Abstract
Salivary glands are innervated by sympathetic and parasympathetic neurons, which release neurotransmitters that promote fluid secretion and exocytosis when they bind to muscarinic and β-adrenergic receptors, respectively. Signaling pathways downstream of these receptors are mainly distinct, but there is cross-talk that affects receptor-dependent events. Here we report that the β-adrenergic ligand isoproterenol blocks increases in extracellular signal-related kinase (ERK) phosphorylation, a protein kinase C-dependent event promoted by the muscarinic receptor ligand carbachol in freshly dispersed rat parotid acinar cells. The inhibitory action of isoproterenol was reproduced by cAMP stimuli (forskolin) and mimetics (dibutyryl-cAMP, 8-(4-chlorophenylthio)-cAMP), including one highly selective for protein kinase A (N6-benzoyl-cAMP). In contrast, Epac (exchange proteins directly activated by cAMP)-selective activators did not mimic the blockade of ERK by isoproterenol, suggesting that inhibition involved protein kinase A. Isoproterenol also blocked ERK downstream of phorbol 12-myristate 13-acetate and the P2X7 and epidermal growth factor receptors. Isoproterenol and forskolin blocked MEK phosphorylation, reduced RAF phosphorylation on a stimulatory site (Ser-338), and increased RAF phosphorylation on an inhibitory site (Ser-259). Inhibitory effects on ERK were also observed in freshly dispersed rat submandibular acinar cells but not in three immortalized/cancer salivary cell lines (Par-C10, HSY, HSG), indicating significant differences between native cells and cell lines. Notably, in native parotid cells isoproterenol enhanced the carbachol-promoted increases in [Ca2+]i and oxygen consumption, events that initiate and accompany, respectively, the stimulation of fluid secretion by muscarinic ligands. Thus, isoproterenol produces opposite effects on prominent events downstream of the muscarinic receptor second messengers diacylglycerol (decrease in ERK phosphorylation) and inositol trisphosphate (increase in [Ca2+]i and fluid secretion).
Keywords: Calcium, Cyclic AMP (cAMP), ERK, Salivary Gland, Secretion, Muscarinic Receptor, Purinergic Receptor, β-Adrenergic Receptor, Parotid Gland, Submandibular Gland
Introduction
Almost all mammalian cells have multiple types of heptahelical G-protein-coupled receptors. The muscarinic receptor and β-adrenergic receptor are G-protein-coupled receptors that affect the physiological activities of many cells, and neurotransmitters binding to these receptors produce well defined and mainly separate functional effects in salivary glands, which are innervated by both sympathetic and parasympathetic neurons (1). Parotid acinar cells express M3 muscarinic receptors (2), which are coupled via Gq to phospholipase C, and acetylcholine and M3R2 ligands binding to this receptor initiate the production of the second messengers inositol trisphosphate (InsP3) and diacylglycerol, which elevate the intracellular Ca2+ concentration ([Ca2+]i) and activate PKC. The increase in intracellular Ca2+ initiates the opening of ion channels, electrolyte and fluid secretion, and subsequently, the formation of saliva in the oral cavity (3). In contrast, the activation of parotid β-adrenergic receptors promotes exocytosis and the release of amylase protein into the oral cavity. β-Adrenergic receptors are coupled via Gs to adenylyl cyclase, and receptor stimulation generates the production of cAMP (cyclic adenosine monophosphate) and the activation of cAMP-dependent protein kinase (protein kinase A (PKA)) and a signaling cascade downstream of PKA. Muscarinic receptors also can promote exocytosis, but this is more limited than that due to β-adrenergic signaling (4). In many cells cAMP also can activate exchange proteins directly activated by cAMP (Epac), which are guanine nucleotide exchange factors for the GTPase Rap. Epac is a cAMP-dependent PKA-independent protein that can mediate various signaling and functional events in salivary glands and other cells (5–7). This can produce complications in evaluating the mechanisms of cross-talk that exist when there is combined muscarinic and β-adrenergic signaling, which is a focus of the present study.
Isoproterenol has other effects on parotid acinar cells in addition to promoting exocytosis of secretory granules. It has been recognized for more than 40 years that the chronic systemic administration of the β-adrenergic ligand isoproterenol to rodents promotes enlargement of parotid salivary glands (8, 9). This is accompanied by increases in DNA synthesis and involves both hyperplasia and hypertrophy. Parotid glands from mice treated with isoproterenol for times up to 24 h display significant changes in the expression patterns of >40 genes, including cell cycle proteins, transcriptional factors, and kinases (10). Treatment of rat parotid glands with isoproterenol (1–4 h) increased the tight junction permeability (11), and acute exposure of dispersed rat parotid acini to isoproterenol enhanced the Na-K-2Cl cotransporter activity (12).
Although muscarinic and β-adrenergic receptors promote chiefly parallel signaling pathways and physiological events in salivary gland cells, there is both positive and negative cross-talk between these two important receptor-mediated signaling pathways. In primary cultures of mouse parotid acinar cells, carbachol and isoproterenol both increased the phosphorylation of the cAMP response element-binding protein (CREB) (13). Isoproterenol and the adenylyl cyclase activator forskolin increased the release of Ca2+ from intracellular stores by the muscarinic and α-adrenergic receptor stimulation in rodent parotid acinar cells, and this was due to the phosphorylation of the InsP3 receptor in a PKA-dependent manner (14–16). This offers an explanation for the stimulatory effect of cAMP production/PKA activation on fluid secretion in parotid and submandibular glands (17, 18) and other epithelial glands (19). Notably, cross-talk between these receptors can go in both directions, as M3 muscarinic receptor stimulation can desensitize β2-adrenergic receptors via a PKC-dependent phosphorylation of the receptor (20). In addition, M3 receptor stimulation by carbachol promotes a reduction in the isoproterenol-promoted production of cAMP in rodent parotid and submandibular cells (2, 21, 22).
Extracellular signal-related kinase (ERK) participates in various functions of normal and cancerous salivary gland tissues (23–25) and plays a vital role in promoting the branching morphogenesis of developing salivary glands (26). In the present study we found that isoproterenol had a substantial negative effect on the carbachol-initiated phosphorylation of ERK in dispersed rat parotid acinar cells. Because cAMP can exert either positive or negative effects on ERK signaling in many cells (27), we examined the cross-talk between β-adrenergic and muscarinic receptors in more detail in several native salivary gland acinar cells and cell lines. We compared the effects of forskolin, cAMP analogs, and Epac-specific activators to the effects of isoproterenol on the stimulation of ERK phosphorylation by carbachol. We also examined the effects of isoproterenol on multiple physiological indices of fluid secretion as well as on ERK phosphorylation downstream of P2X7 and epidermal growth factor (EGF) receptors, neither of which is coupled to G-proteins. The results indicate that isoproterenol produces both positive and negative effects on muscarinic signaling within the same cell and can block ERK activation downstream of multiple types of receptors.
EXPERIMENTAL PROCEDURES
Materials
Carbamylcholine (carbachol) (C4382), isoproterenol (I5627), dibutyryl-cAMP (D0697), 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP, B6396), and propranolol hydrochloride (P0884) were purchased from Sigma. Phorbol 12-myristate 13-acetate (PMA, 524400) was from Calbiochem. The following products were purchased from Biomol: forskolin (CN-100), 8-bromo-cAMP (8-Br-cAMP, CN-115), 8-(4-chlorophenylthio)-cAMP (8-CPT-cAMP, CN-130), N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89, EI-196), and Rp-adenosine-3′,5′-cyclic monophosphothioate (Rp-cAMPS, CN-135). 8-(4-Chlorophenylthio)-2′-O-methyl-cAMP (8-CPT-2′-Me-cAMP, #1645) was purchased from Tocris. 8-(4-Methoxyphenylthio)-2′-O-methyl-cAMP (8-pMeOPT-2′-O-Me-cAMP, M034) and N6-benzoyl-cAMP, acetoxymethyl ester (6-Bnz-cAMP-AM, B079) were from Biolog. EGF (01-107) was purchased from Millipore. Fura-2 AM was from Invitrogen. Polyclonal ERK2 (SC-154), monoclonal ERK2 (SC-1647), and polyclonal c-RAF-1 (SC-133) were purchased from Santa Cruz Biotechnology, Inc. The following antibodies were purchased from Cell Signaling Technology: phospho-Thr-202/Tyr-204-ERK1/2 (9101), phospho-Ser-217/221-MEK (9121), MEK (9122), phospho-c-RAF-Ser-338 (9427), and phospho-c-RAF-Ser-259 (9421). Secondary antibodies used for the Odyssey Infrared Imaging System were IRDye 800-conjugated anti-Rabbit IgG (Rockland, #611-632-122) and Alexa Fluor 680 anti-mouse IgG (Invitrogen A-21058). Anti-rabbit IgG (AP307P) and anti-mouse IgG (AP124P) secondary antibodies used for Western blotting using film were obtained from Chemicon. All other chemicals were reagent grade or better.
Salivary Gland Acinar Cell Preparations and Solutions
Parotid acinar cells were prepared from male Sprague-Dawley rats (Charles River Laboratories, Kingston, NY, 150–200 g) using previously established techniques (28). Rat submandibular acinar cells were prepared in an identical manner. Cells were suspended at ∼0.5–1 mg of protein/ml in Solution A (116.4 mm NaCl, 5.4 mm KCl, 1 mm NaH2PO4, 25 mm Na HEPES, 1.8 mm CaCl2, 0.8 mm MgCl2, 5 mm sodium butyrate, 5.6 mm glucose, pH 7.4). In experiments using BzATP, Solution A was modified to contain 1 mm CaCl2 without MgCl2. For cells used in Western blotting experiments, all treatments of cells were performed at 37 °C in a water-jacketed chamber using a magnetic flea to stir the suspended cells. Aliquots (1.5 ml) of cells were equilibrated for ∼5–10 min before treatments for various times with various agents or vehicles.
Salivary Gland Cell Lines
Par-C10 cells were grown to near confluence in Dulbecco's modified Eagle's medium-F-12 (1:1) medium containing 2.5% fetal bovine serum and supplements similar to those specified elsewhere (29). HSY and HSG cells were grown to near confluence in Dulbecco's modified Eagle's medium-F-12 medium containing 10% fetal bovine serum. Cells were cultured on BD Falcon tissue culture dishes in a humidified atmosphere of 90% air, 10% CO2 at 37 °C.
Western Blot Analysis
At the end of the treatment period, the suspended native parotid cells were collected by rapid sedimentation. Cells were lysed in ice-cold lysis buffer as described previously (30). Lysates were cleared of insoluble proteins by sedimentation at 15,000 × g for 15 min at 4 °C. For experiments conducted using cell lines, the cells were treated as indicated in a 37 °C incubator for the appropriate times, washed 3 times in phosphate-buffered saline solution, lysed in ice-cold lysis buffer, and sedimented at 15,000 × g. The cleared supernatants were diluted with 5× Laemmli sample buffer, boiled for 5 min, and stored at −20 °C before electrophoresis. For samples subjected to RAF immunoprecipitation, 1–2 μg/ml polyclonal anti-RAF-1 antibody (SC-133) and protein A-Sepharose beads were added to the cleared supernatants overnight, and the beads were washed 3 times in phosphate-buffered saline, 1% Igepal solution and then boiled for 5 min in 2× sample buffer. Samples were separated using SDS-polyacrylamide gel electrophoresis with an 8% separating gel and a 3% stacking gel. Proteins were transferred to nitrocellulose. Immunoblots were probed overnight with various antibodies according to the supplier's specifications. Proteins were visualized using chemiluminescence reagents and x-ray film. Alternatively, in some experiments proteins were visualized and quantified by direct infrared fluorescence using an Odyssey Imaging System (Li Cor Biosciences) as reported previously (31).
Quantification of Protein Phosphorylation
The phosphorylation status of proteins visualized on film was quantified by densitometry using the NIH Image J software program. For each sample, the phosphoproteins were normalized to total protein levels (ERK or RAF) to account for gel loading/transfer variations. Blots were probed for phosphoproteins, stripped, and reprobed for total proteins. The phosphorylations for the various conditions were normalized to the phosphorylation under basal (non-stimulated) control (no inhibitors) conditions. In experiments using the Odyssey Imaging System, blots were simultaneously probed for phosphoproteins and total protein levels using polyclonal antibodies and mouse monoclonal antibodies, respectively, and fluorescent anti-rabbit and anti-mouse antibodies were used for visualization and subsequent quantification.
Intracellular Ca2+ Measurements
Alterations in [Ca2+]i were analyzed at room temperature in dispersed parotid cells in suspension by measuring changes in the fluorescence of the Ca2+ indicator dye Fura-2 using a QuantaMaster fluorescence spectrophotometer (Photon Technology International) similar to previous studies (32). Fura-2-loaded cells were washed, suspended in Solution A, and maintained on ice. Generally 2–4 replicates of each condition were examined in each experiment, and the results were averaged as n = 1. Cells suspended in Solution A were exposed to isoproterenol (10−5 m), forskolin (10−5 m), or vehicle for 2–4 min followed by the addition of carbachol (10−6 or 10−5 m). To examine Ca2+ release from intracellular stores and Ca2+ entry, the cells were suspended in Ca2+-free Solution A containing 10 μm EGTA, exposed to isoproterenol (10−5 m) or vehicle for ∼3 min followed by carbachol (10−6 m or 10−5 m), and subsequently exposed to 1 mm CaCl2 when the carbachol-promoted Ca2+ elevation returned to basal levels. The carbachol-promoted peak increases in intracellular Ca2+ were quantified by calculating the differences between the average Fura-2 ratio for ∼5 s after the addition of carbachol and the average ratio immediately before the carbachol addition. The peak increases in Ca2+ due to Ca2+ entry were calculated as the differences between the basal levels immediately before the addition of Ca2+ and the average of the peaks reached 20–30 s after Ca2+ was added. For each experiment, the experimental values (isoproterenol, forskolin) were normalized to control (carbachol alone). Statistics were performed on the results compiled from all independent experiments, and the numbers of separate experiments are reported on each figure.
Oxygen Consumption
Measurements were made at 37 °C similar to those reported previously (33). The rates of O2 consumption (QO2) were normalized to the protein content of the cells. The QO2 was measured for several minutes under basal conditions (control, 10−7 m isoproterenol, 10−5 m isoproterenol) followed by the addition of 10−5 m carbachol. The ΔQO2 values (in nmol O2/mg of protein/min) are the differences between the sustained O2 consumption rates under basal conditions and the maximal linear rate of O2 consumption upon the addition of carbachol. In each experiment, 3–5 individual samples were analyzed for each condition and averaged as n = 1.
Data Analysis
Values were calculated as the mean ± S.E. of n number of independent experiments (each n from a different cell preparation). Differences between control/basal and experimental samples for the accumulated data were evaluated using Student's t test. All experiments including Western blots were performed at least three different times. Representative blots from one experiment are shown in each figure. Within each experiment to be analyzed using Western blotting techniques and/or the Odyssey system, multiple (duplicate or triplicate) cell samples were collected for each condition and subjected to SDS-PAGE, and the average of the values obtained within each individual experiment were treated as n = 1.
RESULTS
Isoproterenol Rapidly Blocks ERK Phosphorylation Promoted by Muscarinic Stimulation
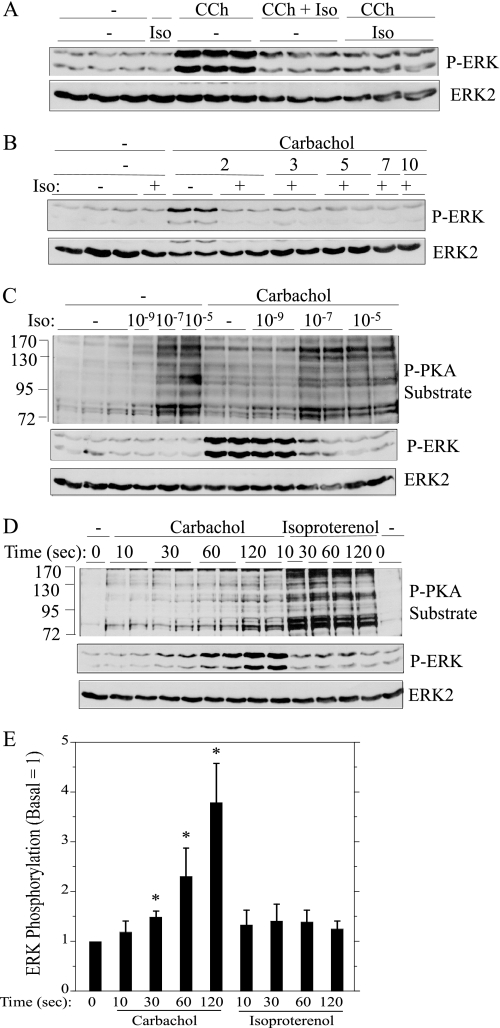
Because parotid acini are innervated by both sympathetic and parasympathetic nerves and because there is a history of functional interactions between muscarinic and β-adrenergic receptors in parotid glands, we evaluated the effects of isoproterenol itself on the activation of ERK as well as its effects on the activation of ERK by carbachol in rat parotid acinar cells. Isoproterenol did not significantly affect the basal level of ERK phosphorylation (Fig. 1A). Remarkably, when isoproterenol was added to parotid acinar cells simultaneously with carbachol (10−5 m), there was nearly a complete inhibition of the phosphorylation of ERK produced by carbachol. Similar inhibitory effects were produced when cells were pretreated with isoproterenol for 1 min before carbachol. The peak increase in carbachol-promoted ERK phosphorylation is at 2 min (not shown), and isoproterenol did not shift the peak from 2 min to a delayed point in time (Fig. 1B). Thus, the reduction of ERK by isoproterenol was due to inhibition and not to a change in the kinetics of ERK phosphorylation in carbachol-treated cells.
FIGURE 1.
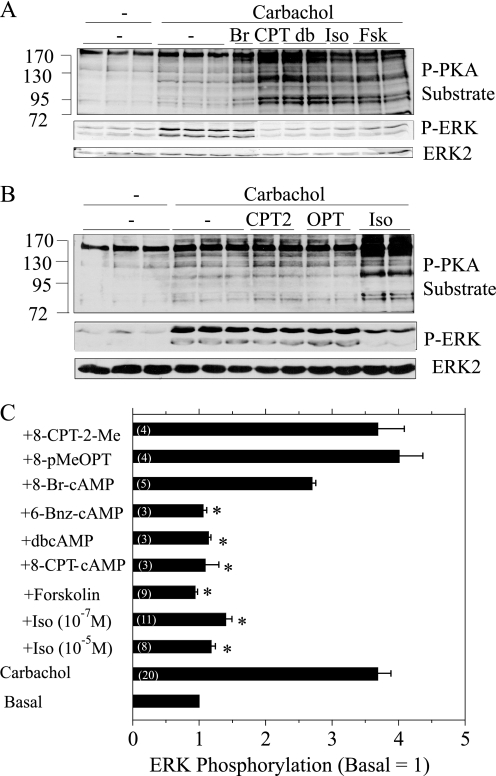
Isoproterenol blocks the phosphorylation of ERK by carbachol in rat parotid acinar cells. A, cells were pretreated (or not) with 10−7 m isoproterenol (Iso) for 1 min and then exposed for 2 min to 10−5 m carbachol (CCh) alone or in combination with 10−7 m isoproterenol. B, cells were pretreated (or not) with 10−7 m isoproterenol for 1 min and then exposed for to 10−5 m carbachol for periods of time between 2–10 min. C, cells were pretreated with 10−9–10−5 m isoproterenol for 1 min and then exposed to 10−5 m carbachol for 2 min. D, cells were exposed to carbachol (10−5 m) and isoproterenol (10−7 m) for the times indicated. E, shown is quantification of ERK phosphorylation relative to the basal control (no addition) for conditions shown in D. *, p < 0.05, n = 3–4. In A–C, cells exposed only to isoproterenol were treated for 3 min. Cell lysates were subjected to immunoblot analysis as indicated.
Our initial observations were conducted using 10−5 m isoproterenol. When we compared the effects of various concentrations of isoproterenol, we found that 10−5 and 10−7 m isoproterenol were equally effective, and 10−9 m isoproterenol was ineffective in blocking ERK phosphorylation (Fig. 1C). Because isoproterenol increases cAMP production and activates PKA in parotid and other cells, we used an antibody that recognizes phosphorylated PKA substrates to evaluate PKA activation downstream of isoproterenol binding to the β-adrenergic receptor. Both 10−5 and 10−7 m isoproterenol produced similar increases in the phosphorylation of PKA substrates, and 10−9 m isoproterenol was without effect, similar to the isoproterenol concentration dependence of carbachol-initiated ERK phosphorylation (Fig. 1C).
We evaluated in more detail how isoproterenol was able to block the effect of carbachol on ERK phosphorylation when both ligands were added simultaneously. To examine this, we compared the time courses (Fig. 1, D and E) of carbachol on ERK phosphorylation and isoproterenol on PKA substrate phosphorylation as an index of β-adrenergic receptor activation. The phosphorylation of ERK was modest after 30 s of carbachol exposure and was greater at 2 min compared with 1 min. In contrast, increases in the phosphorylation of PKA substrates appeared to reach a maximum at ∼10 s, a time at which there was no indication of an increase in ERK phosphorylation by carbachol. Although the phospho-PKA substrate antibody is not the perfect indicator of PKA activation, the results indicate that isoproterenol activates PKA very rapidly and suggest that the rapid increase in signaling downstream of the β-adrenergic receptor blocks the slower increase in ERK phosphorylation by muscarinic receptor activation.
Inhibitory Effects of Isoproterenol Are Mediated by the β-Adrenergic Receptor
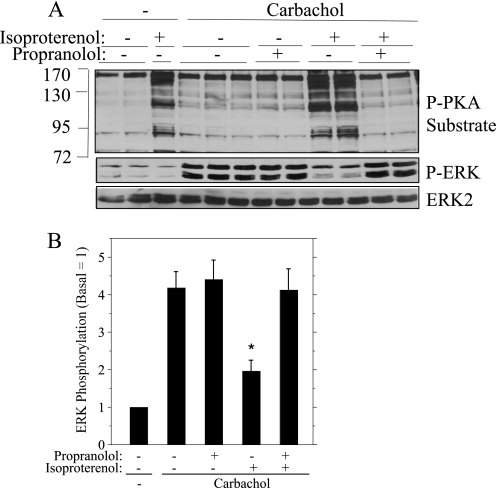
Although isoproterenol is a β-adrenergic receptor ligand known to increase cAMP in rat parotid gland (34), it was possible that its inhibitory actions on ERK phosphorylation were due to an off-target effect. Therefore, we determined whether propranolol, a β-adrenergic receptor antagonist, blocked the inhibitory effects of isoproterenol. Propranolol was very effective in blocking the increase in phospho-PKA substrate bands produced by isoproterenol (Fig. 2A) and was similarly effective in reducing the inhibitory effect of isoproterenol on the phosphorylation of ERK initiated by carbachol (Fig. 2, A and B). Notably, propranolol did not affect the increase in ERK phosphorylation by carbachol, consistent with its action to block β-adrenergic receptors and not affect muscarinic receptors.
FIGURE 2.
The β-adrenergic receptor antagonist propranolol blocks the effects of isoproterenol on rat parotid acinar cells. Where indicated, cells were pretreated (or not) with propranolol (10−6 m) for 3 min before carbachol (10−5 m, 2 min) alone or preceded by isoproterenol (10−7 m, 1 min). A, cell lysates were subjected to immunoblot analysis as indicated. B, quantification of ERK phosphorylation relative to the basal control (no addition) for conditions shown in A. n = 5. *, p < 0.01 versus carbachol (no additions).
cAMP Reduces ERK Phosphorylation Produced by Carbachol and PMA
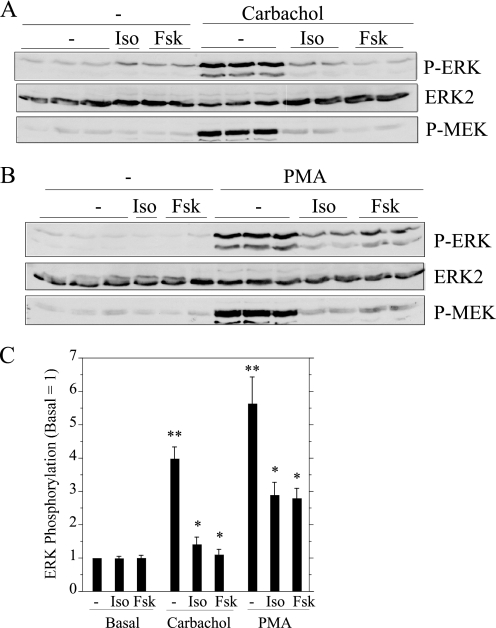
To determine whether the effects of isoproterenol involved the production of cAMP, we examined the effects of forskolin, an agent that directly activates adenylyl cyclase, the enzyme that converts ATP to cAMP. Forskolin produced the same inhibitory effect as isoproterenol on carbachol-promoted ERK phosphorylation (Fig. 3A), suggesting that the effect of isoproterenol was mediated by cAMP production. These cAMP-producing agents also blocked the carbachol-promoted phosphorylation of MEK, the kinase immediately upstream of ERK. Because the increase in ERK phosphorylation by carbachol treatment of rat parotid acinar cells is largely dependent on PKC (23), we examined the effects of isoproterenol and forskolin on ERK phosphorylation in parotid cells treated with PMA to activate PKC directly and bypass receptor activation. These cAMP-producing agents also were effective in blocking the phosphorylation of ERK and MEK when PMA was the stimulus (Fig. 3B). The quantifications of the effects of isoproterenol and forskolin on ERK phosphorylation for carbachol- and PMA-treated cells (Fig. 3C) indicated that cAMP can block ERK downstream of PKC activation in these cells.
FIGURE 3.
Inhibitory effects of isoproterenol and forskolin on the phosphorylation of ERK and MEK in carbachol- and PMA-treated rat parotid acinar cells. Cells were pretreated with 10−5 m isoproterenol (Iso) for 1 min and 10−5 m forskolin (Fsk) for 10 min and then exposed to 10−5 m carbachol (A) and 100 nm PMA (B) for 2 min. Cells not exposed to stimuli were exposed to isoproterenol for 3 min and forskolin for 12 min. Cell lysates were subjected to immunoblot analysis as indicated. C, shown is quantification of ERK phosphorylation relative to the basal control (no addition). n = 7–12. **, p < 0.01 versus basal. *, p < 0.01 versus stimulus control.
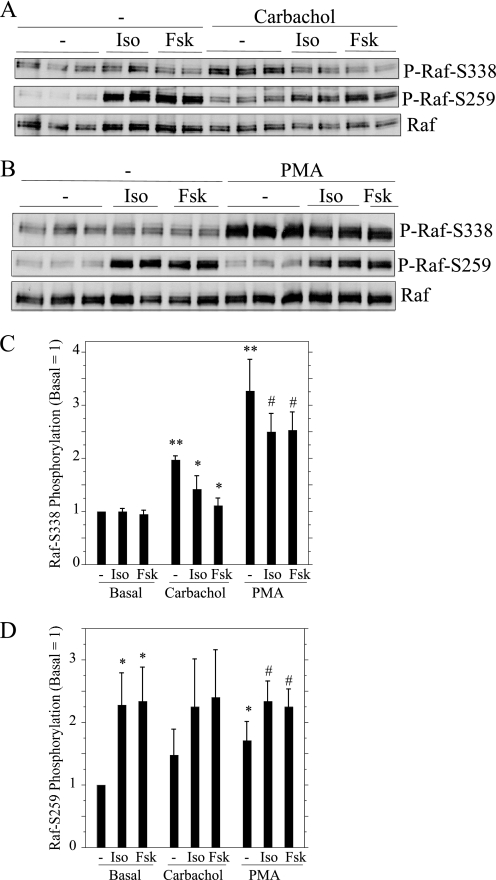
We also examined the effects of isoproterenol and forskolin on the phosphorylation of RAF, the kinase immediately upstream of MEK in the ERK signaling cascade. The regulation of RAF is complicated, as there are multiple sites that can be phosphorylated, and phosphorylations can have positive or negative effects on RAF activity (35, 36). As an indication of changes in RAF activity, we examined the phosphorylation of RAF on two sites: Ser-338, a site which when phosphorylated increases RAF activity, and Ser-259, a site that blocks RAF activity when phosphorylated (37). Because the phospho-RAF-Ser-259 antibody recognized a nonspecific band immediately below RAF in cell lysates (not shown), we evaluated changes in RAF phosphorylation in RAF immunoprecipitates. Carbachol and PMA increased the phosphorylation of RAF on Ser-338, and this phosphorylation was reduced in the prior presence of isoproterenol and forskolin (Fig. 4, A and B). When added alone, isoproterenol and forskolin increased the phosphorylation of RAF on Ser-259. The quantification of the effects of isoproterenol and forskolin on the two RAF phosphorylation sites is shown in Fig. 4, C and D.
FIGURE 4.
Effects of isoproterenol (Iso) and forskolin (Fsk) on the phosphorylation of RAF in carbachol- and PMA-treated rat parotid acinar cells. A and B, cells were exposed to agents as in Fig. 3. Cell lysates were used to immunoprecipitate c-RAF-1, which was subjected to immunoblot analysis using two different phospho-RAF antibodies and c-RAF-1, as indicated. C and D, quantification of RAF phosphorylation on Ser-338 (C) and Ser-259 (D) were calculated relative to the basal control (no additions). For C, n = 6–7. **, p < 0.01 versus basal; *p < 0.01 versus carbachol control; #, p < 0.03 versus PMA control. For D, n = 5. *, p < 0.03 versus basal control; #, p < 0.01 versus basal control.
cAMP Analogs, but Not Epac-specific Activators, Block ERK Phosphorylation
To demonstrate the role of cAMP in this inhibitory process, we also examined the effects of multiple cAMP analogs on the phosphorylation of ERK by carbachol (Fig. 5A). Dibutyryl-cAMP and 8-CPT-cAMP were as effective as forskolin and isoproterenol in increasing PKA substrate phosphorylation and blocking ERK phosphorylation by carbachol. 8-Br-cAMP had little affect on the increase in ERK phosphorylation by carbachol, consistent with the modest effect that it produced on the appearance of putative PKA substrate bands.
FIGURE 5.
Effect of PKA and Epac activators on the phosphorylation of PKA substrates and ERK in rat parotid acinar cells. A, cells were exposed to 8-Br-cAMP (Br, 1 mm, 15 min), 8-CPT-cAMP (CPT, 1 mm, 15 min), dibutyryl-cAMP (db, 1 mm, 15 min), isoproterenol (Iso, 10−7 m, 1 min), and forskolin (Fsk, 10−5 m, 1 min) followed by exposure to carbachol (10−5 m, 2 min). Lysates were subjected to immunoblot analysis as indicated. B, cells were exposed to 8-CPT-2′-Me-cAMP (CPT2, 100 μm, 15–30 min), 8-pMeOPT-2′-O-Me-cAMP (OPT, 100 μm, 30 min), and isoproterenol (Iso, 10−7 m, 1 min) followed by exposure to carbachol (10−5 m, 2 min). Lysates were subjected to immunoblot analysis as indicated. C, quantification of changes in ERK phosphorylation. Shown are the values for basal, carbachol (10−5 m, 2 min), and agents added before carbachol as in A and B in addition to 6-Bnz-cAMP (10 μm, 20 min). 8-CPT-2-Me is 8-CPT-2′-O-Me-cAMP; 8-pMeOPT is 8-pMeOPT-2′-O-Me-cAMP. Changes in ERK phosphorylation are presented relative to the basal control (no additions). Numbers in parentheses indicate the number of experiments. *, p < 0.05 versus carbachol alone.
Because some stimuli and analogs that activate cAMP can also activate Epac1 and Epac2, we used two Epac-selective activators to determine whether these guanine nucleotide exchange factors played a role in the inhibitory effects of isoproterenol and cAMP. Neither 8-pMeOPT-2′-O-Me-cAMP nor 8-CPT-2′-O-Me-cAMP had an inhibitory effect on the basal phosphorylation of ERK (not shown) or on the increase in ERK phosphorylation by carbachol when parotid acinar cells were pretreated with these agents for 15–30 min (Fig. 5B). Also, unlike isoproterenol, these Epac activators did not produce detectible increases in phosphorylated PKA substrate bands. We also employed 6-Bnz-cAMP, a cAMP mimetic that is selective for PKA and does not activate Epac (38), for the purpose of defining the mechanism by which cAMP blocks ERK activation by carbachol. 6-Bnz-cAMP was as effective as isoproterenol in both increasing the phospho-PKA substrate bands (not shown) and blocking the carbachol-promoted ERK phosphorylation (Fig. 5C). A comparison of the relative effects of all of these cAMP analogs and stimuli on the block of ERK phosphorylation is shown in Fig. 5C.
PKA Inhibitors Affect ERK Phosphorylation by Carbachol
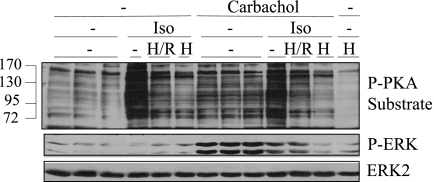
The inhibitory effect of 6-Bnz-cAMP and the lack of effect of Epac activators suggested that cAMP acts on ERK phosphorylation via PKA, so we performed several experiments using PKA inhibitors to try to block the inhibitory effects of cAMP on ERK. Parotid acinar cells treated with a combination of H-89 (10 μm) and Rp-cAMPS (30 μm), an inhibitor combination that was effective in blocking different PKA-mediated effects (34) in mouse parotid acinar cells (14), did not restore the carbachol-promoted increase in ERK phosphorylation in cells treated with isoproterenol (Fig. 6). Most but not all of the phospho-PKA substrate bands were reduced by this inhibitor combination. A 3-fold larger concentration of H-89 (30 μm) was even more effective in blocking increases in phospho-PKA substrate bands in Western blot analysis. However, this produced a substantial inhibition of the increase in ERK phosphorylation produced by carbachol in the absence of isoproterenol, an effect that was probably due to the off-target inhibitions that H-89 can have on other protein kinases (39). This suggests that the lower H-89 concentration (10 μm) may have been ineffective in restoring the carbachol-promoted ERK phosphorylation in isoproterenol-treated cells because it also had a degree of off-target effects, and/or it did not fully inhibit PKA.
FIGURE 6.
Effect of PKA inhibitors on PKA substrate phosphorylation and ERK phosphorylation in rat parotid acinar cells. Where indicated, cells were exposed to 10 μm H-89/30 μm Rp-cAMPs (H/R) or 30 μm H-89 (H) for 20 min, and then exposed to isoproterenol (10−5 m) for 1 min followed by carbachol (10−5 m) for 2 min. Lysates were subjected to immunoblot analysis as indicated.
Isoproterenol Blocks the Phosphorylation of ERK Promoted by P2X7 and EGF Receptor Stimulation
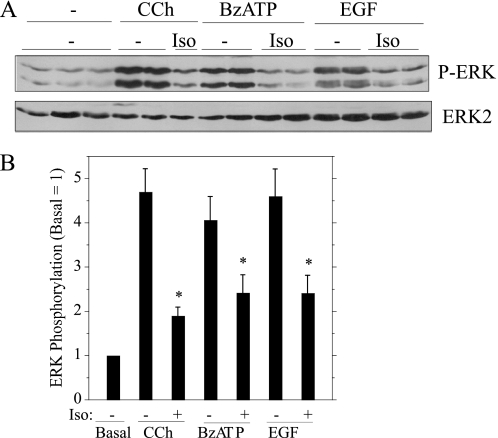
To determine whether the inhibitory effect of isoproterenol on ERK was selective for muscarinic receptor stimulation, we examined whether it blocked ERK phosphorylation when we stimulated other types of receptors. BzATP, which activates P2X7 receptors, and EGF, which activates the EGF receptor, increase ERK phosphorylation in rat parotid acinar cells (23). As was the case for muscarinic receptor stimulation and PMA, isoproterenol reduced the phosphorylation of ERK when P2X7 and EGF receptors were stimulated (Fig. 7). These results demonstrate that isoproterenol has an inhibitory effect on ERK phosphorylation downstream of very different types of receptors (see “Discussion”).
FIGURE 7.
Isoproterenol blocks ERK phosphorylation downstream of different types of receptors in rat parotid acinar cells. A, cells were exposed to isoproterenol (Iso) (10−7 m) for 1 min followed by carbachol (CCh, 10−5 m), BzATP (10 μm), and EGF (100 ng/ml) for 2 min. Lysates were subjected to immunoblot analysis as indicated. B, shown are quantifications of changes in ERK phosphorylation relative to the basal control (no additions). n = 6–8. *, p < 0.01.
Effects of Isoproterenol and Forskolin on Other Salivary Gland Cells
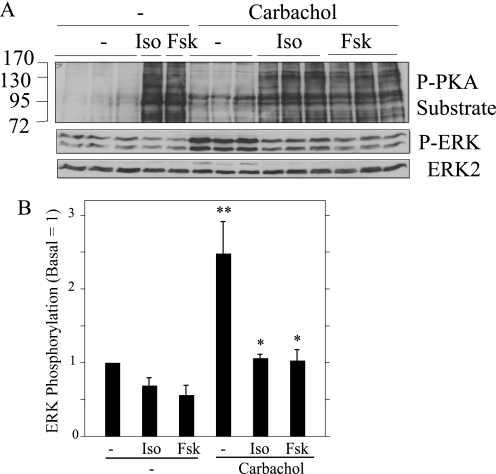
We examined several other salivary gland cell systems to determine whether isoproterenol/cAMP-elevating stimuli blocked the stimulatory effect of carbachol on ERK phosphorylation in other cells. Freshly dispersed acinar cells prepared from rat submandibular glands displayed responses very similar to those of native parotid acinar cells; isoproterenol and forskolin produced significant increases in bands identified using a phospho-PKA substrate antibody, and both these agents blocked the carbachol-promoted increases in ERK phosphorylation (Fig. 8).
FIGURE 8.
Isoproterenol and forskolin block the phosphorylation of ERK by carbachol in rat submandibular acinar cells. A, cells were pretreated (or not) with isoproterenol (Iso, 10−5 m) and forskolin (Fsk, 10−5 m) for 1 min and then exposed to carbachol (10−5 m, 2 min). Cells not exposed to carbachol were exposed to isoproterenol and forskolin for 3 min. Lysates were subjected to immunoblot analysis as indicated. B, shown is quantification of the effects of isoproterenol and forskolin on ERK phosphorylation relative to the basal control (no additions). n = 3. *, p < 0.05 versus carbachol (no addition). **, p < 0.05 versus basal.
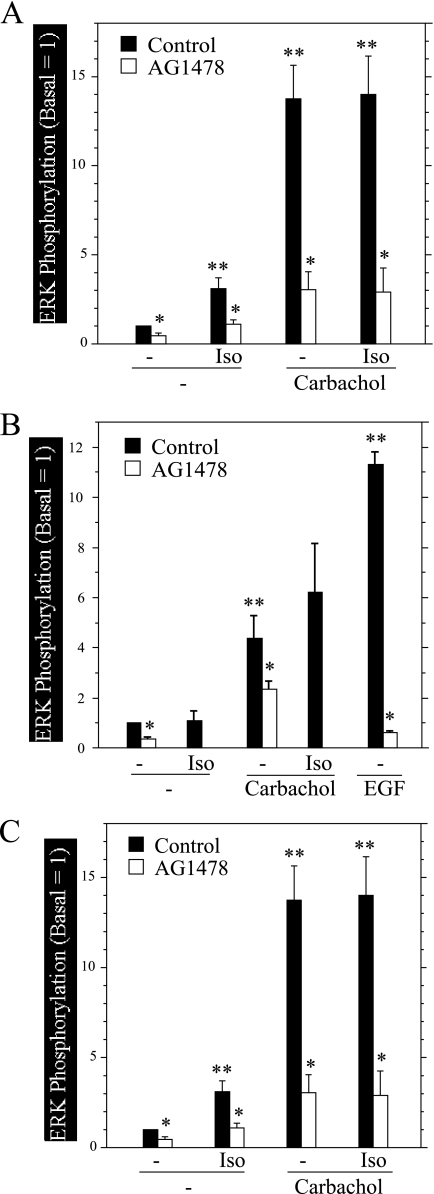
HSY cells are a salivary cell line derived from a human parotid adenocarcinoma of intercalated ductal origin. In these cells isoproterenol did not block the increase in ERK phosphorylation produced by carbachol and itself produced an increase in ERK phosphorylation above basal levels (Fig. 9A). Because it was reported that isoproterenol increased ERK phosphorylation in HSY cells via transactivating the EGFR (40), we investigated the effects of the EGFR inhibitor AG1478 on ERK phosphorylation under various conditions. In addition to reducing the stimulatory effect of isoproterenol on ERK phosphorylation, AG1478 reduced ERK phosphorylation under basal conditions and in cells exposed to carbachol (+isoproterenol), indicating that EGFR activity is critical to the activation of ERK under multiple conditions in these cells.
FIGURE 9.
Effects of isoproterenol, forskolin, and AG1478 on the phosphorylation of ERK by carbachol in rat and human salivary gland cell lines. Cells were exposed to AG1478 (300 nm) for 20 min before stimuli and to isoproterenol (Iso, 10−4 m) and forskolin (10−5 m) for times as indicated below. Cells were exposed to carbachol (10−4 m) and EGF (100 ng/ml) for 3 min. A, HSY cells were treated (or not) with isoproterenol (2 min) followed by carbachol. Cells not exposed to carbachol were treated with isoproterenol for 5 min. n = 3. *, p < 0.05 versus the same conditions without AG1478. **, p < 0.05 versus basal control. B, HSG cells were treated (or not) with isoproterenol for 12 min followed by carbachol. Cells not exposed to carbachol were treated with isoproterenol for 15 min. n = 3–7. *, p < 0.01 versus the same conditions without AG1478. **, p < 0.01 versus basal control. C, C10 cells were treated (or not) with isoproterenol (2 min) and forskolin (10 min) and then exposed to carbachol. Cells not exposed to carbachol were exposed to isoproterenol for 5 min and forskolin for 15 min. n = 3–10. *, p < 0.03 versus same condition with AG1478. p < 0.03 versus basal control.
HSG cells are a neoplastic human submandibular gland intercalated duct cell line. In these cells isoproterenol did not produce an increase in ERK phosphorylation, but it also did not block the carbachol-promoted increases in ERK phosphorylation (Fig. 9B). Because the EGFR activity contributed to ERK phosphorylation in HSY cells, we also examined the effect of AG1478 on HSG cells. Inhibition of the EGFR activity was fully effective in blocking the actions of EGF and partially reduced the basal ERK phosphorylation and the stimulation of ERK phosphorylation by carbachol.
Par-C10 cells are a simian virus 40-transformed cell line derived from rat parotid acinar cells, and they display many signaling and ion transport properties of native rat parotid acinar cells (29). Similar to native rat salivary cells, isoproterenol and forskolin treatment did not affect basal ERK phosphorylation; however, in contrast to native rat cells, these agents did not block the increase in ERK phosphorylation by carbachol (Fig. 9C). In addition to fully blocking the effects of EGF, AG1478 produced a near-complete block of ERK phosphorylation under basal conditions and in cells treated with carbachol. Notably, the same concentration of AG1478 (300 nm) used in these studies for all three salivary cell lines did not block the increase in ERK phosphorylation by carbachol (32), demonstrating an important distinction between cell signaling in native and immortalized cells.
Isoproterenol Increases Intracellular Ca2+ and Fluid Secretion-related Events
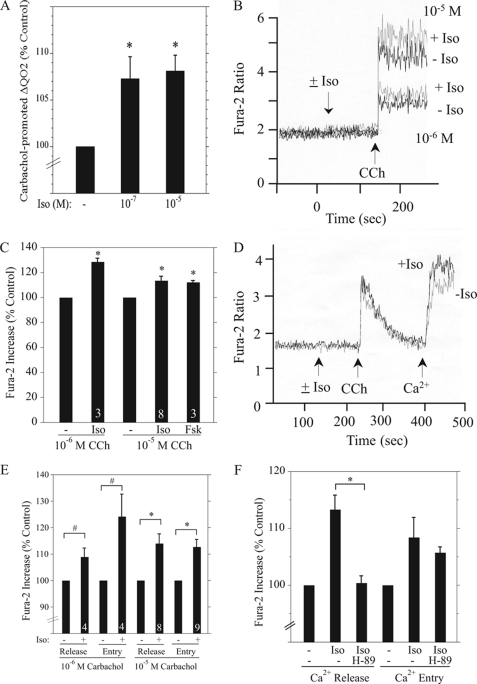
In direct contrast to the negative effects that isoproterenol has on ERK signaling downstream of the muscarinic receptor in native rat salivary cells, isoproterenol produced positive effects on several physiological indices that are initiated downstream of this receptor. The exposure of rat parotid acinar cells to 10−7 and 10−5 m isoproterenol caused significant increases in the carbachol-promoted increase in the O2 consumption rate (ΔQO2) (Fig. 10A). These responses to isoproterenol represent an enhancement of fluid and electrolyte secretion initiated by muscarinic receptor stimulation (see “Discussion”).
FIGURE 10.
Isoproterenol enhances carbachol-promoted increases in QO2 and [Ca2+]i in rat parotid acinar cells. A, shown are increases (ΔQO2) in the rate of O2 consumption of parotid cells exposed to 10−5 m carbachol in cells pretreated (or not) with 10−7 m and 10−5 m isoproterenol (Iso) for 2 min before carbachol. The rates in the presence of isoproterenol were normalized to the control ΔQO2 value (18.9 ± 0.4 nmol of O2/mg of protein/min, n = 3) in the presence of carbachol alone (−). n = 3. *, p < 0.05. B, increases in intracellular Ca2+ in cells suspended in Ca2+-containing Solution A and stimulated with 10−6 and 10−5 m carbachol (CCh) in the presence (+Iso) or absence (−Iso) of 10−5 m isoproterenol. Shown are individual traces from one experiment. The ordinate axis is the 340/380-nm Fura-2 fluorescence excitation ratio. C, cumulative data show the stimulatory effects of isoproterenol (10−5 m) and forskolin (10−5 m) on the initial peak Ca2+ increase produced by carbachol (10−6 m, 10−5 m), as shown in B. Data were normalized to the increases in the Fura-2 ratio produced by carbachol alone (10−6 m, 1.06 ± 0.12, n = 3; 10−5 m, 2.34 ± 0.13, n = 8). Numbers at the bottom of the bars indicate number of experiments. *, p < 0.01 versus control. D, shown is the stimulatory effect of isoproterenol (10−5 m) on intracellular Ca2+ release promoted by 10−5 m carbachol in Fura-2-loaded cells suspended in Ca2+-free Solution A and the entry of Ca2+ when CaCl2 (1 mm) was added to cells after depletion of Ca2+ stores. Shown are individual traces from one experiment. The ordinate axis is the 340/380-nm Fura-2 fluorescence excitation ratio. E, cumulative data show the stimulatory effects of isoproterenol (10−5 m) on the peak Ca2+ increase due to carbachol-promoted Ca2+ release and the subsequent Ca2+ peak due to Ca2+ entry as shown in D. Increases in the Fura-2 ratio from isoproterenol-treated cells were normalized to the increases produced in the absence of isoproterenol (10−6 m, release, 0.74 ± 0.05, n = 4; entry, 1.32 ± 0.21, n = 4; 10−5 m, release, 1.28 ± 0.10, n = 8; entry, 2.05 ± 0.13, n = 9). *, p < 0.01 versus control; #, p < 0.05 versus control. F, cumulative data show the effect of treatment of cells with H-89 (2 μm, 10 min) before initiating the stimulatory effect of isoproterenol on the carbachol (10−5 m)-promoted Ca2+ release and uptake as shown in D. *, p < 0.05. n = 4.
Because fluid secretion by salivary glands is initiated by increases in [Ca2+]i, we also examined the effect of isoproterenol on Ca2+ signaling in rat parotid acinar cells. Isoproterenol (10−5 m) did not change the basal level of [Ca2+]i, but it enhanced the rapid initial elevation of [Ca2+]i (as measured by the changes in the Fura-2 fluorescence excitation ratio) produced by two different concentrations of carbachol (Fig. 10B). Forskolin also enhanced the elevation of [Ca2+]i by 10−5 m carbachol, similar to that of isoproterenol (Fig. 10C), suggesting that these effects were mediated by cAMP. To determine whether these effects were due to the actions of isoproterenol on Ca2+ release or Ca2+ entry, we conducted experiments using parotid cells suspended in Ca2+-free solution to prevent Ca2+ entry. Isoproterenol increased the carbachol-promoted release of Ca2+from intracellular stores, but the cells also displayed enhanced Ca2+ entry when extracellular Ca2+ was added after Ca2+ store depletion (Fig. 10, D and E). To determine whether these isoproterenol effects were mediated by PKA, we repeated these experiments in the presence of the PKA inhibitor H-89 (2 μm). H-89 blocked the isoproterenol-enhanced release of Ca2+ by carbachol (Fig. 10F). However, a significant effect of H-89 on the increase in Ca2+ entry was not observed, most likely due to the relatively small isoproterenol-enhanced increase in Ca2+entry produced in this set of experiments.
DISCUSSION
The present study demonstrates a new aspect of receptor cross-talk in salivary gland acinar cells; cAMP production and signaling proteins downstream of the β-adrenergic receptor exert a significant negative effect on the activation of ERK by M3 muscarinic receptor stimulation. This effect is in contrast to various studies that demonstrated that cAMP/PKA enhances [Ca2+]i elevation and fluid secretion, which are important physiological events downstream of the muscarinic receptor in salivary glands. Acetylcholine, the natural ligand, or carbachol, a cholinergic agonist, bind to the M3 muscarinic receptor in salivary gland acinar cells and promote the production of InsP3 and diacylglycerol, second messengers that affect multiple cellular responses. InsP3 binds to InsP3 receptors on the endoplasmic reticulum, causing the release of stored Ca2+, followed by the entry of Ca2+ across the plasma membrane via proteins contributing to store-operated Ca2+ entry (41). This increases [Ca2+]i, which opens Ca2+-sensitive ion channels and commences the ion movements that result in net fluid and electrolyte secretion to initiate salivation (3). The production of diacylglycerol increases the activity of PKC proteins, and these mediate increases in ERK phosphorylation and activity in parotid acinar cells (30, 42) and many other cells. Notably, the two contrasting actions of isoproterenol (that is, the reduction of the M3R-promoted ERK phosphorylation and the enhancement of the M3R-initiated increase in [Ca2+]i) demonstrate that cAMP has opposite effects on effectors downstream of the two second messengers (diacylglycerol, InsP3) that are produced from M3R-initiated PIP2 hydrolysis. These contrasting effects also indicate that the negative effect of isoproterenol on ERK is not initiated at the level of the M3 receptor in parotid cells, although cAMP can down-regulate some muscarinic receptor subtypes (43). Of interest to our results, recent studies indicate that the signaling downstream of the M3 muscarinic receptor can be complex. Multiple arrestins, G-protein-coupled receptor kinase proteins, and casein kinase 1α can regulate muscarinic receptor-mediated increases in intracellular Ca2+ and ERK phosphorylation in a differential manner (44). The knockdown of these proteins indicated that some regulators, including GRK2, exerted a negative regulation of both ERK and intracellular Ca2+, whereas others were selective for Ca2+.
In addition to blocking ERK and MEK, forskolin and isoproterenol altered RAF phosphorylation in inverse fashion on two known regulatory sites. These cAMP-elevating agents produced a reduction in the carbachol-promoted increase in phosphorylation of RAF on Ser-338, a site within its catalytic domain and one that is required to be phosphorylated for its activation (37). Phosphorylation of RAF on Ser-338 is increased by the stimulation of other G protein-coupled receptors and tyrosine kinase receptors (35, 45), and previously we reported that carbachol increases the phosphorylation of this site in parotid acinar cells (31). In contrast, isoproterenol and forskolin increased the phosphorylation of RAF on Ser-259, a negative regulatory site. cAMP promotes PKA-mediated increases in both Ser-259 and Ser-223 phosphorylation, which stimulates the binding of 14-3-3 and keeps RAF in an inactive state by preventing its recruitment to the plasma membrane (46). When PMA was employed as a stimulus to increase ERK phosphorylation in parotid acinar cells, isoproterenol and forskolin also had inhibitory effects on ERK, MEK, and RAF-Ser-338 phosphorylation, although the relative inhibition was somewhat less than that when carbachol was the stimulus. The inhibitory actions of isoproterenol and other cAMP-elevating stimuli on ERK phosphorylation in carbachol-treated cells may be due to a combination of the increase in Ser-259-RAF phosphorylation and the block of the phosphorylation of Ser-338-RAF. However, a possibility remains that the inhibition of ERK phosphorylation is at least partly independent of RAF, as PKC can activate ERK directly via MEK rather than RAF in some cells (47). ERK phosphorylation by carbachol is nearly completely blocked by inhibition of PKC in parotid cells (23), indicating that PKC is a major upstream regulator of ERK phosphorylation.
Isoproterenol blocked the phosphorylation of ERK downstream of three very different types of receptors, indicating the broad effectiveness of cAMP as an inhibitor of ERK signaling. The P2X7 receptor is a non-selective ion channel that activates some of the same signaling pathways as the G-protein-coupled muscarinic receptor in parotid acinar cells (23), and the EGF receptor is a tyrosine kinase. The actions of isoproterenol on these three different kinds of receptors suggest that the site of inhibition is located at a common part of the ERK signaling cascade that is activated by all three stimuli; most likely, RAF.
PKA can have either positive or negative effects on ERK activity, and it can have affects on multiple proteins that are upstream of ERK signaling (for review, see Ref. 27). cAMP and PKA can have diverse affects on the ERK signaling cascades, and this varies with cell type; therefore, the actions of PKA on ERK signaling are controversial. Forskolin can increase and potentiate the stimulation of various agents on ERK phosphorylation (48), effects that are very different from that on rat parotid acinar cells. In native rat parotid cells, the effects of isoproterenol and forskolin on RAF were consistent, and we did not examine the effects of cAMP-producing stimuli upstream of RAF. Although Epac is involved in many biological and signaling processes, including those in parotid acinar cells, the Epac-selective cAMP analogs were ineffective in reproducing the inhibitory effects of multiple cAMP mimetics and other stimuli on ERK phosphorylation by carbachol (Fig. 5). These compounds are specific for the guanine nuclear exchange factors Epac1 and Epac2 because their structures are highly selective for the cAMP binding domain of Epac compared with the cAMP binding domain of PKA (6, 49). Epac may promote the activation of ERK in some cells (27), but the treatment of rat parotid acinar cells to the Epac analogs alone did not increase ERK phosphorylation. These results are consistent with the inhibitory effects of isoproterenol, forskolin, 8-CPT-cAMP, and dibutyryl-cAMP being mediated by PKA. Moreover, 6-Bnz-cAMP, which activates PKA but not Epac (38), produced inhibitory effects identical to those of the other effective agents, supporting the role of PKA in blocking the phosphorylation of ERK downstream of muscarinic, P2X7, and EGF receptor activation.
Isoproterenol produced three distinct effects on different salivary cell preparations; it inhibited the carbachol-promoted stimulation of ERK phosphorylation in native rat parotid and submandibular acinar cells, it was without effect on carbachol-stimulated ERK phosphorylation in HSG and Par-C10 cells, and it itself stimulated ERK phosphorylation and did not block the stimulation by carbachol in HSY cells. It is unlikely that these varied responses are due to differences in muscarinic receptor subtypes, a family of 5 (M1-M5) isoforms that can all be stimulated by carbachol, a non-selective muscarinic agonist. Native rat parotid acinar cells have >90% M3R and small populations of other subtypes (2, 50, 51). M3Rs also make up the overwhelming majority of muscarinic receptors in HSG cells (52) and Par-C5 cells (50), a rat parotid acinar cell line that is very similar to the Par-C10. However, HSY cells have a mixed population of M1 and M3 receptors, and both receptor subtypes contribute to the stimulation of ERK (53). It was observed previously that the stimulation of ERK by carbachol (53) and isoproterenol (40) in HSY cells are EGFR-dependent, consistent with our finding that AG1478 was also effective on the combination of carbachol and isoproterenol. In fact, the EGFR plays a critical role in ERK signaling in all three cell lines under various stimulated and non-stimulated conditions. In contrast to these findings, the increase in ERK phosphorylation in carbachol-treated native rat parotid acinar cells does not involve the EGFR and is not sensitive to AG1478 (32). This is one illustration of how normal cell signaling can differ between native cells and cell lines, indicating that care must be used in selecting a model cell system that represents native tissue.
Parotid cells respond to secretagogues with rapid increases in the rate of O2 consumption (ΔQO2) due to increases in oxidative metabolism to support the increased energy demands of active ion transport, particularly the ATP demand of the stimulation of the Na,K-ATPase; therefore, this change in O2 consumption is reflective of an increase in fluid and electrolyte secretion (28, 54). The similar enhancing effects that 10−5 and 10−7 m isoproterenol had on the carbachol-promoted ΔQO2 (Fig. 10A) are consistent with the similar increases that these concentrations produced on PKA substrate phosphorylation and in the reduction of the carbachol-initiated ERK phosphorylation (Fig. 1C). Not surprisingly, isoproterenol produced similarly modest (∼10–20%) increases in the changes in [Ca2+]i and O2 consumption initiated by 10−5 m carbachol, consistent with the dependence of ion movement on [Ca2+]i.
The isoproterenol and forskolin enhancements of the carbachol-promoted increases in [Ca2+]i are likely due to the PKA-mediated increased release of Ca2+ from internal Ca2+ stores, as this added release of Ca2+ was blocked by H-89 (Fig. 10F). The stimulatory effects of isoproterenol and forskolin on [Ca2+]i (and ΔQO2) are likely due to the phosphorylation of the InsP3 receptor by PKA. In mouse parotid acinar cells, forskolin promoted the phosphorylation of the InsP3 receptor, and dibutyryl-cAMP enhanced the InsP3-initiated release of Ca2+ from stores in the endoplasmic reticulum (14). Subsequent studies determined that this effect was mediated by the PKA-promoted phosphorylation of Ser-937 on the InsP3 receptor 2 isoform (16). Similar to our results for isoproterenol (Fig. 10, D and E), Bruce et al. (14) reported that forskolin also promoted an increase in Ca2+ entry, and suggested that this may be due to the larger release/depletion of Ca2+ stores under these conditions. On a different note, it recently was reported that cAMP generation and PKA activation can result from the depletion of Ca2+ stores in the endoplasmic reticulum, and the activation of adenylyl cyclase was dependent on stromal interaction molecule 1 (STIM1), the endoplasmic reticulum Ca2+ sensor that plays an integral role in store-operated Ca2+ entry (55).
Inhibitory effects of cAMP on ERK phosphorylation were also found in vasopressin-treated rat renal intermedullary cortical ducts. The binding of a vasopressin analog (dDAVP) to the V2 receptor in intermedullary cortical duct cells reduced the (basal) phosphorylation of ERK and MEK and increased the phosphorylation of RAF on Ser-259, and these effects were mimicked by a cAMP analog (56). dDVAP did not affect the phosphorylation of RAF on Ser-338, but it also accelerated the appearance of aperiodic spikes in [Ca2+]i, which were due to release of Ca2+ by the ryanodine receptor. Thus, there are parallels in intermedullary cortical ducts between some, but not all, of the responses to isoproterenol that we observed in parotid acinar cells.
In conclusion, the present studies document the inhibitory effect of isoproterenol and cAMP on ERK activation by carbachol in rat parotid and submandibular acinar cells. This suggests that the release of neurotransmitter from sympathetic nerves can prevent the activation of ERK by parasympathetic nerve stimulation. The effect on ERK appears to be due, at least in part, to changes in the phosphorylation of RAF on two sites that regulate RAF activity, Ser-259 and Ser-338, which are negative and positive sites, respectively. This negative regulation of ERK occurs in the same cell in which isoproterenol produces a positive effect on physiological events that promote and support muscarinic receptor-promoted fluid secretion. These studies demonstrate that there are multiple interactions between the second messengers and signaling proteins downstream of the β-adrenergic and muscarinic receptors and that the β-adrenergic receptor can exert both positive and negative effects on processes downstream of muscarinic receptor activation. In addition, isoproterenol also blocked increases in ERK phosphorylation downstream of the P2X7 receptor/ion channel and the EGF receptor. The results demonstrate that cAMP can inhibit ERK activation by a wide variety of receptor types. Our results also indicate that there are important differences between native salivary gland acinar cells and immortalized and transformed salivary cells regarding how second messengers affect signal transduction proteins.
This work was supported, in whole or in part, by National Institutes of Health Grant DE-10877 (NIDCR).
- M3R
- muscarinic M3 receptor
- 6-Bnz-cAMP
- N6-benzoyl-cAMP
- 8-Br-cAMP
- 8-bromo-cAMP
- BzATP
- 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate
- [Ca2+]i
- intracellular calcium concentration
- 8-CPT-cAMP
- 8-(4-chlorophenylthio)-2′-O-cAMP
- 8-CPT-2′-Me-cAMP
- 8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- Epac
- exchange proteins directly activated by cAMP
- ERK
- extracellular signal-related kinase
- H-89
- N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide
- InsP3
- inositol 1,4,5-trisphosphate
- MEK
- mitogen-activated protein kinase/ERK kinase
- 8-pMeOPT-2′-O-Me-cAMP
- 8-(4-methoxyphenylthio)-2′-O-methyl-cAMP
- PKA
- protein kinase A
- PKC
- protein kinase C
- PMA
- phorbol 12-myristate 13-acetate
- QO2
- oxygen consumption rate
- Rp-cAMPS
- Rp-adenosine-3′,5′-cyclic monophosphothioate.
REFERENCES
- 1.Proctor G. B., Carpenter G. H. (2007) Auton. Neurosci. 133, 3–18 [DOI] [PubMed] [Google Scholar]
- 2.Dai Y. S., Ambudkar I. S., Horn V. J., Yeh C. K., Kousvelari E. E., Wall S. J., Li M., Yasuda R. P., Wolfe B. B., Baum B. J. (1991) Am. J. Physiol. 261, C1063–C1073 [DOI] [PubMed] [Google Scholar]
- 3.Melvin J. E., Yule D., Shuttleworth T., Begenisich T. (2005) Annu. Rev. Physiol. 67, 445–469 [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura K., Murakami M., Segawa A. (2000) J. Physiol. 522, 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhuri A., Husain S. Z., Kolodecik T. R., Grant W. M., Gorelick F. S. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1403–D1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holz G. G., Kang G., Harbeck M., Roe M. W., Chepurny O. G. (2006) J. Physiol. 577, 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimomura H., Imai A., Nashida T. (2004) Arch. Biochem. Biophys. 431, 124–128 [DOI] [PubMed] [Google Scholar]
- 8.Schneyer C. (1962) Am. J. Physiol. 203, 232–236 [DOI] [PubMed] [Google Scholar]
- 9.Schneyer C. A., Humphreys-Beher M. (1989) Cell Tissue Res. 256, 361–363 [DOI] [PubMed] [Google Scholar]
- 10.Ten Hagen K., Balys M. M., Tabak L. A., Melvin J. E. (2002) Physiol. Genomics 28, 107–114 [DOI] [PubMed] [Google Scholar]
- 11.Mazariegos M. R., Hand A. R. (1984) J. Dent. Res. 63, 1102–1107 [DOI] [PubMed] [Google Scholar]
- 12.Paulais M., Turner R. J. (1992) J. Biol. Chem. 267, 21558–21563 [PubMed] [Google Scholar]
- 13.Yamada K., Inoue H., Kida S., Masushige S., Nishiyama T., Mishima K., Saito I. (2006) Pathobiology 73, 1–7 [DOI] [PubMed] [Google Scholar]
- 14.Bruce J. I., Shuttleworth T. J., Giovannucci D. R., Yule D. I. (2002) J. Biol. Chem. 277, 1340–1348 [DOI] [PubMed] [Google Scholar]
- 15.Tanimura A., Nezu A., Tojyo Y., Matsumoto Y. (1999) Am. J. Physiol. 276, C1282–C1287 [DOI] [PubMed] [Google Scholar]
- 16.Betzenhauser M. J., Fike J. L., Wagner L. E., 2nd, Yule D. I. (2009) J. Biol. Chem. 284, 25116–25125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsson O., Olgart L. (1989) Acta Physiol. Scand. 137, 231–236 [DOI] [PubMed] [Google Scholar]
- 18.Bobyock E., Chernick W. S. (1989) J. Dent. Res. 68, 1489–1494 [DOI] [PubMed] [Google Scholar]
- 19.Martin S. C., Thompson J., Shuttleworth T. J. (1994) Am. J. Physiol. 267, C255–C265 [DOI] [PubMed] [Google Scholar]
- 20.Budd D. C., Challiss R. A., Young K. W., Tobin A. B. (1999) Mol. Pharmacol. 56, 813–823 [PubMed] [Google Scholar]
- 21.Watson E. L., Jacobson K. L., DiJulio D. H., Dowd F. J. (1993) Am. J. Physiol. 265, C1061–C1068 [DOI] [PubMed] [Google Scholar]
- 22.Laniyonu A., Sliwinski-Lis E., Fleming N. (1990) Eur. J. Pharmacol. 188, 171–174 [DOI] [PubMed] [Google Scholar]
- 23.Bradford M. D., Soltoff S. P. (2002) Biochem. J. 366, 745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dang H., Elliott J. J., Lin A. L., Zhu B., Katz M. S., Yeh C. K. (2008) Differentiation 76, 546–557 [DOI] [PubMed] [Google Scholar]
- 25.Handra-Luca A., Bilal H., Bertrand J. C., Fouret P. (2003) Am. J. Pathol. 163, 957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashimata M., Sayeed S., Ka A., Onetti-Muda A., Sakagami H., Faraggiana T., Gresik E. W. (2000) Dev. Biol. 220, 183–196 [DOI] [PubMed] [Google Scholar]
- 27.Gerits N., Kostenko S., Shiryaev A., Johannessen M., Moens U. (2008) Cell. Signal. 20, 1592–1607 [DOI] [PubMed] [Google Scholar]
- 28.Soltoff S. P., McMillian M. K., Cantley L. C., Cragoe E. J., Jr., Talamo B. R. (1989) J. Gen. Physiol. 93, 285–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner J. T., Redman R. S., Camden J. M., Landon L. A., Quissell D. O. (1998) Am. J. Physiol. 275, C367–C374 [DOI] [PubMed] [Google Scholar]
- 30.Benes C., Soltoff S. P. (2001) Am. J. Physiol. Cell Physiol. 280, C1498–C1510 [DOI] [PubMed] [Google Scholar]
- 31.Soltoff S. P., Hedden L. (2008) Am. J. Physiol. Cell Physiol. 295, C590–C599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plourde D., Soltoff S. P. (2006) Am. J. Physiol. Cell Physiol. 290, C702–C710 [DOI] [PubMed] [Google Scholar]
- 33.Soltoff S. P. (2004) J. Biol. Chem. 279, 10910–10918 [DOI] [PubMed] [Google Scholar]
- 34.Fuller C. M., Gallacher D. V. (1984) J. Physiol. 356, 335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dougherty M. K., Müller J., Ritt D. A., Zhou M., Zhou X. Z., Copeland T. D., Conrads T. P., Veenstra T. D., Lu K. P., Morrison D. K. (2005) Mol. Cell 17, 215–224 [DOI] [PubMed] [Google Scholar]
- 36.Wellbrock C., Karasarides M., Marais R. (2004) Nat. Rev. Mol. Cell Biol. 5, 875–885 [DOI] [PubMed] [Google Scholar]
- 37.Dumaz N., Marais R. (2005) FEBS J. 272, 3491–3504 [DOI] [PubMed] [Google Scholar]
- 38.Christensen A. E., Selheim F., de Rooij J., Dremier S., Schwede F., Dao K. K., Martinez A., Maenhaut C., Bos J. L., Genieser H. G., Døskeland S. O. (2003) J. Biol. Chem. 278, 35394–35402 [DOI] [PubMed] [Google Scholar]
- 39.Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh C. K., Ghosh P. M., Dang H., Liu Q., Lin A. L., Zhang B. X., Katz M. S. (2005) Am. J. Physiol. Cell Physiol. 288, C1357–C1366 [DOI] [PubMed] [Google Scholar]
- 41.Cahalan M. D. (2009) Nat. Cell Biol. 11, 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradford M. D., Soltoff S. P. (1998) Eur. J. Morphol. 36, 176–180 [PubMed] [Google Scholar]
- 43.Lee K. M., Toscas K., Villereal M. L. (1993) J. Biol. Chem. 268, 9945–9948 [PubMed] [Google Scholar]
- 44.Luo J., Busillo J. M., Benovic J. L. (2008) Mol. Pharmacol. 74, 338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beeram M., Patnaik A., Rowinsky E. K. (2005) J. Clin. Oncol. 23, 6771–6790 [DOI] [PubMed] [Google Scholar]
- 46.Dumaz N., Marais R. (2003) J. Biol. Chem. 278, 29819–29823 [DOI] [PubMed] [Google Scholar]
- 47.Wen-Sheng W. (2006) Cancer Lett. 239, 27–35 [DOI] [PubMed] [Google Scholar]
- 48.Benes C., Roisin M. P., Van Tan H., Creuzet C., Miyazaki J., Fagard R. (1998) J. Biol. Chem. 273, 15507–15513 [DOI] [PubMed] [Google Scholar]
- 49.Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., Bos J. L. (2002) Nat. Cell Biol. 4, 901–906 [DOI] [PubMed] [Google Scholar]
- 50.Bockman C. S., Bradley M. E., Dang H. K., Zeng W., Scofield M. A., Dowd F. J. (2001) J. Pharmacol. Exp. Ther. 297, 718–726 [PubMed] [Google Scholar]
- 51.Ryberg A. T., Warfvinge G., Axelsson L., Soukup O., Götrick B., Tobin G. (2008) Arch. Oral Biol. 53, 66–74 [DOI] [PubMed] [Google Scholar]
- 52.Nagy K., Szlávik V., Rácz G., Ovári G., Vág J., Varga G. (2007) Acta Physiol. Hung 94, 301–313 [DOI] [PubMed] [Google Scholar]
- 53.Lin A. L., Zhu B., Zhang W., Dang H., Zhang B. X., Katz M. S., Yeh C. K. (2008) Am. J. Physiol. Cell Physiol. 294, C1454–C1464 [DOI] [PubMed] [Google Scholar]
- 54.Murakami M., Miyamoto S., Imai Y. (1990) J. Physiol. 426, 127–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lefkimmiatis K., Srikanthan M., Maiellaro I., Moyer M. P., Curci S., Hofer A. M. (2009) Nat. Cell Biol. 11, 433–442 [DOI] [PubMed] [Google Scholar]
- 56.Pisitkun T., Jacob V., Schleicher S. M., Chou C. L., Yu M. J., Knepper M. A. (2008) Am. J. Physiol. Renal Physiol. 295, F1030–F1043 [DOI] [PMC free article] [PubMed] [Google Scholar]