted in a dramatic increase in both latent and active MMP-9 compared with control skin (Figure 7). In addition, BB-94 treatment significantly increased active and latent MMP-9 in wound margins at day 4 suggesting that BB-94 treatment increased the total amount of MMP-9 in wounded tissues (Figure 7). At the wound margins in control and BB-94 wound tissues both active and latent MMP-9 tended to increase from day 4 to 8 and decrease from day 8 to 14 (Figure 7). In contrast, at the wound center in control and BB-94-treated wounds active and latent MMP-9 increased at each time point measured (Figure 7). These results suggest that activation of MMP-9 and MPP-2 occurs independently of BB-94. In addition, despite that BB-94 increased MMP-9 levels in wound edges day 4 the in situ enzymatic activity was presumably blocked by the MMPI.
Figure 7.
Quantification of MMP-9 levels in wounds. MMP levels in wounds were examined by gelatin zymography and presented as relative to levels in normal skin. BB-94 dramatically increased both active and latent MMP-9 in all samples except day 4 wound center. Control, open bars; BB-94, filled bars. ***p<0.005. Mean ± SEM. n=12 on days 4 and 14, n=6 day 8.
Myofibroblast formation in response to latent TGF-β1 is reduced by MMPI BB-94
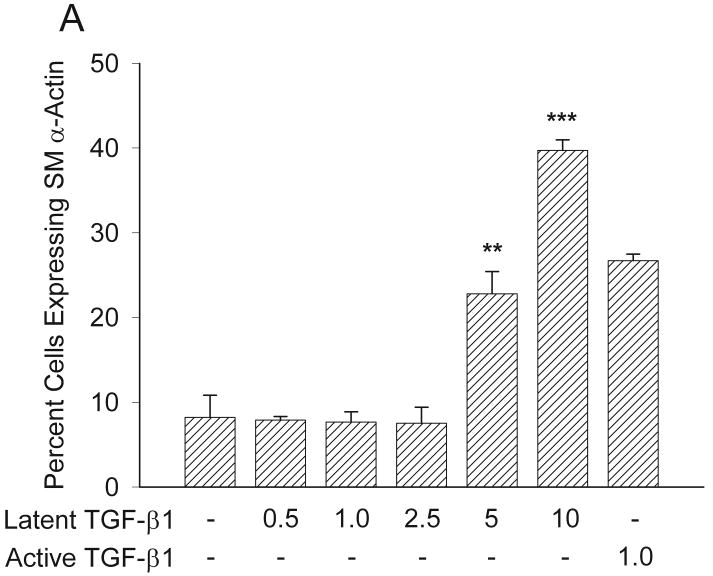
Previous studies have demonstrated that MMPs can activate latent TGF-β1 thereby controlling cellular events.15-18 We have previously demonstrated that inhibiting MMP had no effect upon the ability of active TGF-β1 to promote myofibroblast formation and function.12 Here we examine whether inhibiting endogenous MMP activity with BB-94 would block the ability of latent TGF-β1 to promote myofibroblast formation and function in an in vitro model. First, we examined whether latent TGF-β1 could promote expression of SMAA in human fibroblasts by determining percent cells expressing SMAA.23 Lower concentrations of latent TGF-β1 (0.5-2.5 ng/ml) did not increase expression of SMAA above no TGF-β1 (1 ng/ml); however, addition of higher concentrations of latent TGF-β1 (5-10 ng/ml) resulted in increased expression of SMAA above no TGF-β1 (p<0.01) (Figure 8A). The increased expression of SMAA in response to higher concentrations of latent TGF-β1 (5 ng/ml: 23 ± 3%; 10 ng/ml: 40 ± 1%) was comparable to or greater than the response to active TGF-β1 (1 ng/ml: 27 ± 1%) (Figure 8A). These results demonstrate that latent TGF-β1 can promote expression of SMAA in human fibroblasts similar to active TGF-β1.
Figure 8.
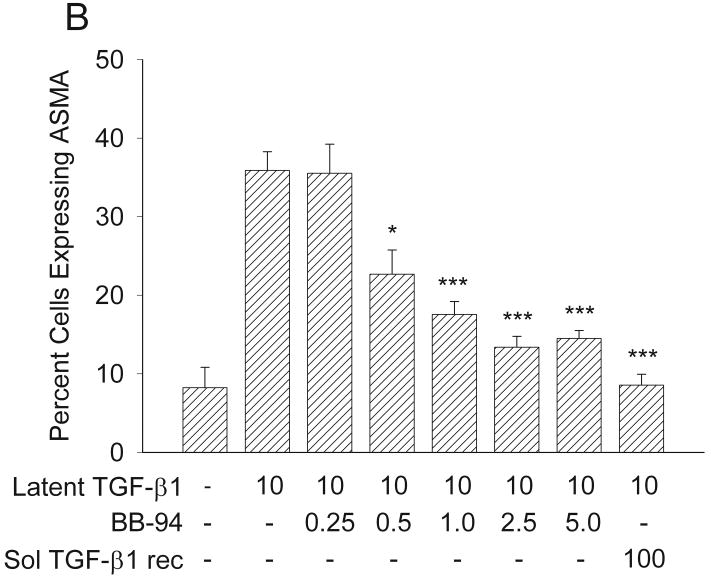
Expression of SMAA in response to TGF-β1 with or without BB-94. (A) Latent TGF-β1significantly induced myofibroblast formation at concentrations of 5 and 10 ng/ml to levels similar to those of active TGF-β1, as assessed by increased expression of SMAA **p<0.01; ***p<0.005. Mean ± SEM. (B) Addition of increasing concentrations of BB-94 (0.5-5.0 μM) significantly inhibited the ability of latent TGF-β1 to promote SMAA expression. The soluble TGF-β receptor antagonist significantly inhibited latent TGF-β1-promoted SMAA expression. The percentage of cells expressing SMAA was determined by counting at least 100 cells per treatment group from three treatment groups. Each experiment was repeated at least three times. *p<0.05; ***p≤0.001. Mean ± SEM.
Next, we examined whether the MMPI BB-94 could inhibit the ability of latent TGF-β1 to promote expression of SMAA in human fibroblasts. SMAA expression was quantified in fibroblasts treated with 10 ng/ml latent TGF-β1 in the presence of varying concentrations of BB-94. The addition of 0.5 μM BB-94 or higher to latent TGF-β1 significantly reduced SMAA expression (0.5 μM: p<0.02; 1-5 μM: p≤0.001) (Figure 8B). To test if the inhibition of SMAA expression was not a TGF-β1 related effect, active TGF- β1 was added together with concentrations of BB-94 that inhibited SMAA expression (0.5-5 μM). All tested BB-94 concentrations (0.25-5 μM) had no inhibitory effect on SMAA expression in the presence of active TGF- β1 (data not shown). To determine whether latent TGF-β1 was acting through the TGF-β receptor a soluble TGF-β receptor antagonist (100 ng/ml) was added with latent TGF-β1 (10 ng/ml). The soluble TGF-β receptor antagonist significantly inhibited the ability of latent TGF-β1 to promote SMAA expression (p<0.001, Figure 8B). These results suggest that increased SMAA expression in fibroblasts resulted from activation of latent TGF-β1 and the binding of this activated TGF-β1 to its cell surface receptor; in addition, BB-94 could inhibit TGF-β1 activation and, thereby, block increased expression of SMAA.
Contraction of stress-relaxed collagen lattices by human fibroblasts in response to latent TGF-β1 is reduced by MMPI BB-94
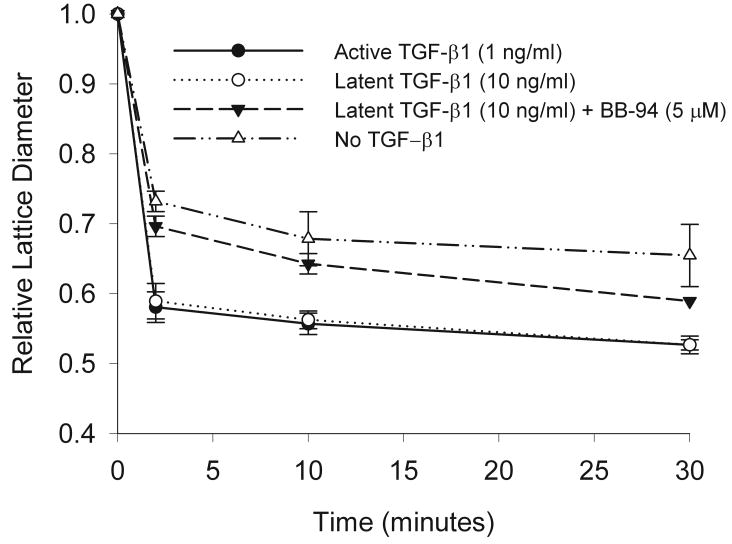
We have previously demonstrated that increased contractile force generation is correlated with the increased expression of SMAA that occurs in response to active TGF-β1.14, 23 To determine whether latent TGF-β1 could promote increased contractile force generation and whether this could be inhibited by BB-94, fibroblast populated collagen lattices were incubated with latent TGF-β1 with or without BB-94. Latent TGF-β1, added at a concentration demonstrated above to increase expression of SMAA (10 ng/ml), promoted a significant increase in contractile force generation similar to active TGF-β1 (Figure 9). BB-94, added at a concentration demonstrated above to inhibit increased expression of SMAA (5 μM), could significantly inhibit the ability of latent TGF-β1 to promote contractile force generation at all time points examined (Figure 9; all times p<0.01). In contrast, BB-94, added at the same concentration, had no effect on active TGF-β1 promoted contractile force generation (not illustrated). These results demonstrate that latent TGF-β1 can promote contractile force generation and this can be inhibited by BB-94 suggesting fibroblasts can activate latent TGF-β1 resulting in myofibroblast formation and this can be inhibited by BB-94.
Figure 9.
Collagen lattice contraction in response to latent TGF-β1 and BB-94. Stressed-relaxed collagen lattices were used to determine contractile force generation. Latent TGF-β1 (10 ng/ml; open circle) could promote collagen contraction significantly greatly than no TGF-β1 (open diamond) (all times p<0.03) and to the same extent as active TGF-β1(1 ng/ml; closed square). Addition of BB-94 (5 μM) to latent TGF-β1 (10 ng/ml; closed triangle) significantly inhibited the promotion of collagen lattice contraction (all times **p≤0.01). All contraction assays were carried out in triplicate, and every experiment was repeated three or more times. Mean ± SEM.
Discussion
The broad-spectrum hydroxamate MMPI BB-94 delayed wound contraction, as well as all other associated events of wound healing examined, including myofibroblast formation, stromal cell proliferation, blood vessel formation, and epithelial wound coverage. Epithelial cell proliferation increased in the epithelial tongue of BB-94 wounds, which may be due to the inability of these cells to migrate in the presence of a MMPI as previously described.11, 12 The MMPI BB-94 dramatically increased the level of latent and active MMP-9. The increased level of active MMP-9 may eventually overcome the ability of BB-94 to inhibit this MMP and may explain why wound contraction and other associated events of wound healing were only delayed and not completely inhibited. MMPI BB-94 was also found to inhibit the ability of latent TGF-β1 to promote the formation and function of myofibroblasts suggesting that the delay in myofibroblast formation and function observed in BB-94-treated wounds may result from a similar inhibition.
Previously we have shown that systemic administration of a broad-spectrum MMPI (GM6001) inhibited, in a similar rat wound model, formation of SMAA positive myofibroblasts, wound contraction, and epithelial wound coverage.12 Unlike in the prior study where GM6001 was applied systemically through the vascular system, in the present study the MMPI BB-94 was applied topically in a hydrogel formulation to avoid systemic effects. Observations with local administration of BB-94 confirmed results seen with systemic application of MMPI with delayed formation of SMAA positive myofibroblasts,12 wound contraction,24 and epithelialization.10, 11 The reason for the observed delay between systemic and topical administration is not clear. It is possible that the systemic administration has additional effects compared with the topical administration; however, both systemic and topical administration wounds closed completely over time, similar to a previous study demonstrating that an MMPI only delayed cutaneous wound closure in a mouse model.25 Interestingly, local BB-94 influenced granulation tissue cell morphology with cells organized as roundish, irregularly arranged cells in the stroma in contrast to spindle shaped cells in parallel to the wound surface in controls confirming previous reports.12, 26, 27 Obviously, topical administration of the hydroxamate MMPI results in sufficient local absorption to influence wound healing.
Different time course with fewer myofibroblasts at days 4 and 8 and more at day 14 in BB-94 treated animals could be interpreted as delayed normal wound healing and with subsequent normal wound closure. Despite the higher numbers of SMAA positive myofibroblasts on day 14 in BB-94 treated wounds, the wounds showed significantly less contraction. Other factors like impaired myofibroblast alignment in and attachment to the extracellular matrix molecules within the wound may result in reduced contractility;28 although BB94 did not reduce collagen lattice contraction in the presence of active TGF-β1. Further studies with longer observation periods will show long-term effects of the MMPI BB-94 treatment on myofibroblast function in wound closure and scar formation.
TGF-β1, fibronectin and mechanical tension of wound tissues are key effectors in triggering fibroblast-myofibroblast transition with expression of SMAA.14, 29 Several recent studies have demonstrated an important role for MMP in activating latent TGF-β1 and controlling TGF-β1-mediated cellular events.15-18 Active TGF-β1 can promote myofibroblast formation and function23 and we have previously demonstrated that blocking MMP has no effect upon this cellular response;12 however, it was not known whether fibroblasts could activate latent TGF-β1 via a MMP-dependent mechanism resulting in promotion of myofibroblast formation and function. In this study we have demonstrated that BB-94 can significantly inhibit the ability of latent TGF-β1 to promote human dermal fibroblasts to form and function as myofibroblasts. This effect was mediated by TGF-β1 because addition of a TGF-β receptor antagonist had the same effect as the inhibition with BB-94. Addition of MMPI to collagen matrices populated with different stromal and epithelial cells reduced lattice contraction in previous studies;27, 30, 31 however, in these studies greater concentrations of the MMPI were used and it was presumed the inhibition was due to altered cell-matrix binding and matrix reorganization. Here we show that at lower concentrations of MMPI the inhibition in myofibroblast formation and function was due to blocked activation of latent TGF-β1 by the MMPI BB-94. These results demonstrate that myofibroblasts are capable of activating latent TGF-β1 in an MMP-dependent manner resulting in increased force generation. Similarly and consistent with our findings Margulis and coworkers32 published during the course of our study that fibroblasts are capable of activating latent TGF-β1 in a MMP-dependent manner resulting in increased matrix reorganization in a floating collagen lattice system.
In this study we have demonstrated that MMPI, by blocking the activation of TGF-β1, can inhibit myofibroblast formation in vitro; however, it is unclear whether the lack of myofibroblast differentiation observed in granulation tissue is the result of MMPI blocking activation of TGF-β1. Active TGF-β1 can promote myofibroblast formation in normal dermis33 and is required for myofibroblast formation and wound closure in cutaneous wound healing.34 Numerous studies have demonstrated that MMPs can activate TGF-β1 in vivo in various tissues and pathologies;15, 17, 18 however, studies of cutaneous wound healing have not yet demonstrated a direct role for MMP activation of TGF-β1 resulting in myofibroblast formation and wound closure. Future studies will need to determine whether during cutaneous wound healing the levels of active TGF-β1 are reduced in the presence of MMPI and whether administration of active TGF-β1 can overcome the effect of MMPI on myofibroblast formation and wound closure.
Topical BB-94 increased epithelial proliferation in the wounds. Several studies point to a possible positive effect of MMPI on cellular proliferation. For example, incubation of psoriatic keratinocytes with BB-9435 or respiratory epithelial cells with GM600136 significantly increased cellular proliferation. BB-3103 increased BrdU incorporation in marginal keratinocytes in wounded human skin explants.22 On the other hand, there is no evidence that MMPs act directly on cellular proliferation or that this effect is mediated indirectly by MMP-activated TGF-β1. In simple model systems, TGF-β is a potent anti-proliferative and a pro-migratory growth factor for keratinocytes. In the more complex skin equivalent model, TGF-β1 profoundly inhibited keratinocyte proliferation but not wound closure.37 Thus, inhibition of activation and/or secretion of TGF-β1 by BB-94 could have resulted in increased epithelial proliferation.
An impressive elevation of MMP-9 levels in response to wounding was found. The MMP-9 elevation was even further increased with BB-94 compared to controls. This observation was reported earlier with hydroxamate MMPI in other very different experimental models.25, 38 In contrast MMP-2 levels in wounds were only slightly elevated over normal skin at specific times post-wound with not differences between control and BB-94 treatment. This is partly at variance with the findings by Schwarz et al.39 who reported increased MMP-2 levels in ischemic wounds in rats treated systemically with another broad spectrum hydroxamate MMPI (SC44463). Higher amounts of MMP-9 with BB-94 could be explained by a rebound effect with increased MMP-9 secretion by immobilized keratinocytes. Maquoi et al.40 observed the same phenomenon in cultured HT1080 cells. They attributed the increased MMP-9 mRNA and protein levels to modulation of the immediate pericellular environment with hydroxamate-type MMP inhibitors.40 It is also possible that the increased MMP-9 is secondary to the delayed wound-healing response.41, 42 On the other hand, persistence of MMP-9 was associated with a scarless skin phenotype.43 Fibroblasts are one of the main dermal source of MMP-220 and are fully motile in spite of the presence of MMPI.28 This might explain why MMP-9 but not MMP-2 was increased with MMPI treatment.
Although BB-94 was present throughout the experiment over time the wound eventually epithelialized. A similar wound-healing pattern was observed with systemic application of GM6001 in murine skin wounds.25 They also noted increased MMP-9 levels with the MMPI.25 It is possible that the large increase in active MMP-9 was able to overcome the presence of the MMPI and epithelialization and wound closure could occur. Intriguingly, wound margins showed higher amounts of MMP-9 compared to wound centers at days 4 and 8; while the wound center had higher amounts of MMP-9 at day 14. In skin wound healing, MMP-9 coordinates and facilitates keratinocyte migration.8, 44 Thus, hyperplastic epithelium in BB-94-treated wound margins seems to compensate abolished migration by increased MMP-9 secretion that may subsequently allow for migration. As described in our previous study,12 we observed a delay in myofibroblast formation and wound closure that correlated with the delay in epithelialization. Previous studies have demonstrated that keratinocyte-fibroblast interactions will dramatically potentiate myofibroblast formation through a TGF-β1-mediated mechanism.45 BB-94 could delay wound closure through a two-fold mechanism; one by blocking keratinocyte migration and thereby blocking necessary keratinocyte-fibroblast interactions needed for myofibroblast formation and two by inhibiting the activation of latent TGF-β1.
In summary, topical application of the synthetic, hydroxamate MMP inhibitor BB-94 impaired wound contraction and epithelialization of full-thickness excisional skin wounds in vivo and myofibroblast formation and function in vitro. We have found a delay in the formation and function of myofibroblasts in BB-94-treated wounds. Our in vitro studies suggest that one mechanism by which BB-94 may delay wound closure is by blocking MMP-mediated activation of latent TGF-β1 resulting in impaired myofibroblast formation and function. Experiments with selective MMPI will show if skin wound contraction can be effectively blocked without impairing epithelial migration and wound closure.
Acknowledgments
This work was financially supported by a grant of the Bundesministerium für Bildung und Forschung Förderinitiative NBL3 – FKZ 01ZZ0407. U.M. received a post-doc scholarship from Nachwuchsförderprogramm, Otto-von-Guericke Universität. J.J.T. was supported by a grant from the National Institutes of Health R01 GM060651. Dr. August Wolff GmbH, Bielefeld, Germany donated the hydrogel. S. Sattelkau is acknowledged for technical assistance and Kyungwon Lee for assistance with in vitro assays.
Abbreviations
- BrdU
5-bromo-2′-deoxyuridine
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- MMPI
MMP inhibitor
- PAGE
polyacrylamide gel electrophoresis
- SMAA
smooth muscle α-actin
- TGF-β1
transforming growth factor-beta 1
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgiadis D, Yiotakis A. Specific targeting of metzincin family members with small-molecule inhibitors: progress toward a multifarious challenge. Bioorg Med Chem. 2008;16:8781–94. doi: 10.1016/j.bmc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Gill SE, Parks WC. Metalloproteinases and their inhibitors: Regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–47. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toriseva M, Kähäri VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009;66:203–24. doi: 10.1007/s00018-008-8388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–5. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, Bornstein P. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol. 2009;28:65–73. doi: 10.1016/j.matbio.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D'Armiento J, Okada Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol. 2009;175:533–46. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ågren MS. Matrix metalloproteinases (MMPs) are required for re-epithelialization of cutaneous wounds. Arch Dermatol Res. 1999;291:583–90. doi: 10.1007/s004030050459. [DOI] [PubMed] [Google Scholar]
- 11.Ågren MS, Mirastschijski U, Karlsmark T, Saarialho-Kere UK. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp Dermatol. 2001;10:337–48. doi: 10.1034/j.1600-0625.2001.100506.x. [DOI] [PubMed] [Google Scholar]
- 12.Mirastschijski U, Haaksma CJ, Tomasek JJ, Ågren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res. 2004;299:465–75. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–9. [PubMed] [Google Scholar]
- 14.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 15.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–76. [PMC free article] [PubMed] [Google Scholar]
- 16.Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, Delaissé JM, Foged NT. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem. 2002;277:44061–7. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- 17.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–9. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 19.Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–4. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madlener M, Parks WC, Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242:201–10. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- 21.Soo C, Shaw WW, Zhang X, Longaker MT, Howard EW, Ting K. Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast Reconstr Surg. 2000;105:638–47. doi: 10.1097/00006534-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Mirastschijski U, Impola U, Karsdal MA, Saarialho-Kere U, Ågren MS. Matrix metalloproteinase inhibitor BB-3103 unlike the serine proteinase inhibitor aprotinin abrogates epidermal healing of human skin wounds ex vivo. J Invest Dermatol. 2002;118:55–64. doi: 10.1046/j.0022-202x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–9. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 24.Klein SA, Anderson GL, Kennedy AB, Bond SJ. The effects of a broad-spectrum matrix metalloproteinase inhibitor on characteristics of wound healing. J Invest Surg. 2002;15:199–207. doi: 10.1080/08941930290085976. [DOI] [PubMed] [Google Scholar]
- 25.Lund LR, Romer J, Bugge TH, Nielsen BS, Frandsen TL, Degen JL, Stephens RW, Danø K. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 1999;18:4645–56. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacCumber MW, Ross CA, Glaser BM, Snyder SH. Endothelin: visualization of mRNAs by in situ hybridization provides evidence for local action. Proc Natl Acad Sci U S A. 1989;86:7285–9. doi: 10.1073/pnas.86.18.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan CM, Occleston NL, Hiscott P, Kon CH, Khaw PT, Grierson I. Matrix metalloproteinases: a role in the contraction of vitreo-retinal scar tissue. Am J Pathol. 2001;159:1555–66. doi: 10.1016/S0002-9440(10)62540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels JT, Cambrey AD, Occleston NL, Garrett Q, Tarnuzzer RW, Schultz GS, Khaw PT. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44:1104–10. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- 31.Scott KA, Wood EJ, Karran EH. A matrix metalloproteinase inhibitor which prevents fibroblast-mediated collagen lattice contraction. FEBS Lett. 1998;441:137–40. doi: 10.1016/s0014-5793(98)01542-7. [DOI] [PubMed] [Google Scholar]
- 32.Margulis A, Nocka KH, Wood NL, Wolf SF, Goldman SJ, Kasaian MT. MMP dependence of fibroblast contraction and collagen production induced by human mast cell activation in a three-dimensional collagen lattice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L236–47. doi: 10.1152/ajplung.90462.2008. [DOI] [PubMed] [Google Scholar]
- 33.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters T, Sindrilaru A, Hinz B, Hinrichs R, Menke A, Al-Azzeh EA, Holzwarth K, Oreshkova T, Wang H, Kess D, Walzog B, Sulyok S, Sunderkötter C, Friedrich W, Wlaschek M, Krieg T, Scharffetter-Kochanek K. Wound-healing defect of CD18(-/-) mice due to a decrease in TGF-beta1 and myofibroblast differentiation. EMBO J. 2005;24:3400–10. doi: 10.1038/sj.emboj.7600809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buisson-Legendre N, Emonard H, Bernard P, Hornebeck W. Relationship between cell-associated matrix metalloproteinase 9 and psoriatic keratinocyte growth. J Invest Dermatol. 2000;115:213–8. doi: 10.1046/j.1523-1747.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 36.Sigurdson L, Sen T, Hall L, 3rd, Rubenfeld A, Hard R, Gardella J, Bright F, Hicks WL., Jr Possible impedance of luminal reepithelialization by tracheal cartilage metalloproteinases. Arch Otolaryngol Head Neck Surg. 2003;129:197–200. doi: 10.1001/archotol.129.2.197. [DOI] [PubMed] [Google Scholar]
- 37.Garlick JA, Taichman LB. Effect of TGF-beta 1 on re-epithelialization of human keratinocytes in vitro: an organotypic model. J Invest Dermatol. 1994;103:554–9. doi: 10.1111/1523-1747.ep12396847. [DOI] [PubMed] [Google Scholar]
- 38.Krüger A, Soeltl R, Sopov I, Kopitz C, Arlt M, Magdolen V, Harbeck N, Gänsbacher B, Schmitt M. Hydroxamate-type matrix metalloproteinase inhibitor batimastat promotes liver metastasis. Cancer Res. 2001;61:1272–5. [PubMed] [Google Scholar]
- 39.Schwarz DA, Thul J, Rees RS, Lindblad WJ. Treatment of delayed healing rat wounds with an inhibitor of matrix metalloproteinases increases tissue gelatinase activity. Wound Repair Regen. 1996;4:A173. [Google Scholar]
- 40.Maquoi E, Munaut C, Colige A, Lambert C, Frankenne F, Noël A, Grams F, Krell HW, Foidart JM. Stimulation of matrix metalloproteinase-9 expression in human fibrosarcoma cells by synthetic matrix metalloproteinase inhibitors. Exp Cell Res. 2002;275:110–21. doi: 10.1006/excr.2002.5489. [DOI] [PubMed] [Google Scholar]
- 41.Ågren MS, Jorgensen LN, Andersen M, Viljanto J, Gottrup F. Matrix metalloproteinase 9 level predicts optimal collagen deposition during early wound repair in humans. Br J Surg. 1998;85:68–71. doi: 10.1046/j.1365-2168.1998.00556.x. [DOI] [PubMed] [Google Scholar]
- 42.Yang C, Zhu P, Yan L, Chen L, Meng R, Lao G. Dynamic changes in matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 levels during wound healing in diabetic rats. J Am Podiatr Med Assoc. 2009;99:489–96. doi: 10.7547/0990489. [DOI] [PubMed] [Google Scholar]
- 43.Manuel JA, Gawronska-Kozak B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biol. 2006;25:505–14. doi: 10.1016/j.matbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065–72. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- 45.Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am J Pathol. 2004;164:2055–66. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]