Abstract
Pathologic accumulation of α-synuclein is a feature of human parkinsonism and other neurodegenerative diseases. This accumulation may be counteracted by mechanisms of protein degradation that have been investigated in vitro but remain to be elucidated in animal models. In this study, lysosomal clearance of α-synuclein in vivo was indicated by the detection of α-synuclein in the lumen of lysosomes isolated from the mouse midbrain. When neuronal α-synuclein expression was enhanced as a result of toxic injury (i.e. treatment of mice with the herbicide paraquat) or transgenic protein overexpression, the intralysosomal content of α-synuclein was also significantly increased. This effect was paralleled by a marked elevation of the lysosome-associated membrane protein type 2A (LAMP-2A) and the lysosomal heat shock cognate protein of 70 kDa (hsc70), two essential components of chaperone-mediated autophagy (CMA). Immunofluorescence microscopy revealed an increase in punctate (lysosomal) LAMP-2A staining that co-localized with α-synuclein within nigral dopaminergic neurons of paraquat-treated and α-synuclein-overexpressing animals. The data provide in vivo evidence of lysosomal degradation of α-synuclein under normal conditions and, quite importantly, under conditions of enhanced protein burden. In the latter, increased lysosomal clearance of α-synuclein was mediated, at least in part, by CMA induction. It is conceivable that these neuronal mechanisms of protein clearance play an important role in neurodegenerative processes characterized by abnormal α-synuclein buildup.
Keywords: Chaperones, Diseases/Neurodegeneration, Protein/Degradation, Subcellular Organelles/Lysosomes, Autophagy, LAMP-2A, Autophagy, Paraquat, Parkinson, Synuclein
Introduction
Several lines of clinical and experimental evidence support a pathogenetic role of α-synuclein in Parkinson disease (PD)2 and other neurodegenerative disorders. The precise mechanisms by which this endogenously expressed protein becomes involved in pathologic processes have yet to be fully elucidated. However, data from both clinical and laboratory studies indicate that enhanced α-synuclein expression is itself capable of triggering a parkinsonian syndrome in humans and PD-like pathology in animal models. Indeed, multiplication mutations of the α-synuclein gene that result in increased expression of the wild-type protein are causally associated with autosomal dominant parkinsonism (1, 2). From the experimental standpoint, evidence of a gain of toxic function of elevated α-synuclein includes the observation of neurodegeneration and neuronal inclusions in the substantia nigra of rats and monkeys that overexpress α-synuclein after viral-mediated neuronal transduction (3, 4).
An important corollary to the concept that enhanced α-synuclein may have deleterious consequences is that intraneuronal mechanisms of protein homeostasis, such as degradation pathways, could well play a key role in maintaining “non-toxic” levels of α-synuclein. Although α-synuclein clearance is likely to occur through different mechanisms, recent reports have underscored the important contribution of lysosomal pathways of protein degradation and, in particular, chaperone-mediated autophagy (CMA) (5–8). CMA targets specific cytosolic proteins that are recognized by the heat shock cognate protein of 70 kDa (hsc70) and, after interacting with the lysosome-associated membrane protein type 2A (LAMP-2A), are translocated into the lysosomal lumen for rapid degradation (9). The amino acid sequence of α-synuclein contains a pentapeptide succession consistent with a CMA recognition motif. Experiments in which purified α-synuclein was added to intact lysosomes revealed that this CMA motif is essential for the internalization of α-synuclein into the lysosomal lumen and degradation by lysosomal proteases (6). Furthermore, inhibition of lysosomal proteolysis and CMA activity has been found to decrease α-synuclein clearance in a variety of in vitro systems, including primary neuronal cultures (6–8).
Despite these compelling in vitro data, the relationship between α-synuclein, lysosomes, and CMA remains unexplored in the brain in vivo. It is unknown if, in this setting, α-synuclein is translocated into lysosomes and if enhanced α-synuclein expression is associated with CMA induction and increased lysosomal levels of the protein. In this study, lysosome-α-synuclein interactions were investigated in normal mice as well as in mice in which increased α-synuclein expression was a consequence of either toxic injury or genetic manipulation. Lysosomes were isolated from midbrain tissue because neuronal cells within this region (i.e. dopaminergic neurons in the substantia nigra) are highly vulnerable to neurodegeneration and α-synuclein pathology in PD. In a first set of experiments, levels of α-synuclein and CMA markers were compared in midbrain lysosomes from control mice versus animals injected with the herbicide paraquat. Paraquat administration was used as a model of enhanced α-synuclein burden because it has previously been shown to induce a marked up-regulation of the protein within nigrostriatal dopaminergic neurons (10, 11). In a second set of experiments, the association between elevated α-synuclein and CMA induction was assessed in transgenic mice overexpressing α-synuclein. Taken together, results of these studies provide in vivo evidence of lysosomal clearance of α-synuclein that is enhanced under conditions of increased protein burden and is mediated, at least in part, by CMA activation.
EXPERIMENTAL PROCEDURES
Animals
Experiments were carried out in 10–12-week old male C57BL/6 mice (Charles River) or 10-week-old male transgenic mice overexpressing wild-type mouse α-synuclein (Jackson Laboratory). C57BL/6 mice received a single intraperitoneal injection of either saline or paraquat (Sigma) at the dose of 10 mg/kg and were sacrificed at day 3 post-treatment. Experimental protocols were in accordance with the NIH guidelines for use of live animals and were approved by the Animal Care and Use Committee at the Parkinson's Institute.
Isolation of Lysosomes and Lysosomal Fractions
For each preparation, midbrain tissues from 10 mice were pooled and homogenized. Lysosomes were isolated from a light mitochondrial-lysosomal fraction in a discontinuous metrizamide density gradient (12, 13). This protocol is optimized to isolate a fraction of lysosomes with high activity for CMA, i.e. lysosomes enriched in the lysosomal chaperone hsc70. Preparations with more than 10% broken lysosomes, as assessed by β-hexosaminidase latency, were discarded. Following isolation, some lysosomal preparations were treated with proteinase K for 15 min at 0 °C, with subsequent addition of 2 mm phenylmethylsulfonyl fluoride (14). Lysosomal matrices and membranes were isolated by ultracentrifugation after hypotonic shock (15). Lysosomes disrupted by hypotonic shock were also used to test α-synuclein proteolysis after incubation for 5 min at 37 °C (7).
Immunoblotting
Analyses were carried out using tissue homogenates (postnuclear fraction), lysosomal preparations (see above), or eluates from immunoprecipitation (see below). Equal amounts of protein were loaded to each gel lane, separated by SDS-PAGE and transferred to nitrocellulose membrane. Blots were blocked with 5% fat-free dry milk in 10 mm phosphate-buffered saline, pH 7.4 (PBS) for 1 h and incubated with primary antibodies against LAMP-2A (1:3000; Invitrogen), hsc70 (1:4000; Abcam), α-synuclein (1:3000; BD Bioscience), LAMP-1 (1:20; Developmental Studies Hybridoma Bank), cathepsin B (1:500; Santa Cruz Biotechnology), cytochrome c (1:1000; Cell Signaling), β-synuclein (1:1000; Chemicon), or β-actin (1:7,500; Sigma) at 4 °C overnight. Blots were then incubated in horseradish peroxidase-conjugated secondary antibody (Pierce) for 1 h, and proteins were detected using SuperSignal West Pico Substrate (Pierce). Membranes were exposed to Hyperfilm ECL, and densitometric quantification was performed with ImageQuant (GE Healthcare).
Immunoprecipitation
For each preparation, ventral mesencephalic tissues from 5 mice were pooled and sonicated in radioimmune precipitation assay buffer with Complete Mini (Roche). After centrifugation (100,000 × g for 30 min), supernatants were collected and precleared in 1% bovine serum albumin (Sigma) containing protein A-Sepharose beads (Pierce). Equal protein aliquots were incubated with an antibody against hsc70 (1:4000; Abcam). To prevent nonspecific binding, immune complexes (pellets) were washed three times with radioimmune precipitation assay buffer. Then, immune complexes were eluted and separated by SDS gel electrophoresis. Proteins were transferred to nitrocellulose membrane, and blots were blocked and incubated with antibodies against hsc70 or α-synuclein.
Quantitative PCR (qPCR)
Total RNA (0.5 μg) was extracted from ventral mesencephalic tissues using the RNeasy lipid tissue mini kit (Qiagen). cDNA was synthesized by reverse transcriptase (SuperScript III, Invitrogen) with oligo(dT) primer. The DNA products of lamp-2a, lamp-2b, lamp-2c, α-synuclein, and β-actin (as an internal control) were amplified using the SYBR Green PCR Master Mix (Applied Biosystems). Primers for LAMP-2A, LAMP-2B, LAMP-2C, and β-actin have been previously reported (16). The primers for α-synuclein were 5′-GATCCTGGCAGTGAGGCTTA-3′ and 5′-GCTTCAGGCTCATAGTCTTGG-3′. Amplification of the DNA products was measured in real time by the 7000 Sequence Detection System (Applied Biosystems). For all genes, the presence of a single amplified product was verified by agarose gel electrophoresis.
Immunohistochemistry
Midbrain blocks were immersion fixed in 4% paraformaldehyde, cryoprotected in sucrose, and frozen. Cryostat-cut coronal sections containing the substantia nigra (40-μm thick) were processed for fluorescence microscopy. For dual immunolabeling, sections were blocked in 5% normal donkey serum followed by incubation in sheep anti-α-synuclein (1:600; Millipore) overnight at 4 °C. Tissues were washed in PBS prior to incubation in donkey anti-sheep IgG conjugated to FITC (1:200; Jackson ImmunoResearch). Sections were then washed in PBS, blocked in 5% normal donkey serum and immersed in either rabbit anti-LAMP-2A (1:200; Invitrogen) or rabbit anti-hsc70 (1:200; Stressgen) overnight at 4 °C. Following washes, tissues were incubated in donkey anti-rabbit secondary antibody conjugated to Cy3 (1:200; Jackson ImmunoResearch), rinsed, and coverslipped. For triple immunolabeling, a separate set of midbrain sections was washed and blocked in 5% normal donkey serum and 5% normal goat serum prior to immersion in sheep anti-α-synuclein (1:500; Millipore) and mouse anti-TH (1:500; Chemicon) overnight at 4 °C. After washing, sections were incubated in donkey anti-sheep-FITC (1:200; Jackson ImmunoResearch) and goat anti-mouse conjugated to Alexa 350 (1:200; Invitrogen). Tissues were washed in PBS and incubated in rabbit anti-LAMP-2A (1:200; Invitrogen) for 2 h. Following rinsing, sections were immersed in donkey anti-rabbit-Cy3 (1:200; Jackson ImmunoResearch), washed, and coverslipped. Tissues were observed using a Nikon light microscope equipped for epifluorescence. Control sections were incubated in normal IgG in lieu of primary antibody and were devoid of staining.
Statistical Analysis
Data analysis was conducted on at least three samples prepared separately from different sets of animals. Differences among means were analyzed using one-way ANOVA. Newman-Keuls post-hoc analysis was used when differences were observed in ANOVA testing (p < 0.05).
RESULTS
Enhanced Lysosomal Content of α-Synuclein after Paraquat Administration to Mice
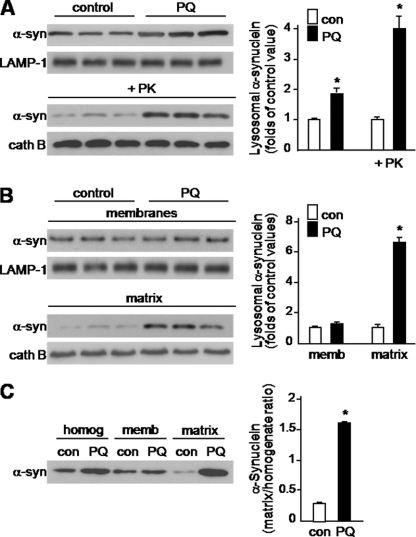
A single intraperitoneal injection of 10 mg/kg paraquat causes an increase in α-synuclein within dopaminergic neurons of the mouse substantia nigra that reaches its maximum at 3-days postexposure (10). To assess lysosome-α-synuclein associations, lysosomes were isolated from the midbrain of mice sacrificed 3 days after a single intraperitoneal injection of either saline or paraquat. The purity of the lysosomal fractions isolated from both groups of animals, their enrichment in lysosomal markers (LAMP-1 and cathepsin B) and the absence of contaminant mitochondria (as assessed by the cytochrome c marker) in the fractions is shown in the supplemental Fig. S1. Immunoblot analysis revealed the presence of α-synuclein protein in isolated lysosomes from both control and paraquat-treated animals (Fig. 1A). In the latter, denser α-synuclein-positive bands indicated a significant increase (2-fold) in α-synuclein content, thus providing in vivo evidence that, as a consequence of paraquat-induced cytosolic elevation of α-synuclein (10), a greater amount of the protein is associated with lysosomes.
FIGURE 1.
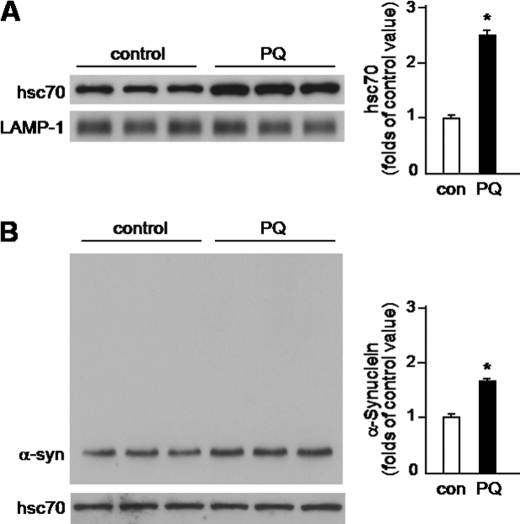
α-Synuclein association with lysosomes is enhanced in the midbrain of paraquat-treated mice. A, lysosomes were isolated from the midbrain of animals sacrificed 3 days after a single intraperitoneal injection of either saline or 10 mg/kg paraquat (PQ). After isolation, they were untreated or incubated with proteinase K (PK). B, lysosomes from the midbrain of saline- and paraquat-treated mice were subjected to hypotonic shock and ultracentrifugation for separation of lysosomal matrices and membranes. A and B, α-synuclein content was measured by Western blot analysis. To ensure equal loading of the gels, immunoblots were probed with an antibody against LAMP-1 (a lysosomal transmembrane protein) or cathepsin B (a marker of lysosomal matrix). Results of the densitometric quantification are the means + S.E. (n = 3) and are expressed as folds of the control (con) value in saline-treated mice. C, homogenate (postnuclear fraction) and lysosomal (membrane and matrix fractions) samples (10 μg) from the midbrain of saline- and PQ-injected mice were assayed in parallel by Western blotting using an anti-α-synuclein antibody. A representative blot is shown. Results of the densitometric quantification are the means + S.E. (n = 3) and are expressed as the ratio of lysosomal matrix/homogenate α-synuclein in control and PQ-treated animals, respectively. *, p < 0.01 versus the corresponding control group.
Increased association of α-synuclein with lysosomes could result from three different scenarios: (i) enhanced binding and translocation of the protein into lysosomes, (ii) decreased proteolysis of the protein once it reaches the lysosomal lumen, and (iii) reduced ability of the protein to cross the lysosomal membrane with consequent accumulation at the membrane. The next set of experiments was designed to evaluate these possibilities. Two separate approaches were used to discriminate between α-synuclein bound to the external surface of lysosomal membranes versus α-synuclein internalized into lysosomes: first, α-synuclein levels were assayed in lysosomal preparations treated with proteinase K and then levels of the protein were compared in lysosomal membrane versus matrix fractions. Incubation of intact lysosomes with proteinase K digests proteins bound at the cytosolic side of the membrane while preserving proteins contained within the lysosomal lumen (14). To ensure that proteinase K treatment did not digest luminal proteins, levels of cathepsin B were assayed (Fig. 1A). Proteinase K treatment reduced but did not completely eliminate lysosome-associated α-synuclein in preparations from saline- and paraquat-injected mice (Fig. 1A). The extent of this reduction was 58 and 36% in the former and latter, respectively (data not shown). Of note, levels of α-synuclein recovered after proteinase K digestion were significantly higher (4-fold) in the lysosomes isolated from paraquat-treated animals (Fig. 1A).
In the second set of experiments, midbrain lysosomes were subjected to hypotonic shock and, after centrifugation, membrane and matrix fractions were evaluated for α-synuclein content. A comparison of protein levels in lysosomal fractions isolated from saline- versus paraquat-treated mice revealed a marked increase (> 6-fold) in α-synuclein in the matrix of lysosomes from animals injected with the herbicide (Fig. 1B). To further evaluate how the relative amounts of extra- and intralysosomal α-synuclein changed as a consequence of paraquat-induced α-synuclein up-regulation, levels of the protein were assayed in samples from midbrain homogenates (postnuclear fraction) and lysosomal fractions in parallel. Data in Fig. 1C show that paraquat administration increased α-synuclein by 1.8-fold in homogenate samples. Under the same conditions, a much greater proportion of the protein was retrieved in the lysosomal matrix fraction; indeed, the ratio of lysosomal matrix/homogenate α-synuclein was 5.5 times higher in paraquat-treated as compared with saline-injected mice (Fig. 1C). Taken together, these findings demonstrate that the association of α-synuclein with lysosomes reflects uptake and internalization of the protein into lysosomes. Furthermore, they suggest that α-synuclein degradation via lysosomal pathways is markedly enhanced when neuronal expression of the protein is increased.
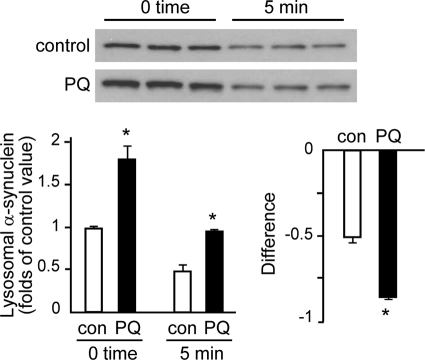
To assess whether the increase in lysosomal α-synuclein caused by paraquat was due to accumulation of protease-resistant forms of the protein in the lysosomal lumen, the rate of α-synuclein degradation was assessed in incubations of disrupted lysosomes from the midbrain of saline- and paraquat-injected mice (7). Immunoblot analysis compared α-synuclein levels at time 0 and after a 5-min incubation. At time 0, lysosomal α-synuclein content was greater in preparations from paraquat- as compared with saline-treated animals, and at 5 min, protein reduction was 47% in the former versus 51% in the latter (Fig. 2). Data indicate therefore that paraquat-induced lysosomal accumulation of α-synuclein is not a consequence of impaired degradation of the protein once it reaches the lysosomal lumen. Because the difference in α-synuclein levels at time 0 versus 5 min was more pronounced in samples from paraquat-injected mice (Fig. 2), data are also consistent with enhanced lysosomal clearance of the protein after toxicant administration.
FIGURE 2.
Lysosomal degradation of α-synuclein. Levels of α-synuclein were measured by Western blot analysis at time 0 and after a 5-min incubation of lysosomes from the midbrain of saline- and PQ-injected mice. After isolation, lysosomes were subjected to hypotonic shock. Results of the densitometric quantification are the means + S.E. of triplicate samples and are expressed as folds of the control (con) value at time 0 in saline-treated mice. Differences in α-synuclein levels (means + S.E.) were calculated by subtracting values at 5 min from the corresponding values at time 0 in preparations from control (saline-injected) and paraquat-treated animals. *, p < 0.005 versus the corresponding control group.
LAMP-2A Up-regulation in the Midbrain of Paraquat-treated Mice
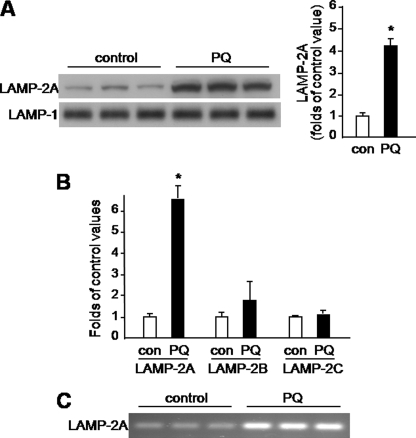
CMA is a pathway of lysosomal protein degradation that has been shown to contribute to α-synuclein clearance in a variety of in vitro cell systems (6–8). We therefore tested if paraquat-induced elevation of lysosomal α-synuclein was accompanied by an increase in levels of the lysosomal receptor LAMP-2A. Binding of cytosolic substrates to LAMP-2A is the rate-limiting step in CMA, and a direct correlation between levels of LAMP-2A at the lysosomal membrane and CMA activity has been previously established (17, 18). LAMP-2A is one of the three spliced variants of a single gene, lamp-2; the other two protein variants coded by this gene, LAMP-2B and -C, are not directly implicated in CMA (17, 19). For this reason, our experiments were carried out using a specific antibody that recognizes LAMP-2A but not LAMP-2B and -C (20). When LAMP-2A levels were compared in the membrane fraction of lysosomes from the midbrain of saline- versus paraquat-injected mice, they were found to be significantly enhanced (4-fold) as a consequence of toxicant exposure (Fig. 3A). To determine if this effect was caused by transcriptional up-regulation, mRNA levels of LAMP-2A, LAMP-2B, and LAMP-2C were assayed by qPCR in homogenates from the mouse ventral mesencephalon. The results showed no change between saline- and paraquat-injected mice in LAMP-2C, a small (not statistically significant) increase in LAMP-2B and a robust elevation (6-fold) of LAMP-2A (Fig. 3B). This significant elevation of LAMP-2A caused by paraquat was also observed when the lamp-2a transcript was amplified by PCR and separated by agarose gel electrophoresis (Fig. 3C).
FIGURE 3.
Paraquat-induced LAMP-2A up-regulation. A, lysosomal membranes were isolated from the midbrain of mice sacrificed 3 days after a single injection of either saline or PQ. Levels of LAMP-2A were measured by Western blot analysis. Blots were probed with an antibody against LAMP-1 to ensure equal loading. B, mRNA levels of LAMP-2A, LAMP-2B, and LAMP-2C were assayed by qPCR in homogenates from the ventral mesencephalon of saline and paraquat-treated mice. A and B, results in the graphs are the means + S.E. of triplicate samples and are expressed as folds of the corresponding control value in saline-treated mice. *, p < 0.01 versus the corresponding control group. C, lamp-2a transcript was amplified by PCR and separated by agarose gel electrophoresis in samples from the ventral mesencephalon of saline and paraquat-injected mice.
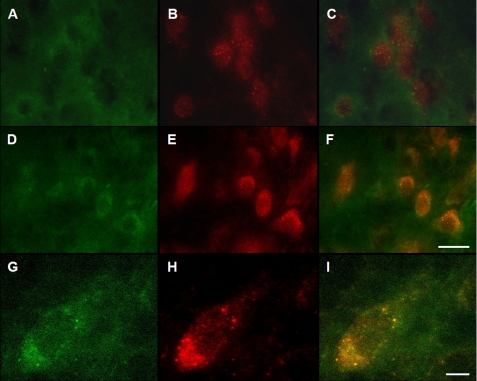
Further evidence of a relationship between paraquat exposure, α-synuclein up-regulation and increased clearance of this protein via CMA was provided by experiments in which midbrain sections from control and paraquat-treated mice were double-immunostained with α-synuclein and LAMP-2A antibodies. Immunoreactivity for both α-synuclein and LAMP-2A was enhanced within nigral neurons in sections from paraquat-injected animals (Fig. 4, A versus D and B versus E), with substantial co-localization of the two proteins (Fig. 4F). α-Synuclein and LAMP-2A antibodies stained neurons in a punctate pattern, most likely reflecting the lysosomal accumulation of α-synuclein that co-localized with the CMA marker (Fig. 4, G–I). Additional midbrain sections were triple-immunostained with antibodies against TH, LAMP-2A, and α-synuclein. In sections from paraquat-treated mice, increased immunoreactivity for LAMP-2A and α-synuclein was observed within neurons that also stained for TH, indicating their dopaminergic phenotype (Fig. 5).
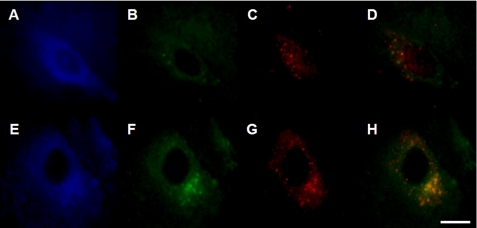
FIGURE 4.
Enhanced LAMP-2A immunoreactivity and LAMP-2A/α-synuclein co-localization within nigral neurons from paraquat-treated mice. A–F, midbrain sections from saline- (A–C) and paraquat- (D–F) injected mice were immunostained with antibodies against α-synuclein (A and D) and LAMP-2A (B and E). Merged images are shown in C and F. Scale bar, 20 μm. G–I, higher magnification images of a representative neuron from the substantia nigra of a paraquat-treated mouse show a punctate pattern of immunostaining for both α-synuclein (G) and LAMP-2A (H) with substantial co-localization of the two proteins (I). Scale bar equals 10 μm.
FIGURE 5.
Paraquat-induced increase in LAMP-2A and α-synuclein immunolabeling within dopaminergic neurons. A–H, midbrain sections from saline- (A–D) and paraquat- (E–H) injected mice were triple-immunolabeled with antibodies against TH (A and E), α-synuclein (B and F), and LAMP-2A (C and G). Merged images of α-synuclein and Lamp-2A immunoreactivity are shown in D and H. Representative images show that, after paraquat treatment, labeling for α-synuclein and Lamp-2A was increased within dopaminergic (TH-positive) neurons. Scale bar equals 10 μm.
Paraquat-induced Increase in Lysosomal hsc70 and hsc70/α-Synuclein Interactions
LAMP-2A up-regulation, enhanced binding of substrates to this receptor, and increased protein translocation across the lysosomal membrane are often associated with increased levels of the lysosomal chaperone hsc70 (12, 21, 22). In the next set of experiments, the content of hsc70 was compared in intact lysosomes isolated from the midbrain of control versus paraquat-injected mice. Data revealed that levels of this chaperone were 2.5-fold higher as a consequence of toxicant administration (Fig. 6A). This finding, together with the observation of LAMP-2A elevation, demonstrates that CMA activity is up-regulated in the midbrain of mice challenged with paraquat.
FIGURE 6.
Paraquat-induced changes in hsc70. A, lysosomes were isolated from the midbrain of animals sacrificed 3 days after a single intraperitoneal injection of saline or PQ. Levels of hsc70 were measured by Western blot analysis. Blots were probed with an antibody against LAMP-1 to ensure equal loading. B, cytosolic fractions of the mouse ventral mesencephalon were immunoprecipitated with an hsc70 antibody, and eluates were then assayed for hsc70 and α-synuclein by Western blot analysis. Immunoprecipitation conditions were adjusted to yield similar levels of hsc70 in tissue eluates from control and paraquat-exposed mice. Results of the densitometric quantification are the means + S.E. of triplicate samples and are expressed as folds of the control (con) value in saline-treated mice. *, p < 0.01 versus the corresponding control group.
If CMA up-regulation plays a role in the clearance of excessive α-synuclein after paraquat exposure, one would expect augmented interactions between α-synuclein and cytosolic hsc70, the chaperone responsible for recognizing CMA substrates and delivering them to the lysosomal membrane (20, 23). To assess hsc70/α-synuclein interactions, cytosolic fractions of the mouse ventral mesencephalon were immunoprecipitated with an hsc70 antibody, and eluates were then assayed for both hsc70 and α-synuclein. Immunoprecipitation conditions were adjusted to yield similar levels of hsc70 in tissue eluates from control and paraquat-exposed mice (Fig. 6B). In agreement with previous findings in cultured cells (6), a portion of cytosolic α-synuclein was recovered bound to cytosolic hsc70 in tissue eluates from control animals (Fig. 6B). To evaluate specificity of this interaction, eluates were also assayed with an antibody against β-synuclein. Immunoblots probed with this antibody showed no immunoreactivity (data not shown). As compared with control mice, levels of α-synuclein were higher in immunoprecipitates from animals injected with paraquat (Fig. 6B), consistent with the interpretation that increased α-synuclein expression resulted in enhanced binding to hsc70 and augmented clearance of the protein via CMA.
CMA Activation in Transgenic Mice Overexpressing α-Synuclein
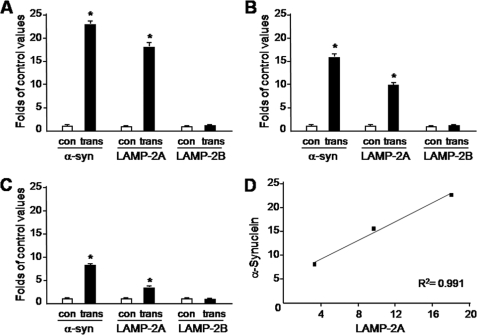
The relationship between increased expression of α-synuclein and CMA activation was further investigated in transgenic mice overexpressing mouse α-synuclein under the regulatory control of the Thy-1 promoter. Earlier work has shown that these transgenics do not develop overt neurodegenerative changes (24). Levels of α-synuclein mRNA were determined in the ventral mesencephalon, hippocampus and frontal cortex of these animals and found to be elevated by 23, 16, and 8 times, respectively, as compared with values in the same brain regions of control littermates (Fig. 7, A–C). α-Synuclein transgenics also displayed a marked increase in LAMP-2A but not LAMP-2B mRNA (Fig. 7, A–C). Interestingly, the extent of LAMP-2A up-regulation was correlated with the degree of α-synuclein overexpression throughout the brain; LAMP-2A levels were increased by 18-fold in the ventral mesencephalon, 10-fold in the hippocampus, and 3-fold in the frontal cortex (Fig. 7D).
FIGURE 7.
Increase in LAMP-2A mRNA in transgenic mice overexpressing α-synuclein. A–C, mRNA levels of α-synuclein (α-syn), LAMP-2A, and LAMP-2B were assayed by qPCR in homogenates from the ventral mesencephalon (A), hippocampus (B), and frontal cortex (C) of control and transgenic mice. Data are the means + S.E. of quadruplicate samples and are expressed as folds of the corresponding control value. *, p < 0.0001 versus the corresponding control group. D, correlation plot for α-synuclein and LAMP-2A mRNAs in the three brain regions of the transgenic mice. R2 = 0.991.
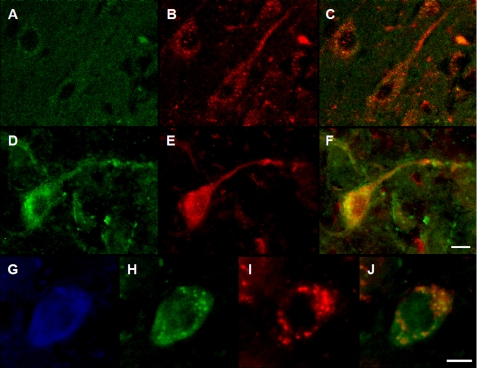
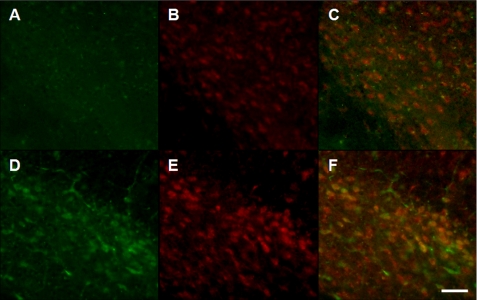
Midbrain sections from transgenic and control animals were immunostained with both α-synuclein and LAMP-2A antibodies. As expected, α-synuclein immunoreactivity was more robust within nigral neurons in samples from overexpressing mice as compared with non-transgenic animals (Fig. 8, D versus A). In the same tissue sections, labeling for LAMP-2A was also more pronounced (Fig. 8, E versus B) and co-localized with α-synuclein immunoreactivity (Fig. 8F), supporting a relationship between α-synuclein overexpression and CMA activation. In a separate set of tissue sections, robust LAMP-2A and α-synuclein staining within dopaminergic neurons of transgenic animals was confirmed by fluorescence microscopy using antibodies against both proteins and TH (Fig. 8, G–J).
FIGURE 8.
Enhanced LAMP-2A labeling in the substantia nigra of α-synuclein-overexpressing mice. A–F, midbrain sections from control (A–C) and transgenic (D–F) mice were immunostained with antibodies against α-synuclein (A and D) and LAMP-2A (B and E). Representative images show increased labeling for both α-synuclein and LAMP-2A in transgenics and substantial co-localization of the two proteins (C and F). G–J, a second set of midbrain sections from transgenic animals was triple-labeled with antibodies recognizing TH (G), α-synuclein (H), and LAMP-2A (I). Merged image of α-synuclein and Lamp-2A immunoreactivity is shown in J. Scale bars equal 15 μm.
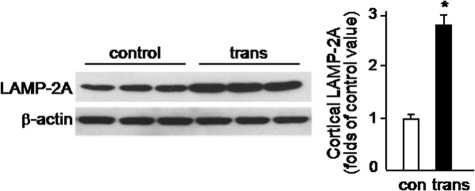
LAMP-2A changes as a consequence of α-synuclein overexpression were also assessed at the protein level by Western blot analysis. Because of limited tissue availability, measurements were not carried out in the substantia nigra. Rather, LAMP-2A protein levels were compared in homogenates from the frontal cortex of control versus transgenic mice. In the latter, in which cortical LAMP-2A mRNA was enhanced by 3-fold (see above), protein levels were also 2.8-fold higher (Fig. 9).
FIGURE 9.
Increase in cortical LAMP-2A in α-synuclein-overexpressing mice. Levels of LAMP-2A were measured by Western blot analysis in homogenates from the frontal cortex of control and transgenic mice. Blots were probed with an antibody against β-actin to ensure equal loading. Results of the densitometric quantification are the means + S.E. of triplicate samples and are expressed as folds of the value in control mice. *, p < 0.001 versus the control group.
To evaluate changes in hsc70 that may arise from α-synuclein overexpression, nigral tissues were dual-labeled with antibodies against these two proteins. Increased α-synuclein immunoreactivity was paralleled by enhanced labeling for hsc70 in transgenic mice (Fig. 10, D versus A and E versus B). Significant co-localization of the two proteins was also observed within nigral neurons (Fig. 10F).
FIGURE 10.
Increased hsc70 immunolabeling in the substantia nigra of α-synuclein-overexpressing mice. A–F, midbrain sections from control (A–C) and transgenic (D–F) mice were immunostained with antibodies against α-synuclein (A and D) and hsc70 (B and E). Merged images are shown in C and F. Representative images of the substantia nigra show increased immunolabeling for α-synuclein and hsc70 and neuronal co-localization of the two proteins in transgenic mice. Scale bar equals 10 μm.
DISCUSSION
Results of this study provide evidence of lysosomal degradation of α-synuclein in vivo by showing internalization of the protein into lysosomes from the midbrain of control mice and mice injected with paraquat. In the latter, selective injury of nigrostriatal dopaminergic neurons is accompanied by an increased expression of α-synuclein (10, 25). Of note, levels of intralysosomal α-synuclein were also markedly enhanced after paraquat exposure, suggesting a relationship between cytosolic up-regulation and lysosomal translocation of the protein. One possible explanation for the increased lysosomal α-synuclein after paraquat exposure may be the formation of post-translationally modified forms of the protein resistant to degradation by lysosomal proteases. In particular, formation of oxidized α-synuclein could be hypothesized under our experimental conditions given the pro-oxidant effects of paraquat on nigral dopaminergic neurons (26, 27). A recent report, however, has demonstrated that the rate of lysosomal clearance is not significantly different between unmodified and oxidized α-synuclein, making it unlikely that paraquat would cause a lysosomal accumulation of protease-resistant forms of the protein (7). Furthermore, we found that ∼50% of α-synuclein was degraded during a 5-min incubation of lysosomal matrix extracts, regardless of whether they were obtained from control or paraquat-treated mice. Thus, lysosomal accumulation of α-synuclein following treatment with the herbicide cannot simply be explained by reduced clearance capability. Rather, it reflects an increase in lysosomal uptake triggered by the cytosolic buildup of the protein.
To elucidate mechanisms involved in the enhanced translocation of α-synuclein into lysosomes after paraquat administration, we assessed changes in lysosomal hsc70 and LAMP-2A as markers of CMA induction. The rationale for investigating the relationship between α-synuclein clearance and CMA in the paraquat model was 2-fold. First, earlier in vitro data are consistent with α-synuclein being a CMA substrate and with CMA playing an important role in preventing neuronal accumulation of the protein (6–8). Second, paraquat administration has previously been shown to upregulate CMA activity in rat liver and cultured fibroblasts (16, 22). Our current findings provide evidence in favor of a contribution of CMA to α-synuclein degradation in the midbrain of paraquat-exposed mice. In these animals, higher levels of lysosomal hsc70 and LAMP-2A paralleled the enhanced cytosolic expression and lysosomal internalization of α-synuclein. Moreover, a punctate α-synuclein and LAMP-2A co-immunoreactivity most likely reflected the lysosomal localization of both these proteins within nigral neurons. Finally, binding of α-synuclein to cytosolic hsc70 was augmented in the midbrain of paraquat-treated mice, further supporting a relationship between CMA induction and increased lysosomal clearance of α-synuclein.
Quite interestingly, paraquat-induced up-regulation of LAMP-2A was achieved through de novo synthesis, as indicated by the dramatic elevation of LAMP-2A mRNA in the ventral mesencephalon of mice injected with the herbicide. At least two mechanisms have been described to increase LAMP-2A under conditions associated with CMA induction. During nutritional stress, the most extensively studied inducer of CMA, enhanced LAMP-2A results from a decrease in its degradation as well as a relocation of the receptor protein from the lysosomal lumen to the lysosomal membrane (18). In contrast, LAMP-2A changes associated with CMA activation during oxidative stress were found to be caused by an actual increase in LAMP-2A synthesis (22). This latter mechanism could explain our present data: the oxidative damage caused by paraquat administration would induce new synthesis of LAMP-2A as a critical CMA factor involved in the lysosomal degradation of α-synuclein and other toxic/damaged proteins.
A causal relationship between intraneuronal α-synuclein levels, CMA induction and enhanced translocation of α-synuclein into lysosomes may be difficult to demonstrate in the in vivo setting. It could be argued that the findings of increased lysosomal α-synuclein and CMA activation in the paraquat model are compatible with but do not necessarily indicate a direct link between these events. In particular, they do not address the issue of whether α-synuclein accumulation is itself a condition leading to CMA induction. To further investigate this possibility, a final set of experiments was carried out in mice in which increased α-synuclein levels resulted from transgenic overexpression of the protein. Data revealed a robust up-regulation of LAMP-2A in the brain of transgenic animals and supported a direct correlation between levels of α-synuclein overexpression and CMA activation. Indeed, the increase in LAMP-2A was greatest in the brain region with the highest α-synuclein level (ventral mesencephalon) and smallest in the area with the lowest protein expression (frontal cortex). CMA induction as a consequence of transgene expression was also indicated by enhanced hsc70 immunoreactivity within nigral neurons laden with α-synuclein.
As suggested by earlier studies, the clearance of wild-type or mutated α-synuclein may involve a variety of mechanisms (5, 6, 8, 28–31). The present findings do not rule out this possibility but clearly reveal the importance of lysosomal pathways and, in particular, CMA in the neuronal degradation of wild-type α-synuclein in vivo. The observation of CMA induction and enhanced lysosomal internalization of α-synuclein under conditions of increased protein expression (i.e. paraquat-induced up-regulation and transgenic overexpression of α-synuclein) bears significant implications. Both clinical and experimental data suggest that higher levels of neuronal α-synuclein may trigger or predispose to pathologic consequences, including aggregate formation and neurodegeneration (2, 4, 32). If so, induction of lysosomal degradation pathways may represent an important mechanism that counteracts α-synuclein-related pathology. Failure of this mechanism, which may arise from normal aging (19), could play a role in the pathogenesis of PD and other α-synucleinopathies, whereas enhancement of these pathways could become a valuable strategy for therapeutic intervention in these human diseases.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant ES12077, the Backus Foundation, and the Parkinson's Disease Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- PD
- Parkinson disease
- LAMP
- lysosome-associated membrane protein
- hsc70
- heat shock cognate protein of 70 kDa
- CMA
- chaperone-mediated autophagy
- qPCR
- quantitative PCR
- Thy-1
- thymus cell antigen 1
- PK
- proteinase K
- PQ
- paraquat
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. (2003) Science 302, 841. [DOI] [PubMed] [Google Scholar]
- 2.Farrer M., Kachergus J., Forno L., Lincoln S., Wang D. S., Hulihan M., Maraganore D., Gwinn-Hardy K., Wszolek Z., Dickson D., Langston J. W. (2004) Ann. Neurol. 55, 174–179 [DOI] [PubMed] [Google Scholar]
- 3.Lo Bianco C., Ridet J. L., Schneider B. L., Deglon N., Aebischer P. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10813–10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eslamboli A., Romero-Ramos M., Burger C., Bjorklund T., Muzyczka N., Mandel R. J., Baker H., Ridley R. M., Kirik D. (2007) Brain 130, 799–815 [DOI] [PubMed] [Google Scholar]
- 5.Webb J. L., Ravikumar B., Atkins J., Skepper J. N., Rubinsztein D. C. (2003) J. Biol. Chem. 278, 25009–25013 [DOI] [PubMed] [Google Scholar]
- 6.Cuervo A. M., Stefanis L., Fredenburg R., Lansbury P. T., Sulzer D. (2004) Science 305, 1292–1295 [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Vicente M., Talloczy Z., Kaushik S., Massey A. C., Mazzulli J., Mosharov E. V., Hodara R., Fredenburg R., Wu D. C., Follenzi A., Dauer W., Przedborski S., Ischiropoulos H., Lansbury P. T., Sulzer D., Cuervo A. M. (2008) J. Clin. Invest. 118, 777–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogiatzi T., Xilouri M., Vekrellis K., Stefanis L. (2008) J. Biol. Chem. 283, 23542–23556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massey A. C., Zhang C., Cuervo A. M. (2006) Curr. Top. Dev. Biol. 73, 205–235 [DOI] [PubMed] [Google Scholar]
- 10.Manning-Bog A. B., McCormack A. L., Li J., Uversky V. N., Fink A. L., Di Monte D. A. (2002) J. Biol. Chem. 277, 1641–1644 [DOI] [PubMed] [Google Scholar]
- 11.Manning-Bog A. B., McCormack A. L., Purisai M. G., Bolin L. M., Di Monte D. A. (2003) J. Neurosci. 23, 3095–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuervo A. M., Dice J. F., Knecht E. (1997) J. Biol. Chem. 272, 5606–5615 [DOI] [PubMed] [Google Scholar]
- 13.Wattiaux R., Wattiaux-De Coninck S., Ronveaux-Dupal M. F., Dubois F. (1978) J. Cell Biol. 78, 349–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aniento F., Roche E., Cuervo A. M., Knecht E. (1993) J. Biol. Chem. 268, 10463–10470 [PubMed] [Google Scholar]
- 15.Ohsumi Y., Ishikawa T., Kato K. (1983) J. Biochem. 93, 547–556 [PubMed] [Google Scholar]
- 16.Massey A. C., Kaushik S., Sovak G., Kiffin R., Cuervo A. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5805–5810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuervo A. M., Dice J. F. (2000) J. Cell Sci. 113, 4441–4450 [DOI] [PubMed] [Google Scholar]
- 18.Cuervo A. M., Dice J. F. (2000) Traffic 1, 570–583 [DOI] [PubMed] [Google Scholar]
- 19.Zhang C., Cuervo A. M. (2008) Nat. Med. 14, 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuervo A. M., Dice J. F. (1996) Science 273, 501–503 [DOI] [PubMed] [Google Scholar]
- 21.Cuervo A. M., Knecht E., Terlecky S. R., Dice J. F. (1995) Am. J. Physiol. 269, C1200–1208 [DOI] [PubMed] [Google Scholar]
- 22.Kiffin R., Christian C., Knecht E., Cuervo A. M. (2004) Mol. Biol. Cell 15, 4829–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang H. L., Terlecky S. R., Plant C. P., Dice J. F. (1989) Science 246, 382–385 [DOI] [PubMed] [Google Scholar]
- 24.Chandra S., Gallardo G., Fernández-Chacón R., Schlüter O. M., Südof T. C. (2005) Cell 123, 383–396 [DOI] [PubMed] [Google Scholar]
- 25.McCormack A. L., Thiruchelvam M., Manning-Bog A. B., Thiffault C., Langston J. W., Cory-Slechta D. A., Di Monte D. A. (2002) Neurobiol. Dis. 10, 119–127 [DOI] [PubMed] [Google Scholar]
- 26.McCormack A. L., Atienza J. G., Johnston L. C., Andersen J. K., Vu S., Di Monte D. A. (2005) J. Neurochem. 93, 1030–1037 [DOI] [PubMed] [Google Scholar]
- 27.McCormack A. L., Atienza J. G., Langston J. W., Di Monte D. A. (2006) Neuroscience 141, 929–937 [DOI] [PubMed] [Google Scholar]
- 28.Bennett M. C., Bishop J. F., Leng Y., Chock P. B., Chase T. N., Mouradian M. M. (1999) J. Biol. Chem. 274, 33855–33858 [DOI] [PubMed] [Google Scholar]
- 29.Lee H. J., Khoshaghideh F., Patel S., Lee S. J. (2004) J. Neurosci. 24, 1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar S., Davies J. E., Huang Z., Tunnacliffe A., Rubinsztein D. C. (2007) J. Biol. Chem. 282, 5641–5652 [DOI] [PubMed] [Google Scholar]
- 31.Yu W. H., Dorado B., Figueroa H. Y., Wang L., Planel E., Cookson M. R., Clark L. N., Duff K. E. (2009) Am. J. Pathol. 175, 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormack A. L., Mak S. K., Shenasa M., Langston W. J., Forno L. S., Di Monte D. A. (2008) J. Neuropathol. Exp. Neurol. 67, 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.