Abstract
The phenotypic modulation of vascular smooth muscle cells (VSMCs) plays a pivotal role in hypertension-induced vascular changes including vascular remodeling. The precise mechanisms underlying VSMC phenotypic modulation remain elusive. Here we test the role of peroxisome proliferator-activated receptor (PPAR)-γ in the VSMC phenotypic modulation during hypertension. Both spontaneously hypertensive rat (SHR) aortas and SHR-derived VSMCs exhibited reduced PPAR-γ expression and excessive VSMC phenotypic modulation identified by reduced contractile proteins, α-smooth muscle actin (α-SMA) and smooth muscle 22α (SM22α), and enhanced proliferation and migration. PPAR-γ overexpression rescued the expression of α-SMA and SM22α, and inhibited the proliferation and migration in SHR-derived VSMCs. In contrast, PPAR-γ silencing exerted the opposite effect. Activating PPAR-γ using rosiglitazone in vivo up-regulated aortic α-SMA and SM22α expression and attenuated aortic remodeling in SHRs. Increased activation of phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling was observed in SHR-derived VSMCs. PI3K inhibitor LY294002 rescued the impaired expression of contractile proteins, and inhibited proliferation and migration in VSMCs from SHRs, whereas constitutively active PI3K mutant had the opposite effect. Overexpression or silencing of PPAR-γ inhibited or excited PI3K/Akt activity, respectively. LY294002 counteracted the PPAR-γ silencing induced proliferation and migration in SHR-derived VSMCs, whereas active PI3K mutant had the opposite effect. In contrast, reduced proliferation and migration by PPAR-γ overexpression were reversed by the active PI3K mutant, and further inhibited by LY294002. We conclude that PPAR-γ inhibits VSMC phenotypic modulation through inhibiting PI3K/Akt signaling. Impaired PPAR-γ expression is responsible for VSMC phenotypic modulation during hypertension. These findings highlight an attractive therapeutic target for hypertension-related vascular disorders.
Keywords: Cellular Regulation, Contractile Protein, Gene Silencing, PI 3-Kinase, Transcription Factors, Transformation, Hypertension, Phenotype, Vascular Smooth Muscle Cell
Introduction
Hypertension and hypertension-induced vascular remodeling underlie numerous cardiovascular disorders. Activated vascular smooth muscle cells (VSMCs)2 are essential contributors to this vascular remodeling (1). Unlike other muscle cells, VSMCs do not terminally differentiate. They can switch from a differentiated phenotype (also known as contractile or quiescent phenotype) to a dedifferentiated phenotype (also known as synthetic or activated phenotype) in response to vascular injury. In this process, VSMCs regain their proliferative and migratory capacities, secrete matrix proteins, and down-regulate smooth muscle contractile proteins, such as α-smooth muscle actin (α-SMA), smooth muscle 22α (SM22α), smooth muscle myosin heavy chain, and calponin (2). Although this phenotypic modulation is undoubtedly required for vascular repair, inappropriate modulation is associated with increased vascular resistance and aggravates vascular injury. However, despite intensive research efforts, the precise mechanisms underlying VSMC phenotypic modulation during hypertension remain unclear.
The peroxisome proliferator-activated receptor (PPAR)-γ is a ligand-activated transcription factor (3). It regulates various genes involved in glucose homeostasis, lipid metabolism, and adipocyte differentiation via binding to the PPAR response elements in target genes, and therefore participates in modulation of several diseases such as diabetes, obesity, and atherosclerosis (4, 5). Convincing evidence indicates the role of PPAR-γ in hypertension. For example, individuals with a dominant-negative PPAR-γ mutation present with early onset hypertension and elements of metabolic syndrome (6).
PPAR-γ is expressed in VSMCs in vivo and in vitro (7) and exerts an important role in the regulation of VSMC viability. In spontaneously hypertensive rat (SHR)-derived VSMCs, PPAR-γ overexpression or treatment with the PPAR-γ agonist thiazolidinedione retards VSMC growth to the level of non-hypertensive rat VSMCs (8, 9). In addition, PPAR-γ inhibits the VSMC proliferation induced by platelet-derived growth factor and angiotensin II (7, 10). It is also reported that PPAR-γ can suppress the VSMC invasion (11) and migration induced by matrix metalloprotease (12, 13). Considering that phenotypic modulation is a prerequisite for VSMCs to regain the proliferative and migratory capacity (14), we postulate that PPAR-γ can negatively regulate the phenotypic modulation of VSMCs.
Phosphoinositide 3-kinase (PI3K)/Akt signaling pathway plays a pivotal role in the regulation of cellular growth, apoptosis, and metabolism (15, 16). Activated PI3K phosphorylates Akt and induces the expression of transcriptional factors involved in multiple processes. The PI3K/Akt signaling is reportedly required for VSMC migration and proliferation, absence of Akt impairs VSMC proliferation and migration (17). A previous study (18) indicated the link between PI3K/Akt signaling and PPAR-γ. PPAR-γ activation can inhibit the Akt phosphorylation induced by vascular endothelial growth factor in endothelial cells (18). However, neither the role of PPAR-γ and PI3K/Akt signaling nor their exact interaction in VSMC phenotypic modulation during hypertension is fully understood. In the present study, we test the hypothesis that PPAR-γ plays an important role in inhibiting VSMC phenotypic modulation through negatively regulating the activity of PI3K/Akt signaling and thus participates in VSMC phenotypic modulation during hypertension.
EXPERIMENTAL PROCEDURES
Reagents
Rosiglitazone and LY294002 were purchased from Sigma. Antibodies targeting PPAR-γ, phospho-Akt (Thr-308), α-SMA, and SM22α were from Santa Cruz Biotechnology. The small interfering RNA (siRNA) duplex targeting PPAR-γ was synthesized by Shanghai Biosia Company and sequenced by Sunbio Biotechnology.
Animals
Eight-week-old SHRs and age-matched normotensive Wistar-Kyoto (WKY) control rats were purchased from Shanghai Experimental Animal Centre and housed at the animal facility in Daping Hospital. Animals were trained for 1 week to minimize any stress-associated blood pressure increases with the tail-cuff method. Both SHRs and WKYs were randomly divided into two groups and treated with vehicle (WKY-veh, SHR-veh) or rosiglitazone (WKY-rsg, SHR-rsg) (10 mg/kg/day) for 12 weeks, administered once per day via gavage. All animals had access to water ad libitum. The systolic blood pressure (SBP) was measured weekly in conscious rats using tail-cuff plethysmography (Letica 5100; PanLab, Barcelona, Spain) according to the supplier's protocol. The SBP values reported are the average of three sequential measurements. The body weight was measured weekly, with the last measurement 24 h before sacrifice.
Cell Culture
Rats were anesthetized by pentobarbital and the VSMCs were isolated from the thoracic aorta. Cells were cultured in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium. Cells in the second to sixth passages were used.
For gene silencing, a siRNA duplex targeting PPAR-γ was transfected into VSMCs using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. A non-related scrambled siRNA was used as a negative control. For PPAR-γ overexpression, adenovirus expressing PPAR-γ was generated using the ViraPower Adenoviral Expression System (Invitrogen) according to the manufacturer's protocol, and then transfected into cultured VSMCs for 24 h. VSMC transfected with the scrambled sequence, siRNA, and PPAR-γ expression adenovirus were named PPAR-γ-scr, PPAR-γ-kd, and PPAR-γ-ov, respectively. Experiments were performed after 60 h of transfection.
For the manipulation of PI3K activity, cultured VSMCs were exposed to the PI3K inhibitor LY294002 (25 μm, 1 h) (19) or transfected with adenovirus encoding constitutively active PI3K mutant. When two-step transfection was needed, VSMCs were first transfected with PPAR-γ-targeted siRNA or PPAR-γ expression adenovirus for 24 h, and then with adenovirus encoding active PI3K mutant. Experiments were performed after 60 h of transfection.
PI3K Activity Assay
The PI3K activity was measured using an enzyme-linked immunosorbent assay kit (Echelon Biosciences) according to the supplier's protocol and a previous study (20). Briefly, cells were lysed with lysis buffer (1% (v/v) Nonidet P-40, 100 mm NaCl, 20 mm Tris, pH 7.4, 10 mm iodoacetamide, 10 mm NaF, 1 mm sodium orthovanadate and protease inhibitors) followed by centrifugation at 12,000 × g for 20 min. Supernatant proteins were incubated with an immobilized anti-p85α antibody overnight. The immunoprecipitates were washed with lysis buffer and then incubated with a reaction mixture containing phosphatidylinositol (PtdIns)-4,5-P2 substrate and ATP. The reaction mixtures were first incubated with an antibody to PtdIns-3,4,5-P3 and then added to the PtdIns-3,4,5-P3-coated microplate for competitive binding. Peroxidase-linked secondary antibody and colorimetric detection were used to detect anti-PtdIns-3,4,5-P3 binding to the plate. The colorimetric signal was inversely proportional to the amount of PtdIns-3,4,5-P3 produced by activated PI3K.
Western Blot Analysis
Western blot analysis was performed as described previously (21). Briefly, protein samples were obtained either from homogenized arteries or cultured cells, and then, the protein concentration was determined. Protein samples (30 mg) were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and incubated with appropriate primary antibodies. After incubation with secondary antibodies, the proteins were detected by enhanced chemiluminescence (PerkinElmer Life Sciences) and quantified using a Gel Doc 2000 Imager (Bio-Rad). Western blot quantification was performed by densitometry and normalization to β-actin.
Cell Proliferation Assay
To measure cell proliferation, VSMCs were seeded (3 × 104 cells/ml) into 96-well plates and cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Cell numbers were determined with a cell counter after 1, 2, or 3 days of culture. MTT assay (22) was also used to analyze cell proliferation after 3 days of culture. Briefly, a 20-μl aliquot of 5 mg/ml of MTT solution was added to each well and incubated for 24 h. Supernatants were aspirated, and the resulting crystals were dissolved in 200 μl of dimethyl sulfoxide. Light absorbance at 570 nm was read on a microplate reader (Bio-Rad).
Cell Migration Assay
Cell migration was examined using modified Boyden chambers, as described previously (23). Dulbecco's modified Eagle's medium containing 0.4% fetal bovine serum and platelet-derived growth factor (20 μg/ml) was added to the lower section of the Boyden chambers. VSMCs suspended in Dulbecco's modified Eagle's medium (5.0 × 104 cells/ml) were added to the upper section of the Boyden chambers. After incubation for 4 h at 37 °C, the top surface of the filters was scraped and rinsed with phosphate-buffered saline. Cells on the undersides of the filters were fixed with methanol and stained with Diff-Quik (Baxter Healthcare Co., Miami, FL), and then counted from five high-power (×400) fields per well. The average was used as the migratory cell number.
Histological Detection
Rats were sacrificed by pentobarbital overdose and subjected to fixed perfusion. The abdominal aortas were removed and fixed in formalin solution, and then embedded in paraffin. Serial 4-μm sections were stained with hematoxylin and eosin, and photomicrographs of the tissue sections were analyzed to determine the internal diameter and thickness of intima and medium.
Statistical Analysis
Values are expressed as mean ± S.E. The significance of the results was assessed by a paired t test between two groups. Differences among three or more groups were analyzed by contrast analysis, using one-way analysis of variance followed by LSD post hoc testing. Statistics were calculated with the SPSS 11.0 software package. Differences were considered statistically significant for p < 0.05.
RESULTS
VSMC Phenotypic Changes during Hypertension
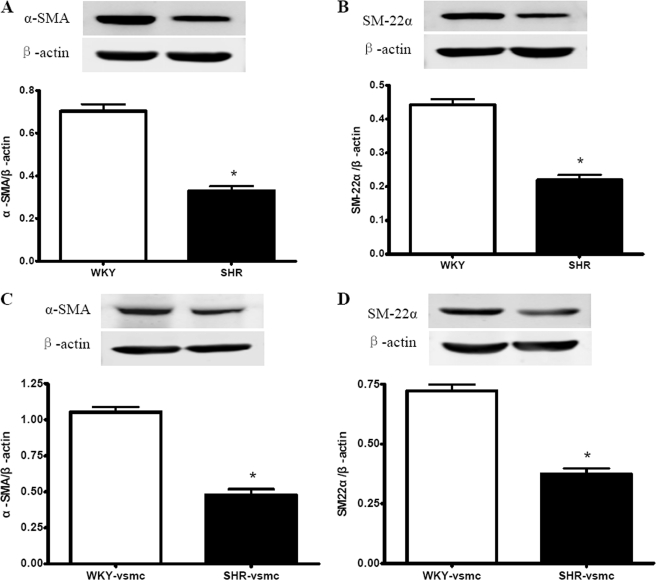
To determine the VSMC phenotypic changes during hypertension, we detected the expression of two smooth muscle contractile proteins, α-SMA and SM22α, in SHR aorta and in cultured VSMCs derived from SHRs. The proliferative and migratory capacities were also measured in cultured VSMCs. It was revealed that the aortic expression levels of α-SMA and SM22α were markedly decreased in SHRs compared with those in WKYs (Fig. 1, A and B). Consistent with this, SHR-derived VSMCs displayed reduced expression of α-SMA and SM22α compared with WKY-derived VSMCs (Fig. 1, C and D).
FIGURE 1.
Expressions of α-SMA and SM22α in rat aorta and cultured VSMCs using Western blot analysis. The SHR aortas exhibited reduced α-SMA (A) and SM22α (B) expression levels compared with WKY aortas. The SHR-derived VSMCs showed a lower level of α-SMA (C) and SM22α (D) expression than WKY-derived VSMCs. Data are normalized to β-actin and presented as mean ± S.E. (n = 5–6). *, p < 0.05 versus WKY or WKY-VSMC.
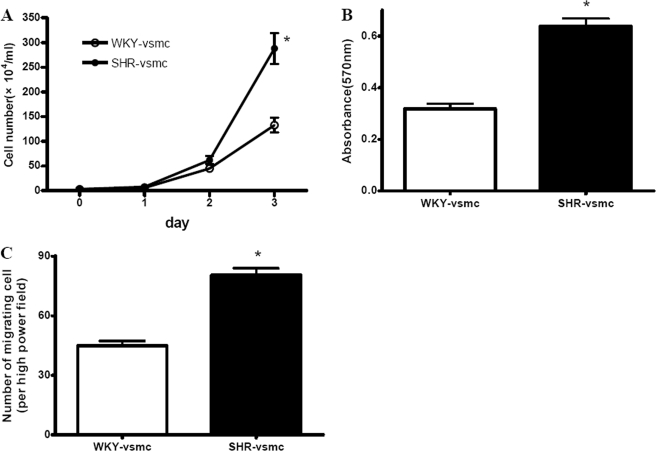
Reportedly, SHR-derived VSMCs display increased proliferative capacities (24). We revealed that after culture for 3 days, the number of VSMC from SHRs was greater than that from WKYs (Fig. 2A). The MTT assay also indicated the increased proliferation in SHR-derived VSMCs compared with WKY-derived VSMCs (Fig. 2B). The migration of VSMCs from the medium to intima is a pivotal step in vascular remodeling. Using a Boyden chamber assay, we observed that, compared with WKY-derived cells, the SHR-derived VSMCs showed enhanced migration in response to platelet-derived growth factor (Fig. 2C). These findings suggested that SHR-derived VSMCs exhibited marked phenotypic modulation, evidenced by enhanced proliferation and migration, and reduced expression of contractile markers α-SMA and SM22α.
FIGURE 2.
Proliferation and migration of cultured VSMCs, as determined by cell counter (A), MTT assay (B), and modified Boyden chamber (C). A, after a 3-day culture, the number of SHR-derived VSMCs was much higher than WKY-derived VSMCs. B and C, the SHR-derived VSMCs showed enhanced proliferative (B) and migratory (C) capacities compared with WKY-derived VSMCs. Data are presented as mean ± S.E. (n = 5). *, p < 0.05 versus WKY-VSMC.
Changes of PPAR-γ Expression during Hypertension
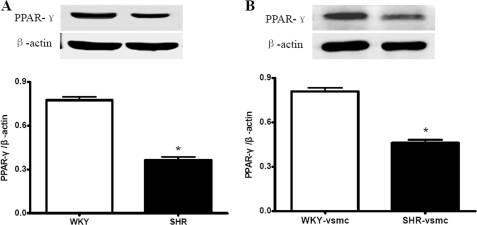
VSMCs express PPAR-γ in vivo and in culture (7), although how PPAR-γ expression changes during hypertension remains controversial (8, 25). Here we found that PPAR-γ expression in SHR aortas was reduced compared with that in WKY aortas (Fig. 3A). Consistent with this, SHR-derived VSMCs also displayed significant reduction in PPAR-γ expression compared with WKY-derived VSMCs (Fig. 3B).
FIGURE 3.
Expressions of PPAR-γ in rat aorta and cultured VSMCs using Western blot analysis. A, the SHR aortas exhibited reduced PPAR-γ expression levels compared with WKY aortas. B, SHR-derived VSMCs exhibited a lower level of PPAR-γ expression than WKY-derived VSMCs. Data are normalized to β-actin and presented as mean ± S.E. (n = 5–6). *, p < 0.05 versus WKY or WKY-VSMC.
Role of PPAR-γ in VSMC Phenotypic Modulation
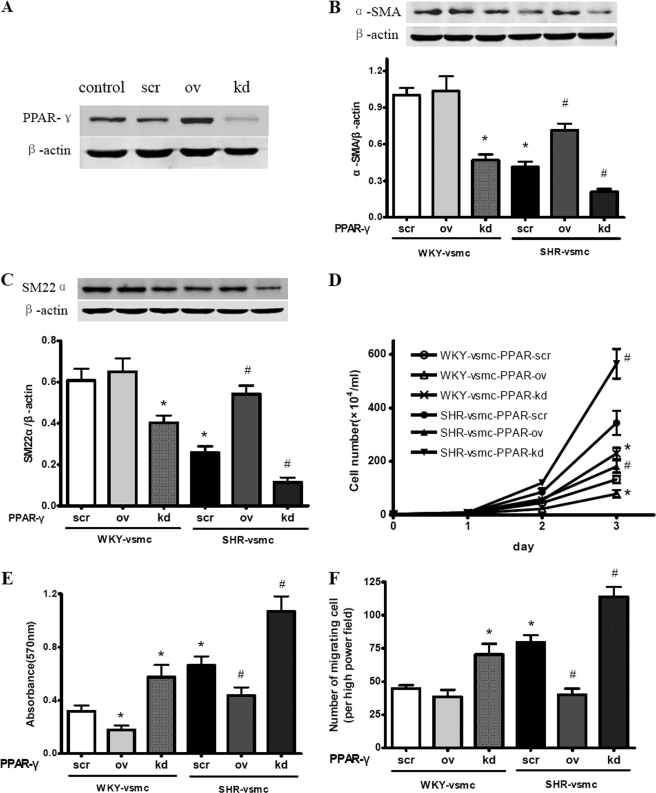
We postulated that impaired PPAR-γ expression is associated with the increased phenotypic modulation of VSMCs during hypertension. To address this, we overexpressed or silenced the PPAR-γ in cultured VSMCs (Fig. 4A), and then measured the expression of contractile proteins and the cell proliferative and migratory capacities. The decreased expression of α-SMA and SM22α in SHR-derived VSMCs was rescued significantly by PPAR-γ overexpression, whereas PPAR-γ silencing further impaired α-SMA and SM22α expression in SHR-derived VSMCs. As for WKY-derived VSMCs, PPAR-γ overexpression had no obvious impact on α-SMA and SM22 expression, whereas PPAR-γ silencing reduced the level of α-SMA and SM22α markedly (Fig. 4, B and C). PPAR-γ overexpression inhibited the increased proliferation and migration observed in SHR-derived VSMCs, whereas PPAR-γ silencing led to a further increased proliferative and migratory capacity. As for WKY-derived VSMCs, PPAR-γ overexpression reduced proliferation but had no obvious effect on migration. In contrast, PPAR-γ silencing led to a significant increase in both proliferation and migration (Fig. 4, D–F). These findings suggest that PPAR-γ serves as an inhibitor of phenotypic modulation by preserving the contractile markers and inhibiting proliferation and migration in cultured VSMCs.
FIGURE 4.
Effect of manipulated PPAR-γ on phenotypic modulation of cultured VSMCs, as determined by Western blot analysis (A–C), cell counter (D), MTT assay (E), and modified Boyden chamber (F). A, manipulating PPAR-γ expression with adenovirus-mediated overexpression or siRNA-mediated gene silencing. B and C, effect of PPAR-γ on α-SMA (B) and SM22α (C) expression in cultured VSMCs. SHR-VSMC showed reduced α-SMA and SM22α expression compared with WKY-VSMC. PPAR-γ overexpression significantly improved the α-SMA and SM22 protein levels in SHR-VSMC, but had no obvious impact on α-SMA and SM22 levels in WKY-VSMC. PPAR-γ silencing reduced the level of α-SMA and SM22α in WKY-VSMC, and exacerbated the impaired α-SMA and SM22α expression in SHR-VSMC. Data are normalized to β-actin and presented as mean ± S.E. (n = 5). D–F, effect of PPAR-γ on proliferation and migration of cultured VSMCs. After a 3-day culture, the number of SHR-VSMC was much more than that of WKY-VSMC. PPAR-γ overexpression decreased, whereas PPAR-γ silencing increased the number of SHR-VSMC. The same effect was also observed in WKY-VSMC, although not as significant as in SHR-VSMC (D). The SHR-VSMC showed enhanced proliferation (E) and migration (F) compared with WKY-VSMC. Overexpression of PPAR-γ markedly impeded proliferation and migration in SHR-VSMC, whereas PPAR-γ silencing had the opposite effects. As to WKY-VSMC, PPAR-γ overexpression inhibited the proliferative capacities but had no obvious impact on migration. In contrast, PPAR-γ silencing significantly increased the proliferation and migration in WKY-VSMC. Data are presented as mean ± S.E. (n = 5). *, p < 0.05 versus WKY-VSMC with PPAR-γ-scr; #, p < 0.05 versus SHR-VSMC with PPAR-γ-scr.
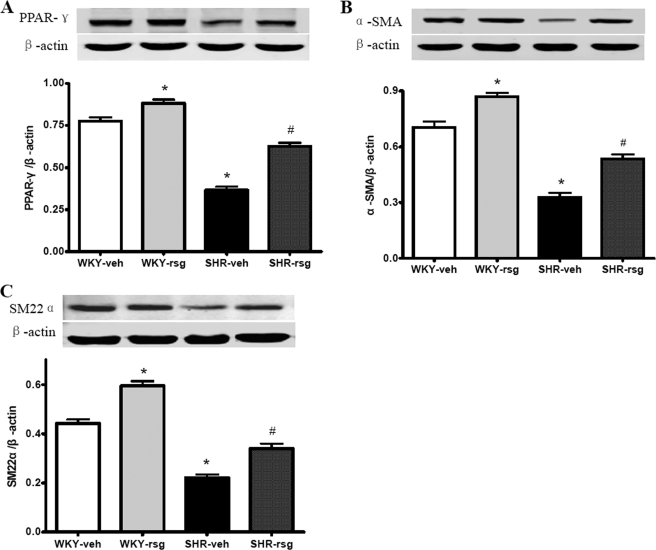
We next activated the PPAR-γ using rosiglitazone in vivo. It showed that rosiglitazone treatment for 12 weeks markedly improved the impaired PPAR-γ expression in SHR aortas (Fig. 5A). Rosiglitazone also reversed the α-SMA and SM22α reduction observed in SHR aorta (Fig. 5, B and C). These findings indicate that increasing PPAR-γ expression with rosiglitazone preserves the aortic contractile proteins during hypertension, confirming the negative modulation of PPAR-γ on VSMC phenotypic modulation during hypertension in vivo.
FIGURE 5.
Effects of rosiglitazone on aortic expressions of PPAR-γ, α-SMA, and SM22α using Western blot analysis. The SHR aortas exhibited reduced PPAR-γ (A), α-SMA (B), and SM22α (C) expression levels compared with WKY aortas. Rosiglitazone treatment improved the expression of PPAR-γ (A), α-SMA (B), and SM22α (C) both in SHR and WKY aortas. Data are presented as mean ± S.E. (n = 6). *, p < 0.05 versus WKY-veh; #, p < 0.05 versus SHR-veh. veh, treated with vehicle; rsg, treated with rosiglitazone.
Effect of Rosiglitazone on Aortic Remodeling
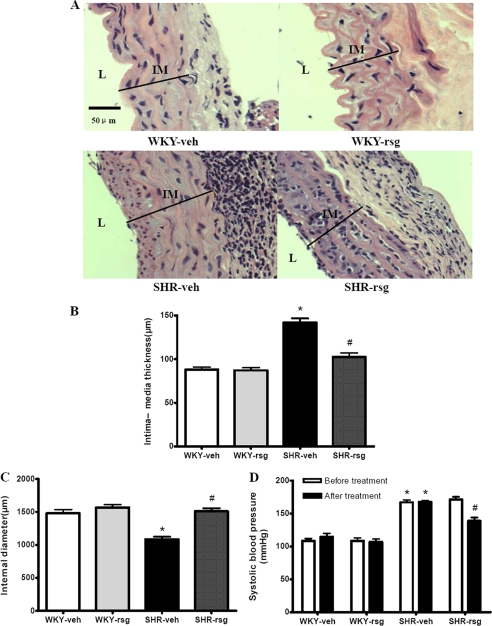
Chronic hypertension is the most important cause of vascular remodeling. In the present study, SHR aortas exhibited obvious remodeling characterized by intima-media thickening (IMT) (Fig. 6, A and B) and reduced internal diameter (Fig. 6C). These changes indicated an inward and hypertrophic remodeling in SHR aortas. Rosiglitazone treatment for 12 weeks reduced IMT and ameliorated the internal diameter in SHR aortas (Fig. 6, A–C), which was consistent with previous studies (26, 27). These data indicate that improving PPAR-γ expression with rosiglitazone ameliorate the aortic remodeling induced by hypertension.
FIGURE 6.
Effect of rosiglitazone on aortic remodeling and SBP in SHRs. A and B, compared with WKYs, SHR aortas showed increased IMT. Using rosiglitazone significantly reduced the IMT in SHR aorta. C and D, SHR exhibited reduced internal diameter (C) and elevated SBP (D). Rosiglitazone treatment significantly improved the internal diameter (C) in SHR aorta and reduced the SBP of SHRs (D). Data are presented as mean ± S.E. (n = 6). For B and C, *, p < 0.05 versus WKY-veh; #, p < 0.05 versus SHR-veh. For D, *, p < 0.05 versus WKY-veh; #, p < 0.05 versus SHR-veh and SHR-rsg before treatment. L, lumen; IM, intima and media; veh, treated with vehicle; rsg, treated with rosiglitazone.
Effect of Rosiglitazone on the SBP of SHRs
The SBP of SHRs was much higher than that of WKYs (167.0 ± 8.81 versus 108.3 ± 8.96 mm Hg). Twelve weeks of rosiglitazone treatment effectively reduced the SBP of SHRs, whereas the vehicle exerted no effect on SBP of SHRs (Fig. 6D), suggesting the beneficial effects of rosiglitazone on blood pressure control.
Similar to previous studies (28), we observed an increased body weight in SHRs compared with WKYs (247.7 ± 4.41 versus 228.8 ± 4.88 g). The body weight was elevated in both WKYs and SHRs after 12 weeks of treatment, whereas rosiglitazone did not show any effect on the body weight of rats in either group (data not shown).
Role of PI3K/Akt Signaling in the Phenotypic Modulation of Cultured VSMCs
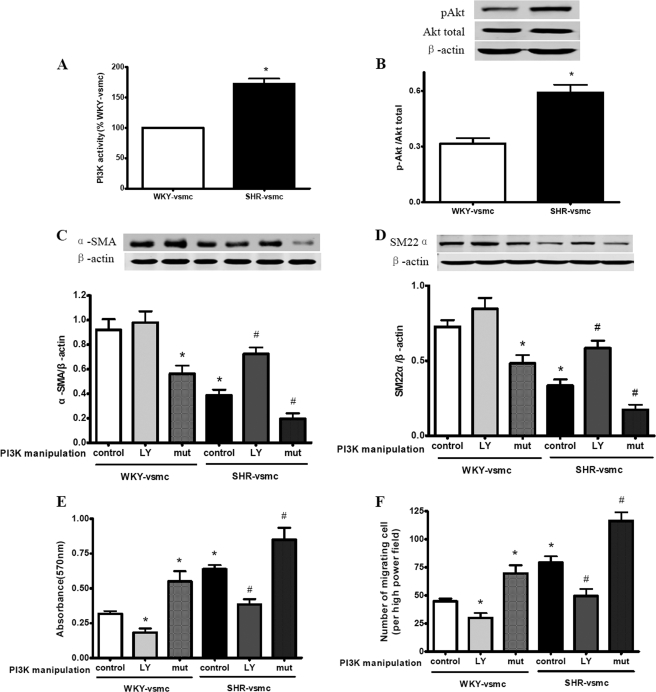
The PI3K/Akt signaling pathway is required for VSMC migration and proliferation (17). To identify the role of PI3K/Akt signaling in hypertension-induced VSMC phenotypic modulation, we determined the PI3K/Akt signaling activity in cultured VSMCs. The results showed that compared with WKY-derived VSMCs, VSMCs derived from SHRs exhibited significantly increased PI3K/Akt signaling activity, as evidenced by enhanced PI3K activity (Fig. 7A) and up-regulated p-Akt expression level (Fig. 7B).
FIGURE 7.
Effect of PI3K on the phenotypic modulation of VSMCs. Enzyme-linked immunosorbent assay and Western blot were used, respectively, to determine the PI3K activity and levels of p-Akt, α-SMA, and SM22α. MTT assay and modified Boyden chamber were used to determine the proliferation and migration of VSMCs. A and B, compared with WKY-VSMC, SHR-VSMC displayed increased PI3K activity (A) and p-Akt expression (B). C and D, effect of PI3K on α-SMA (C) and SM22α (D) expression in VSMCs. Exposure to the PI3K inhibitor LY294002 rescued the impaired expression of α-SMA and SM22α in SHR-VSMC, whereas transfected with adenovirus encoding active PI3K mutant further exacerbated their expression in SHR-VSMC. The expression of α-SMA and SM22α in WKY-VSMC showed no significant changes upon exposure of LY294002, whereas their expression was reduced markedly in WKY-VSMC with active PI3K mutant. E and F, effect of PI3K on the proliferation (E) and migration (F) of VSMCs. Exposure to LY294002 inhibited the enhanced proliferation and migration in SHR-VSMC, whereas the active PI3K mutant led to a further increased proliferation and migration in SHR-VSMC. The same effects were also observed in WKY-VSMC. Data are presented as mean ± S.E. (n = 5–6). *, p < 0.05 versus WKY-VSMC control; #, p < 0.05 versus SHR-VSMC control. LY, LY294002; mut, constitutively active PI3K mutant.
Next, we observed the effect of manipulated PI3K on the phenotypic modulation of cultured VSMCs. Exposure to LY294002 rescued the impaired α-SMA and SM22α expression (Fig. 7, C and D), and reduced the elevated proliferative and migratory capacities (Fig. 7, E and F) in SHR-derived VSMCs, whereas the active PI3K mutant had the opposite effects on these cells (Fig. 7, C–F). In WKY-derived VSMCs, exposure to LY294002 led to reduced proliferation and migration, whereas the expression of α-SMA and SM22α showed no significant changes. In contrast, active PI3K mutant resulted in reduced expression of α-SMA and SM22α and elevated proliferation and migration (Fig. 7, C–F).
These data suggest that PI3K/Akt signaling can promote the phenotypic modulation of cultured VSMCs. Elevated PI3K/Akt signaling in SHR-derived VSMCs may act as an important contributor to increased phenotypic modulation.
Role of PI3K/Akt Signaling in the PPAR-γ-mediated Inhibition of VSMC Proliferation and Migration
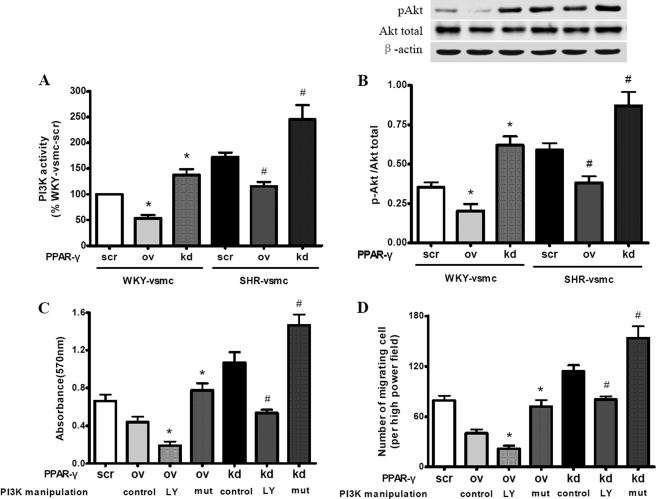
Previous studies have linked PPAR-γ to the PI3K-Akt pathway in various mechanisms (18, 20, 29). To clarify the exact link between PPAR-γ and PI3K/Akt signaling in phenotypic modulation of VSMCs, we first determined the PI3K activity and p-Akt level in the presence of PPAR-γ overexpression or silencing. Overexpression of PPAR-γ reduced PI3K activity and the p-Akt level in SHR-VSMC, whereas PPAR-γ silencing had the opposite effects in these cells. The same effect of manipulated PPAR-γ was also observed in WKY-VSMC (Fig. 8, A and B). These findings indicated that PPAR-γ can inhibit PI3K/Akt signaling in cultured VSMCs. Increased PI3K/Akt signaling activity in SHR-derived VSMCs might be partly due to impaired PPAR-γ expression.
FIGURE 8.
Role of PI3K/Akt signaling in PPAR-γ-mediated regulation of proliferation and migration in VSMCs. Enzyme-linked immunosorbent assay and Western blot were used to determine the PI3K activity and p-Akt level, respectively. MTT assay and a modified Boyden chamber were used to determine the proliferation and migration of VSMCs. A and B, effect of PPAR-γ on PI3K activity (A) and p-Akt level (B). Compared with WKY-VSMC, SHR-VSMC showed markedly enhanced PI3K activity and p-Akt levels. PPAR-γ overexpression reduced PI3K activity and p-Akt levels in SHR-VSMC, whereas PPAR-γ silencing had the opposite effects in these cells. The same effect of manipulated PPAR-γ was also observed in WKY-VSMC. C and D, effect of PI3K on PPAR-γ-mediated regulation of proliferation (C) and migration (D) in SHR-derived VSMCs. LY294002 impeded the elevated proliferation and migration induced by PPAR-γ silencing, whereas active PI3K mutant had the opposite effect. In contrast, reduced proliferation and migration by PPAR-γ overexpression were reversed by active PI3K mutant, and further inhibited by LY294002. Data are presented as mean ± S.E. (n = 5). For A and B, *, p < 0.05 versus WKY-VSMC with PPAR-γ-scr; #, p < 0.05 versus SHR-VSMC with PPAR-γ-scr. For C and D, *, p < 0.05 versus PPAR-γ-ov control; #, p < 0.05 versus PPAR-γ-kd control. LY, LY294002; mut, constitutively active PI3K mutant.
Next, we investigated the effect of manipulated PI3K activity on PPAR-γ-induced inhibition of proliferation and migration in SHR-derived VSMCs. The PPAR-γ overexpression-mediated reduction of proliferation and migration was further inhibited by LY294002 and reversed by active PI3K mutant (Fig. 8, C and D). Inhibition of PI3K with LY294002 counteracted the PPAR-γ silencing-induced enhancement of proliferation and migration in SHR-derived VSMCs, whereas active PI3K mutant had the opposite effect. These findings indicate that PI3K/Akt signaling is negatively modulated by PPAR-γ, and mediates the inhibitory effect of PPAR-γ on VSMC migration and proliferation during hypertension.
DISCUSSION
Here we found that during hypertension, VSMCs exhibit impaired PPAR-γ expression, which is related to excessive phenotypic modulation. PPAR-γ overexpression can inhibit the phenotypic modulation by rescuing the contractile proteins and inhibiting proliferation and migration in SHR-derived VSMCs. Activation of PPAR-γ with rosiglitazone can ameliorate the hypertension-induced aortic remodeling. PI3K/Akt signaling is negatively modulated by PPAR-γ. Impaired PPAR-γ expression in VSMCs accounts for enhanced PI3K/Akt signaling activity, and contributes to the excessive phenotypic modulation during hypertension.
Hypertension can significantly impact the vascular system. VSMCs are susceptible to hypertensive stress, and undergo phenotypic switch from contractile to synthetic phenotype. Reportedly, SHR-derived VSMCs display an exaggerated growth rate, abnormal contact inhibition, and accelerated entry into the cell cycle S phase (8). In the present study, we observed impaired α-SMA and SM22α expression, and enhanced proliferative and migratory capacity in SHR-derived VSMCs, which indicated the VSMC phenotypic modulation during hypertension. Although even normal cultured VSMCs are reported to undergo phenotypic changes by expressing fewer contractile markers and more synthetic phenotype (30), we indeed revealed more obvious phenotypic changes in SHR-derived VSMCs than in WKY-derived VSMCs, which indicated the excessive VSMC phenotypic modulation in hypertension.
Activated VSMCs contribute to thickened vessel wall and vascular remodeling through accumulation in intima and medium (1, 31). As expected, we observed aortic hypertrophic remodeling during hypertension, characterized by intima-media thickening and lumen loss. This finding confirmed the involvement of VSMC phenotypic modulation in hypertension-induced vascular remodeling.
It is well established that PPAR-γ participates in the modulation of multiple diseases including diabetes, atherosclerosis, and hypertension, and plays a critical role in VSMC viability (6–13). Here we hypothesize that PPAR-γ participates in the regulation of VSMC phenotypic modulation, and therefore is associated with increased VSMC phenotypic modulation during hypertension. There are controversies surrounding the specifics of PPAR-γ expression during hypertension. Diep and Schiffrin (25) reported higher PPAR-γ levels in SHR mesenteric arteries compared with WKYs, but no difference between SHR and WKY aortas. In contrast, Xiong et al. (8) observed reduced PPAR-γ protein levels in the SHR aorta, mesenteric arteries, and SHR-derived VSMCs, despite a high mRNA level. The explanation for these conflicting results is unclear. Given that Diep and Schiffrin (25) detected PPAR-γ protein in whole vessels instead of VSMCs, the results might not reflect the exact level of PPAR-γ in VSMCs. In the present study, we observed a significant reduction in PPAR-γ expression in the SHR aorta and SHR-derived VSMCs compared with non-hypertensive rats. We speculated that this impaired PPAR-γ expression was associated with the increased phenotypic modulation of VSMCs in hypertension.
To address this, we manipulated PPAR-γ expression and evaluated the phenotypic modulation of cultured VSMCs. It was found that PPAR-γ overexpression inhibited the proliferation and migration, and rescued the impaired expression of α-SMA and SM22α in SHR-derived VSMCs, whereas PPAR-γ silencing exerted the opposite effect. Furthermore, PPAR-γ silencing in WKY-derived VSMCs led to reduced α-SMA and SM22α expression, and enhanced proliferation and migration. In vivo experiments revealed that PPAR-γ activator rosiglitazone improved the impaired expression of α-SMA and SM22α in SHR aortas. Here we provide the first evidence that PPAR-γ negatively regulates VSMC phenotypic modulation through inhibiting proliferation and migration, and preserving the contractile proteins in cultured VSMCs. The impaired PPAR-γ expression in VSMCs is, at least partly, responsible for the enhanced VSMC phenotypic modulation during hypertension, which in turn contributes to morphological vascular remodeling. As expected, up-regulating PPAR-γ expression with rosiglitazone in vivo ameliorated aortic remodeling by increasing the internal diameter and reducing IMT, which was in line with previous studies (32).
Rosiglitazone treatment also reduced the SBP in SHRs, indicating its potential therapeutic effect on hypertension. It is reported that the decreased endothelin-1 level and amelioration of endothelial function are associated with the effect of rosiglitazone on blood pressure (28). However, due to the lack of direct manipulation of PPAR-γ expression in vivo in the present study, the exact role of PPAR-γ in rosiglitazone-mediated SBP regulation is still not clarified. Rosiglitazone did not show any effects on reducing body weight in SHRs.
To explore the molecular mechanism underlying the inhibition of PPAR-γ on VSMCs phenotypic modulation, we tested the role of PI3K/Akt signaling in this process. The PI3K/Akt signaling pathway is required in multiple cellular processes, including migration and proliferation of numerous cell types (33, 34). Recently, Hegner et al. (35) found that Akt phosphorylation down-regulated VSMC markers in dedifferentiated mesenchymal progenitor cells, whereas inhibition of PI3K induced the VSMC contractile phenotype, with simultaneous calponin, α-SMA, and SM22α expression. In the present study, we observed enhanced PI3K activity and phosphorylation of Akt in SHR-derived VSMCs compared with cells from WKYs. The PI3K inhibitor LY294002 reduced the capacity of proliferation and migration, and rescued the expression of α-SMA and SM22α in SHR-derived VSMCs. In contrast, the active PI3K mutant exerted the opposite effect. Moreover, the active PI3K mutant promoted a synthetic phenotype in WKY-derived VSMCs identified by impaired α-SMA and SM22α expression and elevated proliferation and migration. These findings provide the evidence that enhanced PI3K/Akt signaling is an important contributor to VSMC phenotypic modulation during hypertension.
Growing evidence supports the link between PPAR-γ and the PI3K/Akt signaling pathway. For example, inhibition of PI3K blocks the PPAR-γ-dependent phosphorylation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) 1 Ser-298, extracellular signal-regulated kinase (ERK) 1/2 activation, and the epithelial to mesenchymal modulation (29). PPAR-γ ligand can markedly inhibit the enhancement of PI3K activity induced by lipopolysaccharide/interferon-γ in astrocytes (36). In endothelial cells, PPAR-γ activation inhibits Akt phosphorylation induced by vascular endothelial growth factor (8). Furthermore, PI3K is required for the effects of the PPAR-γ agonist pioglitazone on H2O2-induced endothelial progenitor cell apoptosis (37).
Based on previous studies, we proposed that down-regulation of PI3K/Akt activity may be an important mechanism for PPAR-γ to inhibit VSMC phenotypic modulation. Our results showed that PPAR-γ overexpression reduced, whereas PPAR-γ silencing enhanced the PI3K activity and p-Akt expression in both WKY and SHR-derived VSMCs, indicating the inhibitory effect of PPAR-γ on PI3K/Akt signaling activity. We next observed the proliferation and migration in SHR-derived VSMCs with manipulation of both PPAR-γ and PI3K. Our results showed that LY294002 impeded the elevated proliferation and migration induced by PPAR-γ silencing, whereas the active PI3K mutant had the opposite effect. In contrast, reduced proliferation and migration by PPAR-γ overexpression were reversed by the active PI3K mutant, and further inhibited by LY294002. These findings indicate that PI3K/Akt signaling is inhibited by PPAR-γ, which might be an important mechanism for PPAR-γ to inhibit the VSMC phenotypic modulation. Impaired PPAR-γ in SHR-derived VSMCs is, at least partly, responsible for the elevated PI3K/Akt activity observed in hypertension, and therefore accounts for the increased VSMC modulation from contractile to synthetic phenotype.
There are some limitations to our study. First, multiple mechanisms are reportedly involved in PPAR-γ-mediated regulation of VSMC action. PPAR-γ can inhibit VSMC proliferation through down-regulating inflammatory genes (38), preventing G1/S phase transition (39), and inhibiting telomerase activity (40). Obviously, the suppression of PI3K/Akt is one of these mechanisms. However, we provide convincing evidence that the suppression of PI3K/Akt signaling mediates the PPAR-γ-induced inhibition of VSMC phenotypic modulation, which indicates the important role of PI3K/Akt signaling in the effect of PPAR-γ during hypertension. Second, we realize that some effects of PPAR-γ agonists on VSMCs are PPAR-γ independent. For example, troglitazone can suppress angiotensin II-induced VSMC activation through a PPAR-γ-independent pathway (10). Therefore, we used a genetic approach in vitro as a complementation of rosiglitazone to identify the effect of PPAR-γ on VSMC phenotypic modulation. Third, given that PPAR-γ can also exert a protective effect on VSMCs by improving insulin resistance and dyslipidemia, we propose that the inhibition of phenotypic modulation is one important mechanism for PPAR-γ to protect VSMCs against hypertensive injury.
Taken together, our results provide the first evidence that PPAR-γ inhibits VSMC phenotypic modulation by suppressing PI3K/Akt signaling. During hypertension, impaired PPAR-γ expression in VSMCs accounts for the enhanced activity of PI3K/Akt signaling, and ultimately contributes to excessive VSMC switching from the contractile to synthetic phenotype. These novel findings provide further insight into the mechanism of VSMC phenotypic modulation during hypertension, and offer PPAR-γ as a new target for the prevention and treatment of hypertension-associated vascular disorders.
Acknowledgments
We greatly appreciate the technical assistance of Tao Li and Shaoli Deng.
This work was supported by National Natural Science Foundation of China Grant 30800444.
- VSMC
- vascular smooth muscle cell
- α-SMA
- α-smooth muscle actin
- SM22α
- smooth muscle 22α
- WKY
- Wistar-Kyoto
- SHR
- spontaneously hypertensive rat
- SBP
- systolic blood pressure
- IMT
- intima-media thickening
- PI3K
- phosphoinositide 3-kinase
- siRNA
- small interfering RNA
- PPAR-γ
- peroxisome proliferator-activated receptor-γ
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PtdIns-4,5-P2
- phosphatidylinositol 4,5-phosphate
- scr
- scrambled sequence
- ov
- overexpression
- kd
- knockdown.
REFERENCES
- 1.Hayashi K., Naiki T. (2009) J. Mech. Behav. Biomed. Mater. 2, 3–19 [DOI] [PubMed] [Google Scholar]
- 2.Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Physiol. Rev. 84, 767–801 [DOI] [PubMed] [Google Scholar]
- 3.Kliewer S. A., Xu H. E., Lambert M. H., Willson T. M. (2001) Recent Prog. Horm. Res. 56, 239–263 [DOI] [PubMed] [Google Scholar]
- 4.Marx N., Duez H., Fruchart J. C., Staels B. (2004) Circ. Res. 94, 1168–1178 [DOI] [PubMed] [Google Scholar]
- 5.Hsueh W. A., Bruemmer D. (2004) Hypertension 43, 297–305 [DOI] [PubMed] [Google Scholar]
- 6.Barroso I., Gurnell M., Crowley V. E., Agostini M., Schwabe J. W., Soos M. A., Maslen G. L., Williams T. D., Lewis H., Schafer A. J., Chatterjee V. K., O'Rahilly S. (1999) Nature 402, 880–883 [DOI] [PubMed] [Google Scholar]
- 7.Law R. E., Goetze S., Xi X. P., Jackson S., Kawano Y., Demer L., Fishbein M. C., Meehan W. P., Hsueh W. A. (2000) Circulation 101, 1311–1318 [DOI] [PubMed] [Google Scholar]
- 8.Xiong C., Mou Y., Zhang J., Fu M., Chen Y. E., Akinbami M. A., Cui T. (2005) Life Sci. 77, 3037–3048 [DOI] [PubMed] [Google Scholar]
- 9.Bruemmer D., Law R. E. (2003) Am. J. Med. 115, Suppl. 8A, 87S–92S [DOI] [PubMed] [Google Scholar]
- 10.Hattori Y., Akimoto K., Kasai K. (2000) Biochem. Biophys. Res. Commun. 273, 1144–1149 [DOI] [PubMed] [Google Scholar]
- 11.Goetze S., Kintscher U., Kim S., Meehan W. P., Kaneshiro K., Collins A. R., Fleck E., Hsueh W. A., Law R. E. (2001) J. Cardiovasc. Pharmacol. 38, 909–921 [DOI] [PubMed] [Google Scholar]
- 12.Galis Z. S., Muszynski M., Sukhova G. K., Simon-Morrissey E., Unemori E. N., Lark M. W., Amento E., Libby P. (1994) Circ. Res. 75, 181–189 [DOI] [PubMed] [Google Scholar]
- 13.Marx N., Schönbeck U., Lazar M. A., Libby P., Plutzky J. (1998) Circ. Res. 83, 1097–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung H. J., Eskin S. G., Sakurai Y., Yee A., Kataoka N., McIntire L. V. (2005) Ann. Biomed. Eng. 33, 1546–1554 [DOI] [PubMed] [Google Scholar]
- 15.Kondapaka S. B., Zarnowski M., Yver D. R., Sausville E. A., Cushman S. W. (2004) Clin. Cancer Res. 10, 7192–7198 [DOI] [PubMed] [Google Scholar]
- 16.Choi K. H., Kim J. E., Song N. R., Son J. E., Hwang M. K., Byun S., Kim J. H., Lee K. W., Lee H. J. (2010) Cardiovasc. Res. 859, 836–844 [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Hernando C., József L., Jenkins D., Di Lorenzo A., Sessa W. C. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetze S., Eilers F., Bungenstock A., Kintscher U., Stawowy P., Blaschke F., Graf K., Law R. E., Fleck E., Gräfe M. (2002) Biochem. Biophys. Res. Commun. 293, 1431–1437 [DOI] [PubMed] [Google Scholar]
- 19.Mitra A. K., Jia G., Gangahar D. M., Agrawal D. K. (2009) J. Cell Mol. Med. 13, 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuang C. C., Yang R. S., Tsai K. S., Ho F. M., Liu S. H. (2007) Endocrinology 148, 4267–4275 [DOI] [PubMed] [Google Scholar]
- 21.Xu X., Ha C. H., Wong C., Wang W., Hausser A., Pfizenmaier K., Olson E. N., McKinsey T. A., Jin Z. G. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 2355–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa M., Ohno T., Maruyama R., Okubo M., Nagatsu A., Inoue M., Tanabe H., Takemura G., Minatoguchi S., Fujiwara H. (2007) Biol. Pharm. Bull. 30, 1754–1757 [DOI] [PubMed] [Google Scholar]
- 23.Poon M., Marx S. O., Gallo R., Badimon J. J., Taubman M. B., Marks A. R. (1996) J. Clin. Invest. 98, 2277–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S. J., Kim W. J., Moon S. K. (2009) Int. Immunopharmacol. 9, 837–843 [DOI] [PubMed] [Google Scholar]
- 25.Diep Q. N., Schiffrin E. L. (2001) Hypertension 38, 249–254 [DOI] [PubMed] [Google Scholar]
- 26.Sidhu J. S., Kaposzta Z., Markus H. S., Kaski J. C. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 930–934 [DOI] [PubMed] [Google Scholar]
- 27.Choi D., Kim S. K., Choi S. H., Ko Y. G., Ahn C. W., Jang Y., Lim S. K., Lee H. C., Cha B. S. (2004) Diabetes Care 27, 2654–2660 [DOI] [PubMed] [Google Scholar]
- 28.Potenza M. A., Marasciulo F. L., Tarquinio M., Quon M. J., Montagnani M. (2006) Diabetes 55, 3594–3603 [DOI] [PubMed] [Google Scholar]
- 29.Chen L., Necela B. M., Su W., Yanagisawa M., Anastasiadis P. Z., Fields A. P., Thompson E. A. (2006) J. Biol. Chem. 281, 24575–24587 [DOI] [PubMed] [Google Scholar]
- 30.Sobue K., Hayashi K., Nishida W. (1999) Mol. Cell. Biochem. 190, 105–118 [PubMed] [Google Scholar]
- 31.Wang Z., Rao P. J., Shillcutt S. D., Newman W. H. (2005) Neurosci. Lett. 373, 38–41 [DOI] [PubMed] [Google Scholar]
- 32.Ogihara T., Rakugi H., Ikegami H., Mikami H., Masuo K. (1995) Am. J. Hypertens. 8, 316–320 [DOI] [PubMed] [Google Scholar]
- 33.Morales-Ruiz M., Fulton D., Sowa G., Languino L. R., Fujio Y., Walsh K., Sessa W. C. (2000) Circ. Res. 86, 892–896 [DOI] [PubMed] [Google Scholar]
- 34.Dimmeler S., Dernbach E., Zeiher A. M. (2000) FEBS Lett. 477, 258–262 [DOI] [PubMed] [Google Scholar]
- 35.Hegner B., Lange M., Kusch A., Essin K., Sezer O., Schulze-Lohoff E., Luft F. C., Gollasch M., Dragun D. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 232–238 [DOI] [PubMed] [Google Scholar]
- 36.Giri S., Rattan R., Singh A. K., Singh I. (2004) J. Immunol. 173, 5196–5208 [DOI] [PubMed] [Google Scholar]
- 37.Gensch C., Clever Y. P., Werner C., Hanhoun M., Böhm M., Laufs U. (2007) Atherosclerosis 192, 67–74 [DOI] [PubMed] [Google Scholar]
- 38.Takata Y., Kitami Y., Yang Z. H., Nakamura M., Okura T., Hiwada K. (2002) Circ. Res. 91, 427–433 [DOI] [PubMed] [Google Scholar]
- 39.Wakino S., Kintscher U., Kim S., Yin F., Hsueh W. A., Law R. E. (2000) J. Biol. Chem. 275, 22435–22441 [DOI] [PubMed] [Google Scholar]
- 40.Ogawa D., Nomiyama T., Nakamachi T., Heywood E. B., Stone J. F., Berger J. P., Law R. E., Bruemmer D. (2006) Circ. Res. 98, e50–e59 [DOI] [PubMed] [Google Scholar]