Abstract
In Drosophila, naked cuticle is an inducible antagonist of the Wnt-β-catenin pathway, likely acting at the level of Dishevelled (Dsh/Dvl), an essential component of this pathway. The mechanism by which naked cuticle and its two vertebrate orthologs, Naked1 (NKD1) and Naked2 (NKD2), inhibit Dvl function is unknown. NKD2 is myristoylated, a co-translational modification that leads to its plasma membrane localization. In contrast, myristoylation-deficient G2A NKD2 is cytoplasmic. Herein we show that the ability of Nkd2/NKD2 to antagonize Wnt-β-catenin activity during zebrafish embryonic development and in mammalian HEK293 cells is myristoylation-dependent. NKD2 and Dvl-1 interact and co-localize at the lateral membrane of polarized epithelial cells. In reciprocal overexpression and siRNA knockdown experiments, NKD2 and Dvl-1 destabilize each other via enhanced polyubiquitylation; this effect is also dependent upon Naked2 myristoylation. Cell fractionation and ubiquitylation assays indicate that endogenous NKD2 interacts with a slower migrating, ubiquitylated form of Dvl-1 in plasma membrane fractions. These results provide a mechanism by which NKD2 antagonizes Wnt signaling: myristoylated NKD2 interacts with Dvl-1 at the plasma membrane, and this interaction leads to their mutual ubiquitin-mediated proteasomal degradation.
Keywords: Colon Cancer, Protein Degradation, Protein Myristoylation, Ubiquitylation, Wnt Pathway, Dishevelled, Naked, Wnt Signaling, Myristoylation, Ubiquitylation
Introduction
The Wnt-β-catenin signaling cascade is a central regulator of cell fate determination during embryonic development, and its dysregulation contributes to oncogenesis (1). Upon Wnt binding to its receptor, Dishevelled (Dsh/Dvl)4 becomes activated, leading to the inactivation of the Axin/adenomatous polyposis coli (APC)/glycogen synthase kinase (GSK)3β complex that normally phosphorylates and degrades β-catenin (2). It is presently thought that Dvl recruits Axin from the β-catenin degradation complex to the plasma membrane (3). This results in a stabilized β-catenin that then translocates to the nucleus and binds to members of the TCF/LEF family of transcriptional factors, thus modulating expression of a broad range of target genes.
A reflection of the importance of Wnt-β-catenin signaling is that the cell has evolved a number of negative regulators so as to maintain precise control of this pathway (4–6). Among these are the NKD family, including Drosophila naked cuticle and its two vertebrate orthologs, NKD1 and NKD2. NKDs have been shown to negatively regulate canonical Wnt signaling through binding Dvl, although the mechanism for this inactivation is unknown (7–9). We have identified an additional role for NKD2, that is, to escort transforming growth factor-α (TGFα)-containing vesicles to the basolateral surface of polarized epithelial cells; this action of NKD2 is myristoylation-dependent (10). Myristoylation is an irreversible, co-translational protein modification found in fungi, higher eukaryotes, and viruses in which myristic acid, a 14-carbon saturated fatty acid, is covalently attached via an amide bond to the alpha amino group of an N-terminal glycine residue (11). The modification is catalyzed by the enzyme N-myristoyltransferase (EC 2.3.1.97) and occurs most commonly on glycine residues exposed during co-translational N-terminal methionine removal (12). Myristoylation of the N-terminal glycine residue of NKD2 is required for its plasma membrane localization, because wild-type NKD2 localizes to the plasma membrane, whereas G2A NKD2 mutant protein, which is myristoylation-defective, is trapped in the cytoplasm (10).
In this report, we show that the ability of NKD2 to antagonize Wnt-β-catenin signaling in vitro and in vivo is myristoylation-dependent. NKD2 and Dvl-1 interact and accelerate each other's degradation by promoting mutual polyubiquitylation. In a human colorectal cancer cell line, Caco-2, endogenous NKD2 binds to a specific form of endogenous DVL-1 at the plasma membrane whereupon they both undergo ubiquitin-mediated proteasomal degradation.
EXPERIMENTAL PROCEDURES
Materials
All cell culture reagents were from HyClone (Logan, UT). Anti-HA and anti-β-actin mouse monoclonal antibodies were from Sigma. A rabbit anti-Nkd2a serum was produced with synthesized oligopeptide (DSSSPDADQDPPSRSSHSQSRPH, residues 247–269 of zebrafish Nkd2a) and purified with immunoaffinity chromatography in collaboration with Covance (Denver, PA). VU308, a rabbit anti-NKD2 serum, was produced using the first 217 residues of human NKD2 and purified with immunoaffinity chromatography in collaboration with Cocalico Biologicals (Reamstown, PA). A second rabbit anti-NKD2 serum (R44) has been described previously (10). Human Dvl-1 siRNA and anti-Dvl-2 and -3 monoclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Protein G-agarose and Lipofectamine 2000 were from Invitrogen. A dual luciferase assay kit was from Promega (Madison, WI). Cycloheximide, MG132, ammonium chloride, and anti-α-tubulin monoclonal antibody were from Calbiochem. FuGENE 6 was from Roche Applied Science (Indianapolis, IN). Anti-Dvl-1 polyclonal antibody was kindly provided by Dr. Roel Nusse (Stanford University, Palo Alto, CA). His-ubiquitin construct and rabbit polyclonal anti-ubiquitin antibody were kindly provided by Dr. Allan M. Weissman. A HA-ubiquitin construct was kindly provided by Dr. Michael Freeman (Vanderbilt University). HA-Dvl-1, FLAG-Dvl-1, Wnt3a, and SuperTOPflash constructs were kindly provided by Dr. Ethan Lee (Vanderbilt University).
Cell Culture and Transfection
MDCK Tet-Off cells and HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% bovine growth serum, glutamine, nonessential amino acids, 100 units/ml penicillin, and 100 μg/ml streptomycin. For MDCK cells, NKD2 and G2A NKD2 expression was controlled by an inducible tetracycline system. HA-Dvl-1 was transfected with Lipofectamine 2000 according to the manufacturer's instructions. For HEK293 cells, NKD2, or HA-Dvl-1 was transfected with FuGENE 6 according to the manufacturer's instructions.
NKD2 and Dvl-1 siRNA Transfection
Caco-2 or HEK293 cells were grown in 6-well plates until 30% confluent. Dvl-1 siRNA was transfected with Lipofectamine 2000 according to the manufacturer's instructions.
Cell Fractionation
The plasma membrane, vesicles, and other subcellular organelles in Caco-2-TGFα cells were separated using an Iodixanol gradient fractionation method as described previously (13). Briefly, Caco-2-TGFα cells were washed and lysed in Solution E (0.25 m sucrose, 78 mm KCl, 4 mm MgCl2, 8 mm CaCl2, 10 mm EGTA, and 50 mm Hepes-KOH, pH 7.0). Cell debris was removed by multiple centrifugations at 1,000 × g for 5 min, 5,000 × g for 5 min, 10,000 × g for 10 min, and 15,000 × g for 20 min. The supernatant was further centrifuged for 60 min at 100,000 × g, and the pellet was resuspended in Solution E, applied to gradient iodixanol solutions, and centrifuged at 90,000 × g for 16 h. Successive 500-μl aliquots were taken from the top of the gradient, and each fraction was collected for Western blotting.
Immunoprecipitation and Western Blot Analysis
HEK293 cells cultured in 6-well plates were transfected with HA-Dvl-1 and NKD2-GFP. The cells were washed once with ice-cold phosphate-buffered saline and harvested using immunoprecipitation buffer containing 25 mm Hepes, pH 7.2, 10% glycerol, 1 mm EDTA, 1 mm EGTA, 50 mm KCl, 10 mm NaF, 10 mm Na4P2O7, 1.2 mm Na3VO4, 1% Nonidet P-40, and protease inhibitors mixture. The cell suspensions were sonicated for 10 s and then spun at 120,000 × g for 45 min to pellet the detergent-insoluble fraction. The supernatant was incubated with 2 μl of antibodies and 20 μl of protein G beads overnight. The immunoprecipitates were washed three times with the immunoprecipitation buffer and then resuspended in SDS sample buffer. The samples were analyzed by SDS-PAGE and transferred to nitrocellulose membranes. The blots were then blocked with 5% nonfat milk and incubated with anti-NKD2 or anti-HA antibody and then with a horseradish peroxidase-conjugated secondary antibody. The bands were detected using ECL. Zebrafish Nkd2 antibody was used at 1:1,000 dilution for Western blotting; the secondary antibody was donkey anti-rabbit horseradish peroxidase (Amersham Biosciences NA934V) and was used at a 1:10,000 dilution. The anti-β-actin antibody (Sigma A5441) was used at a 1:2,000 dilution, and secondary donkey anti-mouse horseradish peroxidase (Jackson ImmunoResearch Labs 715-035-150) was used at a 1:10,000 dilution.
Ubiquitylation Assay
HEK293 cells were transfected using the plasmids described in Figs. 4C and 5E. After transfection, the cells were treated with the proteasome inhibitor MG132 for 4 h. Cells were then lysed and immunoprecipitated using either anti-FLAG antibody or anti-NKD2 antibody. The ubiquitylation status of the Dvl-1 and NKD2 were analyzed by Western blot using anti-HA antibody and rabbit polyclonal anti-ubiquitin antibodies.
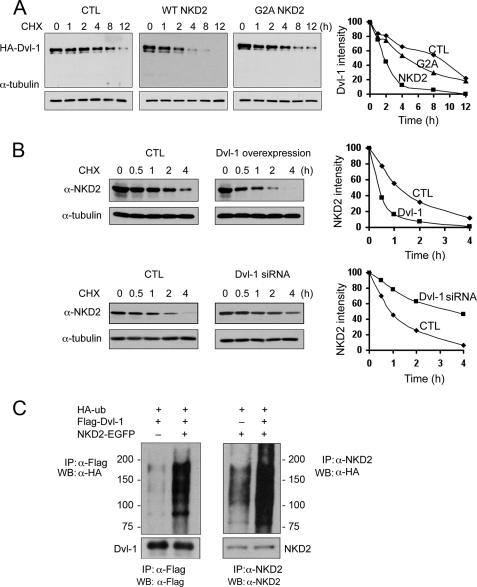
FIGURE 4.
NKD2 and Dvl-1 accelerate each other's degradation via polyubiquitylation. A, MDCK cells were transfected transiently with HA-Dvl-1 and wild-type NKD2-EGFP or G2A NKD2-EGFP. Cycloheximide (CHX, 10 μg/ml) was added for the times indicated to block protein synthesis. Dvl-1 stability was monitored by Western blotting. α-Tubulin served as a loading control. Densitometry was performed, and the intensity of Dvl-1 band was determined after normalization to α-tubulin. B, MDCK cells stably expressing wild-type NKD2-EGFP were transfected transiently with HA-Dvl-1 and control vector (upper panel) or Dvl-1 siRNA and control vector (lower panel). The stability of NKD2 was determined over time following the administration of CHX (10 μg/ml). Levels of NKD2 and α-tubulin were monitored by Western blot analysis. Densitometry was performed, and the intensity of the NKD2 band was determined after normalization to α-tubulin. In A and B, protein levels were normalized to α-tubulin and graphed with time = 0 arbitrarily set to 100%. C, increased ubiquitylation of Dvl-1 (left) or NKD2 (right) in HEK293 cells transiently expressing HA-Ub, FLAG-Dvl-1, or wild-type NKD2. HEK293 cells were treated with MG132 (50 μm) for 4 h before the ubiquitylation assay.
FIGURE 5.
NKD2 specifically recognizes an ubiquitylated form of Dvl-1 at the plasma membrane. A, siRNA knockdown of NKD2 in Caco-2-TGFα cells results in an increased level of Dvl-1 by Western blotting. B, Dvl-1 and NKD2 interaction. Caco-2-TGFα cells were immunoprecipitated with anti-NKD2 antibodies (N-terminal (N-t) R44 or C-terminal (C-t) VU308 NKD2 antibodies) or anti-Dvl-1 antibody and Western blotted with anti-Dvl-1 antibody. C, Caco-2 cells were treated with or without MG132 (50 μm) for 4 h and then lysed, immunoprecipitated with anti-NKD2 antibody, and immunoblotted using anti-Dvl-1 antibody. D, Caco-2-TGFα cells were fractionated over a discontinuous 10–40% iodixanol gradient as described under “Experimental Procedures.” Fractions were blotted with anti-Dvl-1, anti-NKD2, anti-caveolin-1, E-cadherin, and anti-Na+/K+ ATPase α1 antibodies. Slow migrating form of Dvl-1 is found (indicated by arrow) predominantly in early plasma membrane fractions. The additional forms of Dvl-1 detected are similar to those observed from immunoprecipitation and Western blotting of Dvl-1 in B. E, cDNAs of Dvl-1, -2, and -3 and NKD2-EGFP were transiently transfected with a His-tagged ubiquitin cDNA into HEK293T cells. Immunoprecipitations were performed with indicated antibodies. Immunoprecipitates were resolved in the PAGE gel and immunoblotted with anti-ubiquitin and anti-Dvl-1 antibodies. A single slower migrating Dvl-1 band was detected with anti-ubiquitin antibody. Equivalent levels of Dvl-1, -2, and -3 protein were observed by Western blotting in cells transiently transfected with the respective cDNAs (marked as Input in the panel on the right).
Immunostaining and Laser Confocal Microscopy
MDCK cells transfected with HA-Dvl-1 and NKD2-EGFP cDNA were fixed with 4% paraformaldehyde on Transwells for 30 min and then permeabilized with 0.1% Triton X-100 for 10 min, blocked for 1 h in 2% bovine serum albumin TBS, pH 8.0, and incubated with primary anti-HA antibody and Cy3-labeled donkey anti-mouse antibody. Immunofluorescence was visualized by using a Zeiss LSM 510 confocal microscope.
Luciferase Reporter Assay
HEK293 cells cultured in 24-well plates were co-transfected using Lipofectamine 2000 with 100 ng of superTOPflash, 10 ng of TK-Renilla, 50 ng of Wnt-3a, and 50 ng of NKD2 or G2A or Dvl-1 siRNA. A FOPflash assay was used as a control. The assay was performed in accordance with the dual luciferase assay instructions (Promega, Madison, WI) using a luminometer (Molecular Devices, Sunnyvale, CA). Briefly, cells were lysed 24 h after transfection. The TOPflash and Renilla luminescence levels were assayed, respectively, and the ratio was calculated as relative luciferase activity.
Zebrafish Embryo Manipulation
Adult zebrafish and embryos were raised at 28.5 °C and were staged by anatomical criteria for hours or days post-fertilization according to Kimmel et al. (14). Embryos of AB* backgrounds were collected from pairwise matings. Nkd2a was cloned as described in Van Raay et al. (15). Capped mRNA was synthesized using an Ambion mMessage mMachine kit. Following transcription, the RNA was purified over a G-50 Sephadex column (Roche Applied Science) and diluted in RNase-free water containing 1% phenol red, probe quantity was measured by spectrophotometry, and quality was assayed using gel electrophoresis. Two nanoliters of nkd2a mRNA (400 ng/μl) or 1 nl of wnt8 mRNA (25 ng/μl) (15, 16) were pressure-injected into the yolk cell of one- to two-cell stage embryos. Injected embryos were raised at 28.5 °C and assayed at 2 days post-fertilization for presence or absence of eyes.
RESULTS
Myristoylated Zebrafish Nkd2a Inhibits the Wnt-β-Catenin Pathway in Zebrafish Embryos
To examine the ability of Nkd2 to antagonize Wnt-β-catenin signaling in vivo, zebrafish embryos were injected with synthetic RNA encoding Wnt8 at the one- to two-cell stage. Wnt8 activates Wnt-β-catenin signaling, resulting in loss of eyes due to posteriorization of the forebrain (18). Co-expression of wild-type Nkd2a with Wnt8 significantly repressed the eyeless phenotype, whereas co-expression of myristoylation-deficient G2A Nkd2a had no effect (Fig. 1, A and B). Fig. 1C shows equivalent levels of Nkd2a protein in zebrafish embryos by Western blotting after injection of wild-type and G2A Nkd2a RNAs, respectively. Protein samples were taken at 60–70% epiboly, the approximate time in development when Wnt8 posteriorizes the forebrain (17, 19). Thus, the ability of Nkd2 to antagonize Wnt-β-catenin signaling in vivo is myristoylation-dependent.
FIGURE 1.
NKD2 antagonizes zebrafish embryonic development in a myristoylation-dependent manner. A, two days post-fertilization, zebrafish embryos were injected at the one-cell stage with wnt8 (25 pg) mRNA, wnt8 plus Nkd2a (800 pg) mRNAs or wnt8 plus G2A Nkd2a (800 pg) mRNA and then assayed 2 days post-fertilization for the presence or absence of eyes. B, quantification of results from panel A. Results are expressed as the overall mean ± S.D. of five separate experiments. ***, p < 0.0001 for the ability of Nkd2a to rescue the Wnt8 eyeless phenotype. C, levels of Nkd2 by Western blotting from the four experimental conditions in A using a rabbit anti-Nkd2a serum that is described under “Experimental Procedures.” The 50-kDa band represents full-length Nkd2; the nature of the smaller, ∼30-kDa band is uncertain but may represent an N-terminal proteolytic fragment. α-Actin was used as a loading control.
Myristoylated NKD2 Attenuates Wnt-β-Catenin Signaling at the Level of or Downstream of Dvl-1
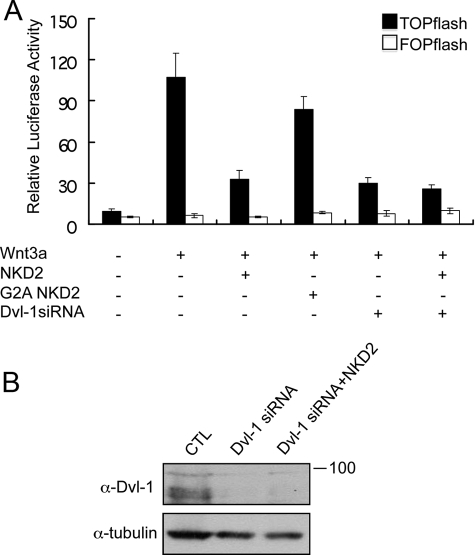
To explore the mechanism by which myristoylated NKD2 antagonizes the Wnt-β-catenin pathway, the effects of human wild-type and G2A NKD2 were studied in HEK293 cells using a commonly used Wnt-β-catenin readout, superTOPflash activity, a luciferase reporter under control of an artificial Wnt-inducible promoter. Because it is known that Nkd/NKDs affect canonical Wnt signaling via Dsh/Dvl (8), we assayed superTOPflash reporter activity in HEK293 cells co-expressing Dvl-1 with wild-type or G2A NKD2. Reducing endogenous Dvl-1 by siRNA (Fig. 2B) markedly reduced Wnt3a-induced TOPflash activity (p < 0.003), indicating that Dvl-1 is a main regulator of Wnt3a-induced activity. The increased luciferase activity observed compared with no treatment (p = 0.002) may be due to incomplete knockdown of Dvl-1 or a contribution of Dvl-2 and Dvl-3 that are also expressed in these cells (data not shown). Whereas wild-type NKD2 significantly inhibited Wnt3a-induced TOPflash activity by at least 70% (p = 0.004), G2A NKD2 reduced activity by only 15% (p = 0.13). Upon siRNA knockdown of endogenous Dvl-1, overexpression of NKD2 had no further inhibitory effect on Wnt signaling (p = 0.3), suggesting that the inhibitory effect of NKD2 on Wnt-β-catenin signaling is via Dvl-1. Taken together, these results indicate that NKD2 inhibits the Wnt-β-catenin pathway activity at the level of or downstream of Dvl-1 and that this action of NKD2 is, in large part, myristoylation-dependent.
FIGURE 2.
NKD2, but not G2A NKD2, antagonizes Wnt-β-catenin signaling in HEK293 cells and this effect is Dvl-1 dependent. A, HEK293 cells were co-transfected with 100 ng of superTOPflash (or FOPflash as control), 10 ng of TK-Renilla, 50 ng of Wnt3a, and 50 ng of NKD2 or G2A NKD2 cDNAs or Dvl-1 siRNA. The assays were carried out as described under “Experimental Procedures.” The results shown are from a representative experiment that was performed in triplicate and repeated at least 10 times. Results are expressed as the mean ± S.D. B, efficient knockdown of Dvl-1 protein by Western blotting of HEK293 cells treated with Dvl-1 siRNA.
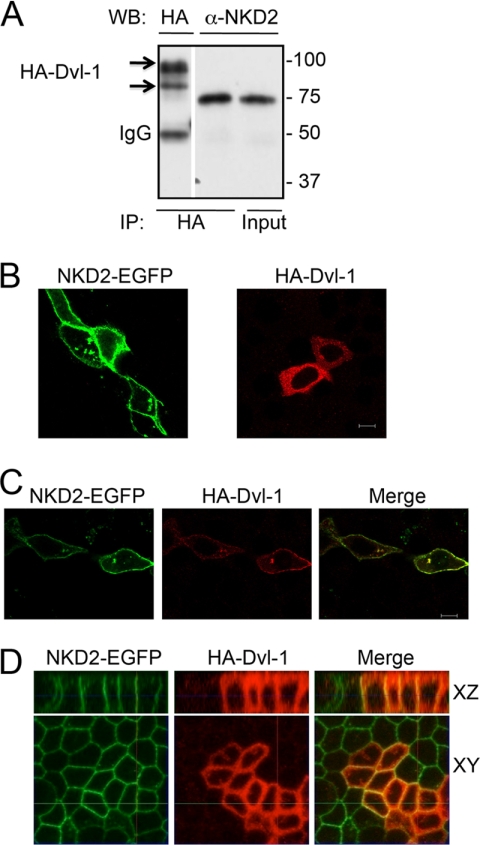
NKD2 Binds Dvl-1 and They Co-localize at the Lateral Membrane of Polarized Epithelial Cells
Fig. 3A shows that NKD2 and Dvl-1 interact as detected by co-immunoprecipitation in HEK293 cells transiently expressing NKD2-EGFP and HA-Dvl-1. NKD2-EGFP itself decorated the plasma membrane and vesicles. Ectopically expressing Dvl-1 was predominantly diffuse in the cytoplasm (Fig. 3B). However, Dvl-1 was co-localized with NKD2 at the plasma membrane when they were co-expressed in MDCK cells (Fig. 3C). In addition, NKD2 and Dvl-1 co-localize at the lateral membrane of polarized MDCK cells stably expressing NKD2-EGFP and transiently expressing HA-Dvl-1 (Fig. 3D).
FIGURE 3.
NKD2 and Dvl-1 interact and co-localize at the plasma membrane. A, HEK293 cells were co-transfected transiently with NKD2-EGFP and HA-Dvl-1 cDNAs. Cells were immunoprecipitated with an anti-HA antibody and immunoblotted using both anti-HA and anti-NKD2 (R44) antibodies. B, NKD2-EGFP and HA-Dvl-1 were expressed in MDCK cells individually. NKD2-EGFP localized to cytoplasmic vesicles and the plasma membrane, whereas HA-Dvl-1 decorated scattered puncta in the cytoplasm. C, HA-Dvl-1 co-localized with NKD2-EGFP at the plasma membrane and in some cytoplasmic puncta in MDCK cells transiently co-expressing these cDNAs. D, polarized MDCK cells stably expressing NKD2-EGFP were transfected transiently with HA-Dvl-1. Cells were immunostained and visualized as described under “Experimental Procedures.”
Myristoylated NKD2 and Dvl-1 Accelerate Each Other's Degradation via Enhanced Polyubiquitylation
Although previous studies suggested that Dvl-1 protein levels might be affected by NKD (20), the precise mechanism underlying this regulation is unknown. The stability of Dvl-1 was examined in parental MDCK cells or MDCK cells stably overexpressing NKD2. Relative to parental MDCK cells, the half-life of Dvl-1 was reduced from 8 to 2 h in cells overexpressing NKD2 (Fig. 4A). In contrast, there was little effect on the rate of Dvl-1 degradation in MDCK cells stably expressing myristoylation-deficient, G2A NKD2. These results indicate that NKD2 regulates Dvl-1 stability in a myristoylation-dependent manner. Because NKD2 can bind to and destabilize Dvl-1, we next examined the stability of NKD2 in the presence of Dvl-1. We transiently overexpressed Dvl-1 in MDCK cells stably expressing NKD2-EGFP and found that the half-life of NKD2 was reduced from 1 h to <30 min (Fig. 4B, upper panel). Moreover, siRNA knockdown of endogenous Dvl-1 in these cells resulted in stabilization of NKD2 (Fig. 4B, lower panel).
To study how Dvl-1 and NKD2 accelerate each other's degradation, we examined whether expression of one would enhance ubiquitylation of the other. In HEK293 cells transiently expressing NKD2 and Dvl-1, NKD2 and Dvl-1 increase each other's polyubiquitylation, thus accelerating their mutual degradation (Fig. 4C).
Endogenous Ubiquitylated DVL-1 Localizes to the Plasma Membrane and Interacts with Endogenous NKD2
Thus far, the relationship between NKD2 and DVL-1 has been examined in heterologous systems. To study endogenous NKD2 and DVL-1, we used a human colorectal cancer cell line, Caco-2, stably overexpressing TGFα; overexpression of TGFα in these cells protects NKD2 from proteasomal degradation (21). As expected, siRNA knockdown of endogenous NKD2 in these Caco-2 cells resulted in increased levels of endogenous DVL-1 (Fig. 5A), providing further evidence that NKD2 regulates DVL-1 stability. Fig. 5B shows by co-immunoprecipitation that NKD2 only interacts with a slower migrating form of DVL-1 and that this form represents a minor proportion of total DVL-1. In this experiment, multiple DVL-1 forms were detected by immunoprecipitation and Western blotting using a DVL-1 antibody compared with immunoprecipitation using two different NKD2 antibodies followed by Western blotting with the DVL-1 antibody. The mobility of this DVL-1 form was unaffected by treatment with alkaline phosphatase or potato acid phosphatase (data not shown), suggesting that the increase in molecular weight is not due to phosphorylation. This slower migrating form of DVL-1 was ubiquitylated in HEK293 transiently overexpressing DVL-1 and NKD2 (data not shown). Moreover, endogenous NKD2 and DVL-1 co-immunoprecipitated in these Caco-2 cells, and the slower migrating form of DVL-1 that interacts with NKD2 accumulated in the presence of MG132 (Fig. 5C).
Overexpressed NKD2 and DVL-1 co-localize at the lateral membrane of polarized MDCK cells (Fig. 3D). To assess biochemically where endogenous NKD2/DVL-1 interact, cell fractionation studies were performed in TGFα-expressing Caco-2 cells using a discontinuous 10–40% iodixanol gradient, a strategy we recently employed to enrich biochemically and then flow sort a plasma membrane-depleted pool of G2A NKD2 vesicles for liquid chromatography-tandem mass spectrometry analysis (13). The early fractions in the gradient were enriched for plasma membrane constituents as demonstrated by the migration patterns of E-cadherin and Na+/K+ ATPase α1. A slower migrating form of DVL-1 was enriched in fractions 3–5 (Fig. 5D, white arrow). In these fractions, full-length 50-kDa NKD2 (marked by the arrow) was not detected; instead, only higher molecular forms were found (marked by the bracket), in a pattern consistent with polyubiquitylation. To further identify the possible modification(s) of this slower migrating form of DVL-1 at the plasma membrane, we compared the ubiquitylation of DVL-1, -2, and -3. Anti-DVL-1 antibody pulled down several forms of DVL-1, but NKD2 only pulled down a single slower migrating form of HA-Dvl-1 (Fig. 5E, Dvl-1 panel). The DVL-1 form pulled down by NKD2 was recognized by an anti-ubiquitin antibody (Fig. 5E, Ub panel). The blot was reprobed with anti-DVL-2 or -3, and we did not observe any HA-DVL-2 or -3 pulled down by NKD2 (Fig. 5E, Dvl-2 and -3 panel). Both DVL-2 and -3 antibodies cross-reacted with DVL-1. The degradation of DVL-2 or -3 was also unaffected by NKD2 (data not shown), suggesting that NKD2 specifically interacts with DVL-1 only. Taken together, these results indicate that NKD2 interacts with an ubiquitylated form of DVL-1 at the plasma membrane, and DVL-1 undergoes ubiquitin-mediated proteasomal degradation.
DISCUSSION
Dvl proteins have been the subject of intensive study because they are at a decisive branch point for the Wnt-β-catenin pathway and Frizzled PCP signaling. Despite this intense scrutiny, their functions remain poorly understood, calling to mind Winston Churchill's assessment of Russia as “a riddle wrapped in a mystery inside an enigma.” A number of factors have complicated the study of Dvl function(s). In zebrafish, mouse, and human, three Dvl genes are expressed to varying degrees in all tissues and cell types. In vitro studies have demonstrated that the three Dvl proteins might exist in a complex and act interdependently, so knocking down any of them will significantly impair Wnt activity (22). However, targeted disruption of all three Dvl genes in the mouse indicates that, whereas they exhibit some overlapping functions, they also display distinct functions (18). Because of the possibility of some redundancy among the three Dvl genes, it is not clear whether Dvl proteins exclusively mediate Wnt-related signaling events or additionally fulfill a more general function essential to cellular viability. The answer to this critical question awaits the construction of a cell lacking all Dvl genes. We did not observe any interactions between NDK2 and Dvl-2 or -3 (data not shown), so the present studies focused on the interaction between Dvl-1 and NKD2.
The view that Dvl at the plasma membrane regulates Frizzled PCP signaling and Dvl in the cytoplasm regulates Wnt-β-catenin signaling is overly simplistic. It is increasingly recognized that Dvl shuttles dynamically between the plasma membrane and cytoplasm; Wallingford and colleagues have pointed out that it is the local concentration of Dvl, rather than its spatial localization, that is central to Wnt-β-catenin signaling (23). Dvls have three modular domains (DIX, PDZ, and DEP) with a long, largely uncharacterized C terminus. Dimerization and vesicle localization have been ascribed to the DIX domain and plasma membrane localization to the DEP domain (24, 25). The PDZ domain of Dvl has greater flexibility in target binding than conventional PDZ domains, which bind type 1 and 2 targets at the C termini of transmembrane proteins (26). In addition to binding a wider range of C-terminal targets, the PDZ domain of Dvl-2 recognizes an internal motif within Frizzled. Finally, in some systems, loss of Dvl function will impair one arm of Wnt signaling but activate the other (the Wnt-β-catenin pathway versus Frizzled PCP), whereas it seems that its loss should adversely affect both arms similarly; in fact, we have shown that Nkd1 and -2 negatively regulate both Wnt-β-catenin pathway activity and Frizzled PCP signaling in zebrafish (15).
Although most of the studies on post-translational modifications of Dvl have focused on phosphorylation (27–30), it is instructive to consider proteins that interact with Dvl and lead to its degradation. Inversin and Dapper1 interact with and degrade Dvl-1 via either proteasomal or lysosomal degradation, respectively (31, 32). NKD2 and Dvl-1 degrade each other via proteasomal degradation (data now shown). Like NKD2, Inversin promotes degradation of Dvl-1 by polyubiquitylation. The effect of inversin on the half-life of Dvl-1 in HEK293 cells is similar to that of NKD2 on Dvl-1 in MDCK cells, reducing it from 8 to 2 h. Differences between the effects of NKD2 and Inversin on Dvl-1 include the site of degradation within the cell and the consequences of that degradation. Inversin was reported to promote degradation of Dvl-1 in the cytoplasm, resulting in inhibition of Wnt-β-catenin activity and activation of Frizzled PCP signaling (31); in fact, attaching a myristoylation tag to Dvl-1 impaired the ability of inversin to degrade this membrane-directed Dvl-1. In contrast, the present work describes how NKD2 promotes degradation of Dvl-1 at the plasma membrane. Also, in contrast to inversin, we previously demonstrated that Nkd2 inhibits both the Wnt-β-catenin pathway and Frizzled PCP signaling in vivo (14).
One must also consider E3 ligases that bind and degrade Dvl. Recent studies showed that Dvl-2 and -3 form a complex with KLHL12 and Cullin3 that leads to their proteasomal degradation (33). We did not detect an interaction between Dvl-1 and Cullin3,5 suggesting that NKD2 and Dvl-1 might form a different degradation complex. Previous studies indicated that a homolog of E6AP C terminus (HECT)-type ubiquitin ligase (NEDL1) interacts with and degrades Dvl-1 (34). However, we were unable to detect NEDL1 in the cells that we studied.5 We recently have reported that the AO-7, a ring finger E3 ligase, constitutively degrades NKD2 in the cytoplasm (21). Overexpression of TGFα reduces binding of AO-7 to NKD2 and protects NKD2 from degradation. To determine whether AO-7 is a possible E3 ligase for Dvl-1, we overexpressed AO-7 in HEK293 cells, which do not express NKD2. We observed no effect on endogenous Dvl-1 stability in these AO-7-expressing HEK293 cells (data not shown), arguing against a direct role for AO-7 in degrading Dvl-1. NKD2 clearly interacts with both Dvl-1 and TGFα, but we have never seen a ternary complex, leading to the model we propose below. Moreover, none of the Dvls were found among the 389 proteins identified in NKD2-associated exocytic vesicles by liquid chromatography-tandem mass spectrometry (13).
A major function of TGFα is to maintain epithelial homeostasis. It is uniquely equipped for this task because it is widely expressed by epithelial cells; it is delivered preferentially to the basolateral surface of polarized epithelial cells where it is rapidly cleaved by TACE and then avidly captured by basolaterally restricted EGFRs. In contrast, although another EGFR ligand, amphiregulin, is also delivered preferentially to the basolateral surface and cleaved by TACE, it is a low affinity EGFR ligand and binds more avidly to heparan sulfate proteoglycans. We have shown that administration of recombinant human TGFα to polarized MDCK cells results in taller, more differentiated cells than recombinant human amphiregulin administration, which leads to an epithelial to mesenchymal-like transition (35). To assure that TGFα is delivered efficiently to its proper destination, it stabilizes its cargo recognition and targeting (CaRT) protein, NKD2 (36). We propose that once NKD2 has discharged this function, it is able to bind and degrade any Dvl-1 it encounters at the plasma membrane. This would ensure tight regulation of Wnt-β-catenin pathway activity and thereby also contribute to the maintenance of epithelial homeostasis. Studies are underway to test this model.
In summary, we provide evidence that myristoylated NKD2 only binds a slower migrating ubiquitylated form of Dvl-1. NKD2 co-localizes with Dvl-1 and regulates Dvl-1 stability at the plasma membrane because a myristoylation-deficient G2A mutant that mainly localizes to the cytoplasm has only a small effect on Dvl-1 degradation and attenuation of Wnt-β-catenin pathway activity. In zebrafish embryos, G2A Nkd2a had very little effect on Wnt activity, and only a modest reduction in Wnt activity was observed in mammalian HEK293T cells overexpressing G2A NKD2 compared with wild-type NKD2. This residual Wnt activity seen with G2A NKD2 may be due, among other possibilities, to endogenous NKD2 (data not shown). These studies demonstrate a mechanism by which Nkd2/NKD2 antagonizes Wnt-β-catenin activity by enhancing ubiquitin-mediated proteasomal degradation of Dvl-1. However, these studies do not address whether Dvl-1 is ubiquitylated prior to the interaction with NKD2. Additional studies are necessary to determine the stoichiometry of the NKD2-Dvl-1 interaction and the complete composition of this complex, including the E3 ligase(s) that execute their degradation (37–40).
Acknowledgment
We thank Jeff Franklin for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants 5R01CA046413 and 5P50CA095103 (to R. J. C.) and 5T32HL007751. This work was also supported by Project Grant 985 from Xiamen University, Ministry of Science and Technology Grants 2009CB522200 and 2006AA02A303, and Research Grant 2009102 from the Key Laboratory of the Ministry of Education for Cell Biology and Tumor Cell Engineering, Xiamen University (to T. H.).
T. Hu and R. J. Coffey, unpublished observation.
- Dsh/Dvl
- Dishevelled
- TGFα
- transforming growth factor-α
- HA
- hemagglutinin
- siRNA
- small interference RNA
- E3
- ubiquitin-protein isopeptide ligase
- EGFR
- epidermal growth factor receptor
- PCP
- planar cell polarity
- TACE
- tumor necrosis factor-α-converting enzymes.
REFERENCES
- 1.Cadigan K. M., Nusse R. (1997) Genes Dev. 11, 3286–3305 [DOI] [PubMed] [Google Scholar]
- 2.Wallingford J. B., Habas R. (2005) Development 132, 4421–4436 [DOI] [PubMed] [Google Scholar]
- 3.Gan X. Q., Wang J. Y., Xi Y., Wu Z. L., Li Y. P., Li L. (2008) J. Cell Biol. 180, 1087–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. (1998) Nature 391, 357–362 [DOI] [PubMed] [Google Scholar]
- 5.Takemaru K., Yamaguchi S., Lee Y. S., Zhang Y., Carthew R. W., Moon R. T. (2003) Nature 422, 905–909 [DOI] [PubMed] [Google Scholar]
- 6.Polakis P. (2000) Genes Dev. 14, 1837–1851 [PubMed] [Google Scholar]
- 7.Zeng W., Wharton K. A., Jr., Mack J. A., Wang K., Gadbaw M., Suyama K., Klein P. S., Scott M. P. (2000) Nature 403, 789–795 [DOI] [PubMed] [Google Scholar]
- 8.Rousset R., Mack J. A., Wharton K. A., Jr., Axelrod J. D., Cadigan K. M., Fish M. P., Nusse R., Scott M. P. (2001) Genes Dev. 15, 658–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan D., Wallingford J. B., Sun T. Q., Nelson A. M., Sakanaka C., Reinhard C., Harland R. M., Fantl W. J., Williams L. T. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 3802–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C., Franklin J. L., Graves-Deal R., Jerome W. G., Cao Z., Coffey R. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 5571–5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resh M. D. (2006) Nat. Chem. Biol. 2, 584–590 [DOI] [PubMed] [Google Scholar]
- 12.Podell S., Gribskov M. (2004) BMC Genomics 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Z., Li C., Higginbotham J. N., Franklin J. L., Tabb D. L., Graves-Deal R., Hill S., Cheek K., Jerome W. G., Lapierre L. A., Goldenring J. R., Ham A. J., Coffey R. J. (2008) Mol. Cell Proteomics 7, 1651–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995) Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- 15.Van Raay T. J., Coffey R. J., Solnica-Krezel L. (2007) Dev. Biol. 309, 151–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly G. M., Greenstein P., Erezyilmaz D. F., Moon R. T. (1995) Development 121, 1787–1799 [DOI] [PubMed] [Google Scholar]
- 17.Erter C. E., Wilm T. P., Basler N., Wright C. V., Solnica-Krezel L. (2001) Development 128, 3571–3583 [DOI] [PubMed] [Google Scholar]
- 18.Etheridge S. L., Ray S., Li S., Hamblet N. S., Lijam N., Tsang M., Greer J., Kardos N., Wang J., Sussman D. J., Chen P., Wynshaw-Boris A. (2008) PLoS Genet. 4, e1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhinn M., Lun K., Luz M., Werner M., Brand M. (2005) Development 132, 1261–1272 [DOI] [PubMed] [Google Scholar]
- 20.Creyghton M. P., Roël G., Eichhorn P. J., Hijmans E. M., Maurer I., Destrée O., Bernards R. (2005) Genes Dev. 19, 376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding W., Li C., Hu T., Graves-Deal R., Fotia A. B., Weissman A. M., Coffey R. J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13433–13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y. N., Gao Y., Wang H. Y. (2008) Cell. Signal. 20, 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park T. J., Gray R. S., Sato A., Habas R., Wallingford J. B. (2005) Curr. Biol. 15, 1039–1044 [DOI] [PubMed] [Google Scholar]
- 24.Capelluto D. G., Kutateladze T. G., Habas R., Finkielstein C. V., He X., Overduin M. (2002) Nature 419, 726–729 [DOI] [PubMed] [Google Scholar]
- 25.Pan W. J., Pang S. Z., Huang T., Guo H. Y., Wu D., Li L. (2004) Cell Res. 14, 324–330 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Appleton B. A., Wiesmann C., Lau T., Costa M., Hannoush R. N., Sidhu S. S. (2009) Nat. Chem. Biol. 5, 217–219 [DOI] [PubMed] [Google Scholar]
- 27.Rothbächer U., Laurent M. N., Deardorff M. A., Klein P. S., Cho K. W., Fraser S. E. (2000) EMBO J. 19, 1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nusse R., Samos C. H., Brink M., Willert K., Cadigan K. M., Wodarz A., Fish M., Rulifson E. (1997) Cold Spring Harbor Symp. Quant. Biol. 62, 185–190 [PubMed] [Google Scholar]
- 29.Cong F., Schweizer L., Varmus H. (2004) Mol. Cell Biol. 24, 2000–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanagawa S., van Leeuwen F., Wodarz A., Klingensmith J., Nusse R. (1995) Genes Dev. 9, 1087–1097 [DOI] [PubMed] [Google Scholar]
- 31.Simons M., Gloy J., Ganner A., Bullerkotte A., Bashkurov M., Krönig C., Schermer B., Benzing T., Cabello O. A., Jenny A., Mlodzik M., Polok B., Driever W., Obara T., Walz G. (2005) Nat. Genet. 37, 537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Gao X., Wen J., Ning Y., Chen Y. G. (2006) J. Biol. Chem. 281, 8607–8612 [DOI] [PubMed] [Google Scholar]
- 33.Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006) Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki K., Fujita T., Ozaki T., Kato C., Kurose Y., Sakamoto M., Kato S., Goto T., Itoyama Y., Aoki M., Nakagawara A. (2004) J. Biol. Chem. 279, 11327–11335 [DOI] [PubMed] [Google Scholar]
- 35.Chung E., Graves-Deal R., Franklin J. L., Coffey R. J. (2005) Exp. Cell Res. 309, 149–160 [DOI] [PubMed] [Google Scholar]
- 36.Li C., Hao M., Cao Z., Ding W., Graves-Deal R., Hu J., Piston D. W., Coffey R. J. (2007) Mol. Biol. Cell 18, 3081–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons M., Gault W. J., Gotthardt D., Rohatgi R., Klein T. J., Shao Y., Lee H. J., Wu A. L., Fang Y., Satlin L. M., Dow J. T., Chen J., Zheng J., Boutros M., Mlodzik M. (2009) Nat. Cell Biol. 11, 286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smalley M. J., Signoret N., Robertson D., Tilley A., Hann A., Ewan K., Ding Y., Paterson H., Dale T. C. (2005) J. Cell Sci. 118, 5279–5289 [DOI] [PubMed] [Google Scholar]
- 39.Narimatsu M., Bose R., Pye M., Zhang L., Miller B., Ching P., Sakuma R., Luga V., Roncari L., Attisano L., Wrana J. L. (2009) Cell 137, 295–307 [DOI] [PubMed] [Google Scholar]
- 40.Schwarz-Romond T., Merrifield C., Nichols B. J., Bienz M. (2005) J. Cell Sci. 118, 5269–5277 [DOI] [PubMed] [Google Scholar]