Abstract
Photorhabdus luminescens is a pathogenic bacterium that produces many toxic proteins. The mono-ADP-ribosyltransferases (mARTs) are an enzyme class produced by numerous pathogenic bacteria and participate in disease in plants and animals, including humans. Herein we report a novel mART from P. luminescens called Photox. This 46-kDa toxin shows high homology to other actin-targeting mARTs in hallmark catalytic regions and a similar core catalytic fold. Furthermore, Photox shows in vivo cytotoxic activity against yeast, with protection occurring when catalytic residues are substituted with alanine. In vitro, enzymatic activity (kcat, 1680 ± 75 min−1) is higher than that of the related iota toxin, and diminishes by nearly 14,000-fold following substitution of the catalytic Glu (E355A). This toxin specifically ADP-ribosylates monomeric α-skeletal actin and nonmuscle β- and γ-actin at Arg177, inhibiting regular polymerization of actin filaments. These results indicate that Photox is indeed an ADP-ribosyltransferase, making it the newest member of the actin-targeting mART family.
Keywords: ADP-ribosylation, Cytoskeleton/Actin, Enzymes, Enzymes/Inhibitors, Enzymes/Structure, Methods/X-ray Crystallography, Toxins, Toxins/Drugs/Xenobiotics/Bacterial
Introduction
Photorhabdus luminescens is a motile, bioluminescent, Gram-negative bacterium belonging to the Enterobacteriaceae family and known to be an insect pathogen (1). Sequenced in 2003 (2), the P. luminescens genome encodes an extensive variety of toxins and hydrolytic enzymes, many of which are being studied as potential virulence factors. These bacteria live in close symbiosis with soil-dwelling Heterorhabditis nematodes. After Photorhabdus colonization of the nematode intestinal tract, the nematodes invade an insect host, and migrate to the hemolymph. Within this open circulatory system, Photorhabdus bacteria are released by regurgitation. An array of toxic compounds released by the bacteria eventually kills the insect host. Both nematode and bacteria feed on the insect cadaver and reproduce to repeat the cycle, each benefiting from their close relationship with the other (3).
Among other toxins, Photorhabdus bacteria produce toxin complexes, high molecular weight, multisubunit, insecticidal toxins (4), some of which show oral toxicity in the same range as Bacillus thuringiensis endotoxins (5), as well as the “makes caterpillars floppy” toxin, responsible for insect midgut destruction (6). Based on its pathogenesis and the high number of virulence factors that it produces, P. luminescens has garnered interest in the area of biopesticides due to increasing resistance against conventional pesticides (7).
Various highly pathogenic bacteria produce toxins that share the enzymatic function of covalently modifying a host protein through addition of an ADP-ribose moiety from NAD+. This covalent attachment of a bulky ADP-ribose group generally inhibits the natural function of the target protein, causing various deleterious effects within a cell. These toxins contribute to a wide variety of diseases in humans including diphtheria, pertussis, and cholera (8, 9). Historically, these mono-ADP-ribosyltransferase (mART)6 toxins have been divided into two groups: the DT group (named for diphtheria toxin) and the CT group (named for cholera toxin). Although the three known toxins of the DT group each target eukaryotic elongation factor 2, the numerous CT toxins are generally further classified depending on their targets within a host. To date, nine mART toxins have been identified that ADP-ribosylate actin and disrupt actin polymerization. Most of these are binary toxins consisting of an A component responsible for binding/translocation and a B component with mART enzymatic activity. The Clostridium toxins, Clostridium perfringens iota (10), Clostridium botulinum C2 toxin (11), Clostridium spiroforme Sa (12), and Clostridium difficile CDTa (13), along with Bacillus cereus vegetative insecticidal protein (14), function in this binary fashion. The remaining actin-targeting mARTs do not fit this architecture. SpvB of Salmonella enterica consists of a single domain, and is thought to gain cell entry via a type III secretion system (15). Likewise, Aeromonas salmonicida AexT uses a type III secretion system for invasion of host cells and carries a second functional domain with Rho-GAP activity (16, 17), reminiscent of the well characterized ExoS expressed by Pseudomonas aeruginosa. Streptococcus pyogenes SpyA (18) is thought to be a single-domain mART with a 30-residue signal sequence for which the mechanism of cell entry is not yet understood and most recently, VgrG1 was found to enter host cells via a type six secretion system (19).
Recently, the structure of a Michaelis complex with iota toxin, actin, and a non-hydrolyzable NAD+ analogue was described (20). Based on this structure, Tsuge et al. (20) provided some insight into substrate recognition. In particular they were able to show that Tyr62 on loop I and Arg248 on loop II play an essential role at the actin-toxin interface. The authors also proposed a common reaction mechanism for the actin-targeting mART toxins whereby an oxocarbenium intermediate is formed following the cleavage of the nicotinamide moiety from NAD+. Rotation then allows for the release of the conformational strain and the formation of a second cationic intermediate. Finally, the nucleophilic attack on Arg177 of the target actin leaves the ADP-ribose group covalently bound to this target protein (20).
Because overall primary sequence identity among mART family members is most often low, identification of new members must rely on a shared core structure (SCOP code d.166.1.1.), sequence identity in several key catalytic regions, and pathogenicity of the organism as a positive indicator. In particular, a region 1 catalytic Arg (His in the DT group), preceeded by an aromatic residue, aids in NAD+ binding and maintaining the active site structure. In region 2 of the CT group toxins, a Ser-Thr-Ser motif on a β-strand, preceded by aromatic hydrophobic residues, forms the scaffold of the active site and stabilizes NAD+ substrate binding. DT group toxins contain a Tyr-X10-Tyr motif, playing a similar role, where X denotes any amino acid. In region 3, the catalytic Glu is found on a β-strand and is responsible for the ADP-ribosyltransferase activity. A second Glu or Gln is found in CT group members two residues away, and may participate in substrate recognition, but the mechanism by which this occurs is not yet known (21, 22).
Increasing our collective knowledge of the mechanism of action of this toxic enzyme family, and the description of new members will provide additional targets for antibacterial interventions and lead to successful inhibitor design against mART enzymatic activity. These efforts will undoubtedly aid in the development of antivirulence strategies and novel therapeutics for the prevention and treatment of bacterial diseases.
Herein we describe a novel putative virulence factor, Photox, produced by P. luminescens, as the newest member of the mART family. This enzyme shows primary sequence homology in key catalytic regions, and the mART overall fold in the catalytic core. It possesses ADP-ribosyltransferase activity and specifically targets Arg177 of actin. Photox enzymatic activity is relatively high (kcat, 1680 ± 75 min−1) among actin-targeting mARTs, and it is shown to abolish actin polymerization upon covalent modification at Arg177.
EXPERIMENTAL PROCEDURES
Identification and Modeling in Silico
The plu0822 gene was identified as a putative mART in silico after searching the Genomic Threading Data base (23) using SCOP code d.166.1.1 (24). The predicted fold was confirmed by metaservers 3D-JURY (25), Genesilico (26), and Pcons (27). The characteristic mART primary sequence pattern was confirmed using ScanProsite (28, 29) and multiple sequence alignments using three-dimensional Coffee (30). Photox was modeled against the SpvB-NAD+ (PDB code 2GWL) using MODELLER (31), and quality assessed by MetaMQAPII (32). The Photox-actin-NAD+ complex was prepared by the superposition of Photox-NAD+ in place of iota toxin (PDB code 3BUZ) using Coot (33).
Overexpression and Purification of Photox in E. coli
The Photox gene was overexpressed in Escherichia coli cells and the protein was purified from inclusion bodies. In brief, the Photox gene was cloned into a pET-28b vector with an N-terminal His6 tag and a tobacco etch virus protease (TEV-8) site. E. coli Rosetta cells were transformed with plasmid and plated onto 2× YT plates containing ampicillin to grow overnight at 37 °C. Cells were grown at 37 °C in 2-liter cultures of 2× YT containing 100 μg/ml of ampicillin to an A600 value of ∼0.6 before induction with 1 mm isopropyl 1-thio-β-d-galactopyranoside. Three hours post-induction cells were harvested by centrifugation at 5,000 × g for 10 min. Cell pellets were resuspended in 20 mm Tris-HCl, pH 7.5, 50 mm NaCl and the cells were lysed using a French press. Lysate was centrifuged for 25 min at 14,000 × g and the pellet was resuspended in 20 ml of inclusion body wash buffer (50 mm Tris-HCl, pH 7.5, 2 mm EDTA, 100 mm NaCl, 0.05% deoxycholate, 0.5 mg/ml of lysozyme). After further centrifugation at 10,000 × g for 20 min the pellet was resuspended in 15 ml of denaturation buffer (50 mm Tris, pH 7.5, 500 mm NaCl, 3 m guanidine hydrochloride). The supernatant from a spin at 20,000 × g for 40 min was diluted to 30 ml with denaturation buffer and spiked to 5 mm imidazole. The sample was loaded to a nickel-charged chelating Sepharose column and eluted with 0–100 mm imidazole, without guanidine hydrochloride. The resulting protein was dialyzed into 10 mm Tris-HCl, pH 7.5, 100 mm NaCl and concentrated to 0.5–1.0 mg/ml using an Amicon Centriprep concentrator (Millipore, Billerica, MA).
Purification of Actin
Chicken skeletal α-actin was purified as described (34) and concentrations were determined by absorbance at 290 nm (ϵM = 67,742 m−1 cm−1). β- and γ-actin were purified using the baculovirus method described previously by Yates et al. (35). The β- and γ-actin mixture was obtained from Cytoskeleton (Denver, CO).
Photox Substrate Assays
Biotinylated ADP-ribosylated (ADPr) actin was detected using a one-step Western blot as described (29), with the exception of purified actin as substrate and lysate from Chinese hamster ovary cells. Fluorescein isothiocyanate (FITC)-ADP-ribose labeling of actin isoforms was obtained by incubating 19 μm FITC-NAD+ with 5 μm Photox and 2 μg of purified β- or γ-actin for 1 h at 25 °C in the dark. Proteins were separated by SDS-PAGE and visualized using UV illumination and a fluorescein filter on a FluorChem 8900 (Alpha Innotech, San Leandro, CA) instrument.
mART Kinetic Assays
Using a stopped-flow spectrometer, model SX20-MV (Applied Photophysics, Leatherhead, UK), 80 nm Photox was mixed rapidly in a 1:1 (v/v) ratio with varying concentrations of etheno-NAD+ (ϵ-NAD+) and actin after equilibration of all samples to ambient temperature. Increasing fluorescence intensity was recorded as the reaction progressed (excitation 305 nm, emission 385 nm cut-off filter). Individual reactions were repeated with actin concentrations ranging from 0 to 15 μm and ϵ-NAD+ concentrations ranging from 0 to 300 μm. All reactions were performed in 10 mm Tris-HCl, pH 7.5, 0.2 mm ATP, 0.2 mm CaCl2, β-mercaptoethanol (β-ME). A calibration curve was created by measuring fluorescence intensity changes upon completion of the reaction at various actin concentrations in the presence of excess ϵ-NAD+, and by assuming the molar amounts of actin and ϵ-NAD+ consumed by the reaction are equal. NAD+ binding and NAD+ glycohydrolase activities were measured as described previously (36).
Mass Spectrometer Analysis of Modified Actin
ADP-ribosylated α-actin was analyzed by LC/MS/MS mass spectrometry following AspN digestion to determine the site of the 541-Da ADP-ribosylation.
Yeast Growth-Inhibition Assay
Saccharomyces cerevisiae haploid strains W303 (MATa leu2 trp1 can1 ura3 ade2 his), DBY6945 (MATa his3 leu2 ura3 tub2 ACT1::LEU2 [pRB668(URA3)-ACT1]), and ACT-RA (MATa his3 leu2 ura3 tub2 ACT1::LEU2 [pJD301(HIS3)-ACT1 R177A]) were cultured at 30 °C on yeast-peptone-dextrose (YPD) or synthetic dextrose (SD) minimal medium missing the appropriate amino acid.
Photox gene constructs were individually cloned into the modified yeast shuttle vector pRS415 and pRS416 containing the yeast CUP1 promoter (37) for expression in yeast. The actin R177A construct was prepared in E. coli in the pET-28a plasmid and subcloned into the pJD301 plasmid, modified from the pRS313 plasmid to contain the ACT1 promoter and terminator sequences.
The ACT-RA yeast strain was prepared by shuffling the pRB668-ACT1 plasmid with the pJD301-ACT1 R177A plasmid in the DBY6945 yeast strain using 5-fluororotic acid selection (S.D. + 0.5% 5-fluororotic acid). All yeast growth inhibition assays were performed as described previously (37) with slight modifications.
Native PAGE
Actin (10 mm Tris-HCl, 0.2 mm CaCl2, 0.2 mm ATP, 0.2 mm β-ME, 0.2 mm ϵ-NAD+) was incubated for 1 h at ambient temperature in the presence or absence of 1 nm Photox. Samples (3 μg) were subjected to native PAGE on 10% non-reducing, non-denaturing acrylamide gels supplemented with 0.2 mm ATP and 0.2 mm CaCl2. Samples were run on ice at low-voltage (<80 V) to prevent protein denaturation. Protein visualization was performed first under UV in a FluorChem 8900 gel-doc system, and subsequently by staining with Coomassie Brilliant Blue R-250.
Polymerization Sedimentation Assay
Polymerization of 10 μm actin and ADPr-actin was monitored using an actin sedimentation assay. Actin and ADPr-actin solutions were brought to 25 mm Tris-HCl, pH 8.0, 50 mm KCl, 1 mm EGTA, 2 mm MgCl2, 0.2 mm ATP, 0.2 mm β-ME using a 10-fold stock of polymerization salts. Sample mixtures were incubated at room temperature for 3 h to allow for complete polymerization. Actin and ADPr-actin were subjected to centrifugation at 392,000 × g for 20 min. Supernatants were carefully removed and pellets were re-suspended in 25 mm Tris-HCl, pH 8.0, 2 mm MgCl2, 0.2 mm ATP, 0.2 mm β-ME. An equal volume of 2× Laemmli buffer was added to the samples. Samples were then subjected to SDS-PAGE and band intensities were determined using ImageJ.
Pyrene Fluorescence
Actin was labeled with pyrene iodoacetamide as previously described (38), frozen in liquid N2, and stored at −80 °C. Actin samples in buffer G (2 mm Tris-HCl pH, 8.0, 0.2 mm ATP, 0.2 mm CaCl2, 0.2 mm β-ME) were diluted and brought to 2.5% pyrene-actin. Polymerization of 10 μm actin was initiated by the addition of polymerization salts with a final concentration of 25 mm Tris-HCl, pH 8.0, 50 mm KCl, 2 mm MgCl2, 0.2 mm ATP, 1.0 mm EGTA. Fluorescence intensity was monitored using a Cary Eclipse spectrophotometer at excitation and emission wavelengths of 347 and 407 nm, respectively. Fluorescence spectra were baseline subtracted and t = 0 was taken at the point of polymerization induction.
Phalloidin-stabilized Actin ADP-ribosylation
Actin (10 μm, 2.5% pyrene, 200 μm NAD+) was polymerized for a period of 160 min in the presence or absence of a 2-fold molar excess of phalloidin. Samples were treated with 1 ng of Photox and the pyrene fluorescence intensity was measured as described previously.
Fluorescent Yeast Labeling
W303a yeast cells grown with 150 μg/ml of adenine to an A600 value of 0.8 were fixed in 3.7% (v/v) formaldehyde. After centrifugation (5,000 × g, 5 min) cells were washed twice with PEM buffer (0.4 m PIPES, pH 6.9, 20 mm EGTA, 20 mm MgCl2) and resuspended in 25% (v/v) methanol in PEM buffer. After addition of 1.5 μm rhodamine phalloidin, cells were stained in the dark for ∼30 min before washing and resuspension in 100 μl of PEM buffer. The 4 μl of stained cell suspension was visualized with a Nikon Eclipse 6600 epifluorescent microscope.
RESULTS AND DISCUSSION
plu0822 Encodes a Putative mART
The gene plu0822 of P. luminescens strain TT01 was found to encode a protein of 408 residues (45.9 kDa) with high identity to other mART toxins. Although a simple BLAST search identifies this protein as a putative mART, this search strategy alone is prone to false positives and benefits from further substantiation by fold-recognition and a pattern-based search. This two-domain protein, named Photox, shares 39% primary structure identity overall with SpvB of S. enterica. Specifically, the C-terminal 200 residues of Photox share 61% identity in primary sequence with the catalytic domain of SpvB. Identity in catalytic signature regions and a predicted mART-fold indicate that plu0822 encodes a putative mART enzyme, and that Photox is likely to have enzymatic activity akin to other toxins of this family. A primary sequence alignment of Photox with several known actin-targeting mART toxins is shown in Fig. 1A. The alignment reveals strong primary sequence identity in region 1 for the catalytic Arg288 preceded by a Tyr, the region 2 Ser-Thr-Ser motif preceded by aromatic and hydrophobic residues, and region 3 primary and secondary glutamate residues, Glu355 and Glu353, respectively. Together these sequence characteristics provide strong evidence that Photox is a new mART toxin member.
FIGURE 1.
Identification and purification of Photox. A, sequence alignment of several mARTs. Characteristic catalytic residues are indicated. Region 1 contains the catalytic arginine, region 2 includes the STS motif, and region 3 contains the catalytic and secondary glutamate residues. General secondary structure for these regions is indicated above the sequences (arrow denotes β-sheet). B, effects of Photox expression on yeast growth. Growth of S. cerevisiae expressing WT or mutant Photox with point mutations to catalytic glutamate residues. The WT (gray), E353A (white), E355A (striped), or E353A/E355A (black) gene in pRS415 was induced with Cu2+ for 48 h. C, purified Photox as analyzed by SDS-PAGE and Coomassie staining. Lane 1, protein molecular mass standards (Bio-Rad) in kDa; lane 2, purified Photox.
ADP-ribosyltransferase Activity Is Responsible for Cytotoxicity in Vivo
The plu0822 gene encoding Photox was cloned into a S. cerevisiae vector under the transcriptional control of the CUP1 promoter on a low-copy number plasmid by homologous recombination, as described by Turgeon et al. (37). Basal expression of Photox in yeast cells was sufficient to incur a severe growth-defective phenotype in yeast (Fig. 1B, gray bars), demonstrating the highly toxic nature of this protein in a eukaryotic cell system. Unfortunately, complete repression of the CUP1 promoter is not possible because Cu2+ is required for yeast cell viability and no repressor is known for this system (39). In contrast, the E353A mutation partially suppresses the lethal effects of the toxin (Fig. 1B, white bars) except at the higher Cu2+ concentration, confirming its accessory role in the catalytic activity of the enzyme. The effects of the E355A mutation restored yeast growth much more significantly than the E353A mutant, identifying Glu355 as the primary catalytic residue (Fig. 1B, striped bars). Importantly, the double mutations, E353A/E355A, almost completely abolished toxin cytotoxicity at all Cu2+ induction levels (Fig. 1B, black bars), indicating both residues are required for full mART activity in vivo and that this activity is entirely responsible for the observed growth-defective phenotype observed in yeast.
Photox Shows mART Activity against Actin
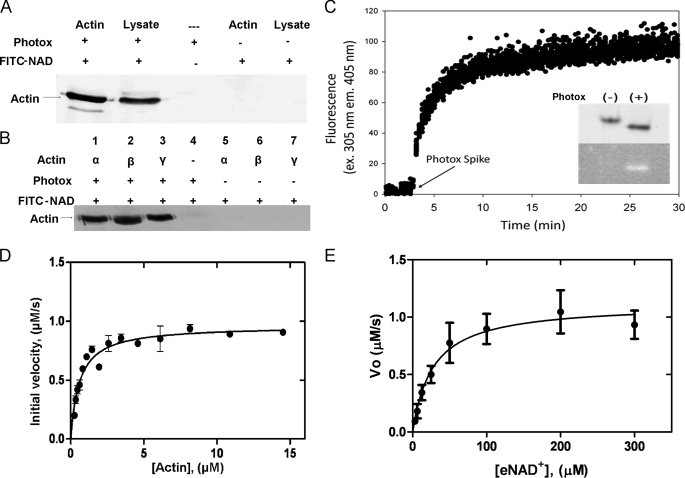
The plu0822 gene was cloned into the E. coli pET-28b vector and overexpressed in E. coli Rosetta cells. Photox was successfully purified from an insoluble state by simultaneous renaturation during immobilized metal affinity chromatography. The purity level and the relative mobility of the protein by SDS-PAGE indicated that we had isolated the plu0822 protein product (Fig. 1C). Photox was conclusively identified as a His-tagged protein by Western blotting using a monoclonal antibody against the poly-His tag (data not shown). The yield of purified Photox was ∼10 mg/liter of culture. The toxin was tested for in vitro mART activity by incubating Photox with biotin-NAD+ and target cell lysate. ADP-ribosylated proteins carrying the biotin label (biotin-ADP-ribose) were detected on a nitrocellulose membrane following SDS-PAGE by a colorimetric reaction as described previously (40). The Photox catalyzed mART activity in the presence of biotin-NAD+ and purified α-actin resulted in biotin-ADP-ribose labeling of actin. A similar reaction with Chinese hamster ovary lysate resulted in a single band of the same molecular weight. The lack of other ADP-ribosylated proteins in the Chinese hamster ovary lysate indicates that actin is the primary target of this mART toxin (Fig. 2A). In addition, the substrate specificity of Photox was observed using various actin isoforms. Incubation of purified muscle α-actin or nonmuscle β- or γ-actin with Photox and FITC-NAD+ allowed for fluorescent visualization of ADP-ribosylated actin following SDS-PAGE. Photox proved to target each of the three actin forms as substrates in the mART reaction (Fig. 2B). Although other actin-targeting mART enzymes have been characterized using a β- and γ-actin mixture (41–43), we show here that all three purified actin isoforms individually serve as targets of this reaction. Like Photox, SpvB, iota, Sa, and CDTa toxins have each been shown to lack specificity toward actin isoforms, which is in sharp contrast to the activity of C2 toxin, specific for β- and γ-nonmuscle forms of actin (44). Based on the structure of the iota-actin complex, Tsuge et al. (20) listed only three residues that are found at the toxin-actin interface and which are different in muscle and nonmuscle actin. One of these, Tyr279 of actin interacts with Tyr62 of iota, which was shown to be an essential residue for mART activity (20). Interestingly, a sequence alignment at this position based on PDB structure files reveals a tyrosine residue for each iota toxin, SpvB, CDTa, and Sa, but a threonine residue in C2. Therefore, this residue may play an important role in discrimination between actin isoforms. However, the N-terminal region of Photox could not be modeled accurately, and a sequence alignment at this position does not reveal any meaningful conclusions. Preliminary kinetic characterizations using purified skeletal α-actin and a mixture of β- and γ-actin showed that mART activity of Photox was only slightly higher (∼3-fold, data not shown) with non-muscle actin as the substrate. As a result, in-depth kinetic characterization was conducted with α-actin as a substrate due to its availability.
FIGURE 2.
ADP-ribosylation of actin by Photox. A, Western blot showing biotin-ADP-ribose labeling of actin. Lane 1, 0.25 μg of purified actin labeled with Photox; lane 2, Chinese hamster ovary lysate labeled by Photox; lanes 3–5, various controls. B, labeling of various actin isoforms by Photox using FITC-NAD+. Lanes 1–3, 0.5 μg of α-, β-, or γ-actin incubated with Photox and FITC-NAD+; lanes 4-7, various controls. C, fluorescence intensity trace of the reaction of α-skeletal actin, ϵ-NAD+ and Photox, showing increasing fluorescence intensity following the addition of Photox. Native-PAGE analysis of actin (inset) in the presence and absence of Photox shows a shift in relative mobility upon ADP-ribosylation. Ultraviolet visualization (lower panel) shows detectable fluorescence only in the presence of Photox. Michaelis-Menten plots of Photox mART activity. D, dependence of initial velocity on actin substrate concentration. Measurements were obtained using 200 μm ϵ-NAD+ and 40 nm Photox at 25 °C, and fit to a standard Michaelis-Menten model. E, activity as a function of ϵ-NAD+ concentration. Measurements were obtained using 3.5 μm actin and 40 nm Photox at 25 °C, and were fit to a substrate inhibition model. Error bars indicate S.E.
Kinetic Characterization of Photox mART Activity
A fluorescence-based assay was used to characterize the mART activity of Photox using highly purified α-actin as protein substrate. Using a stopped-flow spectrophotometer to determine initial reaction rates, Photox (40 nm) was incubated with varying concentrations of actin and etheno-NAD+. In the presence of excess ϵ-NAD+, the reaction proceeded to completion after 30 min (Fig. 2C), and a band-shift assay involving native PAGE revealed that at equilibrium nearly all of the actin is ADP-ribosylated (Fig. 2C, gel inset).
Photox mART activity exhibited Michaelis-Menten kinetic behavior with respect to the actin substrate (Fig. 2D). The Michaelis-Menten constant, Km, was found to be 0.60 μm for α-actin. As shown in Table 1, Photox is a highly efficient and active mART enzyme (kcat/Km = 107–109 m−1 min−1) with a substrate turnover number, kcat, of approximately 2200 min−1. This measure of activity is comparable with that of iota toxin (kcat = 1680 ± 75 min−1) (46). Fig. 2E displays the kinetic data at various ϵ-NAD+ concentrations fit to the Michaelis-Menten model. The Michaelis-Menten constant of Photox with respect to ϵ-NAD+ was found to be 45 μm, which is only slightly higher than that observed for iota toxin (6.0 μm) (46).
TABLE 1.
Kinetic parameters for Photox mART activity
Kinetic parameters were measured as described under “Experimental Procedures.” All measurements represent the mean ± S.D. of at least 5 replicates.
| Parameter | Varied substrate |
|
|---|---|---|
| ϵ-NAD+ | Actin | |
| Km (μm) | 27.4 ± 4.4 | 0.60 ± 0.082 |
| Vmax (μm min−1) | 67.2 ± 3.0 | 57.6 ± 1.86 |
| kcat (min−1) | 1680 ± 75 | –a |
| kcat/Km (m−1 min−1) | 6.13 × 107 | 2.4 × 109 |
a kcat could not accurately be determined using varied concentrations of actin due to propensity of actin to polymerize at high concentrations under the conditions required for this reaction.
Additionally, significant impairment in Photox mART activity was observed when signature catalytic residues were replaced with alanine. The most dramatic effect was evident when the region 1 conserved Arg was substituted with Ala (R288A showed greater than 20,000-fold reduction in kcat; Table 2). Replacement of the primary and secondary region 3 Glu residues with Ala resulted in 13,000- and 600-fold reduction in mART activity, respectively. The conversion of the region 2 Ser-Thr-Ser motif to Ala-Thr-Ala decreased the activity by 2000-fold. These dramatic effects on mART activity indicate that these residues do indeed play an important catalytic role in the enzymatic activity of Photox, which strongly correlates with other actin-targeting members of this toxin family. Previous studies have shown comparative relative activity levels for mutations in these signature catalytic residues using C2I from C. botulinum and iota toxin from C. perfringens (46, 47). Importantly, none of these mutations affected the NAD+-binding activity of Photox in the absence of the actin protein substrate, because all catalytic signature mutants had similar NAD+ binding constants (KD) to the wild-type (WT) enzyme (Table 2).
TABLE 2.
Folded stability, kinetic activity, and substrate affinity of hallmark Photox mutants
Measurements were obtained as described under “Experimental Procedures” and represent the mean ± S.D. Trp λ emission measurements represent at least 3 replicates; mART activity, at least 5 replicates.
| Photox mutant | Trp λ emission maximum | Relative kcat | Relative KD |
|---|---|---|---|
| nm | mART activity | NAD+ | |
| Wild-type | 344 ± 0.6 | 1.00 | 1.00 ± 0.03 |
| E353A | 343 ± 0.6 | 0.00156 | 1.22 ± 0.08 |
| E355A | 344 ± 0.1 | 0.0000737 | 3.67 ± 0.14 |
| R288A | 343 ± 0.1 | ≈0a | 4.28 ± 0.45 |
| STS/ATA | 343 ± 0.1 | 0.000470 | 5.41 ± 1.07 |
a Measurement could not be accurately determined as it was near background levels.
Notably, NAD+-glycohydrolase activity was not detectable for WT and mutant Photox enzymes, although most mARTs are known to possess this residual activity to hydrolyze the NAD+ substrate. Among actin-targeting mARTs, both C2I and iota toxin show NAD+-glycohydrolase activity (43, 47), whereas SpvB does not (42, 48) further highlighting the similarity of this toxin with Photox.
We used Trp fluorescence wavelength emission maximum (λem maximum) values to assess the folded integrity of WT and mutant Photox enzymes and the data indicated that all of the mutants were folded similar to the WT enzyme (λem maximum = 343–344 nm; Table 2). This was further corroborated by circular dichroism spectroscopic analysis of the proteins (supplemental Fig. S1).
Photox-catalyzed ADP-ribosylation on Arg177 of Globular Actin Inhibits Actin Polymerization
Polymerization of actin was monitored using a pyrene fluorescence assay (Fig. 3A). In the presence of polymerization salts, globular actin showed an increase in pyrene fluorescence due to polymerization, and filament elongation achieves >95% completion over 60 min. In the absence of polymerization salts no increase in fluorescence was seen, implying the lack of actin polymerization and that it remains in its monomeric form. ADPr-actin was tested under similar conditions and was found to be polymerization deficient, indicating that ADP-ribosylation inhibits regular actin polymerization. In addition, sedimentation assays showed >95% pelleting of actin in the presence of polymerization salts and conversely >95% ADPr-actin remained in the supernatant under similar conditions (Fig. 3B). Densitometry of four similar sedimentation assays indicated that ADPr-actin remains within the supernatant under polymer-forming conditions (data not shown). Pyrene fluorescence was further used to observe the effect of Photox on actin filaments (Fig. 3C). The addition of Photox to F-actin showed a dramatic fluorescence decrease, which could be prevented by filament stabilization with phalloidin. These results suggest that Photox is unable to ADP-ribosylate stabilized actin filaments and that the reduction in pyrene fluorescence in the absence of phalloidin likely occurs through a treadmilling type of mechanism.
FIGURE 3.
Photox catalyzed ADP-ribosylation on Arg177 of globular actin inhibits actin polymerization. A, pyrene fluorescence assay of actin polymerization. Actin (circle) or ADPr-actin (triangle) was supplemented with either G-buffer (open) or polymerization salts (closed). B, sedimentation assays of actin polymerization. Left, pellet and supernatant fractions showing >95% pelleting of actin in the presence of polymerization salts. Right, >95% ADPr-actin remains in the supernatant under similar conditions. C, effect of Photox on actin treadmilling. Pyrene fluorescence intensity of polymerized actin (×), F-actin stabilized with a 2 m excess of phalloidin to prevent treadmilling (squares), F-actin stabilized with phalloidin and treated with Photox (triangles), and F-actin treated with Photox (circles). D, growth recovery in yeast producing R177A mutant actin (gray) as compared with wild-type actin (black) when treated with Photox. Error bars show the S.D. of 8 replicates. The 100% horizontal line represents growth of yeast expressing a non-toxic aminotransferase protein, as described by Turgeon et al. (37).
R177A Actin Mutant Confers Resistance to Photox in Vivo
The specific site of actin modification was analyzed in vivo using a yeast assay. A mutant yeast strain (ACT-RA) harboring a single plasmid-encoded copy of the ACT1 gene that codes for the R177A ACT1 mutant protein was employed. The W303 WT yeast strain was sensitive to Photox upon toxin gene induction with Cu2+ (Fig. 3D, black bars), but the ACT-RA strain showed resistance to Photox (Fig. 3D, gray bars). These results support the conclusion that Photox specifically ADP-ribosylates actin at Arg177, the same target residue of other actin-targeting mART toxins. Replacement of this residue with Ala inhibits the ADP-ribosylation of α-actin by Photox. Furthermore, this single point mutant of actin is sufficient to completely reverse the cytotoxic effects of Photox, suggesting that ADP-ribosylation of actin at Arg177 is the only cytotoxic activity linked with Photox. The site of ADP-ribosylation on α-actin was confirmed by LC/MS/MS mass spectrometry of ADPr-actin following AspN digestion and it was revealed that the only modification site was at Arg177. Fig. 4A shows the fragmentation pattern of the ADP-ribose group. Fig. 4B displays the b- and y-ions resulting from fragmentation of the peptide containing this modification. For this peptide, b-ions are easily identifiable up to b-20, and no modifications are seen on any of these first 20 amino acids, leaving only Arg or Leu as the site of modification. Because Leu is not ionizable, the modification cannot exist here. A single site of modification on the protein is confirmed by mass spectrometry analysis of labeled and unlabeled actin show a difference of 541.28 Da (supplemental Fig. S2), equivalent to a single ADP-ribose group. Additionally, greater than 99% ADP-ribosylation efficiency was confirmed by mass spectrometry.
FIGURE 4.
Mass spectrometry analysis of the ADP-ribosylation site on α-actin. A, fragmentation of the ADP-ribose group as produced by LC/MS/MS following AspN digestions. B, spectrum showing b- and y-ions for the peptide fragment containing an ADP-ribose modification. Peaks are labeled by mass and the primary sequence is shown at the top, including Arg177, the site of ADP-ribosylation.
Photox Co-localization with Actin
To visualize the Photox-actin interactions within the cell, fluorescence microscopy of yeast cells expressing green fluorescent protein-Photox was used to observe the possible co-localization with actin (Fig. 5A). Visualization of actin using rhodamine-phalloidin resulted in images that could be overlaid with those depicting green fluorescent protein-Photox. Yellow regions of overlapping intensity indicate some co-localization of actin with Photox within the cell. Some areas where co-localization is not seen may be due to Photox association with G-actin, which would have been detected by rhodamine-phalloidin.
FIGURE 5.
Structural model and localization of Photox. A, localization of Photox and actin within yeast cells. Fluorescent microscopy images of yeast (W303a) expressing green fluorescent protein-Photox and stained with rhodamine-phalloidin. (i) Green fluorescent protein-Photox expression, (ii) rhodamine-phalloidin staining of F-actin, (iii) overlay of panels i and ii where yellow regions indicate co-localization. Arrows highlight several of these regions. B, homology model of the Photox-actin-NAD+ complex. Schematic representation of Photox (gray) and actin (blue) with NAD+ shown in pink and Arg177 in green. The structure of Photox was modeled on SpvB-NAD+ (PDB code 2GWL), which was then aligned with iota toxin in an iota-actin complex (PDB code 3BUZ) using Coot. Inset, important active site residues of the Photox-actin complex. NAD+ (black) is seen in the active site of the modeled complex of Photox (gray) and actin (blue surface representation). Catalytically important residues are shown in stick representation, colored by atom, and Arg177 of actin is shown in stick representation in blue. Important loops are in schematic and colored yellow (ARTT loop) and green (PN loop), and the region B active site loop is seen behind the actin in red. C, Oda model of an F-actin trimer (40). Distinct actin subunits are shown in shades of blue. Arg177 site of ADP-ribosylation is indicated by green spheres. Inset shows a magnification of residues at the subunit interface at Arg177 shown in spheres in shades of purple. These residues include Val194, Glu195, Ser199, Phe200, and Val201.
Photox Homology Modeling
Due to limited solubility, Photox proved recalcitrant to x-ray crystallography. Thus, we employed a homology modeling approach to help understand the structure-function relationships of Photox and its interactions with NAD+ and actin. A model of the Photox catalytic domain (Fig. 5B) was built on the 1.9-Å x-ray structure of NAD+-bound SpvB from Salmonella typhimurium (PDB code 2GWL), and spans 200 residues in length. High identity (61%) between these two proteins allows for very reliable and accurate modeling. Importantly, there are no insertions or deletions in the alignment between these two proteins, and Photox includes a homologous region to the 30-residue segment previously unique to SpvB. Quality assessment on the model estimates GDT_TS at 45.62, a respectable score for homology modeling (GDT_TS stands for Global Distance Test_TotalScore and is a measure, or estimate, of the position difference between Cα atoms in theoretical and experimental models; further quality assessment can be found in supplemental Fig. S3).
The catalytic domain of Photox is an α/β protein primarily containing anti-parallel β-sheets and having separate α and β regions characteristic of the ADP-ribosylation fold (SCOP code d.166.1.1, CATH code 3.90.176.10). This model contains two sheets, two β hairpins, two β bulges, eight strands, nine helices, six helix-helix interactions, and 10 β turns.
As with other members of this toxin family, NAD+ binding is expected to occur through both hydrogen bonds and hydrophobic interactions. Hydrogen bonds are predicted as follows: Glu313 binds to adenine, Lys291 and Lys294 both bind to adenine-ribose, Arg231 and Asp227 bind to adenine phosphate, Arg288 binds to adenine and/or nicotinamide phosphate, Ser219 binds to nicotinamide ribose, and Gly289 binds to nicotinamide. The active site is stabilized by Ser318, which hydrogen bonds to Glu355. Thr319 forms hydrogen bonds to an adjacent β-sheet to maintain active site integrity. Ser320 orients the ARTT loop, including Glu353. These proposed interactions help to explain the observed decreases in activity after replacement of signature mART catalytic residues with alanine as reported above. Gly289 was not substituted because its main chain atoms are involved in interactions within the active site, and any residue would be expected to have a similar effect. Ala replacement of those residues indicated, but not substituted in this study, are also expected to negatively affect mART activity to varying degrees based on conservation among C2- and C3-like mARTs.
The Photox homology model was assembled into a complex with actin based on the recent iota-actin 2.81-Å x-ray structure (PDB code 3BUZ). In the proposed complex, 22 Photox residues interact with 27 actin residues over an ∼1,300 Å2 area. Notably, Tyr223 and Phe350 of Photox are in place to potentially interact with Arg177 of actin through non-bonded van der Waals contacts.
Photox, like other mARTs, likely recognizes actin through the region B active site loop (residues 238–254), the PN loop (residues 321–329), and the ARTT loop (residues 344–354) (48). The reaction is expected to proceed through an Sn1-alleviated strain mechanism (20). In this regard, Glu355 is poised to hydrogen bond to the nicotinamide ribose, whereas phosphate electrostatic interactions hold the NAD+ in a conformation favoring oxocarbenium cation formation. Tyr223 is in place to possibly assist bond rotation about the nicotinamide phosphate single bond to perhaps reposition the nicotinamide ribose. Actin Asp179 may stabilize the nicotinamide ribose and Photox Glu353 is in range to stabilize actin Arg177 so it can proceed with nucleophilic attack of the oxacarbenium cation.
Using the Holmes model of filamentous actin, it has been previously shown (49) that the location of the ADP-ribosylation on actin Arg177 is an important region of intersubunit interactions. Because crystal structures of ribosylated actin monomers reveal no large structural changes due to this modification, it has been suggested that polymerization is impeded due to steric hindrance, and not by allosteric changes (49). Residues involved in intersubunit interactions and predicted to be affected by the ADP-ribosylation include Glu195, Ser199, Phe200, and Val201. Recently, the Oda model (50) has replaced the Holmes model of filamentous actin. Inspection of the current Oda model reveals few differences in the interaction of these residues with Arg177 of an adjacent subunit. Using this new model, Val194 is also predicted to be involved in subunit interactions with Arg177, and all such residues are highlighted in Fig. 5C. We suggest that the change in proposed actin structure should not present large changes for studies of actin-targeting mARTs.
Photox varies considerably from SpvB in domain organization. Unlike the single-domain SpvB, Photox includes an N-terminal domain from residue 1 to approximately 185. The Photox N-terminal domain is presently an enigma, as it is predicted to be disordered by GeneSilico (26). A BLAST search did not identify any close homologs to the B component of other actin-targeting mARTs indicating that Photox may not function in the typical binary fashion. Based on the disorder, it is predicted that this domain may participate in chaperone binding in a secretion system, or perhaps entry of the toxin into the host cell, but details are not yet known. Intriguingly, genetic neighborhood and gene fusion evidence collected on the STRING database suggests interactions between plu0822 (encoding Photox) and plu0826 (encoding a VgrG-type protein). VgrG may penetrate target cells during type VI secretion to serve as a translocator or an effector (51), presenting the possibility that Photox may infect cells via type VI secretion.
In summary, actin-targeting ADP-ribosyltransferase enzymes have been studied since C. botulinum C2 toxin was first reported in 1986 (23). The most recent addition to this group of toxins was made when SpyA from Streptococcus pyogenes was introduced in 2004 (18). Improved in silico search strategies including limited consensus primary sequence identification, fold-prediction, and secondary structure prediction have allowed for more accurate detection of new putative members of this family. Here we present convincing evidence that Photox is the newest addition to these actin-targeting toxins. We show that Photox shares high homology in the catalytic domain with several other known toxins, but has a domain organization unique from other mARTs. Photox shows catalytic activity both in vitro and in vivo and specifically targets all actin isoforms, inhibiting polymerization. Additionally, Photox is the latest toxic protein in the arsenal of those secreted by P. luminescens. Increased understanding of the mechanism of action of Photox and a complete description of Photox structure and function relationships will provide broader insight to this family of mARTs. This in turn will aid in the eventual discovery of potent inhibitors against these toxic enzymes, which could lead to novel therapeutics for the prevention and treatment of a wide range of bacterial diseases in humans.
Acknowledgments
We thank Dawn White and Gerry Prentice for excellent technical support during this research. We are grateful for help with yeast microscopy provided by Shawn Chafe, Manoja Eswara, and Andy McGuire, mass spectrometer analysis by Suya Liu, and valuable interpretation of results with Dyanne Brewer. We thank Ryan Demers for the generous donation of β- and γ-actin. We also thank Andres Nieto and Aimee Hutton for preliminary work.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- mART
- mono-ADP-ribosyltransferase
- β-ME
- β-mercaptoethanol
- CT
- cholera toxin
- DT
- diphtheria toxin
- ϵ-NAD+
- etheno-NAD+
- FITC
- fluorescein isothiocyanate
- WT
- wild-type
- PIPES
- 1,4-piperazinediethanesulfonic acid
- ADPr
- ADP-ribosylated.
REFERENCES
- 1.Fischer-Le Saux M., Viallard V., Brunel B., Normand P., Boemare N. E. (1999) Int. J. Syst. Bacteriol. 49, 1645–1656 [DOI] [PubMed] [Google Scholar]
- 2.Duchaud E., Rusniok C., Frangeul L., Buchrieser C., Givaudan A., Taourit S., Bocs S., Boursaux-Eude C., Chandler M., Charles J. F., Dassa E., Derose R., Derzelle S., Freyssinet G., Gaudriault S., Médigue C., Lanois A., Powell K., Siguier P., Vincent R., Wingate V., Zouine M., Glaser P., Boemare N., Danchin A., Kunst F. (2003) Nat. Biotechnol. 21, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 3.Waterfield N. R., Ciche T., Clarke D. (2009) Annu. Rev. Microbiol. 63, 557–574 [DOI] [PubMed] [Google Scholar]
- 4.Waterfield N. R., Bowen D. J., Fetherston J. D., Perry R. D., ffrench-Constant R. H. (2001) Trends Microbiol. 9, 185–191 [DOI] [PubMed] [Google Scholar]
- 5.ffrench-Constant R. H., Bowen D. J. (2000) Cell Mol. Life Sci. 57, 828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daborn P. J., Waterfield N., Silva C. P., Au C. P., Sharma S., Ffrench-Constant R. H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10742–10747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehlers R. U. (2001) Appl. Microbiol. Biotechnol. 56, 623–633 [DOI] [PubMed] [Google Scholar]
- 8.Deng Q., Barbieri J. T. (2008) Annu. Rev. Microbiol. 62, 271–288 [DOI] [PubMed] [Google Scholar]
- 9.Holbourn K. P., Shone C. C., Acharya K. R. (2006) FEBS J. 273, 4579–4593 [DOI] [PubMed] [Google Scholar]
- 10.Vandekerckhove J., Schering B., Bärmann M., Aktories K. (1987) FEBS Lett. 225, 48–52 [DOI] [PubMed] [Google Scholar]
- 11.Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. (1986) Nature 322, 390–392 [DOI] [PubMed] [Google Scholar]
- 12.Popoff M. R., Boquet P. (1988) Biochem. Biophys. Res. Commun. 152, 1361–1368 [DOI] [PubMed] [Google Scholar]
- 13.Gülke I., Pfeifer G., Liese J., Fritz M., Hofmann F., Aktories K., Barth H. (2001) Infect. Immun. 69, 6004–6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han S., Craig J. A., Putnam C. D., Carozzi N. B., Tainer J. A. (1999) Nat. Struct. Biol. 6, 932–936 [DOI] [PubMed] [Google Scholar]
- 15.Browne S. H., Hasegawa P., Okamoto S., Fierer J., Guiney D. G. (2008) FEMS Immunol. Med. Microbiol. 52, 194–201 [DOI] [PubMed] [Google Scholar]
- 16.Fehr D., Burr S. E., Gibert M., d'Alayer J., Frey J., Popoff M. R. (2007) J. Biol. Chem. 282, 28843–28852 [DOI] [PubMed] [Google Scholar]
- 17.Vilches S., Wilhelms M., Yu H. B., Leung K. Y., Tomás J. M., Merino S. (2008) Microb. Pathog. 44, 1–12 [DOI] [PubMed] [Google Scholar]
- 18.Coye L. H., Collins C. M. (2004) Mol. Microbiol. 54, 89–98 [DOI] [PubMed] [Google Scholar]
- 19.Suarez G., Sierra J. C., Erova T. E., Sha J., Horneman A. J., Chopra A. K. (2010) J. Bacteriol. 192, 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuge H., Nagahama M., Oda M., Iwamoto S., Utsunomiya H., Marquez V. E., Katunuma N., Nishizawa M., Sakurai J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7399–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han S., Arvai A. S., Clancy S. B., Tainer J. A. (2001) J. Mol. Biol. 305, 95–107 [DOI] [PubMed] [Google Scholar]
- 22.Vogelsgesang M., Aktories K. (2006) Biochemistry 45, 1017–1025 [DOI] [PubMed] [Google Scholar]
- 23.McGuffin L. J., Street S. A., Bryson K., Sørensen S. A., Jones D. T. (2004) Nucleic Acids Res. 32, D196–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murzin A. G., Brenner S. E., Hubbard T., Chothia C. (1995) J. Mol. Biol. 247, 536–540 [DOI] [PubMed] [Google Scholar]
- 25.Ginalski K., Elofsson A., Fischer D., Rychlewski L. (2003) Bioinformatics 19, 1015–1018 [DOI] [PubMed] [Google Scholar]
- 26.Kurowski M. A., Bujnicki J. M. (2003) Nucleic Acids Res. 31, 3305–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallner B., Larsson P., Elofsson A. (2007) Nucleic Acids Res. 35, W369–W374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Castro E., Sigrist C. J., Gattiker A., Bulliard V., Langendijk-Genevaux P. S., Gasteiger E., Bairoch A., Hulo N. (2006) Nucleic Acids Res. 34, W362–W365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fieldhouse R. J., Merrill A. R. (2008) Trends Biochem. Sci. 33, 546–556 [DOI] [PubMed] [Google Scholar]
- 30.Armougom F., Moretti S., Poirot O., Audic S., Dumas P., Schaeli B., Keduas V., Notredame C. (2006) Nucleic Acids Res. 34, W604–W608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eswar N., Eramian D., Webb B., Shen M. Y., Sali A. (2008) Methods Mol. Biol. 426, 145–159 [DOI] [PubMed] [Google Scholar]
- 32.Pawlowski M., Gajda M. J., Matlak R., Bujnicki J. M. (2008) BMC Bioinformatics 9, 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 34.Spudich J. A., Watt S. (1971) J. Biol. Chem. 246, 4866–4871 [PubMed] [Google Scholar]
- 35.Yates S. P., Otley M. D., Dawson J. F. (2007) Arch. Biochem. Biophys. 466, 58–65 [DOI] [PubMed] [Google Scholar]
- 36.Li M., Dyda F., Benhar I., Pastan I., Davies D. R. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 6902–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turgeon Z., White D., Jørgensen R., Visschedyk D., Fieldhouse R. J., Mangroo D., Merrill A. R. (2009) FEMS Microbiol. Lett. 300, 97–106 [DOI] [PubMed] [Google Scholar]
- 38.Cooper J. A., Walker S. B., Pollard T. D. (1983) J. Muscle Res. Cell. Motil. 4, 253–262 [DOI] [PubMed] [Google Scholar]
- 39.Arnoldo A., Curak J., Kittanakom S., Chevelev I., Lee V. T., Sahebol-Amri M., Koscik B., Ljuma L., Roy P. J., Bedalov A., Giaever G., Nislow C., Merrill A. R., Merrill R. A., Lory S., Stagljar I. (2008) PLoS Genet. 4, e1000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jørgensen R., Purdy A. E., Fieldhouse R. J., Kimber M. S., Bartlett D. H., Merrill A. R. (2008) J. Biol. Chem. 283, 10671–10678 [DOI] [PubMed] [Google Scholar]
- 41.Hochmann H., Pust S., von Figura G., Aktories K., Barth H. (2006) Biochemistry 45, 1271–1277 [DOI] [PubMed] [Google Scholar]
- 42.Sakurai J., Nagahama M., Hisatsune J., Katunuma N., Tsuge H. (2003) Adv. Enzyme Regul. 43, 361–377 [DOI] [PubMed] [Google Scholar]
- 43.Schleberger C., Hochmann H., Barth H., Aktories K., Schulz G. E. (2006) J. Mol. Biol. 364, 705–715 [DOI] [PubMed] [Google Scholar]
- 44.Gill D. M., Meren R. (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 3050–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deleted in proof
- 46.Barth H., Preiss J. C., Hofmann F., Aktories K. (1998) J. Biol. Chem. 273, 29506–29511 [DOI] [PubMed] [Google Scholar]
- 47.Otto H., Tezcan-Merdol D., Girisch R., Haag F., Rhen M., Koch-Nolte F. (2000) Mol. Microbiol. 37, 1106–1115 [DOI] [PubMed] [Google Scholar]
- 48.Sun J., Maresso A. W., Kim J. J., Barbieri J. T. (2004) Nat. Struct. Mol. Biol. 11, 868–876 [DOI] [PubMed] [Google Scholar]
- 49.Margarit S. M., Davidson W., Frego L., Stebbins C. E. (2006) Structure 14, 1219–1229 [DOI] [PubMed] [Google Scholar]
- 50.Oda T., Iwasa M., Aihara T., Maéda Y., Narita A. (2009) Nature 457, 441–445 [DOI] [PubMed] [Google Scholar]
- 51.Pukatzki S., Ma A. T., Revel A. T., Sturtevant D., Mekalanos J. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 15508–15513 [DOI] [PMC free article] [PubMed] [Google Scholar]