Abstract
Cellular cholesterol balance induces changes in the inflammatory status of macrophages, and low grade chronic inflammation is increasingly being recognized as one of the key steps in the development of atherosclerosis as well as insulin resistance. Cholesteryl ester hydrolase (CEH) catalyzes the hydrolysis of intracellular stored cholesteryl esters (CEs) and thereby enhances free cholesterol efflux and reduces cellular CE content. We have earlier reported reduced atherosclerosis and lesion necrosis in macrophage-specific CEH transgenic mice on a Ldlr−/− background. In the present study, we tested the hypothesis that reduced intracellular accumulation of CE in macrophages from CEH transgenic mice will attenuate expression of proinflammatory mediators, thereby reducing infiltration into adipose tissue, alleviating inflammation, and resulting in improved insulin sensitivity. Western diet fed Ldlr−/−CEH transgenic mice showed improved insulin sensitivity as assessed by glucose and insulin tolerance tests. Macrophages from CEH transgenic mice expressed significantly lower levels of proinflammatory cytokines (interleukin-1β and interleukin-6) and chemokine (MCP-1; monocyte chemoattractant protein). Attenuation of NF-κB- and AP-1-driven gene expression was determined to be the underlying mechanism. Infiltration of macrophages into the adipose tissue that increases inflammation and impairs insulin signaling was also significantly reduced in Ldlr−/−CEH transgenic mice. In the OP-9 adipocyte peritoneal macrophage co-culture system, macrophages from CEH transgenic mice had a significantly reduced effect on insulin signaling as measured by Akt phosphorylation compared with nontransgenic macrophages. Taken together, these studies demonstrate that macrophage-specific overexpression of CEH decreases expression of proinflammatory mediators and attenuates macrophage infiltration into the adipose tissue, resulting in decreased circulating cytokines and improved insulin sensitivity.
Keywords: Diseases/Diabetes, Diseases/Metabolic, Gene/Transgene, Lipid/Cholesterol, Macrophage, Adipose Tissue, Inflammation
Introduction
Accumulation of lipids, specifically cholesteryl esters (CEs)3, in macrophages is central to foam cell formation and development of atherosclerosis. In addition to contributing to the increasing lipid burden of the developing plaque, foam cells also add to associated inflammation by secreting proinflammatory cytokines and chemokines. Intracellular accumulation of CE or cholesterol is considered to enhance the expression of proinflammatory mediators. Fazio and Linton (1) proposed a feedback loop-linking macrophage cholesterol balance and inflammatory mediators and suggested that a primary defect in cellular cholesterol balance may induce changes in the inflammatory status of the macrophage. Consistently, under conditions of enhanced cholesterol accumulation, for example by deficiency of cholesterol transporter ABCA1, there was a marked increase in TNFα secretion from macrophages (2). In contrast, overexpression of apoA1 that enhances cholesterol removal and decreases cellular cholesterol levels resulted in attenuated response to proinflammatory insult by LPS (3). However, the underlying mechanisms linking cellular cholesterol content and expression of proinflammatory mediators are not completely defined. Furthermore, the cause or effect relationship of inflammation to atherosclerosis is also not completely understood (4).
Chronic low grade inflammation is now also recognized as a key step in the pathogenesis of obesity-induced insulin resistance and type 2 diabetes mellitus. Adipose tissue was initially recognized as the site of production of proinflammatory mediators (5, 6) responsible for this low grade inflammation. However, recent studies have demonstrated that majority of adipose tissue-derived cytokines (TNFα, IL-6, and IL-1β) actually originate in nonfat cells, and, among them, infiltrated macrophages play the most prominent role, and this low grade inflammation is mediated by the activation and recruitment of macrophages into expanding adipose tissue (7). The level of macrophages within a tissue represents a balance between recruitment, survival/expansion, and emigration. Adipocytes as well as resident macrophages produce several chemokines and growth factors that facilitate macrophage infiltration in expanding adipose tissue, namely MCP-1 (8), macrophage colony-stimulating factor and granulocyte macrophage colony-stimulating factor (9) that recruit and assist in expansion or colonization of macrophages, respectively. Whether accumulation of cholesterol in macrophages can affect macrophage recruitment into the adipose tissue due to increased production of these proinflammatory mediators has not been explored. Subramanian et al. (10) have recently reported that addition of a relatively small amount (0.15%) of dietary cholesterol resulted in a marked increase in accumulation of macrophages in adipose tissue. Although this study provides the first evidence that cholesterol plays an important role in macrophage infiltration into the adipose tissue, the role of macrophage cholesterol balance in regulating this process remains undefined.
We recently developed transgenic mice with macrophage-specific overexpression of cholesteryl ester hydrolase (CEH). Macrophages from these mice stored less CEs as a result of CEH-mediated CE mobilization, and this led to a significant attenuation of diet-induced atherosclerosis in a Ldlr−/− background (11). The present study was undertaken to test the hypothesis that reduced intracellular accumulation of CE in macrophages from CEH transgenic mice will attenuate expression of proinflammatory mediators, thereby reducing infiltration into adipose tissue, alleviating inflammation, and resulting in improved insulin sensitivity. The data presented here support this hypothesis and also identify attenuated NF-κB activation with overexpression of CEH as one of the potential underlying mechanisms.
EXPERIMENTAL PROCEDURES
Animals and Diets
Development and characterization of macrophage-specific CEH transgenic mice has been described elsewhere (11). Littermates with or without CEH transgene on a Ldlr−/− background (Ldlr−/−CEHTg and Ldlr−/−) were used for all studies. Where indicated, mice were fed a high fat, high cholesterol (Western) diet (TD88137, Harlan Teklad) for 16 weeks.
Intraperitoneal Glucose Tolerance Tests
10-week-old Ldlr−/− and Ldlr−/−CEHTg littermates were fed a Western diet for 16 weeks. After an overnight fast, a single bolus of glucose (2 mg/g body weight) was given intraperitoneally. Blood glucose levels were determined by commercially available glucometer using tail vein blood at 0, 15, 30, 60, 120, and 180 min.
Intraperitoneal Insulin Tolerance Tests
10-week-old Ldlr−/− and Ldlr−/−CEHTg littermates were fed a Western diet for 16 weeks. After a 6-h morning fast, a single bolus of insulin (0.75 units/kg body weight) was given intraperitoneally. Blood glucose levels were determined by commercially available glucometer using tail vein blood at 0, 15, 30, 60, 120, and 180 min.
Glucose-induced Plasma Insulin Levels
10-week-old Ldlr−/− and Ldlr−/−CEHTg littermates were fed a Western diet for 16 weeks. After an overnight fast, a single bolus of glucose (2 mg/g body weight) was given intraperitoneally. Blood was collected from the tail vein at 0, 15, 30, 45, and 60 min, and plasma insulin levels were determined by ELISA (ultra-sensitive rat/mouse insulin assay from Crystal Chem, Inc.).
Transient Transfections and Luciferase Reporter Assays
agmACAT1 cells were obtained from Dr. Lawrence L. Rudel. Cells were maintained in Ham's F12 medium supplemented with 10% fetal bovine serum, penicillin/streptomycin, and 200 μg/ml GeneticinTM. Cells were plated in 24-well tissue culture plates at a density of 2 × 105 cells/well and were either transfected with vector alone (pCMV) or CEH expression vector, pCMV-CEH using EffecteneTM and the optimized conditions described earlier (12). In addition, cells were also co-transfected with a luciferase reporter vector driven by tandem NF-κB- or AP-1-binding sites. Growth medium was replaced every 24 h, and luciferase activity was determined in the cell lysates 48 h after transfection. To correct for transfection efficiencies, luciferase activity was normalized to β-galactosidase activity. To determine the effects of CEH overexpression on NF-κB- or AP-1-driven gene expression, data are presented as percent control (cells transfected with empty pCMV vector) for three independent experiments performed in triplicate. In some experiments, NF-κB inhibitor (MG132, 10 μm) was added to cells 24-h post-transfection with pCMV vector, and cell lysates were assayed for luciferase activity after an additional 24 h.
Isolation of Peritoneal Macrophages
Thioglycollate-elicited peritoneal macrophages were harvested, and nonadherent cells were removed after 2 h, and medium was replaced with fresh growth medium (11). For lipid loading, cells were incubated with loading medium containing 50 μg/ml acetylated low density lipoprotein for 24 h. For determination of gene expression, in some experiments, total RNA was isolated (using the RNeasy kit from Qiagen) from the adherent macrophages 2 h after plating.
Isolation of Adipose Tissue Macrophages
Adipose tissue macrophages were isolated by the method described by Brown et al. (13). In brief, 1 gm of visceral (perigonadal) adipose tissue was washed and then minced in phosphate-buffered saline. Following centrifugation, the washed tissue was collected and digested with collagenase type II (Sigma-Aldrich) for 1 h at 37 °C with gentle shaking. The undigested tissue was removed by straining through a cell strainer (100 μm), and cells were collected by centrifugation. Following a wash in RPMI medium, cells were plated in appropriate tissue culture dishes.
Activation of NF-κB
NF-κB DNA-binding activity was assessed with TransAMTM NF-κB p50 transcription factor assay kit (Active Motif) using nuclear extracts from peritoneal or adipose tissue macrophages. Briefly, nuclear extracts were added to each well of a 96-stripwell plate to which the consensus NF-κB-binding site oligonucleotide had been immobilized. A primary antibody specific for an epitope on the bound and active form of the transcription factor was then added, followed by subsequent incubation with secondary antibody and developing solution. Intensity of the color developed was quantified and is a measure of activated and DNA-bound NF-κB in the tested nuclear extract.
OP-9 Adipocyte Cultures
OP-9 cells were obtained from Dr. Nancy Webb and were grown in OP-9 propagation medium: minimum Eagle's medium with 20% fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were replated every 3 days at a density of at least 7,000 cells/cm2 and differentiated into adipocytes by serum replacement method as described earlier (14). In brief, the cells were grown to confluence and then cultured for additional 2 days in propagation medium. Cells were then cultured for 2 more days in serum replacement medium: minimum Eagle's medium with 15% KnockOutTM SR, 100 units/ml penicillin, and 100 μg/ml streptomycin. Medium was then replaced with the propagation medium, and, 2 days after differentiation, 0.4-μm cell culture inserts (Millipore) were placed in the well. Thioglycollate elicited macrophages (1 × 106 cells) were plated in the inserts, and these co-cultures were incubated for 24 h. The macrophage-containing inserts were removed, and adipocytes washed three times with phosphate-buffered saline containing Ca2+ and Mg2+ to remove the bound insulin and rinsed once with minimum Eagle's medium with 0.2% bovine serum albumin and then incubated in this medium for 2.5 h. Following two washes with phosphate-buffered saline containing Ca2+ and Mg2+ buffer and a 30-min incubation, insulin (50 nm) was added, and total cell lysates were prepared after 15 min as described below. Changes in insulin signaling were determined by measuring insulin-dependent Akt phosphorylation by Western blot analyses as described below.
Western Blot Analyses
The cells were washed twice with ice-cold phosphate-buffered saline and then lysed by incubating in lysis buffer (150 mm NaCl, 50 mm Tris, pH 7.5, 1% Triton X-100, protease and phosphatase inhibitor mixture (Sigma-Aldrich, St. Louis, MO)) for 30 min at 4 °C. Following centrifugation at 14,000 rpm for 10 min at 4 °C, the supernatant or the total cell lysates were collected and used for Western blot analyses. The proteins (20 μg) were separated by 10% SDS-PAGE (Bio-Rad Laboratories), transferred to polyvinylidene difluoride membrane, immunoblotted with primary antibodies (AKT1/2/3 or p-AKT1/2/3, Santa Cruz Biotechnology) and followed by species-specific horseradish peroxidase-conjugated secondary antibodies. Positive immunoreactivity was detected by chemiluminescence and quantified by densitometry using Quantity One 4.4.0 software (Bio-Rad Laboratories).
Real Time PCR
Total RNA was extracted using RNeasy kit (Qiagen). Complementary DNA was synthesized using a high capacity cDNA reverse transcription kit (Applied Biosystems). Real time PCR was performed on Stratagene Mx3000P machine, using TaqMan Universal PCR Master Mix and optimized probe and primer sets from Applied Biosystems. Following probes were used CD-68 (Mm00839636_g1), SR-A (Mm00446214_m1), IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), MCP-1 (Mm00441242_m1), Pref1 (Mm00494477_m1), and aP2 (FABP4, Mm00445880_m1).
Cytokine Measurements
Cytokines in plasma as well as cell culture supernatants were determined using the BeadlyteTM Multiplex assay system (Millipore).
Histological Analyses of Adipose Tissue
Visceral (perigonadal) adipose tissue was fixed in buffered formalin and paraffin-embedded, and three to four sections (5-mm thick) were transferred to numbered slides. Slides were then stained with hematoxylin and eosin or immunostained for macrophages using F4/80 as the primary antibody (eBioscience). Positive immunoreactivity was detected using Alexa 546-conjugated secondary antibody (Invitrogen). Images were acquired using a Zeiss Observer A1 inverted microscope and analyzed using AxioVision software.
RESULTS
Macrophage-specific Transgenic Expression of CEH Improves Glucose and Insulin Tolerance Tests
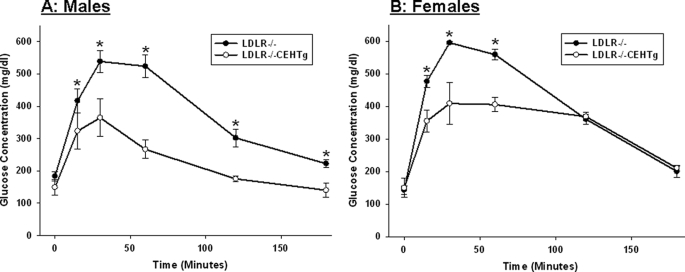
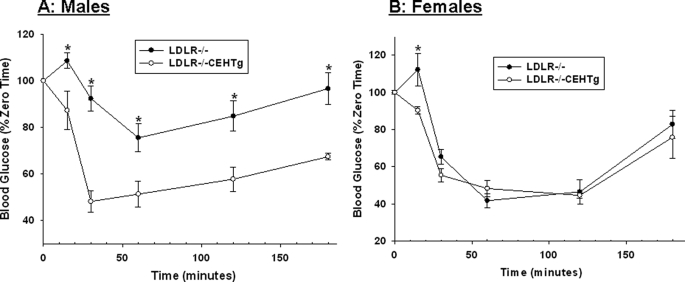
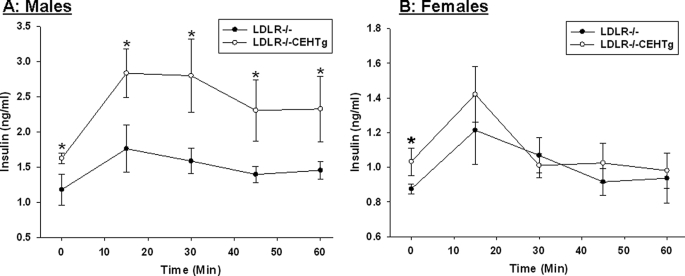
Intraperitoneal glucose and insulin tolerance tests were performed in Western diet-fed Ldlr−/− and Ldlr−/−CEHTg mice. As shown in Fig. 1, significant improvement in glucose tolerance was noted in Ldlr−/−CEHTg mice. Although there was a significant difference at all time points in males (Fig. 1A), the differences were significant only at earlier time points in females (15, 30, and 60 min, Fig. 1B). However, a significant difference in insulin tolerance was only apparent in male mice (Fig. 2A); the significant difference in females was apparent only at the earliest time point of 15 min. These data suggest that macrophage-specific CEH expression leads to improved insulin sensitivity in Ldlr−/−CEHTg mice compared with Ldlr−/−, and the differences are more significant in male mice. Consistently, glucose-induced plasma insulin levels were significantly higher in Ldlr−/−CEHTg male mice (Fig. 3A).
FIGURE 1.
Intraperitoneal glucose tolerance test. 10-week-old Ldlr−/− and Ldlr−/−CEHTg mice (littermates) were fed a Western diet for 16 weeks. After an overnight fast, blood glucose levels at time 0 were determined using a commercial glucometer. Subsequently, mice were given an intraperitoneal bolus of glucose (2 mg/g body weight), and blood glucose levels were determined at indicated times. Data are expressed as mean ± S.D. (n = 4 for males (A) and females (B). *, p < 0.05.
FIGURE 2.
Intraperitoneal insulin tolerance test. 10-week-old Ldlr−/− and Ldlr−/−CEHTg mice (littermates) were fed a Western diet for 16 weeks. After a 6-h morning fast, blood glucose levels were determined at time 0 using a commercial glucometer. Insulin (0.75 units/kg body weight) was then injected intraperitoneally, and blood glucose levels were determined at indicated times. Data (mean ± S.D.; n = 4 for males (A) and females (B)) are expressed as percent of glucose levels at time 0. *, p < 0.05.
FIGURE 3.
Plasma insulin levels following an intraperitoneal glucose challenge. 10-week-old Ldlr−/− and Ldlr−/−CEHTg mice (littermates) were fed a Western diet for 16 weeks. After an overnight fast, mice were given an intraperitoneal bolus of glucose (2 mg/g body weight), and blood was collected at indicated times. Plasma insulin levels were determined by ELISA. Data are expressed as mean ± S.D. (n = 4 for males (A) and females (B). *, p < 0.05.
CEH Overexpression Leads to Attenuation of Cytokine Expression in Macrophages
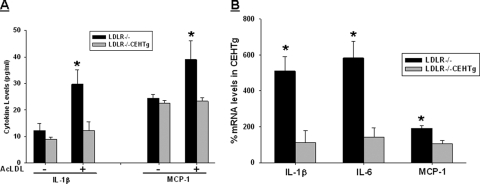
Systemic as well as adipose tissue inflammation affects insulin sensitivity and cellular cholesterol content is thought to regulate the inflammatory status of macrophages. Transgenic expression of CEH reduces intracellular cholesterol levels in macrophages (11), and we hypothesized that CEH-mediated reduction in cellular cholesterol content will reduce expression of inflammatory cytokines. This reduction in inflammation could be one of the underlying mechanisms for the observed improvement in insulin sensitivity in CEHTg mice. Expression of primary master cytokine IL-1β and chemokine MCP-1, which regulates macrophage recruitment into other tissues, was examined in peritoneal macrophages isolated from Ldlr−/− and Ldlr−/−CEHTg mice. Upon lipid loading with acetylated low density lipoprotein, there was a significant increase in IL-1β as well as MCP-1 secretion from macrophages isolated from Ldlr−/− mice (Fig. 4A). However, this lipid loading-induced increase in cytokine and chemokine secretion was significantly attenuated in macrophages from Ldlr−/−CEHTg mice. Consistently, significantly higher mRNA levels of IL-1β, IL-6, and MCP-1 were observed in freshly isolated peritoneal macrophages from Western diet-fed Ldlr−/− mice compared with those from Ldlr−/−CEHTg mice (Fig. 4B). These data indicate that macrophages from CEHTg mice have attenuated proinflammatory cytokine and chemokine expression.
FIGURE 4.
CEH overexpression decreases the proinflammatory cytokine production from macrophages. A, thioglycollate-elicited macrophages were obtained from Ldlr−/− and Ldlr−/−CEHTg mice and lipid loaded with acetylated low density lipoprotein (AcLDL; 50 μg/ml). IL-1β and MCP-1 levels in the conditioned medium were determined by ELISA. Data are expressed as mean ± S.D. (n = 3). B, thioglycollate-elicited macrophages were obtained from Western diet-fed Ldlr−/− and Ldlr−/−CEHTg mice, and total RNA was isolated from adherent cells 2 h after plating. Expression of IL-1β, IL-6, and MCP-1 was determined by real time reverse transcription-PCR, and data (mean ± S.D.; n = 3) are expressed as percent mRNA levels in Ldlr−/−CEHTg mice. *, p < 0.05.
Macrophages with Transgenic Expression of CEH Have Reduced NF-κB Activation
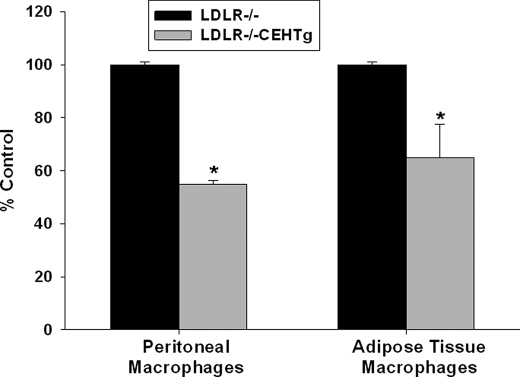
NF-κB is the key transcription factor regulating the expression of majority of proinflammatory cytokines. To test the hypothesis that reduced activation of NF-κB may be responsible for the observed decrease in proinflammatory cytokine expression in macrophages from CEHTg mice, activated NF-κB levels in nuclear extracts prepared from freshly isolated peritoneal macrophages and adipose tissue macrophages from Western diet-fed mice were determined. As shown in Fig. 5, NF-κB levels were significantly attenuated in macrophages from CEHTg mice, suggesting that reduced activation/nuclear translocation of proinflammatory transcription factor NF-κB is likely the underlying mechanism for reduced cytokine production.
FIGURE 5.
Reduced activation of NF-κB in CEHTg macrophages. Thioglycollate-elicited peritoneal macrophages and adipose tissue macrophages were obtained from Western diet-fed Ldlr−/− and Ldlr−/−CEHTg mice and nuclear extracts were prepared as described under “Experimental Procedures.” DNA binding of activated or nuclear NF-κB levels was determined by TransAmTM Assay. Data (mean ± S.D., n = 3) are presented as percent LDLR−/− control. *, p < 0.05.
CEH Overexpression Attenuates Reporter Gene Expression Driven by Proinflammatory Transcription Factors NF-κB and AP-1
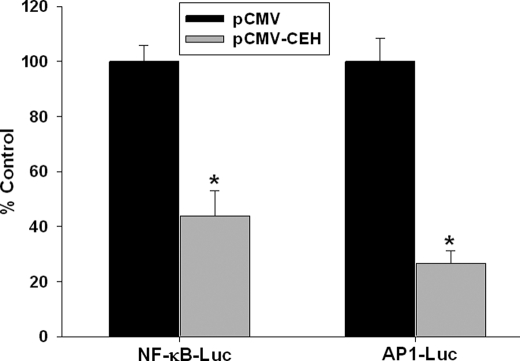
To evaluate the direct effects of CEH overexpression on NF-κB- or AP-1-mediated gene transcription, a previously standardized cell culture system was used. These cells (agmACAT-1) accumulate reproducible amounts of CEs due to stable overexpression of ACAT-1, and CEH overexpression in these cells efficiently mobilizes cellular CE (12). Using optimal conditions where significant reduction in cellular CE is achieved following transient transfection with CEH expression vector (pCMV-CEH) (12), we investigated the effects on NF-κB- or AP-1-dependent reporter gene expression. As shown in Fig. 6, NF-κB- or AP-1-driven luciferase expression was significantly reduced when agmACAT1 cells were co-transfected with CEH expression vector (pCMV-CEH). These data suggest that CEH overexpression attenuates proinflammatory transcription factor (NF-κB or AP-1) driven gene expression. In the presence of NF-κB inhibitor MG132, there was a dramatic decrease in luciferase activity (2.63 ± 2.19% of vehicle control, data not shown) in cells transfected with pCMV, suggesting that the NF-κB inhibitor can mimic the effects of CEH overexpression, albeit in an appreciably more potent manner.
FIGURE 6.
Transient overexpression of CEH in agmACAT-1 cells decreases NF-κB- and AP-1-driven luciferase expression. agmACAT1 cells were plated in 24-well tissue culture plates and transfected with either NF-κB- or AP-1-driven luciferase reporter vector. The cells were also co-transfected with either an empty vector pCMV or CEH expression vector pCMV-CEH. The medium was replaced every 24 h, and cells were lysed 48 h post-transfection, and luciferase activity was determined and normalized to transfection efficiency control (β-galactosidase). Data (mean ± S.D.; n = 3) are expressed as percent pCMV control. *, p < 0.05.
Reduced Levels of Circulating Cytokines in Ldlr−/−CEHTg Mice
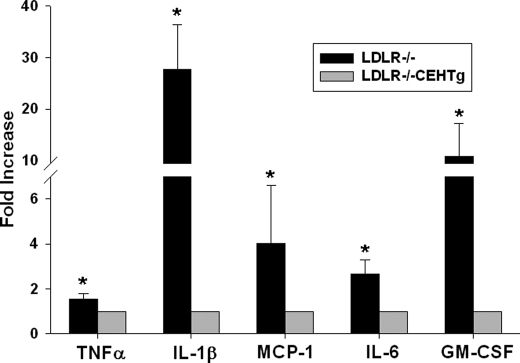
To evaluate whether reduced activation of cellular NF-κB or AP-1 and thereby reduced macrophage inflammation leads to decreased systemic inflammation, circulating levels of plasma cytokines were determined in Western diet-fed Ldlr−/− and Ldlr−/−CEHTg mice. Consistent with attenuated activation of proinflammatory transcription factors, significantly reduced levels of circulating cytokines were observed in Ldlr−/−CEHTg mice when compared with Ldlr−/− mice (Fig. 7). It is noteworthy that the levels of master cytokine IL-1β were almost 20-fold higher in Ldlr−/− mice.
FIGURE 7.
Decreased levels of circulating cytokines in Ldlr−/−CEHTg mice. Ldlr−/− and Ldlr−/−CEHTg mice were fed a Western diet for 16 weeks. Plasma samples were collected at the time of sacrifice, and circulating cytokine levels were determined by BeadlyteTM multiplex assay system. Data (mean ± S.D., n = 5) are expressed as percent respective cytokine levels in Ldlr−/−CEHTg mice. *, p < 0.05. GM-CSF, granulocyte macrophage colony-stimulating factor.
Decreased Infiltration of Macrophages into Adipose Tissue in CEH Transgenic Mice
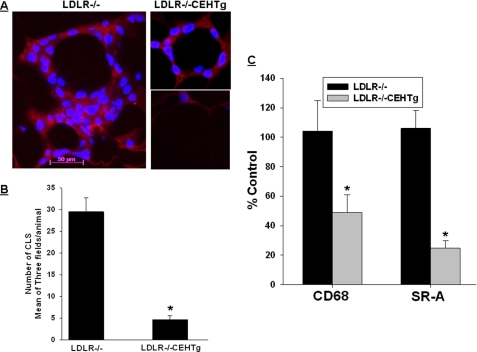
In obesity, adipose tissue becomes a major contributor to circulatory cytokines. Furthermore, infiltration of macrophages into the adipose tissue is increasingly being considered as the determinant of adipose tissue inflammation. To determine whether decreased macrophage inflammation leads to reduced infiltration into the expanding adipose tissue, presence of macrophages in perigonadal adipose tissue was evaluated either by immunohistochemistry or by monitoring the expression of macrophage-specific genes (CD-68 and SR-A). Consistent with the pattern of macrophage distribution in adipose tissue, adipocytes were surrounded by macrophages forming a “crown-like” structure in adipose tissue from Western diet-fed Ldlr−/− mice. In contrast, in adipose tissue obtained from Western diet-fed Ldlr−/−CEHTg mice, either there were no macrophages surrounding the adipocytes, or a smaller number of macrophages were present around the adipocytes (Fig. 8A). Quantification of these crown-like structures demonstrated a significantly attenuated infiltration of macrophages into the adipose tissue of Ldlr−/−CEHTg mice. These data were confirmed by a corresponding reduction in the expression of macrophage-specific genes (CD-68 and SR-A) in adipose tissue obtained from Western diet-fed Ldlr−/−CEHTg mice.
FIGURE 8.
Transgenic overexpression of CEH reduces macrophage infiltration into adipose tissue. Infiltration of macrophages into perigonadal adipose tissue was assessed by immunohistochemistry as well as by measuring macrophage-specific gene expression as described under “Experimental Procedures.” A, macrophages stained with F4/80 (red) surround the adipocytes. Nuclei are stained with DAPI (blue). The magnification for all the three images is the same (scale bar shown in one of the images), although the field size is different. B, quantification of crown-like structures (CLS). Data are expressed as mean ± S.D. for n = 3; a minimum of three fields were analyzed for each animal. C, total RNA from adipose tissue was used to determine the levels of macrophage-specific genes (CD-68 and SR-A) by real time reverse transcription-PCR. Data (mean ± S.D., n = 3) are presented as percent Ldlr−/− control. *, p < 0.05.
Attenuated Effect on Insulin Signaling in Adipocytes by Macrophages from Ldlr−/−CEHTg Mice
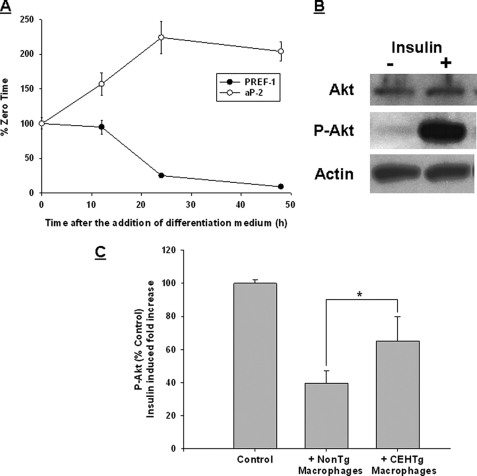
In addition to increasing the adipose tissue inflammation, the infiltrated macrophages also affect insulin signaling in adipocytes, leading to insulin resistance. OP-9 adipocyte culture was optimized, and differentiation was assessed by the decrease in the expression of preadipocyte factor-1 and increase in the expression of adipocyte marker aP2 (Fig. 9A). These differentiated OP-9 adipocytes were responsive to insulin, as shown by an increase in insulin-dependent Akt phosphorylation (Fig. 9B). A co-culture system was used to examine the effects of macrophages on insulin signaling in OP-9 adipocytes. Insulin signaling as measured by activation/phosphorylation of Akt was attenuated when OP-9 adipocytes were co-cultured with macrophages from Ldlr−/− mice. This attenuation was significantly reduced when macrophages from Ldlr−/−CEHTg were used (Fig. 9C). Although incubation of OP-9 adipocytes with medium conditioned by macrophages that were isolated from Ldlr−/−CEHTg mice did not noticeably affect insulin-induced Akt phosphorylation, a decrease (∼30%) was noted when adipocytes were incubated with medium conditioned by macrophages from Ldlr−/− mice (data not shown). Taken together with the results shown in Fig. 8, these data demonstrate that macrophage-specific transgenic expression of CEH not only reduces infiltration of macrophages into the expanding adipose tissue, but the negative effect of infiltrated macrophages on insulin signaling in adipocytes is also reduced. Decreased systemic and adipose tissue inflammation, as well as reduced effect on adipocyte insulin signaling, are therefore the potential mechanisms for the observed improvement in insulin sensitivity in Ldlr−/−CEHTg mice.
FIGURE 9.
CEHTg macrophages have reduced effect on insulin signaling in adipocytes compared with nontransgenic macrophages. A, differentiation of OP-9 cells. Preadipocyte factor-1 (Pref-1, marker gene for preadipocyte) and aP2 (adipocyte-specific marker) mRNA levels were monitored to confirm the differentiation into adipocytes. Data are presented as percent of undifferentiated cells (zero time) prior to the addition of the differentiation medium. B, insulin responsiveness of differentiated OP-9 adipocytes was assessed by monitoring insulin-mediated Akt phosphorylation. Cells were stimulated with insulin for 30 min and Akt, phosphorylated Akt (P-Akt) were examined by Western blot analyses. C, differentiated OP-9 adipocytes were co-cultured with control nontransgenic or CEH transgenic macrophages, and insulin-induced fold increase in Akt phosphorylation was determined as described under “Experimental Procedures.” Data (mean ± S.D., n = 3) are presented as percent control; insulin-induced fold increase in Akt phosphorylation observed in OP-9 adipocytes cultured without macrophages was used as control. *, p < 0.05. CLS, crown-like structures.
DISCUSSION
Inflamed adipose tissue due to increased infiltration of macrophages is being considered as an important factor underlying the metabolic syndrome and atherosclerosis (15). Using macrophage-specific CEH transgenic mice, we have earlier demonstrated that overexpression of CEH and resulting reduction in macrophage cholesterol accumulation and increased reverse cholesterol transport leads to attenuation of diet-induced atherosclerosis and lesion necrosis in Ldlr−/− mice (11). Our present study demonstrates that transgenic expression of CEH also results in decreased expression of proinflammatory mediators due to reduced activation of NF-κB, attenuated infiltration of macrophages into the adipose tissue of Ldlr−/−CEHTg mice, and significantly lower levels of circulating cytokines. In addition, attenuation of insulin signaling in adipocytes by CEHTg macrophages was significantly lower when compared with nontransgenic macrophages. Collectively, these effects result in improved glucose and insulin tolerance in Western diet-fed Ldlr−/− mice.
Macrophage cholesterol balance is directly linked to the inflammatory status, and the close relationship between macrophage cholesterol efflux and inflammation is exemplified by attenuation of in vitro efflux and in vivo reverse cholesterol transport by endotoxins (16). In addition, proinflammatory cytokines such as IL-1β and TNFα enhance lipid accumulation and foam cell formation by reducing lipid catabolism (17). CEH-mediated hydrolysis of intracellular CE represents the obligatory first step in cholesterol efflux, and the data presented here establishes that overexpression of CEH attenuates proinflammatory gene expression. This is consistent with the reported role of the ATP-binding cassette transporter A1 (ABCA1), which exports cholesterol from cells to apoA1, in suppressing macrophage inflammation and genetic manipulations of ABCA1 expression in mice affect inflammation and pancreatic beta cell function (18). It is noteworthy that macrophages from Ldlr−/−ABCA1−/− mice with an 80-fold increase in cellular CE content display an exaggerated inflammatory response (19). A recent clinical study demonstrated beneficial effects of reconstituted high density lipoprotein infusions that enhance cholesterol efflux from cells on suppression of inflammation (20), underscoring the importance of cholesterol removal in modulating inflammatory processes.
Adipose tissue inflammation and circulating cytokines, namely IL-1β (21), IL-6 (22), and TNFα (23), collectively contribute to insulin resistance associated with obesity. IL-1β is the primary cytokine that not only regulates its own production by an autofeed forward loop but also regulates the production of other cytokines including IL-6 (24). IL-1β and IL-6 are thought to perturb insulin signaling, including insulin-dependent Akt phosphorylation (25, 26), and recent evidence suggests that some of the effects of TNFα may be mediated by its ability to induce IL-6 (27). Macrophages are the primary source of IL-1β, and macrophage-derived IL-1β production in insulin-sensitive organs such as adipose tissue, leads to progression of inflammation and induction of insulin resistance in obesity (28). IL-1β production and secretion have also been reported from pancreatic islets and insulin-producing beta cells within pancreatic islets are specifically prone to IL-1β-induced destruction and loss of function (28). Taken together with our data showing that CEH transgenic mice have dramatically reduced circulating IL-1β levels along with reduced levels of other cytokines and chemokines, attenuated levels of circulating IL-1β and/or other cytokines likely contribute to the observed improvement in insulin sensitivity in these mice. Reduction in macrophage infiltration into the adipose tissue due to reduced chemokine (MCP-1 and granulocyte macrophage colony-stimulating factor) levels further add to this beneficial effect.
Transcription of proinflammatory cytokines is largely regulated by transcription factors NF-κB and/or AP-1, and increased levels of activated NF-κB and AP-1 are observed in macrophages from diabetic patients (29). Our studies show that there is reduced activation of NF-κB in peritoneal as well as adipose tissue macrophages from CEH transgenic mice and in vitro overexpression of CEH directly affects NF-κB- and AP-1-mediated reporter gene transcription. These data are consistent with the observed increase in the expression of proinflammatory cytokines and increased activation of the NF-κB in macrophages isolated from macrophage-specific ABCA1 knock-out mice that accumulate high levels of intracellular CE (30). Thus, cellular cholesterol levels and activation of proinflammatory transcription factors are probably linked by a mechanism not completely understood as yet. In this regard, it is noteworthy that macrophage specific inactivation of NF-κB reduces foam cell formation (31).
In conclusion, transgenic overexpression of CEH results in reduced systemic and adipose tissue inflammation, and collectively, these two processes lead to improved insulin sensitivity. CEH overexpression mediated attenuation of NF-κB and AP-1 activation is one of the underlying mechanisms. Future studies will establish the specific mechanism(s) or pathways linking cellular cholesterol balance and inflammation.
Recent studies have suggested a tentative link between cellular cholesterol content, membrane lipid rafts, and TLR4 signaling, suggesting that these changes underlie the cholesterol accumulation-mediated increase in inflammation (2, 30). Ongoing studies in the laboratory will establish whether CEH-mediated reduction in cellular cholesterol content has the opposite effect and whether changes in lipid rafts/TLR4 signaling or other intracellular events lead to the observed attenuation of NF-κB activation as well as decreased expression of proinflammatory mediators in CEH-overexpressing cells.
Acknowledgment
The transgenic mice used in these studies were developed by Virginia Commonwealth University/Massey Cancer Center Transgenic/Knock-out Mouse Core, which is supported by National Institutes of Health Grant P30 CA16059.
This work was supported, in whole or in part, by National Institutes of Health Research Grant HL-069946 from NHLBI. This work was also supported by Research Award 7-08-RA-51 from the American Diabetes Association (to S. G.).
- CE
- cholesteryl ester
- CEH
- CE hydrolase
- TNF
- tumor necrosis factor
- ELISA
- enzyme-linked immunosorbent assay
- CMV
- cytomegalovirus
- ABCA1
- ATP-binding cassette transporter A1
- SR-A
- scavenger receptor A
- IL
- interleukin.
REFERENCES
- 1.Fazio S., Linton M. F. (2001) Am. J. Cardiol. 88, 12E–15E [DOI] [PubMed] [Google Scholar]
- 2.Koseki M., Hirano K., Masuda D., Ikegami C., Tanaka M., Ota A., Sandoval J. C., Nakagawa-Toyama Y., Sato S. B., Kobayashi T., Shimada Y., Ohno-Iwashita Y., Matsuura F., Shimomura I., Yamashita S. (2007) J. Lipid Res. 48, 299–306 [DOI] [PubMed] [Google Scholar]
- 3.Levine D. M., Parker T. S., Donnelly T. M., Walsh A., Rubin A. L. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 12040–12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chait A., Han C. Y., Oram J. F., Heinecke J. W. (2005) J. Lipid Res. 46, 389–403 [DOI] [PubMed] [Google Scholar]
- 5.Trayhurn P., Wood I. S. (2004) Br. J Nutr. 92, 347–355 [DOI] [PubMed] [Google Scholar]
- 6.Wisse B. E. (2004) J. Am. Soc. Nephrol. 15, 2792–2800 [DOI] [PubMed] [Google Scholar]
- 7.Bouloumié A., Curat C. A., Sengenès C., Lolmède K., Miranville A., Busse R. (2005) 8, 347–354 [DOI] [PubMed] [Google Scholar]
- 8.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., Kasuga M. (2006) J. Clin. Invest. 116, 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D. H., Sandoval D., Reed J. A., Matter E. K., Tolod E. G., Woods S. C., Seeley R. J. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E1038–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian S., Han C. Y., Chiba T., McMillen T. S., Wang S. A., Haw A., 3rd., Kirk E. A., O'Brien K. D., Chait A. (2008). Arterioscler. Thromb. Vasc. Biol. 28, 685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B., Song J., Chow W. N., St. Clair R. W., Rudel L. L., Ghosh S. (2007). J. Clin. Invest. 117, 2983–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh S., St. Clair R. W., Rudel L. L. (2003) J. Lipid Res. 44, 1833–1840 [DOI] [PubMed] [Google Scholar]
- 13.Brown J. M., Boysen M. S., Chung S., Fabiyi O., Morrison R. F., Mandrup S., McIntosh M. K. (2004) J. Biol. Chem. 279, 26735–26747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolins N. E., Quaynor B. K., Skinner J. R., Tzekov A., Park C., Choi K., Bickel P. E. (2006) J. Lipid Res. 47, 450–460 [DOI] [PubMed] [Google Scholar]
- 15.Gustafson B., Hammarstedt A., Andersson C. X., Smith U. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 2276–2283 [DOI] [PubMed] [Google Scholar]
- 16.McGillicuddy F. C., de la Llera Moya M., Hinkle C. C., Joshi M. R., Chiquoine E. H., Billheimer J. T., Rothblat G. H., Reilly M. P. (2009) Circulation. 119, 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson J., Nilsson J., Lindholm M. W. (2008) BMC Immunol. 9, 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang C., Oram J. F. (2009) Biochim. Biophys. Acta 1791, 563–572 [DOI] [PubMed] [Google Scholar]
- 19.Francone O. L., Royer L., Boucher G., Haghpassand M., Freeman A., Brees D., Aiello R. J. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1198–1205 [DOI] [PubMed] [Google Scholar]
- 20.Patel S., Drew B. G., Nakhla S., Duffy S. J., Murphy A. J., Barter P. J., Rye K. A., Chin-Dusting J., Hoang A., Sviridov D., Celermajer D. S., Kingwell B. A. (2009) J. Am. Coll. Cardiol. 53, 962–971 [DOI] [PubMed] [Google Scholar]
- 21.Besedovsky H. O., Del Rey A. (1990) Ann. N.Y. Acad. Sci. 594, 214–221 [DOI] [PubMed] [Google Scholar]
- 22.Vozarova B., Weyer C., Hanson K., Tataranni P. A., Bogardus C., Pratley R. E. (2001) Obes. Res. 9, 414–417 [DOI] [PubMed] [Google Scholar]
- 23.Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993) Science 259, 87–91 [DOI] [PubMed] [Google Scholar]
- 24.Taishi P., Churchill L., De A., Obal F., Jr., Krueger J. M. (2008) Brain Res. 1226, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagathu C., Bastard J. P., Auclair M., Maachi M., Capeau J., Caron M. (2003) Biochem. Biophys. Res. Commun. 311, 372–379 [DOI] [PubMed] [Google Scholar]
- 26.Jager J., Grémeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J. F. (2007) Endocrinology 148, 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez B., Quinn L. S., Busquets S., Quiles M. T., Lopez-Soriano F. J., Argiles J. M. (2002) Biochim. Biophys. Acta 1542, 66–72 [DOI] [PubMed] [Google Scholar]
- 28.Maedler K., Dharmadhikari G., Schumann D. M., Størling J. (2009) Expert Opin. Biol. Ther. 9, 1177–1188 [DOI] [PubMed] [Google Scholar]
- 29.Nam J. S., Cho M. H., Lee G. T., Park J. S., Ahn C. W., Cha B. S., Lim S. K., Kim K. R., Ha H. J., Lee H. C. (2008) Diabetes Res. Clin. Pract. 81, 25–32 [DOI] [PubMed] [Google Scholar]
- 30.Zhu X., Lee J. Y., Timmins J. M., Brown J. M., Boudyguina E., Mulya A., Gebre A. K., Willingham M. C., Hiltbold E. M., Mishra N., Maeda N., Parks J. S. (2008) J. Biol. Chem. 283, 22930–22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira V., van Dijk K. W., Groen A. K., Vos R. M., van der Kaa J., Gijbels M. J., Havekes L. M., Pannekoek H. (2007) Atherosclerosis 192, 283–290 [DOI] [PubMed] [Google Scholar]