Abstract
The control of resting free Ca2+ in skeletal muscle is thought to be a balance of channels, pumps, and exchangers in both the sarcolemma and sarcoplasmic reticulum. We explored these mechanisms using pharmacologic and molecular perturbations of genetically engineered (dyspedic) muscle cells that constitutively lack expression of the skeletal muscle sarcoplasmic reticulum Ca2+ release channels, RyR1 and RyR3. We demonstrate here that expression of RyR1 is responsible for more than half of total resting Ca2+ concentration ([Ca2+]rest) measured in wild type cells. The elevated [Ca2+]rest in RyR1-expressing cells is not a result of active gating of the RyR1 channel but instead is accounted for by the RyR1 ryanodine-insensitive Ca2+ leak conformation. In addition, we demonstrate that basal sarcolemmal Ca2+ influx is also governed by RyR1 expression and contributes in the regulation of [Ca2+]rest in skeletal myotubes.
Keywords: Calcium, Calcium Channels, Calcium Imaging, Calcium Transport, Ryanodine, Bastadin 5, Calcium Entry
Introduction
In skeletal muscle active Ca2+ efflux from the sarcoplasmic reticulum (SR)2 occurs fundamentally through RyR1 via an orthograde signal from DHPR. In the absence of stimuli the open probability of RyR1 is very low, and [Ca2+]rest is maintained near 100 nm in frog (1), mammalian (2), and human skeletal muscle (3) and in mammalian skeletal myotubes (4). This stems from the fact that in the absence of depolarization, the DHPR appears to suppress spontaneous RyR1 activity (5, 6) as evidenced higher RyR1 activity in mdg myotubes that lack expression of the α1s-DHPR (7, 8).
In addition to the “classical” release pathway mediated by RyR1 activation, at rest there is ample evidence for the existence of a second less defined SR Ca2+ efflux pathway that has been referred to as ryanodine (Ry)-insensitive “Ca2+ leak” (4, 9, 10). This Ca2+ leak can be broadly defined as a passive efflux of Ca2+ from the SR under resting or quiescent conditions. Part if not all of the Ry-insensitive Ca2+ leak pathway has been proposed to represent a conformation of RyR1 with a low conductance that is constitutively open (PO∼1) and represents a distinct conformation from that of actively gated RyR1 channels involved in excitation contraction coupling (4, 10). It has been shown that Ry-insensitive Ca2+ leaks may contribute significantly to SR Ca2+ loading capacity and that they may have a significant contribution to regulation of [Ca2+]rest in skeletal muscle. If this is correct, then RyR1 leak may have relevance in physiological and pathological regulation of muscle Ca2+ homeostasis.
Macrocyclic bastadins isolated from the marine sponge Lanthella basta are novel modulators of RyR1. Bastadin 5 (B5) has been shown to prolong dramatically both open and closed time constants of single RyR1 channels reconstituted in bilayer lipid membranes without changing their unitary conductance or overall open probability (11). Importantly, under conditions where RyR1 channels are pharmacologically blocked (with micromolar ryanodine or ruthenium red) both B5 and its related congener bastadin 10 have been shown to increase significantly the Ca2+ loading capacity in SR vesicles and increase the capacity of SR membranes to bind [3H]Ry ∼4-fold (Bmax) (10).
We hypothesized that expression of RyR1 in RyR-null (NullRyR) myotubes would increase [Ca2+]rest and that this increase would be secondary to passive Ca2+ efflux from SR stores mediated by Ry-insensitive Ca2+ leak. As expected, expression of RyR1 in NullRyR myotubes increased [Ca2+]rest to concentrations typically found in wild type myotubes, and complete blockade of caffeine sensitive RyR1 Ca2+ release by ryanodine did not modify [Ca2+]rest levels. When B5 was added to examine the contribution of RyR1 leaks toward the [Ca2+]rest, we found that Ry+B5 in combination reduced resting [Ca2+]rest to essentially dyspedic levels in RyR1-expressing cells, but had no effect in NullRyR cells. [Ca2+]rest was further reduced when Ry+B5-pretreated NullRyR and wild type (WtRyR) myotubes were exposed to low external Ca2+ solution. Similar results were obtained in primary myotubes generated from RyR1/3-null dyspedic mice and their wild type littermates. These results show that a fraction of RyR1 within the SR membrane exists in a Ry-insensitive conformation that mediates Ca2+ leak that determines [Ca2+]rest levels in skeletal muscle. In addition, RyR1 expression also regulates basal sarcolemmal Ca2+ influx, which also contributes to [Ca2+]rest in skeletal myotubes.
MATERIALS AND METHODS
Isolation of B5
B5 was extracted from lyophilized Ianthella basta sponge collected from Guam using methods described previously (11).
Cell Culture and Infection with RyR1 Herpes Simplex Virus Virions
1B5 cells that lack expression of RyR-1, RyR-2, and RyR-3 (NullRyR) were cultured on Matrigel- (BD Bioscience) coated 10-cm dishes as described previously (4, 12, 13) and allowed to differentiate for 5 days. Grid plates containing differentiated myotubes were infected with helper-free herpes simplex virus type 1 virion particles containing WtRyR1 cDNA at a multiplicity of infection of 0.5 for 2 h, and then cultured for 48 h in differentiation media prior to being used experimentally (10, 14). Transduced cells were identified after making measurements of resting [Ca2+] using immunofluorescence with Ab34C.
Primary myoblast cell lines were generated from the hindlimb and forelimb muscles of E18 RyR1/RyR3 double-null dyspedic mice and their Wt littermates (15, 16). Myoblasts were differentiated into myotubes by withdrawal of growth factors as described previously (14).
Ca2+-selective Microelectrodes
Single- and Double-barreled Ca2+-selective microelectrodes were prepared using thin walled borosilicate glass capillaries (WPI 2B150F-4, and WPI PB-150F-4 Sarasota, FL) as described previously (4). They were back-filled first with the neutral carrier ETH 129 (Fluka, Ronkontioma, NY) and then with pCa7 solution. Each Ca2+-selective microelectrode was individually calibrated as described previously (1), and only those with a linear relationship between pCa3 and pCa7(Nernstian response, 29.5 mV/pCa unit) and at least 25 mV between pCa7 and pCa 8 were used experimentally.
To better mimic intracellular ionic conditions, all calibration solutions were supplemented with 1 mm Mg2+. After making measurements of resting [Ca2+], all electrodes were then recalibrated, and if the two calibration curves did not agree within 3 mV, the data from that microelectrode were discarded. Before starting the studies, we determined by direct calibration that the calcium sensitivity of the Ca2+ microelectrodes was not affected by any of the drugs used in the present study.
Microelectrode recordings were performed as described previously (1, 4). The potential from the 3 m KCl microelectrode (Vm) was subtracted electronically from potential of the Ca2+ electrode (VCaE), to produce a differential Ca2+-specific potential (VCa) that represents the [Ca2+]rest. Vm and VCa were filtered (30–50 KHz) to improve the signal-to-noise ratio and stored in a computer for further analysis.
Mn2+ Quench
Primary NullRyR and WtRyR myotubes were loaded with 5 μm fura-2/AM to measure the rate of dye quench by Mn2+ entry (Molecular Probes, Eugene, OR) at 36 °C for 20 min in imaging buffer, pH 7.4. The myotubes were then washed three times with imaging buffer and transferred to the stage of a Nikon TE2000 inverted microscope and illuminated at the isosbestic wavelength for fura-2 (360 nm). Fluorescence emission was captured from regions of interest within each myotube from 3–10 individual cells at 5 frames/s using an Olympus 40 × oil 1.3 NA objective. Mn2+ influx into myotubes was measured as described previously with minor modification (17, 18). Final concentrations of 500 μm MnCl2 and 1.2 mm Mg2+ were added to a nominally Ca2+-free (∼7 μm free Ca2+).
SR Ca2+ Loading Content Determination
Relative SR Ca2+ content levels of primary NullRyR and WtRyR myotubes were estimated from the magnitude of the Ca2+ release induced by 5 μm ionomycin in cells loaded with 5 μm fluo-5N/AM for 20 min at 37 °C. Cells were incubated in Ca2+-free solution to avoid Ca2+ entry from the extracellular medium. Total SR calcium content was expressed as the area under the curve of the Ca2+ release transient.
Membrane Vesicle Preparation and Immunoblotting
Microsomal vesicles were prepared from cultured myotubes. Myotubes were homogenized in a Polytron cell disrupter in 5 mm imidazole, pH 7.4, 300 mm sucrose supplemented with protease inhibitor (CompleteTM; Roche Applied Science) and collected as described previously (19). Proteins were separated using SDS-PAGE (20) and transferred to polyvinylidene difluoride membranes. Expression of specific proteins was assessed by incubation of the membranes with poly- or monoclonal antibodies against; RyR1 (34C; Sigma-Aldrich), SR Ca2+-ATPase 1 (SERCA-1) (ABR-Affinity BioReagents, Rockford, IL), Na+-Ca2+ exchange 3 (NCX3) (a gift from Dr. Kenneth Philipson and 95209 Swant, Bellinzona, Switzerland), plasma membrane sarcolemmal Ca2+-ATPase (PMCA) (sc-28765 Santa Cruz Biotechnology, Santa Cruz, CA), myosin (sc-20641 Santa Cruz Biotechnology), and glyceraldehyde-3-phosphate dehydrogenase (FL-335, Santa Cruz Biotechnology).
Solutions
Ionic composition of the mammalian Ringer solution was: 125 mm NaCl, 5 mm KCl, 1 mm MgSO4, 25 mm HEPES, 6 mm glucose, 2 mm CaCl2. Ryanodine (500 μm) and B5 (20 μm) solutions were prepared by adding these compounds to the desired concentration in normal mammalian Ringer solution. The low Ca2+ solution was prepared using the same protocol as the corresponding regular Ringer solution, but Ca2+ was omitted, and Mg2+ (2 mm) was added. Ca2+-free solution was prepared by omitting Ca2+ and adding Mg2+ (2 mm) and EGTA (1 mm). All solutions were adjusted to pH 7.4. Solution exchange was realized by gentle aspiration of the media and application of the new media with a transfer pipette. Solution replacement was repeated several times to assure the complete exchange of media. Experiments were performed at 23 °C.
Drug Treatments
In the drug treatment studies, all cells were first incubated with 500 μm Ry for 45 min before [Ca2+]rest measurements were made. In studies of the effects of B5, 20 μm B5 was added to the cells for 10 min prior to [Ca2+]rest measurements. For studying the effect of Ry and B5 together, the myotubes were first incubated with Ry for 45 min followed by an additional 10-min incubation with both Ry and B5 before [Ca2+]rest measurements were made. To explore the effect of low Ca2+ solution in cells treated with Ry and B5 together, the myotubes were first incubated with Ry 45 min followed by an additional 10-min incubation with both Ry and B5 and then were incubated in low Ca2+ in the presence of Ry and B5 for 2 min before [Ca2+]rest measurements were made. We avoided making [Ca2+]rest measurements after long incubations in low Ca2+ solution (more than 10 min) because despite the fact that 2 mm Mg2+ was added to the low Ca2+ solution all myotubes began to show a significant depolarization after this interval (>7 mV).
Statistics
All values are expressed as mean ± S.D. Paired and unpaired t tests were used to compare the [Ca2+]rest in single myotubes and groups of myotubes before and after drug treatment(s); p < 0.05 was considered significant.
RESULTS
RyR1 Expression Significantly Increases [Ca2+]rest in NullRyR Myotubes
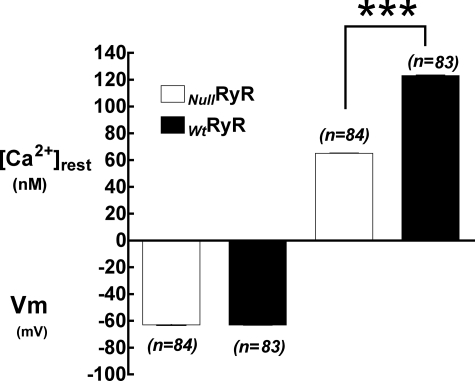
Resting membrane potentials and [Ca2+]rest were measured in differentiated NullRyR 1B5 myotubes and those transduced with WtRyR1 virions. The [Ca2+]rest observed in myotubes expressing WtRyR1 was significantly higher than that observed in NullRyR myotubes (123 ± 4.7 nm, n = 83 versus 65 ± 4 nm, n = 84, p < 0.0001) (Fig. 1). There were no differences in membrane potential of WtRyR1-expressing myotubes compared with NullRyR myotubes (63 ± 2.1 mV, n = 84 versus 63 ± 1.8 mV, n = 83, p > 0.05) (Fig. 1).
FIGURE 1.
Resting membrane potentials (left) and resting intracellular free Ca2+ concentrations (right) measured using double-barreled microelectrodes in RyR-null and Wt RyR1-expressing 1B5 myotubes. Data are expressed as mean ± S.D., n = 20 cells/group.
Ry Does Not Lower [Ca2+]rest
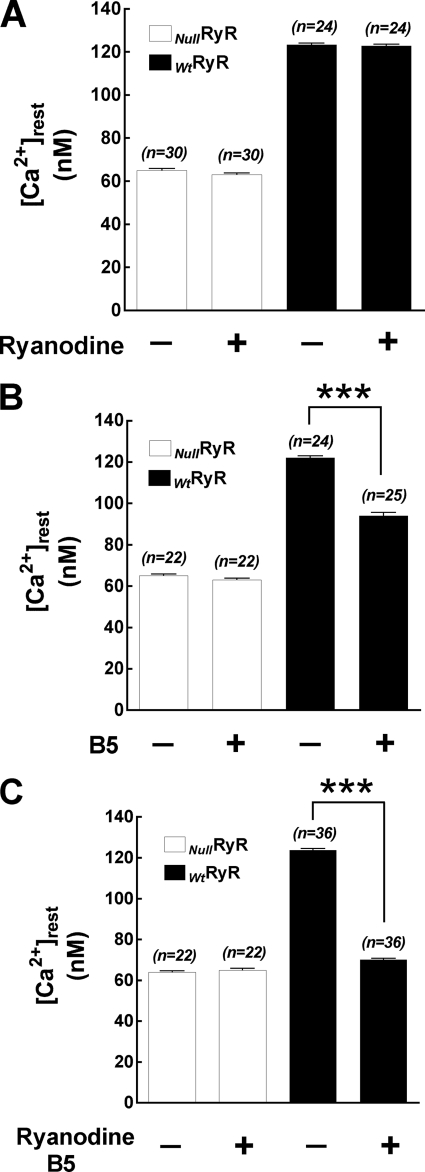
It is well established that low concentrations Ry can enhance the open probability of RyR1 and that high micromolar concentrations (such as the conditions used in the present study) fully block channel conductance (21–23) and prevent Ca2+ release induced by direct RyR agonists (e.g. caffeine, 4 CmC). Incubation of myotubes expressing WtRyR1 with 500 μm Ry completely abolished Ca2+ responses to the first and any subsequent exposure to caffeine (20 mm) (data not shown). Incubation of WtRyR1-expressing myotubes and NullRyR myotubes in 500 μm Ry for 45 min did not modify [Ca2+]rest in either group of cells. In NullRyR myotubes[Ca2+]rest was 65 ± 4.8 nm (n = 30) before and 63 ± 4 nm (n = 30) (p > 0.05) after Ry treatment, and in WtRyR1-expressing myotubes, [Ca2+]i was 123 ± 3.6 nm (n = 24) before and 122 ± 3.2 nm (n = 24) (p > 0.05) after Ry incubation (Fig. 2A). There was no change in Vm in NullRyR and WtRyR1myotubes after the treatment with Ry.
FIGURE 2.
Resting intracellular free Ca2+ concentrations measured in RyR-null and Wt RyR1-expressing 1B5 myotubes. A, after treatment with 500 μm ryanodine. B, after treatment with 20 μm B5. C, after treatment with 500 μm ryanodine and 20 μm B5. Data are expressed as mean ± S.D., n = 20 cells/group.***, p < 0.0001.
B5 Reduces [Ca2+]rest in Myotubes Expressing RyR1
Incubation of NullRyR myotubes for 10 min with B5 did not affect the levels of [Ca2+]rest (65 ± 4 nm, n = 22 before versus 63 ± 4 nm, n = 22, p > 0.05) (Fig. 2B). Interestingly, B5 alone diminished [Ca2+]rest in WtRyR1-expressing myotubes by 25% in relation to control, from 121 ± 5.3 nm (n = 24) to 94 ± 8.1 nm (n = 25) p < 0.0001 (Fig. 2B). There was no change in Vm in NullRyR and WtRyR1 myotubes during the treatment with B5 from pretreatment values (data not shown).
Ry and B5 in Combination Restore [Ca2+]rest to Dyspedic Levels in RyR1-expressing Cells
Incubation of NullRyR myotubes with Ry+B5 had no effect on [Ca2+]rest (64 ± 3 nm (n = 22) before and 65 ± 4.2 nm (n = 22), p > 0.05 after). However in WtRyR1-expressing myotubes, incubation with Ry+B5 significantly decreased [Ca2+]rest in myotubes to a level not significantly different from that measured in NullRyR myotubes, from 123 ± 4.8 nm (n = 36) to 70 ± 4.7 nm (n = 44, p < 0.0001) (Fig. 2C). As seen when treated with either agent alone, there was no change in Vm in NullRyR and WtRyR1 myotubes during the incubation with Ry+B5 (data not shown).
Effect of Ry+B5 in low [Ca2+]e
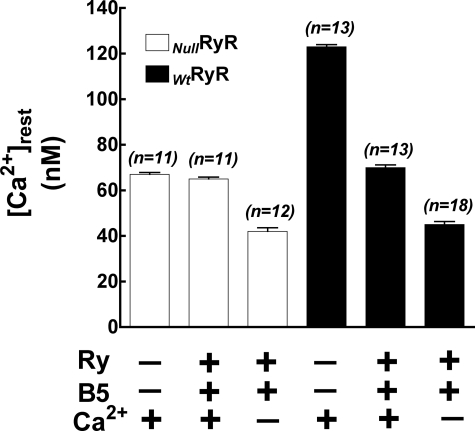
To explore the contribution of extracellular Ca2+ to [Ca2+]rest in skeletal myotubes, NullRyR and WtRyR myotubes were incubated in Ry+B5 (see under “Materials and Methods”), and then the bath solution was then substituted with one containing low Ca2+ in the presence of Ry+B5. In NullRyR myotubes, [Ca2+]rest in normal Ringer was 67 ± 2.9 nm (n = 11), and after Ry+B5 incubation [Ca2+]rest was 65 ± 2.6 nm (n = 11, p > 0.05) After substitution with the Ca2+ solution [Ca2+]rest declined to 42 ± 5.6 nm (n = 12, p < 0.0001). Following the same experimental protocol in WtRyR-expressing myotubes resulted in [Ca2+]rest values that shifted from 123 ± 3.4 nm (n = 13) in normal Ringer to 70 ± 4.2 nm (n = 13, p < 0.001) after Ry+B5 incubation, and to 45 ± 5.8 nm (n = 18, p < 0.0001) after incubation in low Ca2+ solution. The presence of Ry+B5 and low [Ca2+]e resulted in [Ca2+]rest in WtRyR and NullRyR myotubes that were nearly identical (45 ± 5.8 nm versus 42 ± 5.6 nm, respectively) (Fig. 3).
FIGURE 3.
Effects of removal of extracellular Ca2+ on intracellular free Ca2+ concentrations after pretreatment with ryanodine and B5. Data are expressed as mean ± S.D., n = 20 cells/group.
[Ca2+]rest in NullRyR and WtRyR1 Primary Myotubes
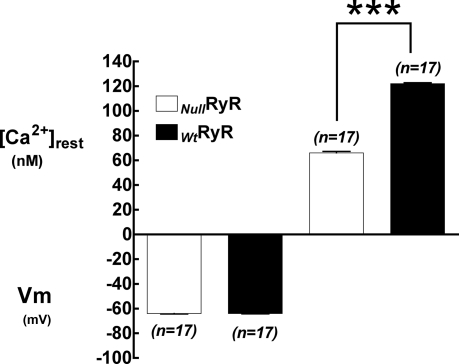
Resting membrane potentials and [Ca2+]rest were measured in differentiated dyspedic and wild type primary myotubes. Similar to the data for null and RyR1-transduced 1B5 myotubes, [Ca2+]rest observed in WtRyR1 primary myotubes was significantly higher than that observed in dyspedic myotubes (122 ± 3.6 nm, n = 17 versus 66 ± 5.2 nm, n = 17, p < 0.001) (Fig. 4) with no difference in the resting membrane potential (64 ± 1.6 mV, n = 17 versus 64 ± 1.5 mV, n = 17, p > 0.05) between the two groups. In addition, these measurements were not different from the values seen in corresponding 1B5 cells.
FIGURE 4.
Resting membrane potentials (left) and resting intracellular free Ca2+ concentrations (right) measured using double-barreled microelectrodes in Wt and RyR-null primary myotubes. Data are expressed as mean ± S.D., n = 20 cells/group.
Ca2+ Entry in NullRyR and WtRyR1 Primary Myotubes
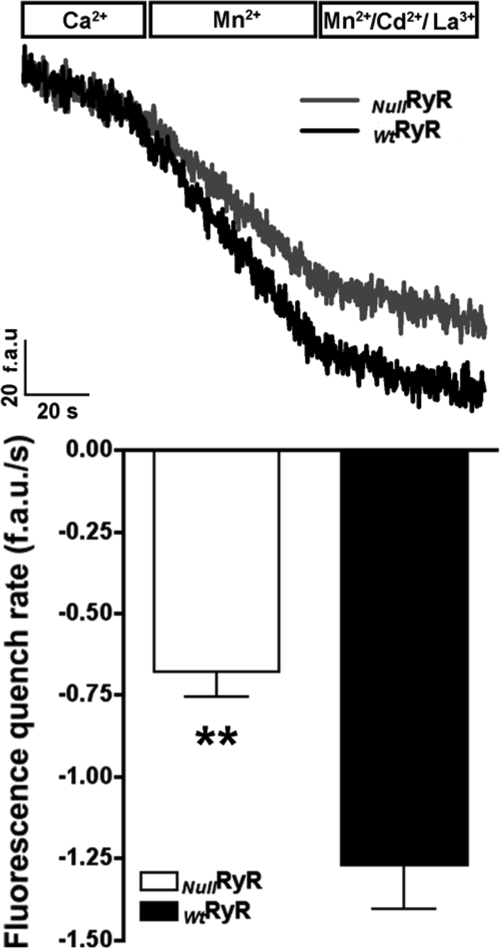
Using the Mn2+ quench technique, the quenching of fura-2 fluorescence was measured at its isosbestic point in primary NullRyR and WtRyR myotubes at rest in a nominally Ca2+-free external solution containing 500 μm Mn2+. Under these assay conditions, the rate of Mn2+ quench of fura-2 signal can be attributed to Ca2+ entry (Fig. 5, upper). The rate of Mn2+ quench at rest was ∼2-fold greater in WtRyR than in NullRyR myotubes (1.26 versus 0.67 fluorescence (arbitrary units)−s respectively, p < 0.0001) (Fig. 5, lower). These data describe for the first time that resting Ca2+ entry is greater in WtRyR1 compared with NullRyR myotubes.
FIGURE 5.
Measurements of resting cation entry using Mn2+ quench in RyR-null and Wt primary myotubes. Upper, fura-2 fluorescence raw traces from representative myotubes in the presence of extracellular Ca2+, Mn2+ in the absence of Ca2+, and Mn2+ in the absence of Ca2+ after the addition of extracellular Cd2+ and La3+. Lower, comparison of the rate of Mn2+ quench between Wt and RyR-null primary myotubes. Data are shown as mean ± S.D., n = 15 cells/group. **, p < 0.0001.
SR Ca2+ Content
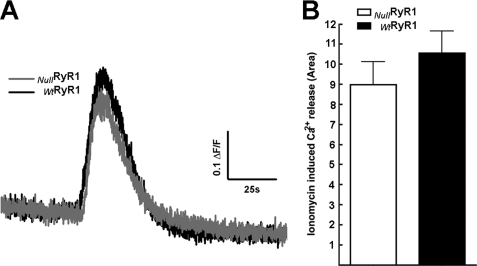
To quantify the level of SR Ca2+ content, we exposed NullRyR and WtRyR1 primary myotubes loaded with Fluo-5N/AM and exposed to 5 μm ionomycin in Ca2+ free solution, to avoid Ca2+ entry from the extracellular medium. Fig. 6 shows that under these conditions the there is no significant difference in amplitude of the fluorescence signal (Fig. 6A) or the total Ca2+ released (area under the curve, Fig. 6B) from NullRyR myotubes compared with WtRyR1 myotubes.
FIGURE 6.
Fluo-5N fluorescence signals after the addition of 5 μm inomycin to Wt and RyR-null primary myotubes in the presence of nominal free extracellular Ca2+ buffer. A, representative curve of Wt and RyR-null responses. B, average area under the curve of the inomycin-induced Ca2+ release. Data are shown as mean ± S.D., n = 20 cells in each group. p > 0.05.
Ca2+ Handling Protein Expression
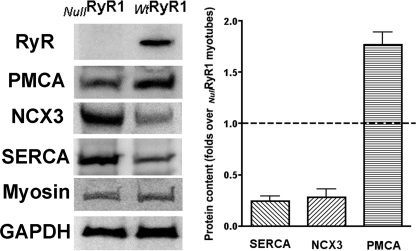
Western blot analysis for expression of RyR1, PMCA, NCX3, and SERCA performed on membranes isolated from NullRyR and WtRyR1 primary myotubes is shown in Fig. 7 (representative blot, Fig. 7, left; densitometry analysis, Fig. 7, right). Expression of RyR1 was accompanied with increased expression of PMCA and a decreased expression of NCX3 and SERCA.
FIGURE 7.
Expression of Ca2+-handling proteins in Wt and RyR-null primary myotubes. Left, representative Western blots using antibodies directed against RyR1, PMCA, NCX3, SERCA1, myosin (to demonstrate similar differentiation state), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control. Right, expression of RyR1 associated with a significant decrease in the expression of SERCA and NCX3 and a significant up-regulation of the expression of PMCA. Data are expressed as mean ± S.D., n = 5 Western blots/group.
DISCUSSION
The purpose of this study was to examine whether the expression of RyR1 has any effect on [Ca2+]rest, the resting Ca2+ entry, and SR Ca2+ loading in skeletal muscle. Our study demonstrates that expression of WtRyR1 is associated with a significant increase in [Ca2+]rest, in resting Ca2+ entry, with no significant change in SR Ca2+ loading compared with NullRyR myotubes. The fact that we observed the same results with RyR1-transduced 1B5 myotubes and in primary myotubes rules out the possibility that the observed difference in [Ca2+]rest was related to overexpression of RyR1 when the differentiated myotubes were transduced with virion particles containing wild type RyR1 cDNA. A significant part of this elevation in [Ca2+]rest appears to be related to the presence of RyR1 leaks because Ry+B5 was able to reduce [Ca2+]rest to levels similar to those observed in NullRyR myotubes. In addition, our results show clearly that extracellular Ca2+ also plays an important role in maintaining [Ca2+]rest at physiological levels.
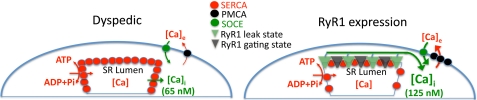
From these results it is clear that in addition to the control of well known intracellular Ca2+ regulatory mechanisms (PMCA, NCX, SERCA) on steady-state [Ca2+]rest in skeletal muscle a significant portion of [Ca2+]rest is also set by passive Ca2+ release, which appears to be the result of a fraction of WtRyR1s within SR that are in a Ry-insensitive Ca2+ leak conformation and by the Ca2+ influx via the sarcolemma that is modulated by the presence of RyR1. In fact, the combined effect of expression of RyR1 elevated the [Ca2+]rest by ∼2-fold. The precise mechanism underlying how RyR1 leaks and the expression of the RyR1 lead to a chronic elevation [Ca2+]rest needs further study. If RyR1 leak exceeds Ca2+ uptake by the SR and extrusion by sarcolemmal mechanisms, a new dynamic equilibrium of Ca2+ mobilization must be established that allows the higher [Ca2+]rest. If the currently proposed mechanism that Ca2+extrusion processes of the plasma membrane (PMCA, SERCA, and NCX) control [Ca2+]rest they should be stimulated by elevations in resting [Ca2+]i, and in the steady state, such as those defined by our experimental conditions, these transport mechanisms should be sufficient to compensate for the increased Ca2+ leak/sarcolemmal Ca2+ entry, resulting in a return of [Ca2+]rest toward that measured in NullRyR myotubes. Because this does not happen, then the changes in [Ca2+]rest observed with the expression of RyR1 must involve a modification of the set points of the activity of both SERCA and these sarcolemmal Ca2+ transport mechanisms and/or the amount of expression of such proteins at the SR and plasma membrane. In fact, it was found that expression of RyR1 was accompanied by an increase in the expression of PMCA and a decreased expression of NCX3 and SERCA, all of them linked to the regulation of intracellular [Ca2+]. As modeled in Fig. 8, the decreased expression of SERCA, an increased expression of PMCA, elevated resting Ca2+ entry, and an elevated cytoplasmic Ca2+ are the costs for expressing RyR1 and maintaining SR stores at levels equal to that found in dyspedic cells. One explanation is that RyR1-expressing cells down-regulate SERCA as a compensatory adaptation to limit the consumption of ATP that would be needed to offset RyR1-mediated Ca2+ leak from SR. If the level of SERCA expression was maintained in the face of a sizable Ca2+ leak, futile cycling of Ca2+ between the SR lumen and the extracellular space would come at a great energy cost. Conversely, dyspedic cells express higher levels of SERCA because without the RyR1 Ca2+ leak there is a reduced energy cost. One intriguing discovery in the present study is that RyR1 expression appears to confer significant regulation of the density of SERCA protein found in SR membranes. These results are consistent with previous findings that indicated up-regulation of SERCA levels in skeletal muscle membranes isolated from dyspedic mice compared with those isolated from wild type (Fig. 6 in Ref. 24).
FIGURE 8.
Model showing the changes in expression of Ca2+-handling proteins and the consequent changes in myoplasmic Ca2+ concentration, rate of resting Ca2+ entry, and Ca2+ removal.
Second, the higher PMCA expression could help offset decreased rates of Ca2+ transient recovery (relaxation) in light of lower SERCA capacity by removing a larger fraction of released Ca2+ during excitation to the extracellular space. Why NCX protein is down-regulated and how it contributes to maintenance of resting Ca2+ are unclear.
B5, through its modulatory actions on the FKBP12·RyR1 complex, has been previously shown to increase SR Ca2+ loading capacity and concomitantly attenuate RyR1 Ca2+ leak. This property of the bastadins is the result of their ability to convert Ry-insensitive leak states (RyR1 leak) into ryanodine-sensitive channels (RyR1 Ca2+ channels) (10) and is demonstrated by their ability to increase Bmax of Ry binding/mg of protein (10). Therefore, in the present study B5 was used to examine the relationship between Ry-sensitive and Ry-insensitive Ca2+ efflux pathways that coexist in the SR of WtRyR1-expressing myotubes. We found that B5 in combination with blocking concentrations of Ry decreased [Ca2+]rest in WtRyR-expressing myotubes by 43% but had no effect in NullRyR myotubes. These data are consistent with the hypothesis that bastadins can promote the conversion of RyR1 in the Ry-insensitive Ca2+ leak conformation into Ry-sensitive RyR1 channels. B5 alone, also reduced [Ca2+]rest in WtRyR1-expressing myotubes but to a lesser degree (25%) compared with its effect in combination with Ry (43%) probably because the RyR1 leaks converted into gating channels do not have the same degree of negative control by the DHPR as normal gating channels.
Another interesting result is that the resting Ca2+ entry is greater in WtRyR than NullRyR myotubes, suggesting that the magnitude of this entry is modulated by the presence of RyR1. The physiological role of this resting Ca2+ entry is poorly understood, but appears to be independent of resting membrane potential (myotubes polarized based on the Nernst equation for 23 °C) and/or the degree of SR depletion as we showed in Fig. 6, as has been postulated by Kurebayashi and Ogawa (25), since the experiments were conducted in unstimulated myotubes.
The existence of a RyR1-mediated Ca2+ leak pathway in the SR may have some implications for the pathophysiology of two well characterized disorders of skeletal muscle, malignant hyperthermia and central core disease. In muscle cells from the majority of patients with either disorder, there is a global elevation of [Ca2+]rest, which can be partially reversed by treating the muscle cells with B5 in combination with blocking concentrations of Ry (4). In summary, our results demonstrate that that expression of WtRyR1 is associated with an increase in [Ca2+]rest and that in addition to traditionally proposed mechanisms involving SERCA, NCX, and PMCA, [Ca2+]rest in skeletal muscle is determined in part by passive Ca2+ leak through WtRyR1 and increased basal sarcolemmal Ca2+ entry.
Acknowledgments
We thank Dr. Kenneth Philipson for antibodies against NCX3 and Dr. Peter Schupp (University of Guam, Marine Laboratory) for I. basta. Monoclonal antibody 34C was developed by J. A. Airey and J. L. Sutko and was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biology, University of Iowa, Iowa City, IA 52240.
This work was supported, in whole or in part, by National Institutes of Health Grants 2R01AR43140 and 5P01 AR052354 (to P. D. A. and I. N. P.).
- SR
- sarcoplasmic reticulum
- DHPR
- dihydropyridine receptor
- Ry
- ryanodine
- [Ca2+]rest
- resting Ca2+ concentration
- B5
- bastadin 5
- NullRyR
- RyR-null
- Wt
- wild type
- SERCA
- SR Ca2+-ATPase
- PMCA
- plasma membrane sarcolemmal Ca2+-ATPase
- NCX
- Na+-Ca2+ exchanger.
REFERENCES
- 1.López J. R., Alamo L., Caputo C., DiPolo R., Vergara S. (1983) Biophys. J. 43, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López J. R., Linares N., Pessah I. N., Allen P. D. (2005) Am. J. Physiol. Cell Physiol. 288, C606–C612 [DOI] [PubMed] [Google Scholar]
- 3.López J. R., Medina P., Alamo L. (1987) Muscle Nerve 10, 77–79 [DOI] [PubMed] [Google Scholar]
- 4.Yang T., Esteve E., Pessah I. N., Molinski T. F., Allen P. D., López J. R. (2007) Am. J. Physiol. Cell Physiol. 292, C1591–C1598 [DOI] [PubMed] [Google Scholar]
- 5.Ward C. W., Protasi F., Castillo D., Wang Y., Chen S. R., Pessah I. N., Allen P. D., Schneider M. F. (2001) Biophys. J. 81, 3216–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward C. W., Schneider M. F., Castillo D., Protasi F., Wang Y., Chen S. R., Allen P. D. (2000) J. Physiol. 525, 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee E. H., Lopez J. R., Li J., Protasi F., Pessah I. N., Kim D. H., Allen P. D. (2004) Am. J. Physiol. Cell Physiol. 286, C179–C189 [DOI] [PubMed] [Google Scholar]
- 8.Zhou J., Yi J., Royer L., Launikonis B. S., González A., Garcia J., Ríos E. (2006) Am. J. Physiol. Cell Physiol. 290, C539–C553 [DOI] [PubMed] [Google Scholar]
- 9.Masuno M. N., Pessah I. N., Olmstead M. M., Molinski T. F. (2006) J. Med. Chem. 49, 4497–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pessah I. N., Molinski T. F., Meloy T. D., Wong P., Buck E. D., Allen P. D., Mohr F. C., Mack M. M. (1997) Am. J. Physiol. Cell Physiol. 272, C601–C614 [DOI] [PubMed] [Google Scholar]
- 11.Mack M. M., Molinski T. F., Buck E. D., Pessah I. N. (1994) J. Biol. Chem. 269, 23236–23249 [PubMed] [Google Scholar]
- 12.Moore R. A., Nguyen H., Galceran J., Pessah I. N., Allen P. D. (1998) J. Cell Biol. 140, 843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Protasi F., Franzini-Armstrong C., Allen P. D. (1998) J. Cell Biol. 140, 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang T., Ta T. A., Pessah I. N., Allen P. D. (2003) J. Biol. Chem. 278, 25722–25730 [DOI] [PubMed] [Google Scholar]
- 15.Rando T. A., Blau H. M. (1994) J. Cell Biol. 125, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang T., Riehl J., Esteve E., Matthaei K. I., Goth S., Allen P. D., Pessah I. N., Lopez J. R. (2006) Anesthesiology 105, 1164–1175 [DOI] [PubMed] [Google Scholar]
- 17.Fessenden J. D., Chen L., Wang Y., Paolini C., Franzini-Armstrong C., Allen P. D., Pessah I. N. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2865–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quattrini A., Lorenzetti I., Sciorati C., Corbo M., Previtali S. C., Feltri M. L., Canal N., Wrabetz L., Nemni R., Clementi E. (2001) J. Neuroimmunol. 114, 213–219 [DOI] [PubMed] [Google Scholar]
- 19.Perez C. F., López J. R., Allen P. D. (2005) Am. J. Physiol. Cell Physiol. 288, C640–C649 [DOI] [PubMed] [Google Scholar]
- 20.King J., Laemmli U. K. (1971) J. Mol. Biol. 62, 465–477 [DOI] [PubMed] [Google Scholar]
- 21.Meissner G. (1986) J. Biol. Chem. 261, 6300–6306 [PubMed] [Google Scholar]
- 22.Pessah I. N., Zimanyi I. (1991) Mol. Pharmacol. 39, 679–689 [PubMed] [Google Scholar]
- 23.Buck E., Zimanyi I., Abramson J. J., Pessah I. N. (1992) J. Biol. Chem. 267, 23560–23567 [PubMed] [Google Scholar]
- 24.Buck E. D., Nguyen H. T., Pessah I. N., Allen P. D. (1997) J. Biol. Chem. 272, 7360–7367 [DOI] [PubMed] [Google Scholar]
- 25.Kurebayashi N., Ogawa Y. (2001) J. Physiol. 533, 185–199 [DOI] [PMC free article] [PubMed] [Google Scholar]