Abstract
Gq-coupled G protein-coupled receptors (GPCR) mediate the actions of a variety of messengers that are key regulators of different cellular functions. These receptors can regulate a highly interconnected network of biochemical routes that control the activity of several members of the mitogen-activated protein kinase (MAPK) family. The ERK5 MAPK has been shown to be activated by Gq-coupled GPCR via unknown mechanisms. We find that the atypical protein kinase C (PKCζ), previously reported to interact with the ERK5 activator MEK5 and to be involved in epidermal growth factor-mediated ERK5 stimulation, plays a crucial role in the activation of the ERK5 pathway by Gq-coupled GPCR. Stimulation of ERK5 by Gq-coupled GPCR is abolished upon pharmacological inhibition of PKCζ as well as in embryonic fibroblasts obtained from PKCζ-deficient mice. Both PKCζ and MEK5 associate to Gαq upon activation of GPCR, thus forming a ternary complex that seems essential for the activation of ERK5. These data put forward a novel function of Gαq as a scaffold protein involved in the modulation of the ERK5 cascade by GPCR that could be relevant in Gq-mediated physiological functions.
Keywords: Adaptor Proteins, G Protein-coupled Receptors (GPCR), G Proteins, MAP Kinases (MAPKs), Signal Transduction, ERK5, G&αq, MEK5, PKC&ζ
Introduction
The activation of the mitogen-activated protein kinase (MAPK)3 superfamily plays an important role in a wide variety of signaling pathways involved in embryogenesis, cell proliferation, differentiation, migration, apoptosis, and gene expression. The MAPK superfamily includes the well known extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK1–3), and p38 (α, β, γ, and δ) families. In addition, ERK3, ERK4, ERK5 (also termed big MAPK1 or BMK1), and ERK7 are other more recently described MAPK family members that display distinct regulatory mechanisms.
A plethora of extracellular stimuli have been found to modulate MAPK cascades (1, 2). Although many aspects remain to be detailed, several studies have described the specific mechanisms by which G protein-coupled receptors (GPCR) can activate the main MAPK families (2–4), which can be modulated by different Gα or βγ subunits, in a stimulus- or context-specific manner (2, 5).
The ERK5 MAPK has been reported to be activated by mitogens (EGF, granulocyte-colony-stimulating factor), agonists of GPCR, cytokines (leukemia inhibitory factor, cardiotrophin-1), and stress (6–8). ERK5 is selectively activated by the upstream kinase MEK5, which in turn is stimulated by MEKK2 and MEKK3, in a process that appears to involve Src tyrosine kinase activation or the scaffolding function of Gab1 or Lck-associated adaptor (LAD) adaptors depending on the stimuli (7, 9). It has also been described that EGF-mediated ERK5 stimulation requires a direct association between PKCζ and MEK5 (10) through their respective PB1 domains (11), in a process that may involve scaffold proteins such as p62, to which both MEK5 and PKCζ can bind (12–14). However, the mechanisms by which GPCR stimulate ERK5 are largely unknown. It has been reported that ERK5 stimulation can be triggered by GPCR coupled to the Gq and G12/13 families of heterotrimeric G proteins independent of Ras, Rho, Rac, and Cdc42 stimulation (15–17), but the biochemical mechanisms involved in this cascade have not been identified.
In this study, we show a novel functional interaction between Gαq, PKCζ, and MEK5 that accounts for the activation of the ERK5 cascade upon Gq-coupled GPCR stimulation. The direct association of Gαq with both PKCζ and MEK5 puts forward these proteins as ”bona fide“ Gq effectors and also reveals for the first time an unforeseen role of Gαq as an adaptor protein that facilitates the recruitment of key players in the ERK5 stimulation cascade.
EXPERIMENTAL PROCEDURES
Materials
The cDNAs of the M1-muscarinic acetylcholine receptor, Gαq, and the constitutively active Gαq-R183C mutant were kindly provided by Dr. Anna Aragay (University of Bergen, Norway). The constitutively active Gαq mutant that lacks the ability to interact with PLCβ (Gαq Q209L/R256A/T257A (Q209L-AA)) was provided By Dr. Richard Lin (Stony Brook University, NY). The cDNAs encoding HA-ERK5, GST-MEK5, GST-MEK5ΔPB1, GST-PKCζPB1, HA-PKCζ, and HA-PKCλ and the purification of recombinant full-length His-PKCζ have been previously described by our laboratories (10). The Gαs, Gα12, and Gαi3 constructs were purchased from the Missouri S&T cDNA Resource Center. Gαq recombinant protein, purified from baculovirus-infected Sf9 insect cells, was kindly provided by Dr. Elliot Ross (University of Texas Southwestern Medical Center, Dallas, TX). GST-MEK5 recombinant protein was purchased from Abnova (Walnut, CA). COS-7 cells were from the American Type Culture Collection (Manassas, VA), and the NIH 3T3 fibroblasts expressing ∼20,000 human m1-muscarinic receptors per cell, designated 3T3-m1 cells, were kindly provided by J. S. Gutkind (National Institutes of Health, Bethesda, MD). Culture media and Lipofectamine were from Invitrogen. The affinity-purified rabbit polyclonal antibodies Gαq/11 (C19), Gαs (K20), Gα12 (S-20), Gαi1 (I-20), hemagglutinin (HA) (Y-11), or PKCζ (C-20), as well as the mouse monoclonal antibody (H1) raised against the carboxyl terminus of PKCζ and the affinity-purified rabbit polyclonal antibody against GST (Z5), were all purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The monoclonal 12CA5 anti-HA antibody was from Roche Applied Science. The rabbit polyclonal antibody that recognizes ERK5 was from Upstate Biotech Millipore (Lake Placid, NY). The MEK5 polyclonal antibody was purchased from Abcam (Cambridge, UK). Polyclonal C-16 and C-14 antibodies that recognize ERK1 and ERK2 were obtained from Santa Cruz Biotechnology. The anti-phospho-ERK1/2 polyclonal antibody was purchased from Cell Signaling Technologies (Beverly, MA). Mouse monoclonal anti-His tag clone HIS1, EGF, sphingosine 1 phosphate, and carbachol were obtained from Sigma. Different anti-phospho-ERK5 antibodies were purchased from Invitrogen, Abcam, Cell Signaling, Santa Cruz Biotechnology, or Upstate Biotech Millipore. The Src inhibitor PP2 and the EGF receptor-specific tyrosine kinase inhibitor AG1478 were obtained from Calbiochem. Myristoylated PKCζ pseudosubstrate peptide (Myr-SIYRRGARRWRKL) was obtained from BIOSOURCE (Camarillo, CA). G protein-Sepharose and ProBond resins were obtained from Invitrogen. Pertussis toxin was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). All other reagents were of the highest commercially available grades.
Cell Culture and Treatment
COS-7 and NIH 3T3-ml cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum (Sigma-Aldrich) or newborn serum (Invitrogen), respectively, at 37 °C in a humidified 5% CO2 atmosphere. Mouse embryonic fibroblasts (MEFs) obtained from wild-type or PKCζ−/− mice were cultured as described previously (18). The desired cell type was stimulated with carbachol (10 μm) or sphingosine-1-phosphate (100 nm) at 37 °C in serum-free Dulbecco's modified Eagle's medium during the indicated time periods. The cells were serum-starved for 5–6 h before ligand addition to minimize basal kinase activity. Treatments with the Src inhibitor PP2 (10 μm), AG1478 (250 nm), the PLCβ inhibitor U73122 (10 μm), or the PKCζ pseudosubstrate inhibitor (10 μm) were initiated 30 min before agonist stimulation. For the inactivation of Gi proteins, cells were pretreated with pertussis toxin (100 ng/ml) for 16 h. COS-7 or 3T3 cells (70–80% confluent monolayers in 60- or 100-mm dishes) were transiently transfected with the desired combinations of cDNA constructs using the Lipofectamine Plus method, following the manufacturer's instructions. Empty vector was added to keep the total amount of DNA per dish constant. Assays were performed 48 h after transfection. Transient expression of the desired proteins was confirmed by immunoblot analysis of whole-cell lysates using specific antisera, as described below.
Determination of MAPK Stimulation
The activation state of ERK1/2 and ERK5 was measured by Western blot analysis of cell lysates by using anti-phospho-ERK1/2 (1:500) as reported previously (19) or anti-ERK5 (1:500) antibodies, respectively. In the latter case, the stimulation of ERK5 can be detected by the presence of a band with slower electrophoretic mobility that represents the active, phosphorylated form of the protein (20) or by using specific anti-phospho ERK5 antibodies. To obtain cell lysates, cells were washed with ice-cold phosphate-buffered saline buffer plus 1 mm sodium orthovanadate and subsequently solubilized in lysis buffer (50 mm Tris-HCl, 150 mm NaCl, 1% (w/v) Nonidet P-40, 0.25% (w/v) sodium deoxycholate, 1 mm EGTA, 1 mm NaF, supplemented with 1 mm sodium orthovanadate plus a mixture of protease inhibitors). Lysates were resolved by 6–10% SDS-PAGE and subjected to immunoblot analysis as described (19). Bands were quantified by laser-scanner densitometry, and the amount of phosphorylated ERK1/2 or phosphorylated ERK5 protein was normalized to the amount of the total ERK1/2 or ERK5 protein, as assessed by the specific antibodies. Statistical analysis was performed using the two-tailed Student's t test, as indicated.
Immunoprecipitation
Immunoprecipitation assays of co-transfected proteins were performed 48 h after transfection. Cells were scraped and washed twice with ice-cold phosphate-buffered saline, solubilized in 500 μl/100-mm dish of radioimmune precipitation buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 0.5% (w/v) sodium deoxycholate, 1% (v/v) Triton X-100, 0.1% SDS) supplemented with a mixture of protease inhibitors. The lysates were clarified by centrifugation, and an aliquot (30 μl) was used to assess protein expression levels. The immunoprecipitation reactions were performed by incubating the supernatants with 1 mg/ml bovine serum albumin and the specific antibodies for HA (12CA5, 4 μg), Gαq (C19, 2 μg), PKCζ (H1, 0.6 μg), or GST (Z5, 2 μg) at 4 °C overnight followed by reincubation with protein G-Sepharose for 1 h, as reported previously (21). For immunoprecipitation of endogenous proteins, 80% confluent monolayers from two 100-mm dishes of cultured cells were used. Cell lysates were tested for protein expression by using the required specific antibodies. Additionally, to identify MEK5 interaction partners, lysates from cells expressing GST-MEK5 (or GST alone as a negative control) were subjected to GST pulldown assays with glutathione-Sepharose 4B as reported previously (21). All blots were developed using the chemiluminescence method (ECL, Amersham Biosciences). When required, bands were quantified by laser-scanner densitometry, and the amount of co-precipitated protein was normalized to the amount of the immunoprecipitated protein, as assessed by the specific antibodies. Statistical analyses were performed using the two-tailed Student's t test, as indicated.
Protein Interaction Assays
Purified recombinant Gαq (10–20 nm) was incubated at 4 °C with purified His-PKCζ (20 nm) or GST-MEK5 (100 nm) fusion proteins (or GST 100 nm as a negative control) in a final volume of 100 μl of binding buffer (50 mm Tris-HCl, pH 7.9, 70 mm NaCl, 0.6 mm EDTA, 0.01% Lubrol plus a mixture of protease inhibitors). Subsequently, ProBond (for His PKCζ) or glutathione-Sepharose 4B (for GST-MEK5) resins was added for 2 h at 4 °C, after which the affinity matrix was pelleted and washed four times with 500 μl of ice-cold binding buffer (in the presence of 10 mm imidazole in experiments involving His-PKCζ). Proteins retained on the matrix were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Western blot analysis was then performed with the anti-Gαq (C-19, (1:1000)), anti-histidine (1:1000), or anti-GST (1:500) antibodies, depending on the experiment.
RESULTS
Stimulation of Gq-coupled GPCR Promotes ERK5 Activation in NIH 3T3 Cells
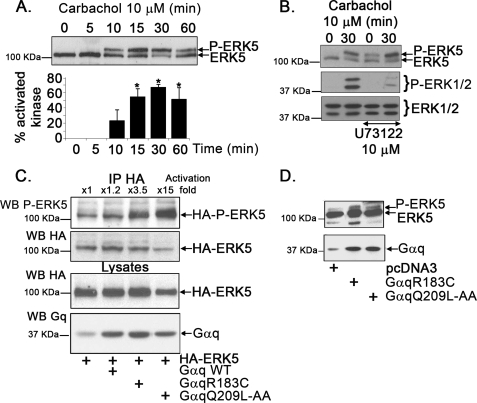
Previous studies indicated that GPCR that can couple to the Gq family of heterotrimeric G proteins, such as m1-muscarinic and thrombin receptors, were able to promote ERK5 activation in COS-7 or NIH 3T3 cells (15, 17). To explore the mechanisms involved, NIH 3T3 cells stably expressing the human m1-muscarinic acetylcholine receptor (NIH 3T3-m1R) (22) were stimulated with carbachol for different periods of time. This agonist promoted a clear, time-dependent increase in endogenous ERK5 activation (Fig. 1A) that can be detected by immunoblot analysis with an ERK5 antibody by the appearance of a band of slower electrophoretic mobility (corresponding to the phosphorylated, stimulated kinase), which comigrated with the band detected with different ERK5-phosphospecific antibodies (supplemental Fig. S1). Because the latter method was, in our hands, less sensitive (supplemental Fig. S1, lower panel), the band-shift method was routinely used to assess ERK5 stimulation.
FIGURE 1.
ERK5 pathway activation by Gq-coupled GPCR. A, NIH 3T3-m1R cells, stably expressing the human m1-muscarinic acetylcholine receptor, were incubated with 10 μm carbachol for the indicated times, and endogenous ERK5 activation was determined with an antibody that recognizes both the phosphorylated (P-ERK5) and the unphosphorylated forms of ERK5 and analyzed as detailed under ”Experimental Procedures.“ The band of slower electrophoretic mobility corresponds to the stimulated kinase. Blot bands were quantified by laser-scanner densitometry, and data were expressed as the percentage of activated kinase (P-ERK5) versus total ERK5. Data are mean ± S.E. of 3 independent experiments. *, p < 0.05 when compared with 0 min. B, NIH 3T3-m1 cells were incubated with the PLCβ inhibitor U73122 (10 μm) or vehicle prior to stimulation with carbachol. The pattern of ERK5 activation by carbachol is not affected by this inhibitor (upper panel), whereas ERK1/2 stimulation is clearly impaired (lower panel). C, NIH 3T3 cells were transiently transfected with a plasmid encoding HA-tagged ERK5 and with constitutively active Gαq mutants able (Gαq R183C) or unable (Gαq Q209L-AA) to interact with the Gαq effector PLCβ. Then, HA-ERK5 was immunoprecipitated (IP), and ERK5 activation was assessed with an ERK5-phosphospecific antibody. The normalized -fold stimulation of ERK5 activity versus control conditions is indicated above the representative blot. Gαq and HA-ERK5 expression was monitored by immunoblot analysis (WB) of cell lysates (lower panel). D, endogenous ERK5 activation in 3T3m1R cells is induced upon overexpression of either Gαq R183C or Gαq Q209L-AA. Migration of unphosphorylated and phosphorylated forms of ERK5 or ERK1/2 and of molecular weight makers is indicated in all panels. Blots are representative of at least 3 independent experiments.
Activation of ERK5 by different mitogens may involve Src tyrosine kinase (9) and can also be triggered by EGF (10). However, stimulation of ERK5 by muscarinic agonists was not affected in the presence of the Src inhibitor PP2 (supplemental Fig. S2A) or the EGF receptor tyrosine kinase inhibitor AG1478 (supplemental Fig. S2B). On the other hand, ERK5 activation by carbachol was not affected by the presence of the PLCβ inhibitor U73122, whereas ERK1/2 stimulation was markedly decreased (Fig. 1B). Moreover, expression of a GTPase-deficient, constitutively active Gαq mutant (GqR183C) mimicked ERK5 activation by Gq-coupled GPCR in NIH 3T3-m1R cells, and the same was true for the other constitutively active construct (Gq Q209L-AA), previously shown to be unable to interact with the known Gαq effector PLCβ (Fig. 1, C and D) (23). Overall, these data suggested that Gq-coupled GPCR trigger the stimulation of the ERK5 cascade by biochemical routes involving Gαq but not its classical effector PLCβ, nor cytoplasmic tyrosine kinases nor EGF receptor transactivation.
PKCζ Is Required for ERK5 Activation by Gαq-coupled GPCR
It has been previously shown that the atypical PKC isoform PKCζ interacts with MEK5 in a growth factor-inducible manner and that such interaction is required and sufficient for the activation of the MEK5/ERK5 pathway (10, 13). Interestingly, some groups had reported that agonists acting through Gq-coupled GPCR such as angiotensin (24–27) or phenylephrine (28) were able to promote PKCζ translocation, although the mechanisms involved and the potential triggering of downstream cascades were not explored in detail. Therefore, we sought to determine whether PKCζ could be involved in Gαq-mediated GPCR stimulation of the ERK5 pathway.
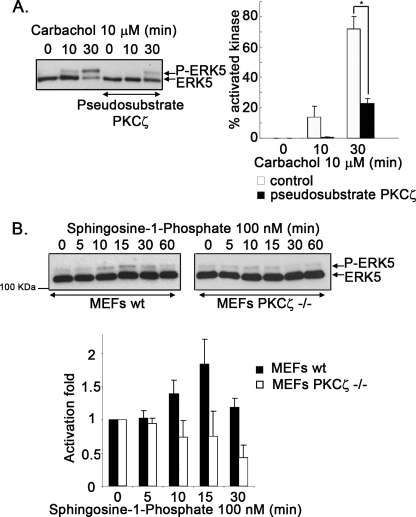
To test this hypothesis, NIH 3T3-m1 cells were pretreated or not with a cell-permeable myristoylated-PKCζ pseudosubstrate peptide inhibitor (27, 28). Fig. 2A shows that the presence of the inhibitor promotes a marked decrease in the percentage of phosphorylated endogenous ERK5 in response to the agonist carbachol. Similar results were obtained when overexpressing a dominant-negative PKCζ-mutant (11.1 ± 2.6% of phosphorylated HA-ERK5 after a 30-min stimulation with carbachol in its absence versus 6.5 ± 0.9% in its presence).
FIGURE 2.
PKCζ is required for Gq-coupled GPCR stimulation of the ERK5 pathway. A, NIH 3T3-m1R cells, preincubated or not with a myristoylated PKCζ pseudosubstrate inhibitor (10 μm), were challenged with the agonist carbachol, and ERK5 activation (P-ERK5) was determined as detailed under ”Experimental Procedures.“ Blot bands were quantified by laser-scanner densitometry, and data (mean ± S.E. of 3 independent experiments) were expressed as a percentage of activated kinase (P-ERK5) versus total ERK5. B, MEFs obtained from WT or PKCζ-deficient mice (PKCζ−/−) were challenged with S1P, and ERK5 activation was assessed at different times as in previous panels. Data (mean ± S.E. of 3 independent experiments) were expressed as -fold activation when compared with the absence of agonist.
To further establish that PKCζ is required for ERK5 stimulation by Gq-coupled GPCR in a physiological setting, we investigated this pathway in cells derived from PKCζ-deficient mice (18). Using MEFs from wild-type mice, we found that sphingosine-1-phosphate (S1P), an agonist that can stimulate both Gi-coupled and Gq-coupled endogenous receptors (29), promoted a clear increase in ERK5 activation that was not affected by the presence of pertussis toxin (supplemental Fig. S3A), thus indicating that this process did not involve G proteins of the Gi subfamily. In contrast, S1P-mediated ERK1/2 stimulation was markedly inhibited by pertussis toxin (supplemental Fig. S3B). Interestingly, although S1P significantly increased ERK5 activity in MEFs from wild-type (WT) mice (1.89 ± 0.38-fold over basal at 15 min of treatment, p < 0.05, two-tailed t test), no ERK5 activation over basal levels (0.74 ± 0.37-fold) could be observed when MEFs obtained from PKCζ knock-out mice were used (Fig. 2B). In contrast, the stimulation of ERK1/2 was detected in these cells to an extent similar to that observed in WT MEFs (supplemental Fig. S3B), indicating that the effect of PKCζ deficiency is specific to the ERK5 pathway and does not lead to a general decrease in receptor signaling.
Gαq Subunits Associate with Protein Kinase Cζ
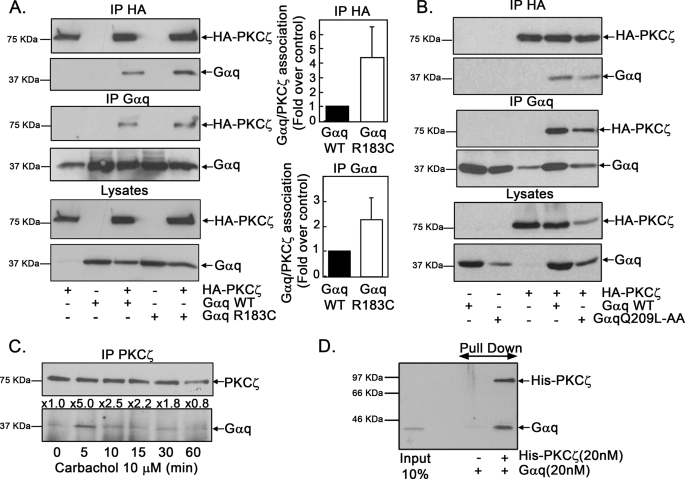
To investigate how Gαq and PKCζ functionally interact to promote ERK5 stimulation, we expressed an HA-tagged PKCζ construct together with wild-type or active Gαq (the GTPase-deficient Gαq R183C mutant) and performed immunoprecipitation assays using either anti-HA-monoclonal (Fig. 3A, upper panel) or anti-Gαq polyclonal antibodies (Fig. 3A, middle panel). A clear association of PKCζ and Gαq upon co-expression is observed using both approaches. Interestingly, a clear association with PKCζ was also observed for the Gq Q209L-AA mutant unable to activate and interact with PLCβ (Fig. 3B), again suggesting that this process is independent of the PLCβ pathway. Moreover, the association between PKCζ and Gαq appears to be specific because PKCζ does not co-immunoprecipitate with co-expressed Gαs, Gαi, or Gα12 subunits, nor does Gαq associate with PKCλ, another atypical PKC isoform with high sequence similarity to PKCζ (supplemental Fig. S4).
FIGURE 3.
Gαq associates with PKCζ. A, COS-7 cells were transiently transfected with the indicated combinations of plasmids encoding HA-tagged-PKCζ, wild-type Gαq (WT) or a constitutively active Gαq mutant (Gαq R183C). Expression of the different proteins was confirmed by immunoblot analysis of cell lysates (lower panel). Cell lysates were subjected to immunoprecipitation (IP) with an anti-HA monoclonal antibody or an anti-Gαq polyclonal antibody as indicated. Immunoprecipitates were resolved by SDS-PAGE, and the presence of PKCζ and Gαq in the immunocomplexes was determined by Western blot analysis with specific antibodies. To compare the association of PKCζ with WT Gαq and Gαq R183C, band quantification was normalized by total HA-PKCζ (upper panel) or total Gαq (middle panel), and the PKCζ/WT Gαq association was taken as control conditions. Data are mean ± S.E. of 3–4 independent experiments. Representative blots are shown. B, COS-7 cells were transiently transfected with the indicated combinations of plasmids encoding HA-tagged-PKCζ, WT Gαq, or a constitutively active Gαq mutant unable to activate and interact with PLCβ (Gαq Q209L-AA). Immunoprecipitation and SDS-PAGE procedures were carried out as in panel A. C, stimulation of Gq-coupled GPCR promotes the association between endogenous Gαq and PKCζ proteins. NIH 3T3-m1R cells were challenged with 10 μm carbachol for different times as in Fig. 1A, and endogenous Gαq/PKCζ co-immunoprecipitation was assessed with specific antibodies. The normalized -fold stimulation of co-immunoprecipitation versus basal conditions is indicated above the representative blot. In 3 independent experiments, an average stimulation of association of 3.44 ± 1.5- and 2.06 ± 0.36-fold over basal at 5 and 10 min after carbachol challenge, respectively, was obtained. D, direct interaction between Gαq and PKCζ. Purified recombinant Gαq (20 nm) was incubated in the absence or presence of purified His-tagged PKCζ fusion protein (20 nm), and the mixture was subjected to affinity chromatography using a ProBond nickel resin. Proteins retained in the matrix were resolved by SDS-PAGE, and the presence of His-PKCζ or Gαq was analyzed by immunoblot analysis with specific antibodies, including the input (20%) of Gαq as a control. This experiment was repeated twice with similar results.
The fact that the Gαq/PKCζ association was markedly increased (from 2.5- to 4-fold over control, Fig. 3A) when expressing the active Gαq mutant when compared with wild-type Gαq subunit suggested that Gαq/PKCζ co-immunoprecipitation would be regulated upon Gq protein activation by GPCR, as is the case for other Gα protein subunit effectors. Consistently, carbachol stimulation of Gαq-coupled m1-muscarinic receptors promoted a clear increase in the association of either co-transfected (not shown) or endogenous PKCζ and Gαq (3.1–5-fold over basal conditions at 5 min of agonist challenge, Fig. 3C), indicating that the functional interaction between these proteins takes place in physiological conditions upon activation of GPCR.
To determine whether the Gαq/PKCζ association was direct or mediated by other cellular proteins, we performed an “in vitro” binding assay using purified recombinant Gαq and a His-PKCζ fusion protein. Fig. 3D shows a clear, direct interaction between both proteins.
Gαq Interacts with MEK5
In agreement with the notion that the Gαq/PKCζ pathway is relevant for the activation of the ERK5 cascade, we were able to detect the presence of MEK5, the well known PKCζ interactor and upstream activator of ERK5 (7, 10), in Gαq immunocomplexes (Fig. 4A) upon co-expression of active Gαq, HA-PKCζ, and GST-MEK5 constructs in COS-7 cells. Surprisingly, MEK5 co-immunoprecipitated with Gαq even in the absence of co-expressed PKCζ, whereas the presence of extra MEK5 decreased the extent to which PKCζ associated with Gαq (Fig. 4A). The same was observed when PKCζ immunocomplexes were analyzed in similar assays using a HA immunoprecipitating antibody (Fig. 4B). However, the presence of extra Gαq does not reduce, but appears to even enhance PKCζ/MEK5 association (Fig. 4B). To further characterize such Gαq/MEK5 functional interaction, we performed co-immunoprecipitation assays using an anti-GST-MEK5 polyclonal antibody. Gαq/MEK5 association was clearly detected and markedly increased (4-fold over control, Fig. 5A) when expressing an active Gαq mutant when compared with wild-type Gαq subunit, consistent with a stimulus-dependent interaction.
FIGURE 4.
Analysis of PKCζ-Gαq-MEK5 macromolecular complexes. COS-7 cells were transfected with HA-PKCζ, the constitutively active Gαq mutant (Gαq R183C) and GST-MEK5. Cell lysates were subjected to immunoprecipitation (IP) with an anti-Gαq monoclonal antibody (A) or an anti-HA polyclonal antibody (B). Immunoprecipitates were resolved by SDS-PAGE, and the presence of HA-PKCζ, Gαq, and GST-MEK5 in the immunocomplexes was determined by Western blot (WB) analysis with specific antibodies. To compare the association of HA-PKCζ with Gαq or GST-MEK5, in the presence or absence (taken as control conditions) of the indicated proteins, band densities were normalized to total Gαq (upper panel) or HA-PKCζ (lower panel). Data are mean ± S.E. of 3 independent experiments. Representative blots are shown.
FIGURE 5.
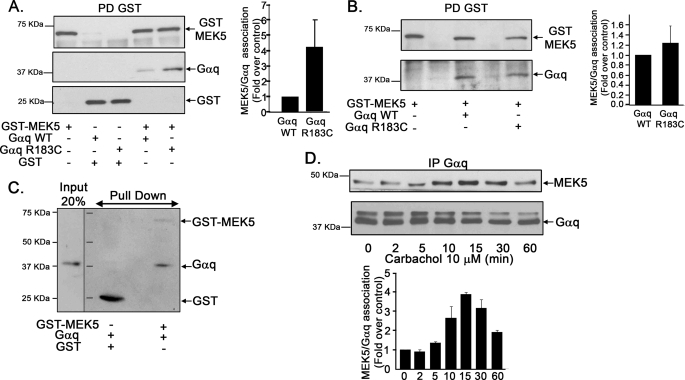
Gαq associates with MEK5. A, COS-7 cells were transiently transfected with the indicated combinations of plasmids encoding GST-MEK5, GST, wild-type Gαq (WT), or a constitutively active Gαq mutant (Gαq R183C). Cell lysates were subjected to pulldown (PD) with glutathione-Sepharose 4B resin as detailed under ”Experimental Procedures.“ Proteins retained in the matrix were resolved by SDS-PAGE, and the presence of GST, GST-MEK5, and Gαq was determined by Western blot analysis with specific antibodies. To compare the association of MEK5 to WT Gαq and Gαq R183C, blot bands were quantified and normalized by total GST-MEK5. The MEK5/WT Gαq association was taken as the control condition. Data are mean ± S.E. of 3–4 independent experiments. Representative blots are shown. B, MEFs obtained from PKCζ−/− mice cells were transiently transfected with the indicated combinations of plasmids, and cell lysates were analyzed using a pulldown assay as in the previous panel. C, direct interaction between Gαq and GST-MEK5. Purified recombinant Gαq (10 nm) was incubated in the absence or presence of purified GST-MEK5 fusion protein (100 nm), and the mixture was subjected to affinity chromatography using a glutathione-Sepharose 4B resin. Proteins retained in the matrix were resolved by SDS-PAGE, and the presence of GST-MEK5 or Gαq was analyzed by immunoblot analysis with specific antibodies, including the input (20%) of Gαq as a control. This experiment was repeated twice with similar results. D, stimulation of Gq-coupled GPCR promotes the association of endogenous Gαq and MEK5 proteins. NIH 3T3-m1R cells were challenged with 10 μm carbachol for different times as in Fig. 1A, and endogenous Gαq/MEK5 co-immunoprecipitation (IP) was assessed with specific antibodies. Data are mean ± S.E. of 3 independent experiments.
In principle, the observed association between Gαq and MEK5 could be either direct or mediated by endogenous PKCζ, able to interact with both proteins. To discriminate between these possibilities, we carried out similar co-immunoprecipitation assays in MEFs obtained from PKCζ knock-out mice (Fig. 5B). Under these conditions, a clear association between Gαq and MEK5 was also observed, indicating that this process does not strictly require PKCζ. Consistently, an in vitro binding assay using purified recombinant Gαq and a GST-MEK5 fusion protein shows a clear, direct interaction between both proteins (Fig. 5C). However, it is interesting to note that in the cell milieu, active and wild-type Gαq display a similar association to MEK5 in the absence of PKCζ (Fig. 5B), suggesting that PKCζ may facilitate the binding of MEK5 to active Gαq. In agreement with this notion, stimulation of Gq-coupled m1-muscarinic receptors promoted a clear increase in the association of endogenous MEK5 and Gαq (Fig. 5D) with a time course slightly retarded when compared with the Gαq/PKCζ association (Fig. 3C).
Dynamic Gαq-PKCζ-MEK5 Complexes Are Essential for ERK5 Activation by GPCR
Overall, our data suggested that by recruiting both PKCζ and MEK5 to the same macromolecular complex, Gαq would lead to ERK5 activation by GPCR. In such a model, receptor stimulation would promote association of activated Gαq to PKCζ, which would in turn facilitate MEK5 binding to Gαq and the subsequent formation of a PKCζ-MEK5 complex, resulting in ERK5 stimulation (Fig. 6A).
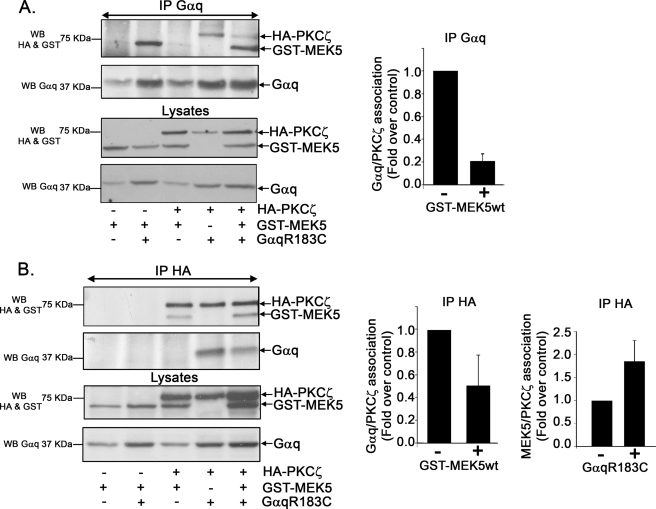
FIGURE 6.
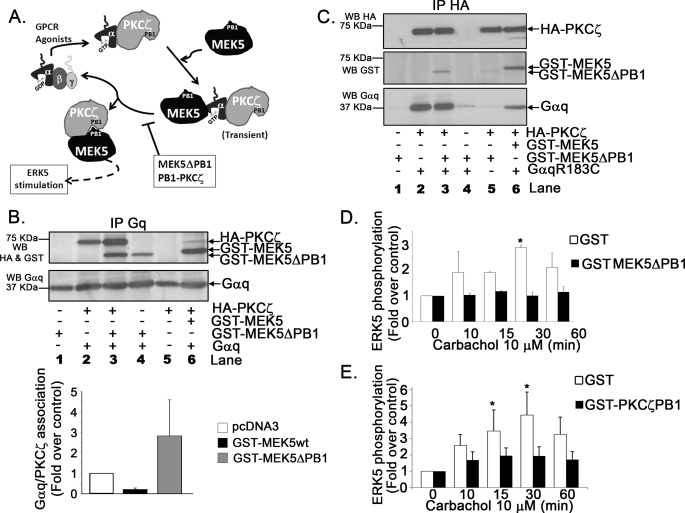
The formation of dynamic Gαq-PKCζ-MEK5 complexes is essential for ERK5 activation. A, model for the proposed dynamics of the Gαq-PKCζ-MEK5 complexes. See ”Results“ for detailed explanation and discussion. B, the MEK5ΔPB1 mutant stabilizes PKCζ/Gαq association and impairs the formation of the MEK5-PKCζ complex. COS-7 cells were transiently transfected with the indicated combinations of plasmids encoding HA-tagged-PKCζ, the constitutively active Gαq mutant (Gαq R183C), wild-type GST-MEK5, and the GST-MEK5ΔPB1 mutant. Cell lysates were subjected to immunoprecipitation (IP) with an anti-Gq polyclonal antibody. Immunoprecipitates were resolved by SDS-PAGE, and the presence of PKCζ, MEK5, and Gαq in the immunocomplexes was determined by Western blot (WB) analysis with specific antibodies. To compare the association of HA-PKCζ with GαqR183C in the presence of MEK5WT or MEK5ΔPB1, band densities were normalized to total Gαq. Data are mean ± S.E. of 3 independent experiments. A representative blot is shown. C, the presence of extra Gαq rescues the lack of association between PKCζ and the GST-MEK5ΔPB1 mutant. Cell lysates as in panel B were subjected to immunoprecipitation with an anti-HA monoclonal antibody to analyze PKCζ complexes. D and E, effect of the expression of GST-MEK5ΔPB1 or the independent PKCζ PB1 domain on ERK5 activation by Gq-coupled GPCR. NIH 3T3-m1R cells were transiently transfected with GST-MEK5ΔPB1 (D) or GST-PKCζ PB1 (E) and GST as a control. 48 h after transfection, cells were challenged with 10 μm carbachol for different times as in Fig. 1A. Endogenous ERK5 activation was then determined and expressed as a mean ± S.E. of 2–3 independent experiments. *, p < 0.05, analysis of variance followed by Fischer's least significant difference.
The association between PKCζ and MEK5 has been reported to involve PB1 domains in both proteins (11, 30), and the MEK5ΔPB1 mutant (which does not have a functional PB1 domain) has been shown to be unable to interact with PKCζ upon EGF stimulation (10). We thus used this mutant to further dissect the dynamics of the Gαq-PKCζ-MEK5 complexes.
Consistent with the notion that Gαq associates to MEK5 independently of PKCζ, MEK5ΔPB1 was detected in Gαq immunocomplexes (Fig. 6B, lane 4). Interestingly, although wild-type MEK5 appears to “displace” PKCζ from Gαq, this effect was not observed with MEK5ΔPB1, which even increases the extent of PKCζ/Gαq association (Fig. 6B, compare lanes 2, 3, and 6), as predicted by our model. Accordingly, Fig. 6C shows that when analyzing PKCζ immunocomplex in such experimental conditions, the lack of association between MEK5ΔPB1 and PKCζ (Fig. 6C, lane 5) can be “rescued” in the presence of extra Gαq (Fig. 6C, lane 3) in line with a scaffold role for Gαq in this process. The inability of MEK5ΔPB1 to associate to PKCζ would stabilize the usually transient MEK5-Gαq-PKCζ complexes and therefore block activation of GPCR-mediated ERK5. Consistent with this notion, overexpression of MEK5ΔPB1 completely abrogates carbachol-mediated endogenous ERK5 stimulation in cells (Fig. 6D). The same effect is observed upon expression of an independent GST-PKCζ PB1 construct, known to inhibit PKCζ/MEK5 association (Fig. 6E).
DISCUSSION
In this report, we show that PKCζ plays a key role in the activation of the ERK5 pathway by Gq-coupled GPCR in epithelial cells and that Gαq displays a scaffold-like role in this process by independently interacting with both PKCζ and MEK5 (see model in Fig. 6A). This is, to our knowledge, the first demonstration that G protein α-subunits can serve as a scaffold, bringing two proteins into close proximity and proper relative orientation to promote their transient interaction and subsequent stimulation of a signal transduction cascade. Several lines of evidence support this model. First, ERK5 stimulation by carbachol does not appear to require the activity of EGF receptors or cytosolic tyrosine kinases, known to participate in ERK5 activation in response to different mitogens (7, 9, 10), thus indicating that potential GPCR/EGF receptor transactivation mechanisms (31) are not involved. Second, overexpression of a constitutively active Gαq subunit mutant promotes ERK5 stimulation “per se,” independently of its ability to interact with the classical Gαq effector PLCβ. Third, stimulation of ERK5 by Gq-coupled GPCR is blocked by PKCζ pharmacological inhibitors and is absent in MEFs derived from PKCζ-deficient mice. Fourth, Gαq (and not other Gα subunits) associates with PKCζ in cells, and co-immunoprecipitation of these endogenous proteins can be promoted upon Gq-coupled activation of GPCR. Moreover, a direct Gαq/PKCζ interaction can be observed using purified proteins. Fifth, Gαq, PKCζ, and MEK5 (the upstream ERK5 activator) appear to form dynamic complexes to trigger ERK5 activation, involving direct interactions between Gαq and both PKCζ and MEK5 and a PKCζ/MEK5 association mediated by their respective PB1 domains.
Previous reports have shown that GPCR able to couple to Gq proteins can regulate the activity of ERK5 in epithelial cells. This process was mimicked by expression of activated forms of Gαq (but not of Gαs or Gαi or upon overexpression of βγ subunits) and was independent of the activation of Ras or Rho signaling pathways (16, 17), although the mechanisms linking Gαq to ERK5 were not identified. The primary downstream actions of Gαq have been tied to activation of its classic effector PLCβ. However, because we find that pharmacological inhibition of PLCβ does not affect ERK5 activation by Gq-coupled GPCR and because the expression of an activated form of Gαq that does not interact with PLCβ (23) is still able to stimulate ERK5, we demonstrate that PLCβ is not involved in this pathway. Instead, we show that the functional interactions of Gαq with PKCζ and MEK5 underlie its ability to trigger the ERK5 cascade.
Consistent with the notion that PKCζ is a novel Gαq effector, agonists acting through Gq-coupled GPCR such as angiotensin II, phenylephrine, platelet-activating-factor, or thromboxane A2 have been shown to promote PKCζ translocation and activation in several cell types (24–26, 32, 33), and PKCζ has been suggested to participate in GPCR-mediated control of cell proliferation (25–27), eosinophil degranulation (32), or smooth muscle cell adhesion, spreading, and hypertrophy (25). Several authors have suggested a role for PKCζ in ERK1/2 activation by GPCR (34, 35), although another recent report indicates that inhibition of PKCζ in adult cardiomyocytes has no effect in ERK1/2 activation by Gq-coupled GPCR (36). However, this is the first report to show a direct link between Gαq and PKCζ and to establish a role for such association in the stimulation of the ERK5 MAPK cascade by GPCR.
The Btk and Csk kinases or the nucleotide exchange factor, p63RhoGEF, have also been reported to be PLCβ-independent Gαq effectors (23, 37, 38). Besides that effector diversity, our report puts forward a novel scaffold role for Gαq in ERK5 signaling based on its ability to directly interact with both PKCζ and MEK5. Our data suggest that activation of GPCR would first promote Gαq/PKCζ association followed by direct binding or MEK5 to Gαq, which would in turn favor PKCζ/MEK5 interaction through their respective PB1 domains (39, 40), leading to ERK5 activation (see model in Fig. 6A). In agreement with this model, we find that wild-type MEK5 decreases the extent of Gαq/PKCζ association, whereas the presence of extra Gαq enhances PKCζ/MEK5 co-immunoprecipitation. Also consistent with this notion, the time course of endogenous MEK5/Gαq co-immunoprecipitation is slightly delayed when compared with that of Gαq and PKCζ. The use of the MEK5ΔPB1 mutant, reported to be unable to interact with PKCζ (10), and of PKCζ-deficient cells has provided further insight into the dynamics of these complexes. On the one hand, the fact that the MEK5ΔPB1 mutant can associate to Gαq indicates that endogenous PKCζ does not act as a “bridge” between these two proteins, also showing that the MEK5 PB1 domain does not play a role in the interaction with Gαq. The ability of MEK5 to directly bind to Gαq is further established using purified proteins. Interestingly, although MEK5 and Gαq can co-immunoprecipitate in PKCζ-deficient MEFs, such association is not sufficient to trigger ERK5 activation, nor is it increased upon expression of activated when compared with wild-type Gαq, suggesting that the activated Gαq-PKCζ complex is the preferred recruiting site for MEK5.
Finally, it is worth noting that the presence of the MEK5ΔPB1 mutant (contrary to the wild-type kinase) does not displace Gαq from PKCζ and blocks GPCR-mediated ERK5 stimulation. This suggests that the stabilization of a “non-productive” Gαq-mutant MEK5-PKCζ complex is taking place instead of the transient ternary complex that would normally lead to MEK5/PKCζ association (see model in Fig. 6A). Consistent with the scaffold role of Gαq in the process, the presence of extra Gαq rescues the lack of association between PKCζ and MEK5ΔPB1.
Scaffold proteins bring together specific kinases or other components of signaling cascades for selective activation and localization. Both MEK5 and PKCζ have been shown to bind scaffold proteins such as p62 or Par-6 (12, 30). In fact, p62 has been described as an important factor in MEK5/ERK5-mediated activation of the transcription factors MEF2C and Sap1a following EGF stimulation (14). Moreover, p62 knockdown can block nerve growth factor-mediated activation of ERK5 (13). Lamark et al. (14) have postulated that the interaction between PKCζ and MEK5 is stabilized by p62. In this context, our data strongly suggest that Gαq plays a similar scaffold role for PKCζ and MEK5 in GPCR-mediated ERK5-mediated activation. Interestingly, other routes of MEK5/ERK5 stimulation also appear to require adaptor proteins. The Lck-associated adaptor (LAD) may be responsible for facilitating MEKK2/MEK5 binding and recruitment to the growth factor receptor complex (41), and Gab-1 participates in leukemia inhibitory factor-mediated ERK5 modulation (reviewed in Ref. 7).
It has been recently suggested that activated Gαq subunits would display specific membrane orientations that would unmask binding surfaces ready for the docking of structurally different effectors (38). Although a scaffold role for Gαq has not been reported to our knowledge, it is worth noting that different regions of this protein can specifically associate with the distinct Dbl homology (DH) and RGS homology (RH) domains of the Gαq effector p63RhoGEF (38). Moreover, recent studies have shown the formation of RGS-Gαq-p63RhoGEF or and RGS-Gαq-GRK2 ternary complexes (42), suggesting the occurrence of two independent binding surfaces in Gαq. In vitro studies have also shown the ability of Gαq to simultaneously bind to both PLCβ and phosphatidylinositol 3-kinase (43). Our preliminary data indicate that both Gαq/PKCζ and Gαq/MEK5 association are inhibited in the presence of the GRK2 RH domain,4 which has been reported to interact with Gαq and block the interaction with its effector PLCβ (21, 44, 45). However, the Gαq sites required for the interactions with both PKCζ and MEK5 appear to be different from those involved in PLCβ binding because a Gαq mutant that is unable to interact with the latter promotes ERK5 activation and readily associates to PKCζ. The detailed architecture of the Gαq-PKCζ-MEK5 complexes and the mechanisms underlying their spatial and temporal assembly await further investigation.
ERK5 has been implicated in the regulation of many cellular functions, such as differentiation, proliferation, migration, survival, and cardiovascular development (2, 7, 46, 47). The triggering of such ERK5 cascade by association of Gq-coupled GPCR may thus play relevant roles in several cell types and physiological settings, which are being actively investigated in our laboratory. Finally, whether this novel Gαq/PKCζ interaction may also be involved in modulating signaling pathways downstream of PKCζ other than the ERK5 cascade (39) upon activation of Gq-coupled GPCR also deserves to be explored in future studies.
Acknowledgments
We thank Drs. A. Aragay, R. Lin, J. S. Gutkind, and E. Ross for the indicated reagents and tools.
This work was supported by grants from Ministerio de Educación y Ciencia (Grant SAF2008-00552), Fundación Ramón Areces, The Cardiovascular Network (RECAVA) of Instituto de Salud Carlos III (Grant RD06-0014/0037), Comunidad de Madrid (Grant S-SAL-0159-2006), and the Migration and Inflammation (MAIN) European Network (Grant LSHG-CT-2003-502935) (to F. M.) and Instituto de Salud Carlos III (Grant PI080461) (to C. R.).
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
C. García-Hoz, G. Sánchez, C. Ribas, and F. Mayor, manuscript in preparation.
- MAPK
- mitogen-activated protein kinase
- GPCR
- G protein-coupled receptors
- PKC
- protein kinase C
- EGF
- epidermal growth factor
- MEF
- mouse embryonic fibroblast
- S1P
- sphingosine-1-phosphate
- PLC
- phospholipase C
- HA
- hemagglutinin
- GST
- glutathione S-transferase
- WT
- wild-type.
REFERENCES
- 1.Turjanski A. G., Vaqué J. P., Gutkind J. S. (2007) Oncogene 26, 3240–3253 [DOI] [PubMed] [Google Scholar]
- 2.Goldsmith Z. G., Dhanasekaran D. N. (2007) Oncogene 26, 3122–3142 [DOI] [PubMed] [Google Scholar]
- 3.Rozengurt E. (2007) J. Cell. Physiol. 213, 589–602 [DOI] [PubMed] [Google Scholar]
- 4.May L. T., Hill S. J. (2008) Int. J. Biochem. Cell Biol. 40, 2013–2017 [DOI] [PubMed] [Google Scholar]
- 5.Gutkind J. S. (2000) Sci. STKE 40, re1. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M., Lee J. D. (2004) J. Mol. Med. 82, 800–808 [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Tournier C. (2006) Cell Signal 18, 753–760 [DOI] [PubMed] [Google Scholar]
- 8.Obara Y., Nakahata N. (2010) Mol. Pharmacol. 77, 10–16 [DOI] [PubMed] [Google Scholar]
- 9.Sun W., Kesavan K., Schaefer B. C., Garrington T. P., Ware M., Johnson N. L., Gelfand E. W., Johnson G. L. (2001) J. Biol. Chem. 276, 5093–5100 [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Meco M. T., Moscat J. (2001) Mol. Cell Biol. 21, 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumimoto H., Kamakura S., Ito T. (2007) Sci. STKE 401, re6. [DOI] [PubMed] [Google Scholar]
- 12.Moscat J., Diaz-Meco M. T. (2000) EMBO reports 1, 399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geetha T., Wooten M. W. (2003) J. Biol. Chem. 278, 4730–4739 [DOI] [PubMed] [Google Scholar]
- 14.Lamark T., Perander M., Outzen H., Kristiansen K., Øvervatn A., Michaelsen E., Bjørkøy G., Johansen T. (2003) J. Biol. Chem. 278, 34568–34581 [DOI] [PubMed] [Google Scholar]
- 15.Marinissen M. J., Chiariello M., Pallante M., Gutkind J. S. (1999) Mol. Cell Biol. 19, 4289–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuhara S., Marinissen M. J., Chiariello M., Gutkind J. S. (2000) J. Biol. Chem. 275, 21730–21736 [DOI] [PubMed] [Google Scholar]
- 17.Marinissen M. J., Servitja J. M., Offermanns S., Simon M. I., Gutkind J. S. (2003) J. Biol. Chem. 278, 46814–46825 [DOI] [PubMed] [Google Scholar]
- 18.Leitges M., Sanz L., Martin P., Duran A., Braun U., García J. F., Camacho F., Diaz-Meco M. T., Rennert P. D., Moscat J. (2001) Mol. Cell 8, 771–780 [DOI] [PubMed] [Google Scholar]
- 19.Elorza A., Sarnago S., Mayor F., Jr. (2000) Mol. Pharmacol. 57, 778–783 [DOI] [PubMed] [Google Scholar]
- 20.Xu B. E., Stippec S., Lenertz L., Lee B. H., Zhang W., Lee Y. K., Cobb M. H. (2004) J. Biol. Chem. 279, 7826–7831 [DOI] [PubMed] [Google Scholar]
- 21.Mariggiò S., García-Hoz C., Sarnago S., De Blasi A., Mayor F., Jr., Ribas C. (2006) Cell. Signal. 18, 2004–2012 [DOI] [PubMed] [Google Scholar]
- 22.Crespo P., Xu N., Daniotti J. L., Troppmair J., Rapp U. R., Gutkind J. S. (1994) J. Biol. Chem. 269, 21103–21109 [PubMed] [Google Scholar]
- 23.Fan G., Ballou L. M., Lin R. Z. (2003) J. Biol. Chem. 278, 52432–52436 [DOI] [PubMed] [Google Scholar]
- 24.Takeishi Y., Jalili T., Ball N. A., Walsh R. A. (1999) Circ. Res. 85, 264–271 [DOI] [PubMed] [Google Scholar]
- 25.Parmentier J. H., Zhang C., Estes A., Schaefer S., Malik K. U. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H1602–H1613 [DOI] [PubMed] [Google Scholar]
- 26.Muscella A., Greco S., Elia M. G., Storelli C., Marsigliante S. (2003) J. Cell Physiol. 197, 61–68 [DOI] [PubMed] [Google Scholar]
- 27.Godeny M. D., Sayeski P. P. (2006) Am. J. Physiol. Cell Physiol. 291, C1297–1307 [DOI] [PubMed] [Google Scholar]
- 28.Parmentier J. H., Smelcer P., Pavicevic Z., Basic E., Idrizovic A., Estes A., Malik K. U. (2003) Hypertension 41, 794–800 [DOI] [PubMed] [Google Scholar]
- 29.Spiegel S., Milstien S. (2003) Nat. Rev. Mol. Cell Biol. 4, 397–407 [DOI] [PubMed] [Google Scholar]
- 30.Moscat J., Diaz-Meco M. T., Albert A., Campuzano S. (2006) Mol. Cell 23, 631–640 [DOI] [PubMed] [Google Scholar]
- 31.Liggett S. B. (2006) J. Clin. Invest. 116, 875–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato M., Yamaguchi T., Tachibana A., Suzuki M., Izumi T., Maruyama K., Hayashi Y., Kimura H. (2005) Immunology 116, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cogolludo A., Moreno L., Bosca L., Tamargo J., Perez-Vizcaino F. (2003) Circ. Res. 93, 656–663 [DOI] [PubMed] [Google Scholar]
- 34.Hirai T., Chida K. (2003) J. Biochem. 133, 1–7 [DOI] [PubMed] [Google Scholar]
- 35.Jiménez E., Montiel M. (2005) J. Cell Physiol. 204, 678–686 [DOI] [PubMed] [Google Scholar]
- 36.Olson E. R., Shamhart P. E., Naugle J. E., Meszaros J. G. (2008) Hypertension 51, 704–711 [DOI] [PubMed] [Google Scholar]
- 37.Ma Y. C., Huang X. Y. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 12197–12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutz S., Shankaranarayanan A., Coco C., Ridilla M., Nance M. R., Vettel C., Baltus D., Evelyn C. R., Neubig R. R., Wieland T., Tesmer J. J. (2007) Science 318, 1923–1927 [DOI] [PubMed] [Google Scholar]
- 39.Moscat J., Rennert P., Diaz-Meco M. T. (2006) Cell Death Differ. 13, 702–711 [DOI] [PubMed] [Google Scholar]
- 40.Hirano Y., Yoshinaga S., Ogura K., Yokochi M., Noda Y., Sumimoto H., Inagaki F. (2004) J. Biol. Chem. 279, 31883–31890 [DOI] [PubMed] [Google Scholar]
- 41.Sun W., Wei X., Kesavan K., Garrington T. P., Fan R., Mei J., Anderson S. M., Gelfand E. W., Johnson G. L. (2003) Mol. Cell Biol. 23, 2298–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shankaranarayanan A., Thal D. M., Tesmer V. M., Roman D. L., Neubig R. R., Kozasa T., Tesmer J. J. (2008) J. Biol. Chem. 283, 34923–34934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golebiewska U., Scarlata S. (2008) Biophys. J. 95, 2575–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penela P., Ribas C., Mayor F., Jr. (2003) Cell. Signal. 15, 973–981 [DOI] [PubMed] [Google Scholar]
- 45.Ribas C., Penela P., Murga C., Salcedo A., García-Hoz C., Jurado-Pueyo M., Aymerich I., Mayor F., Jr. (2007) Biochim. Biophys. Acta 1768, 913–922 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y. (2007) Circulation 116, 1413–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimoto S., Nishida E. (2006) EMBO Rep. 7, 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]