Abstract
Canonical Wnt ligands are secreted by several cell types in the adipose tissue. We examined if mature adipocytes can also be target cells and found that canonical Wnt activation by Wnt3a induced a marked dedifferentiation of both 3T3-L1 and human adipocytes. Typical adipogenic markers were reduced while undifferentiated cell markers like Pref-1/Dlk1, Wnt10b, and Gata2 were increased. The cells also became insulin-resistant with impaired upstream insulin signaling and reduced glucose uptake. Wnt3a stabilized β-catenin in the absence of the LRP6 receptor and with maintained axin and Dickkopf-1 protein expression. PPARγ was repressed and PPARγ ligands could not restore the adipogenic markers or reduce the β-catenin levels. The dedifferentiated adipocytes expressed the myofibroblast marker α-smooth muscle actin and were also susceptible to osteogenic transdifferentiation. These results identify a novel pathway in mature adipose cells that is critical for maintaining the normal adipocyte phenotype and insulin sensitivity.
Keywords: Adipose Tissue, Diabetes, Differentiation, Glucose Transport, PPAR, Transformation, Tumor Necrosis Factor
Introduction
The wingless-type MMTV integration site family (Wnt)2 signaling pathway plays a fundamental role during embryogenesis and normal cell and organ development. Recent studies in humans have shown that genetic variants in the Wnt signaling molecules are associated with obesity, coronary heart disease, hyperlipidemia, and type 2 diabetes (1–3). Wnt proteins signal through both canonical and non-canonical pathways involving several intracellular pathways. The canonical Wnt/β-catenin pathway is highly active in mesenchymal precursor cells and directs pluripotent cells toward adipogenic, osteogenic, or myogenic differentiation. One of the mediators of the canonical signaling in mesenchymal precursor cells is Wnt10b, and constitutive Wnt/β-catenin signaling favors expression of osteogenic genes at the expense of adipogenic genes (4). Ectopic expression of Wnt10b impairs the development of the adipose tissue and transgenic mice are resistant to diet-induced obesity (5, 6). It has also been shown that Wnt10b deficiency plays a role for the increased intra-myocellular lipid accumulation that is associated with aging in mice (7). Active canonical Wnt signaling stabilizes and increases total cellular and nuclear β-catenin levels, which repress adipogenesis (4), and inhibition of Wnt signaling is necessary for PPARγ induction and preadipocyte differentiation. Wnt10b, which is highly expressed in dividing and confluent preadipocytes, is also rapidly down-regulated during differentiation (5).
Non-canonical Wnt signaling has been less well characterized, but at least two non-canonical Wnt signaling pathways have been proposed: the planar cell polarity pathway and the Wnt/Ca2+ pathway (8). The non-canonical Wnt signals are transduced through receptors such as RYK and ROR2 that cross-link with the Frizzled receptors and transduce the signal either to the Dishevelled pathway or to the Ca2-dependent Nemo-like kinase pathway, both of which are implicated in activation of the transcription factors T-cell factor/lymphoid enhancer factor (9, 10). Canonical Wnt ligands such as Wnt1 and Wnt3a can also activate the non-canonical pathway. Activation of the RYK receptor induces both activation of the mitogen-activated protein kinase pathway through the Raf/Ras pathway and through Dishevelled (11). The role of the non-canonical Wnt signaling pathways for preadipocyte differentiation has, however, not been much studied.
The mechanisms for the commitment of pluripotent stem cells into the adipose lineage are poorly understood (12). However, once committed, the preadipocytes undergo the adipogenic program, which requires a coordinate activation of several pathways, including C/EBPα and PPARγ. Impaired preadipocyte differentiation (13), including terminal differentiation to mature adipocytes, has been demonstrated in obesity characterized by enlarged adipose cells and insulin-resistance. The impaired (pre)adipocyte differentiation in hypertrophic adipose tissue is also associated with an altered pattern of adipokine secretion, increased lipolysis, and free fatty acid release (14–16).
The adipose tissue contains several Wnt-producing cells, i.e. preadipocytes, endothelial cells, and macrophages (17–19). In general, Wnt proteins are produced by cells that have not undergone full terminal differentiation. Wnt proteins function either as short- (autocrine) or long-range (paracrine or, possibly, endocrine) signaling proteins. At present, nothing is known about the ability of Wnt proteins to affect fully differentiated adipocytes, because all focus has been on their importance for precursor cell differentiation.
In the present study, we examined the effects of the canonical Wnt ligand, Wnt3a, on differentiation, Wnt signal activation, insulin sensitivity, and action in fully differentiated adipose cells. For comparison, we also included TNFα, because exposure of mature adipocytes to TNFα results in insulin resistance combined with a reduced expression of adipogenic genes such as PPARγ, C/EBPα, GLUT4, and APM1 and, instead, promotion and local secretion of inflammatory cytokines and chemokines (19–21). Remarkably, we find that fully differentiated adipocytes are highly responsive to the canonical Wnt ligand-promoting β-catenin stabilization, cell dedifferentiation associated with insulin resistance, and ability to undergo transdifferentiation.
EXPERIMENTAL PROCEDURES
3T3-L1 Adipocyte Differentiation
3T3-L1 cells were induced to differentiate as described in (19). Wnt3a (10% of cell culture supernatant, comparable to 50 ng/ml) and TNFα (20 ng/ml) were added to the cell culture media at day 8 of differentiation for the indicated time. Oil Red O staining was performed as described (22). The adipocytes were counterstained with Mayer hematoxylin. Oil Red O was quantified by dissolving the Oil Red O stain in 2-propanol and measuring optical density at λ510 nm.
Wnt3a-conditioned Medium
Mouse L cells (control cells and Wnt-3a-expressing cells) were obtained from ATCC (CRL-2648 and CRL-2647, Manassas, VA). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum and G-418 at 37 °C. At confluency, the cells were split 1:10 and cultured without G-418. Cell culture media were repeatedly collected each 3 days. 3T3-L1 preadipocytes were differentiated in the presence of 0.5–80% Wnt3a-conditioned medium and using L-cell medium as control. Percent Wnt3a-conditioned medium that caused total inhibition of differentiation based on APM1, FABP4, and PPARγ2 mRNA expression was determined. In parallel recombinant mouse Wnt3a (1324-WN, R&D Systems, UK) was analyzed for quantification.
Osteocyte Differentiation and Mineral Detection
The osteocyte differentiation was induced as described by adding 0.1 μm dexamethasone, 50 μm ascorbic acid-2-phosphate, and 10 mm β-glycerolphosphate into Dulbecco's modified Eagle's medium with 10% (v/v) fetal bovine serum (23). Extracellular matrix mineralization was detected with the von Kossa method (23).
Human Subjects
Abdominal subcutaneous adipose tissue was obtained from six different subjects by needle biopsies. The Ethical Committee of the University of Gothenburg approved the procedure. The subjects were between 27 and 57 years of age and had a mean body mass index of 23.4 ± 3.1 kg/m2.
Human Adipocyte Differentiation
Preadipocytes were isolated from human subcutaneous adipose tissue and differentiated as described (13). At day 12, when 10% Wnt3a-conditioned medium and/or 20 ng/ml TNFα was added, pioglitazone was reduced to 0.1 μm. The cells were than left to differentiate for a further 12 days with change of medium every third day.
Whole Cell Extracts and Western Blots
Whole cell protein lysates were prepared, and Western blot analyses were performed as described (19). Total protein, 25–40 μg, was loaded on SDS-PAGE (Cambrex, Biosciences Inc.) gels for each sample. Immunoblots were performed with the following antibodies: Axin (H-98), WIF-1 (sc-25520), α-SMA (sc-32251), pY (99), and IR (sc-711) (all from Santa Cruz Biotechnology, Santa Cruz, CA); LRP6 (AF1505) and DKK1 (MAB 1765) (R&D Systems, Inc., UK); β-catenin (C19220) and GSK3β (G22320) (BD Transduction Laboratories); PKB (9272), PKBser473 (9271), GSK3βser9 (9331), IRS-1 (2382), pERK1/2 (9106), and β-cateninser675 (9567) (all from Cell Signaling, New England Biolabs Ltd., UK); ERK1/2 (V803A, Promega Biotech AB, Stockholm, Sweden); PPARγ2 (MAB3630, Chemicon International, Inc., Millipore); and GSK3βY214 (05–413, Upstate Biotechnology, Millipore AB, Sweden). For detection, the membranes were incubated with secondary antibody for 1 h at room temperature. The bands were visualized either with ECL reagent (Amersham Biosciences) for x-ray films or with an Immun-Star HRP Chemiluminescent Kit (Bio-Rad Laboratories) for quantification with ChemiDocTM XRS System (Bio-Rad).
Real-time PCR
Total RNA was isolated from the adipocytes with RNeasy (Qiagen Nordic, Solna, Sweden). Quantitative real-time PCR was performed by using the TaqMan system (Applied Biosystems, Foster City, CA). Gene-specific primers and probes were either designed using Primer Express software (sequences used are available on request), or purchased as Assay-on-Demand (α-SMA, Runx2, and ET-1, Applied Biosystems). The real-time PCR reaction was essentially performed as described (19). Relative quantification of mRNA levels was plotted as -fold change, generally compared with the day 8 (=1) when additions were made. 18 S ribosomal RNA was used as endogenous control (Applied Biosystems). Analyses were performed in duplicates, and all experiments were repeated at least three times.
Glucose Transport into 3T3-L1 Adipocytes
Cellular uptake of 2-deoxy-d-[1-3H]glucose (TRK383, Amersham Biosciences) was measured after incubation of the adipocytes in low glucose medium with 0.1% (wt/v) bovine albumin for 3 h. Glucose transport was initiated after stimulation with 100 nm insulin for 30 min. The preincubation medium was removed by washing the adipocytes with warm (37 °C) phosphate-buffered saline/0.1% (w/v) bovine serum albumin, and 1 μCi of [3H]glucose was added to the incubation media (phosphate-buffered saline/0.1% (w/v) bovine serum albumin) for 10 min. Glucose transport was stopped by adding phloretin (Sigma-Aldrich) to a final concentration of 50 μm. The adipocytes were washed carefully with ice-cold phosphate-buffered saline and lysed in 0.2 m NaOH. Part of the lysate was counted in a scintillation counter, and the remaining part was used for protein determination (Bradford Reagent, Sigma-Aldrich).
Statistical Analyses
Conventional statistical methods were used to calculate means ± S.E. Student's paired t test was used to compare differential gene expression between untreated and Wnt3a/cytokine-treated samples. Differences were considered statistically significant at p < 0.05 level.
RESULTS
Wnt3a Promotes Dedifferentiation of 3T3-L1 Adipocytes
Morphological Changes
Within 24 h, 3T3-L1 adipocytes stimulated with Wnt3a-conditioned medium showed a pronounced increase in migration rate with a massive cluster formation (Fig. 1, B and G) that was not seen in control cells (Fig. 1A). This was also associated with a marked decrease in number and size of the lipid droplets (Figs. 1D, 1E, and 3A). Furthermore, Wnt3a induced the appearance of a large number of proliferating cells containing many small lipid droplets and with the morphology of fibroblasts migrating from the clusters (Fig. 1, G–I).
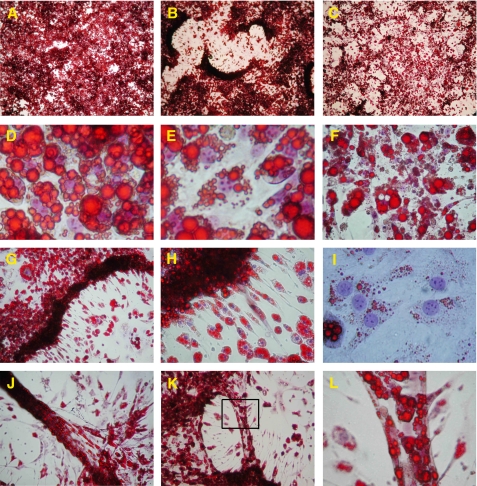
FIGURE 1.
Wnt3a promotes morphological changes of mature 3T3-L1 adipocytes. Microscopy was performed on Oil Red O- and hematoxylin-stained 3T3-L1 adipocytes stimulated with either 10% Wnt3a-CM or 10% of L-cell medium (control medium) or 20 ng/ml TNFα for different time periods: A–C, 9 days; D–I, 6 days; and J–L, 3 days. A, adipocytes with control medium; B, Wnt3a-induced cluster formation; and C, TNFα-stimulated adipocytes. D, close examination of control adipocytes showing filled lipid droplets and E, Wnt3a-stimulated adipocytes with irregular morphology of the lipid droplets and a decrease in lipid droplets size. F, enlarged view of the TNFα-stimulated adipocytes showing the remainder of many dead cells. G and H, from the clusters formed by Wnt3a migration, many elongated small cells that had lost most of their lipids. I, the Wnt3a-dedifferentiated adipocytes filled up the empty spaces between the cluster formations. J and K, Wnt3a-induced formations similar to tubules. L, magnification of marked area in K.
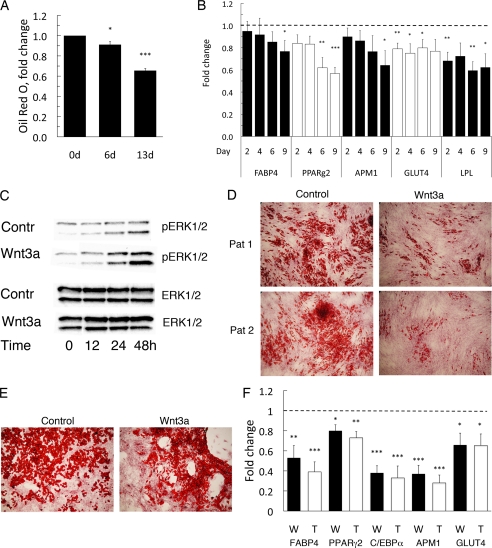
FIGURE 3.
Wnt3a-induced dedifferentiation of mouse 3T3-L1 adipocytes and human in vitro differentiated adipocytes. A, Wnt3a decreased lipid accumulation in 3T3-L1 adipocytes. Oil Red O uptake (n = 3). The data are normalized against control values (=1) at each time point. B, quantitative real-time PCR of the adipogenic differentiation markers LPL, FABP4, adiponectin (APM1), GLUT4, and PPARγ2 in Wnt3a-treated 3T3-L1 adipocytes. Expression levels of the genes was first normalized to 18 S rRNA and then normalized to expression levels in the control sample (=1) at each time point (n = 6, LPL n = 4). Data are presented as the mean ± S.E. *, p < 0.05; **, p < 0.02; and ***, p < 0.002, compared with untreated adipocytes. C, cell lysates from Wnt3a-incubated 3T3-L1 adipocytes were blotted with antibodies as indicated. Wnt3a induced activation of ERK1/2. Blots from replicate experiments are analyzed. D, human preadipocytes isolated from subcutaneous adipose tissue were first differentiated to adipocytes for 12 days and then incubated with 10% Wnt3a-CM containing 0.1 μm pioglitazone for additional 12 days (Oil Red O staining). E, human in vitro differentiated adipocytes treated with Wnt3a for 21 days, added at differentiation day 12, showing cluster formation. F, Wnt3a reduced several genes related to mature adipocytes. Quantitative gene expression for FABP4, PPARγ2, adiponectin (APM1), and C/EBPα in human Wnt3a- and TNFα-treated adipocytes, Wnt3a (W) and TNFα (T). The data were fist normalized to 18 S rRNA and then normalized to expression levels in the control sample (=1). Data indicate mean ± S.E. (n = 6); *, p < 0.05; **, p < 0.02; and ***, p < 0.002, compared with untreated adipocytes.
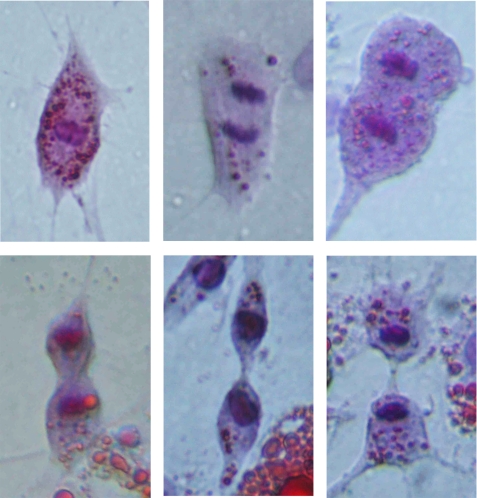
Astonishingly, the proliferating cells also formed structures resembling tubuli formation (Fig. 1, J–L). These structures were induced after ∼3 days with Wnt3a. Close examination of the Wnt3a-treated cells showed lipid-containing cells with a fibroblast-like morphology and that divided symmetrically (Fig. 2). The increased proliferation was also verified with bromodeoxyuridine incorporation (see supplemental Fig. 1). Thus, a canonical Wnt ligand rapidly alters both the morphology and migration of fully differentiated adipose cells and promotes proliferation of cells containing lipid droplets; i.e. it induces a pro-oncogenic pattern in fully differentiated cells. It is likely that Wnt 3a also exerted an anti-apoptotic effect, but this was not examined in detail. In contrast, TNFα induced a rapid loss of cellular lipids (Fig. 1C) and, after 4–6 days, a marked apoptosis of the adipocytes as also previously reported (Fig. 1F) (25). The massive migration and cluster formation caused by Wnt3a was not induced in the presence of TNFα, and neither tubuli formation nor lipid-containing dividing cells were seen (data not shown).
FIGURE 2.
Wnt3a-induced proliferation of dedifferentiated 3T3-L1 adipocytes. Cells containing small lipid droplets undergo mitosis in the presence of Wnt3a. Oil Red O and hematoxylin staining of fully differentiated 3T3-L1 adipocytes stimulated with 10% Wnt3a-CM for 72 h.
Although Wnt3a could hypothetically promote myogenic differentiation with fusion of cells, the hypothesis of transformation of adipocytes into a myocyte lineage was not confirmed, because MyoD gene expression was not induced in the Wnt3a-treated adipocytes (data not shown).
Taken together, Wnt3a promotes loss of lipid droplets, increased mitogenesis, and mobility as well as cluster formation in 3T3-L1 adipose cells while TNFα promotes apoptosis. The gradual reduction of the total cellular lipid content measured with Oil Red O is summarized in Fig. 3A.
Gene Expression
These results are consistent with the induction of a dedifferentiation process in the fully differentiated 3T3-L1 adipocytes. To examine this, we also analyzed the expression of adipogenic genes in the cells. In the presence of Wnt3a, the expression of lipoprotein lipase was decreased with ∼40% (Fig. 3B), and Wnt3a also induced an overall reduction of all tested adipogenic markers, including PPARγ2, FABP4, APM1, and GLUT4 (Fig. 3B). Interestingly, addition of the PPARγ ligand, pioglitazone, was unable to prevent this effect (see supplemental Fig. 2, A and B). Taken together, these results are consistent with a dedifferentiation of mature adipose cells by the canonical Wnt ligand.
Wnt3a Induces Activation of the MAPKs ERK1 and ERK2
As expected, the presence of Wnt3a increased the phosphorylation of ERK1/2, and this effect was not related to altered ERK protein levels (Fig. 3C). However, in contrast to TNFα, Wnt3a did not increase the phosphorylation of either JNK1 or p38 (data not shown). Thus, the mitogenic effect of Wnt3a is also reflected by the ERK1/2 activation in agreement with what has been shown in NIH 3T3 cells (26).
Dedifferentiation of Human Adipocytes
To establish whether Wnt3a also induced dedifferentiation of human adipocytes, we differentiated isolated preadipocytes from human subcutaneous tissue into adipocytes for 12 days and, subsequently, incubated the adipocytes with Wnt3a for another 12 days in the presence of the PPARγ ligand pioglitazone in the culture medium. Wnt3a induced a marked decrease in the number and size of the lipid droplets also in these cells (Fig. 3D). Increased motility and migration was also seen but required a longer incubation with Wnt3a (3 weeks, Fig. 3E), but human preadipocytes also grow much more slowly than 3T3-L1 cells. To confirm the induction of dedifferentiation, we analyzed adipogenic markers and, as shown in Fig. 3F, all genes were significantly repressed 25–60%. This was also seen in the presence of TNFα (Fig. 3F). Thus, activation of the canonical Wnt signaling pathway with Wnt3a suppresses adipogenic markers in both human and 3T3-L1 adipocytes consistent with the induction of dedifferentiation. These effects were not prevented by the continued presence of a PPARγ ligand in the incubation medium showing that the ability of PPARγ to become activated by ligands is impaired in the presence of Wnt3a.
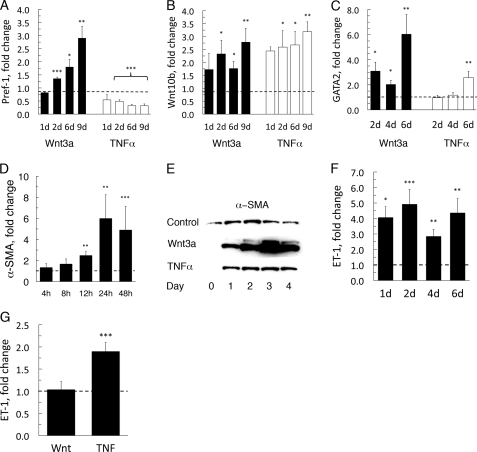
Induction of Dlk1 (Pref-1), Wnt10b, and Gata2 in Dedifferentiated 3T3-L1 Adipocytes
Dlk1 (preadipocyte factor-1, Pref-1) is only expressed in preadipocytes and is rapidly and, as suggested, irreversibly down-regulated when preadipocytes undergo adipose conversion (26). Accordingly, addition of TNFα does not reverse the normal decrease in Dlk1 expression in differentiating 3T3-L1 preadipocytes or induce Dlk1 in adipocytes (Fig. 4A) as also previously reported (19, 27). In contrast to TNFα, Wnt3a markedly increased the expression of Dlk1 in mature 3T3-L1 adipocytes (Fig. 4A) further supporting a dedifferentiation of the cells. Wnt10b, another marker of undifferentiated cells, was also increased in the presence of Wnt3a (Fig. 4B). Similarly, Gata2, was strongly induced by Wnt3a (Fig. 4C).
FIGURE 4.
Wnt3a promotes an undifferentiated phenotype in adipocytes. Gene expression of Wnt3a- and TNFα-incubated 3T3-L1 adipocytes. A, Pref-1 (Dlk1); B, Wnt10b; and C, Gata2. D, Wnt3a-induced α-SMA expression. A–D, F, and G, the data were fist normalized to 18 S rRNA then normalized to expression levels in the control sample (=1). Data are presented as the mean ± S.E. (n = 4); *, p < 0.05; **, p < 0.02; ***; and p < 0.002, compared with untreated. E, 3T3-L1 adipocytes were stimulated with Wnt3a and TNFα. Western blotting was performed to determine relative levels of α-SMA protein expressed in adipocytes. F, quantitative ET-1 gene expression in the presence of Wnt3a. RNA was extracted at the indicated times, and mRNA levels were determined with real-time PCR; for evaluation see above. G, quantitative real-time PCR analysis for human ET-1 gene expression; for evaluation of data, see above.
One important mechanism for dedifferentiation of adipose cells is inhibition of PPARγ activation, although this is, by itself, not sufficient to induce a marked dedifferentiation (28). Wnt ligands prevent PPARγ activation during preadipocyte differentiation (19, 29), and the effects of Wnt3a were not prevented by the presence of a PPARγ ligand. As expected, we also found that both TNFα and Wnt3a inhibited PPARγ activation by thiazolidinedione in a reporter assay with 3T3-L1 preadipocytes (see supplemental Fig. 2, C and D). However, because both Wnt3a and TNFα inhibited ligand activation of PPARγ, although Wnt3a induced a more pronounced dedifferentiation, PPARγ inhibition was not sufficient by itself to explain the pronounced effect of canonical Wnt activation.
Induction of α-SMA in 3T3-L1 Adipocytes
α-Smooth muscle actin (α-SMA) is found in pericytes, vascular smooth muscle cells, and mesenchymal stem cells. Wnt3a induced a rapid and marked (∼40-fold) increase in α-SMA expression in the 3T3-L1 adipocytes, whereas this effect was marginal for TNFα (Fig. 4, D and E). To further confirm activation of genes that could be responsible for a myofibroblastic phenotype we analyzed the expression of endothelin-1 (ET-1/Edn1), which was induced 4-fold with Wnt3a (Fig. 4F). In human adipocytes, ET-1 was already highly expressed and did not increase further in the presence of Wnt3a (Fig. 4G), whereas TNFα significantly increased the expression of ET-1. Thus, a differential response was seen between human and murine cells in the activation of the ET-1 gene by TNFα and Wnt3a.
Induction of Osteogenesis in Dedifferentiated 3T3-L1 Adipocytes
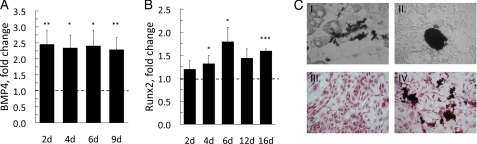
Induction of osteogenesis in progenitor cells requires suppression of C/EBPα and PPARγ to alter cell fate (30). The pleiotrophic bone morphogenetic protein (BMP) 4 is mainly related to the ability to induce ectopic bone formation, but it has also been shown to recruit mesenchymal precursor cells to the adipocyte lineage (31), whereas Runx2 is essential for ossification and placed downstream of BMPs (30). Because canonical Wnt signaling promotes osteogenesis in osteogenic cells, we investigated if exposure to Wnt3a could allow transdifferentiation of the dedifferentiated adipocytes to the osteocyte lineage. Both Bmp4 and Runx2 were increased in the presence of Wnt3a (Fig. 5, A and B), and the addition of osteogenic medium induced mineralization visualized with von Kossa staining (Fig. 5C, I, II, and IV), which was not seen in the absence of Wnt3a (Fig. 5C, III). Positive von Kossa staining was particularly seen around cells with a few lipid droplets showing that these cells were dedifferentiated and most prone to transdifferentiation. Taken together, these data show that a canonical Wnt ligand induces dedifferentiation of mature adipocyte, which is sufficient to allow them to enter the pathways required for an osteogenic phenotype.
FIGURE 5.
Wnt3a promotes osteogenesis in 3T3-L1 adipocytes. A, 3T3-L1 adipocytes were incubated with Wnt3a-CM for the indicated time points. Quantitative real-time PCR of the osteogenic transcription factors Bmp4 and Runx2 (B). The data were first normalized to 18 S rRNA and then normalized to expression levels in the control sample (=1). Data in A and B represent means ± S.E. from four independent experiments; *, p < 0.05; **, p < 0.02; ***, p < 0.002, compared with untreated. In C: I, II, and IV, adipocytes were incubated in osteocyte mineralization medium in the presence of Wnt3a-CM; III, Wnt3a-CM was omitted; I–IV, on day 12, cellular mineralization was visualized with von Kossa staining; and III–IV, double staining with Oil Red O.
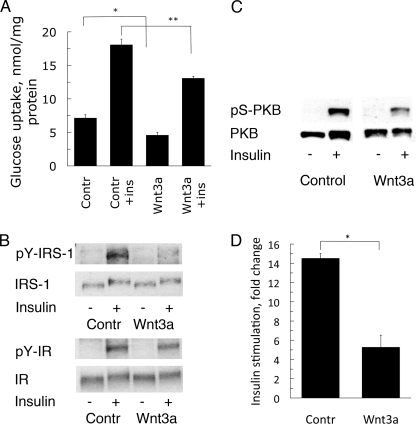
Wnt3a Induces Insulin Resistance in 3T3-L1 Adipocytes
Because the 3T3-L1 adipocytes underwent dedifferentiation in the presence of Wnt3a, we also evaluated the effect on insulin sensitivity and action. 3T3-L1 adipocytes were incubated with Wnt3a for 72 h prior to the glucose transport assay. Wnt3a significantly decreased both basal and insulin-stimulated glucose transport in the adipocytes (Fig. 6A). There was also a marked decrease in the insulin-stimulated tyrosine phosphorylation of the insulin receptor (IR) as well as the insulin receptor substrate-1 (IRS-1) (Fig. 6B), whereas there were no differences in the IR or IRS-1 proteins (Fig. 6B). pS307 of IRS-1 was also not increased in the presence of Wnt3a (data not shown). We also examined insulin-induced phosphorylation of PKBser473 and found a significant reduction with Wnt3a (Fig. 6, C and D).
FIGURE 6.
Wnt3a-induced insulin resistance in 3T3-L1 adipocytes. A, adipocytes were incubated with/without Wnt3a-CM for 72 h before stimulation with 100 nm insulin for 30 min. Glucose uptake was initiated for 10 min. *, p < 0.05; **, p < 0.02. B, Wnt3a caused a reduction in the insulin-stimulated phosphorylation of both IR and IRS-1. Expression of IR, IRS-1 proteins, and tyrosine phosphorylation was analyzed by Western blotting. C, Wnt3a-induced reduction of PKBser473 phosphorylation. The adipocytes were incubated with/without Wnt3a for 72 h before stimulation with 100 nm insulin for 30 min. Cell lysates were prepared and blotted with the indicated antibodies. D, quantitative protein immunoblot analyses from four independent experiments were performed to determine relative levels (mean ± S.E.) of PKBser473 phosphorylation from C.
Taken together, Wnt3a gradually dedifferentiates mature 3T3-L1 adipocytes, and this is accompanied by insulin resistance with a decrease in Glut4 expression, insulin-stimulated tyrosine phosphorylation of IR and IRS-1, and activation of PKB at an early stage (72 h).
Wnt3a Increases β-Catenin in Mature 3T3-L1 Adipocytes through an Impaired Degradation
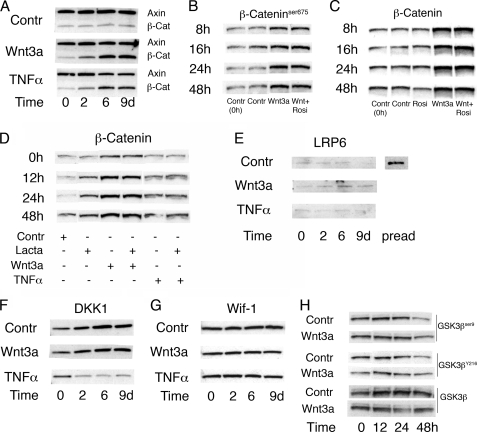
We asked if Wnt3a could induce stabilization of β-catenin in mature adipocytes. To address this question, we incubated mature 3T3-L1 adipocytes with Wnt3a-conditioned medium and measured β-catenin protein and key molecules involved in the canonical Wnt signaling pathway. Wnt3a-conditioned medium increased the cellular β-catenin levels after 8 h and maintained elevated β-catenin levels throughout the 16 days of incubation (Fig. 7A and supplemental Fig. 3). TNFα also increased the cellular β-catenin levels, although to a lesser extent and after a delay of ∼24 h (Fig. 7A and data not shown). The increased cellular β-catenin levels were not due to an increased transcription of the gene, because β-catenin mRNA levels were only slightly and transiently increased; i.e. no increase was seen by either Wnt3a or TNFα after 4 days of exposure (see supplemental Fig. 4). The increased β-catenin levels following Wnt3a and TNFα were also targeted for nuclear localization because serine β-cateninser675 was also increased (Fig. 7B).
FIGURE 7.
Stabilization of β-catenin by Wnt3a or TNFα despite down-regulation of the canonical Wnt-signaling pathway in 3T3-L1 adipocytes. A, Wnt3a-induced stabilization of β-catenin in the presence of axin. Western blotting was performed on extracts from Wnt3a- and TNFα-incubated adipocytes with the indicated antibodies. B, rosiglitazone had no effect on Wnt3a-induced stabilization of β-cateninser675 phosphorylation, important for nuclear translocation, or total β-catenin protein levels (C). Visualization of β-cateninser675 and total β-catenin was analyzed with Western blotting. D, the proteasomal inhibitor lactacystin had no effect on Wnt3a- or TNFα-stabilized β-catenin. Adipocytes were harvested at the indicated times points, and Western blot was performed on cell lysates. E, Western blotting was performed on extracts from Wnt3a- and TNFα-incubated adipocytes to test for changes in the LRP6 receptor when β-catenin was stabilized. Cell lysates from preadipocytes were included to show LRP6 protein expression. F, Wnt3a did not affect the protein levels of the Wnt inhibitors DKK1 or Wif-1 (G). Western blotting was carried out with the indicated antibodies. H, Western blotting was performed on extracts from Wnt3a- and TNFα-incubated adipocytes to test for changes in activating GSK3βY216 and inactivating GSK3βser9 phosphorylations. A–H, blots from replicate experiments were analyzed.
Thus, Wnt3a increased total cellular and β-cateninser675 levels in the absence of any clear changes in mRNA expression, which suggests a reduced β-catenin degradation and/or a stabilization of β-catenin through a restitution of the upstream canonical Wnt signaling cascade. To address the possibility that Wnt3a increased the β-catenin levels through repressed PPARγ, because PPARγ activation increases β-catenin degradation (32), we incubated the dedifferentiated 3T3-L1 cells with the PPARγ ligand, rosiglitazone. As shown in Fig. 7C, PPARγ activation decreased β-catenin levels in the control cells but had no effect in the Wnt3a-incubated cells. This was verified in three separate experiments where rosiglitazone significantly reduced β-catenin levels in control cells by 30% (p = 0.003), whereas no reduction at all was seen in the presence of Wnt3a.
Taken together, these results are consistent with a decreased proteasomal degradation of β-catenin that could be a consequence of PPARγ repression. To further validate this, we incubated control, Wnt3a-expoased, and TNFα-exposed cells with the proteasomal inhibitor lactacystin. As shown in Fig. 7D, lactacystin increased β-catenin levels in control cells while no difference was seen in cells incubated with Wnt3a or TNFα. Because the ability of a PPARγ ligand to decrease cellular β-catenin levels also was inhibited in the presence of Wnt3a, these results support an important role of PPARγ activation in maintaining the adipocyte phenotype by promoting β-catenin degradation.
To further address the mechanisms for the stabilized β-catenin levels, we examined if also the canonical Wnt-signaling pathway was restituted and activated. However, the Wnt co-receptor LRP6 that is degraded during differentiation of preadipocytes to adipocytes remained absent (Fig. 7E). Axin, the rate-limiting protein in the canonical Wnt signaling pathway, is rapidly degraded following activation of the canonical Wnt signaling cascade. Again, axin was present and not affected by Wnt3a-stabilized β-catenin or by TNFα (Fig. 7A). TNFα, but not Wnt3a, reduced the expression of the Wnt antagonist DKK1 (Fig. 7F). DKK1 binds to the LRP receptors and, thereby, negatively regulates Wnt-signaling. Also other inhibitors of Wnt signaling, Wif-1, sFRP1, and sFRP2, that bind to the Frizzled receptors were unaffected by both Wnt3a and TNFα (Fig. 7G and data not shown). To exclude that the stabilization of β-catenin was due to inactivation of GSK3β, which is required for targeting β-catenin for proteasomal degradation, we examined GSK3β phosphorylation and activity. However, neither the inactivating GSK3βser9 nor the activating GSK3βY214 phosphorylations were changed by the presence of Wnt3a (Fig. 7H). We also measured total GSK3β activity in cell lysates but saw no effect of Wnt3a on GSK3β activity in differentiated 3T3-L1 cells (see supplemental Fig. 5).
Induction of Inflammation
We examined the effect of Wnt3a and TNFα on inflammatory genes in the adipocytes. Unlike TNFα, which dramatically induces expression of chemo- and cytokines, Wnt3a was virtually without any effect on inflammatory molecules. A small (4-fold) increase in monocyte chemoattractant protein-1 (MCP-1/Ccl2) was seen with Wnt3a while TNFα increased Mcp-1 > 100-fold (see supplemental Fig. 6). Furthermore, Wnt3a did not induce expression of the pro-inflammatory genes Il-1β or Il-6 (see supplemental Fig. 5).
Thus, Wnt3a like TNFα, represses PPARγ activity and stabilizes β-catenin levels in mature adipocytes leading to a dedifferentiation of the cells. However, it is also clear that there are major differences in the effects of these ligands. TNFα induces apoptosis and promotes a marked pro-inflammatory and macrophage-like profile of the cells. In contrast, Wnt3a promotes mitogenesis and allows the transdifferentiation of the cells to a myofibroblast phenotype or, when induced by an appropriate mixture, to an osteogenic phenotype. The differences between TNFα and Wnt3a may, in part, be due to the fact that the canonical Wnt ligand did not induce a pro-inflammatory phenotype of the dedifferentiated adipocytes, which was the case for TNFα. PPARγ inhibition is likely to play an important role, although this cannot alone account for the dedifferentiation as also recently shown (28). Wnt3a also induces other effects like ERK1/2 activation and increases Dlk1, Gata2, and Wnt10b leading to a real and pronounced dedifferentiation of the cells, the extent of which is sufficient to allow them to both assume a myofibroblast phenotype and, under appropriate conditions, to become sensitive to osteogenic stimuli with mineralization.
DISCUSSION
A key novel finding of the present study is that fully differentiated 3T3-L1 and human adipose cells are responsive to a canonical Wnt ligand and that this is associated with pro-oncogenic changes, including elevated β-catenin levels, increased mitogenesis, motility, and dedifferentiation of the cells associated with a potential for transdifferentiation. However, this effect is not due to a restitution of the normal canonical Wnt signaling pathway, because it remained virtually unchanged to that of normally differentiated adipocytes with maintained axin protein and degraded LRP6. GSK3β activity, like β-catenin mRNA levels, was also unchanged suggesting that other downstream degradation pathways were impaired. This was further supported by the finding that the increased degradation of β-catenin seen in the presence of a PPARγ ligand was abolished by Wnt3a.
Furthermore, proteasomal inhibition by lactacystin increased the β-catenin levels in control cells, but had no effect in cells incubated with Wnt3a or TNFα supporting that this pathway was already inhibited. Taken together, our results support the conclusion that the increased β-catenin levels were mainly a result of the repressed PPARγ leading to a reduced degradation of β-catenin and induction of dedifferentiation of the cells. This, in turn, means that PPARγ activation is required to keep the adipocytes differentiated and that the effect on β-catenin proteasomal degradation plays a important role for this. Although an effect of PPARγ activation on β-catenin degradation has been shown previously (32), the detailed molecular mechanisms for this are still unclear and require further studies.
PPARγ activation was markedly repressed, but Wnt3a only had a minor effect on PPARγ protein levels. In agreement with this, it has been shown that Wnt signaling prevents activation but does not alter translation or stability of the PPARγ protein (32). It has also been shown that β-catenin can interact with PPARγ and that this is associated with a repression of PPARγ (33, 34). In addition, Wnt ligands, through β-catenin, have recently been shown to activate the anti-adipogenic repressor, COUP-TFII, which recruits SMRT to repress PPARγ activation (29).
It is also intriguing that TNFα induces a similar effect on the β-catenin levels in the mature adipocytes and the associated PPARγ repression. This link between the canonical Wnt pathway and inflammation has previously been documented in 3T3-L1 preadipocytes (19, 35) and seems to be specific for TNFα, because we have not seen any similar cross-talk for monocyte chemoattractant protein-1 or resistin (36), whereas IL-6 does induce some activation in 3T3-L1 cells (19). Taken together, these results show that a canonical Wnt ligand, as well as TNFα, increases β-catenin levels in both undifferentiated preadipocytes and differentiated adipocytes.
It is clear that Wnt3a can dramatically force mature 3T3-L1 adipocytes, like human adipocytes, into an immature phenotype with reduced expression of the typical adipocyte- and PPARγ-associated genes like C/EBPα, APM1, FABP4, and GLUT4 while, instead, genes such as Dlk1, Gata2, and Wnt10b, which are characteristic of and highly expressed in undifferentiated preadipocytes, are induced. Moreover, the dedifferentiated adipocytes have the ability to enter the cell cycle and proliferate (Fig. 2). Proliferation of NIH3T3 fibroblasts has been shown to be regulated both by the ERK- and β-catenin-signaling pathways in accordance with our results (37).
Adipocytes are derived from mesenchymal stem cells, and conversion of stem cells to preadipocytes involves steps where the cell has lost its potential to differentiate into other cell types. One important prerequisite is down-regulation of the Wnt signaling. The last step in the commitment is when the preadipocyte enters terminal differentiation to the adipocyte. Terminally differentiated cells have, therefore, been considered incapable of reversing this process (38). Reversal of differentiation is a process that, so far, has only has been documented in amphibian cells. However, a recent study indicates that human mature adipocytes, exposed to the dedifferentiation procedure of ceiling culture, can gain capacity to proliferate again (39). We observed that <5% of the 3T3-L1 cells did not accumulate significant amounts of lipids at differentiation day 8, and it is unlikely that these potentially less differentiated cells could affect the result. In fact, the cells undergoing mitosis in the presence of Wnt3a contained lipid droplets (Fig. 2), and there is no differentiation at all in preadipocytes exposed to Wnt3a in the differentiation mixture (13, 19, 40).
Directional cell migration requires establishment of cell polarity to create a leading edge, including establishment of new matrix contacts, whereas adhesion is disassembled at the trailing edge. Migration also usually requires matrix remodeling (41). The impressive migration and cluster formation induced by Wnt3a is more reminiscent of a myofibroblastic or a smooth muscle phenotype rather than adipocytes. Adipocyte migration was also noticeably one-sided into the clusters (Fig. 1, B and G).
Our striking finding, that the contractile protein α-SMA, highly expressed in myofibroblasts and a marker for pericytes and induced by ET-1, was markedly up-regulated in the adipocytes, further shows that the adipocytes were able to adopt an immature phenotype (42). Wnt3a has previously been shown to induce ET-1 in murine fibroblasts and induce proliferation of melanocytes (43, 44). However, there was a clear species difference in this regard, because TNFα, rather than Wnt3a, activated ET-1 in the human cells. The mechanisms for this difference are currently unclear.
Wnt3a induced a marked decrease in number and size of the lipid droplets as well as in genes important for maintaining adipocyte metabolism, insulin sensitivity, and action. This is likely to be secondary to the repressed PPARγ2, but a more pronounced dedifferentiation requires additional restitution of important early molecules related to the undifferentiated state like Gata2 (28) and Dlk1. Consistent with this, the dedifferentiated cells rapidly (72 h) became insulin-resistant, measured as a decreased insulin-stimulated glucose transport as well as decreased tyrosine phosphorylation of the insulin receptor, IRS-1, and PKBser473 phosphorylation. The mechanism for the reduced upstream tyrosine phosphorylation is unclear, but it could be due to the increased ERK1/2 activation (45) and/or increased phosphotyrosine phosphatase activity such as PTP1b (46). Whether Wnt 3a inhibits the receptor tyrosine kinase activity also remains to be established. Importantly, however, the effect of Wnt3a differs from that of TNFα, because IRS-1 protein levels were unchanged further supporting our finding that Wnt3a does not increase the pro-inflammatory response in the adipocytes. Consistent with this, Wnt3a did not activate JNK1/2 or p38. In contrast, TNFα increases serine phosphorylation of IRS-1 following activation of JNK kinases leading to its degradation and insulin resistance (47).
Intriguingly, we found that mature adipocytes exposed to Wnt3a, but not TNFα, have the potential to assume an immature phenotype and express genes related to osteogenic cells with activation of Runx2 and Bmp4. Activation of Wnt signaling rapidly suppresses expression of the transcription factors PPARγ and C/EBPα, and, in mesenchymal stem cells, this suppression allows an increase in osteogenic transcription factors (30). Suppression of PPARγ is necessary for osteogenesis, because the interaction with PPARγ prevents Runx2 from binding to DNA (30). So far, Wnt10b is a likely candidate for activation of osteogenic factors, but other candidates are Wnt7b and Wnt3a, also expressed in mesenchymal precursor cells (48, 49). Surprisingly, the expression of Wif-1, sFRP1, and sFRP2 as well as DKK1 remained in the presence of Wnt3a in the adipocytes. DKK1 is generally considered to be an inhibitor of osteoblastogenesis (24). In contrast, TNFα reduced DKK1 expression, which may contribute to its ability to activate the Wnt pathway.
In conclusion, we here show that the canonical Wnt ligand, Wnt3a, induces dedifferentiation of mature adipocytes and that this is associated with increased β-catenin levels, PPARγ repression, insulin resistance as well as increased mitogenesis and migration of the cells. Increased β-catenin levels and PPARγ repression are also seen with TNFα and, thus, are likely common effects of repressors of PPARγ. This suggests that PPARγ activation is critical for the maintained adipocyte phenotype, including for β-catenin degradation. However, transdifferentiation requires additional steps than just PPARγ repression such as re-expression of markers of the undifferentiated state like DLK1 and GATA2. It is also intriguing that there is cross-talk between TNFα and the canonical Wnt pathway in both preadipocytes (19, 35) and mature adipocytes. Thus, the adipose tissue in obesity with enlarged adipose cells and concurrent inflammation may be characterized by Wnt activation with impaired adipogenesis. In fact, we have recently found that several markers of canonical Wnt activation are increased and that preadipocyte differentiation is reduced in human obesity characterized by enlarged adipocytes (13).
Wnt3a lacks a pro-inflammatory effect and allows transdifferentiation to myofibroblasts or, after appropriate stimuli, to an osteogenic lineage. These results further support the plasticity of adipocytes and suggest that these cells can be used for cellular engineering. Finally, the finding that a canonical Wnt ligand can induce dedifferentiation of fully differentiated cells also raises the question whether newly induced mutations or other perturbations in this signaling cascade, as seen in colon cancer and several other cancers, allows differentiated cells to regress to an undifferentiated pro-mitogenic and pro-oncogenic phenotype such as the cancer stem cells.
Supplementary Material
Acknowledgment
We thank Lachmi Jenndahl for performing the PPARγ reporter assay.
This work was supported by the Swedish Research Council, the Swedish Diabetes Association, the Novo Nordisk Foundation, the Swedish Foundation for Strategic Research, and the Torsten and Ragnar Söderberg Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. 1–6.
- Wnt
- wingless-type MMTV integration site family
- PPAR
- peroxisome proliferator-activated receptor
- CEBP
- CCAAT/enhancer-binding protein
- Dvl
- Dishevelled
- TNF
- tumor necrosis factor
- GLUT
- glucose transporter
- FABP
- fatty acid-binding protein
- JNK
- c-Jun N-terminal kinase
- ERK
- extracellular regulated MAPK
- PKB
- protein kinase B
- GSK
- glycogene synthase kinase
- α-SMA
- α-smooth muscle actin
- MAPK
- mitogen-activated protein kinase
- Pref-1
- preadipocyte factor-1
- ET-1
- endothelin-1
- BMP
- bone morphogenetic protein
- IR
- insulin receptor
- IRS-1
- insulin receptor substrate-1.
REFERENCES
- 1.Guo Y. F., Xiong D. H., Shen H., Zhao L. J., Xiao P., Guo Y., Wang W., Yang T. L., Recker R. R., Deng H. W. (2006) J. Med. Genet. 43, 798–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mani A., Radhakrishnan J., Wang H., Mani A., Mani M. A., Nelson-Williams C., Carew K. S., Mane S., Najmabadi H., Wu D., Lifton R. P. (2007) Science 315, 1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant S. F., Thorleifsson G., Reynisdottir I., Benediktsson R., Manolescu A., Sainz J., Helgason A., Stefansson H., Emilsson V., Helgadottir A., Styrkarsdottir U., Magnusson K. P., Walters G. B., Palsdottir E., Jonsdottir T., Gudmundsdottir T., Gylfason A., Saemundsdottir J., Wilensky R. L., Reilly M. P., Rader D. J., Bagger Y., Christiansen C., Gudnason V., Sigurdsson G., Thorsteinsdottir U., Gulcher J. R., Kong A., Stefansson K. (2006) Nat. Genet. 38, 320–323 [DOI] [PubMed] [Google Scholar]
- 4.Krishnan V., Bryant H. U., Macdougald O. A. (2006) J. Clin. Invest. 116, 1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longo K. A., Wright W. S., Kang S., Gerin I., Chiang S. H., Lucas P. C., Opp M. R., MacDougald O. A. (2004) J. Biol. Chem. 279, 35503–35509 [DOI] [PubMed] [Google Scholar]
- 6.Huang X., Charbeneau R. A., Fu Y., Kaur K., Gerin I., MacDougald O. A., Neubig R. R. (2008) Diabetes 57, 77–85 [DOI] [PubMed] [Google Scholar]
- 7.Vertino A. M., Taylor-Jones J. M., Longo K. A., Bearden E. D., Lane T. F., McGehee R. E., Jr., MacDougald O. A., Peterson C. A. (2005) Mol. Biol. Cell 16, 2039–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González-Sancho J. M., Brennan K. R., Castelo-Soccio L. A., Brown A. M. (2004) Mol. Cell Biol. 24, 4757–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katoh M. (2005) Oncol. Rep. 14, 1583–1588 [PubMed] [Google Scholar]
- 10.Katoh M., Katoh M. (2007) Clin. Cancer Res. 13, 4042–4045 [DOI] [PubMed] [Google Scholar]
- 11.Lu W., Yamamoto V., Ortega B., Baltimore D. (2004) Cell 119, 97–108 [DOI] [PubMed] [Google Scholar]
- 12.Otto T. C., Lane M. D. (2005) Crit. Rev. Biochem. Mol. Biol. 40, 229–242 [DOI] [PubMed] [Google Scholar]
- 13.Isakson P., Hammarstedt A., Gustafson B., Smith U. (2009) Diabetes 58, 1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansson P. A., Pellmé F., Hammarstedt A., Sandqvist M., Brekke H., Caidahl K., Forsberg M., Volkmann R., Carvalho E., Funahashi T., Matsuzawa Y., Wiklund O., Yang X., Taskinen M. R., Smith U. (2003) FASEB J. 17, 1434–1440 [DOI] [PubMed] [Google Scholar]
- 15.Yang X., Jansson P. A., Nagaev I., Jack M. M., Carvalho E., Sunnerhagen K. S., Cam M. C., Cushman S. W., Smith U. (2004) Biochem. Biophys. Res. Commun. 317, 1045–1051 [DOI] [PubMed] [Google Scholar]
- 16.Dubois S. G., Heilbronn L. K., Smith S. R., Albu J. B., Kelley D. E., Ravussin E. (2006) Obesity 14, 1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tickenbrock L., Schwäble J., Strey A., Sargin B., Hehn S., Baas M., Choudhary C., Gerke V., Berdel W. E., Müller-Tidow C., Serve H. (2006) J. Leukocyte Biol. 79, 1306–1313 [DOI] [PubMed] [Google Scholar]
- 18.Zerlin M., Julius M. A., Kitajewski J. (2008) Angiogenesis 11, 63–69 [DOI] [PubMed] [Google Scholar]
- 19.Gustafson B., Smith U. (2006) J. Biol. Chem. 281, 9507–9516 [DOI] [PubMed] [Google Scholar]
- 20.Xu H., Hirosumi J., Uysal K. T., Guler A. D., Hotamisligil G. S. (2002) Endocrinology 143, 1502–1511 [DOI] [PubMed] [Google Scholar]
- 21.Stephens J. M., Pekala P. H. (1992) J. Biol. Chem. 267, 13580–13584 [PubMed] [Google Scholar]
- 22.Tang Q. Q., Lane M. D. (1999) Genes Dev. 13, 2231–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda S., Nobukuni T., Shimo-Onoda K., Hayashi K., Yone K., Komiya S., Inoue I. (2002) J. Cell Physiol. 193, 73–79 [DOI] [PubMed] [Google Scholar]
- 24.Gregory C. A., Gunn W. G., Reyes E., Smolarz A. J., Munoz J., Spees J. L., Prockop D. J. (2005) Ann. N.Y. Acad. Sci. 1049, 97–106 [DOI] [PubMed] [Google Scholar]
- 25.Prins J. B., Niesler C. U., Winterford C. M., Bright N. A., Siddle K., O'Rahilly S., Walker N. I., Cameron D. P. (1997) Diabetes 46, 1939–1944 [DOI] [PubMed] [Google Scholar]
- 26.Smas C. M., Kachinskas D., Liu C. M., Xie X., Dircks L. K., Sul H. S. (1998) J. Biol. Chem. 273, 31751–31758 [DOI] [PubMed] [Google Scholar]
- 27.Xing H., Northrop J. P., Grove J. R., Kilpatrick K. E., Su J. L., Ringold G. M. (1997) Endocrinology 138, 2776–2783 [DOI] [PubMed] [Google Scholar]
- 28.Schupp M., Cristancho A. G., Lefterova M. I., Hanniman E. A., Briggs E. R., Steger D. J., Qatanani M., Curtin J. C., Schug J., Ochsner S. A., McKenna N. J., Lazar M. A. (2009) J. Biol. Chem. 284, 9458–9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamura M., Kudo H., Wakabayashi K., Tanaka T., Nonaka A., Uchida A., Tsutsumi S., Sakakibara I., Naito M., Osborne T. F., Hamakubo T., Ito S., Aburatani H., Yanagisawa M., Kodama T., Sakai J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 5819–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang S., Bennett C. N., Gerin I., Rapp L. A., Hankenson K. D., Macdougald O. A. (2007) J. Biol. Chem. 282, 14515–14524 [DOI] [PubMed] [Google Scholar]
- 31.Bowers R. R., Lane M. D. (2007) Cell Cycle 6, 385–389 [DOI] [PubMed] [Google Scholar]
- 32.Liu J., Wang H., Zuo Y., Farmer S. R. (2006) Mol. Cell Biol. 26, 5827–5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansson E. A., Are A., Greicius G., Kuo I. C., Kelly D., Arulampalam V., Pettersson S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1460–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma C., Pradeep A., Pestell R. G., Rana B. (2004) J. Biol. Chem. 279, 16927–16938 [DOI] [PubMed] [Google Scholar]
- 35.Cawthorn W. P., Heyd F., Hegyi K., Sethi J. K. (2007) Cell Death Differ. 14, 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammarstedt A., Isakson P., Gustafson B., Smith U. (2007) Biochem. Biophys. Res. Commun. 357, 700–706 [DOI] [PubMed] [Google Scholar]
- 37.Kim S. E., Lee W. J., Choi K. Y. (2007) Cell Signal 19, 511–518 [DOI] [PubMed] [Google Scholar]
- 38.Rosen E. D., MacDougald O. A. (2006) Nat. Rev. Mol. Cell Biol. 7, 885–896 [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto T., Kano K., Kondo D., Fukuda N., Iribe Y., Tanaka N., Matsubara Y., Sakuma T., Satomi A., Otaki M., Ryu J., Mugishima H. (2008) J. Cell Physiol. 215, 210–222 [DOI] [PubMed] [Google Scholar]
- 40.Ross S. E., Hemati N., Longo K. A., Bennett C. N., Lucas P. C., Erickson R. L., MacDougald O. A. (2000) Science 289, 950–953 [DOI] [PubMed] [Google Scholar]
- 41.Berrier A. L., Yamada K. M. (2007) J. Cell Physiol. 213, 565–573 [DOI] [PubMed] [Google Scholar]
- 42.Tang W., Zeve D., Suh J. M., Bosnakovski D., Kyba M., Hammer R. E., Tallquist M. D., Graff J. M. (2008) Science 322, 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S., McLean S., Carter D. E., Leask A. (2007) J. Cell Commun. Signal 1, 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirobe T., Shinpo T., Higuchi K., Sano T. (2009) J. Dermatol. Sci. 57, 123–131 [DOI] [PubMed] [Google Scholar]
- 45.Fujishiro M., Gotoh Y., Katagiri H., Sakoda H., Ogihara T., Anai M., Onishi Y., Ono H., Abe M., Shojima N., Fukushima Y., Kikuchi M., Oka Y., Asano T. (2003) Mol. Endocrinol. 17, 487–497 [DOI] [PubMed] [Google Scholar]
- 46.Galic S., Hauser C., Kahn B. B., Haj F. G., Neel B. G., Tonks N. K., Tiganis T. (2005) Mol. Cell Biol. 25, 819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotamisligil G. S., Peraldi P., Budavari A., Ellis R., White M. F., Spiegelman B. M. (1996) Science 271, 665–668 [DOI] [PubMed] [Google Scholar]
- 48.Bennett C. N., Longo K. A., Wright W. S., Suva L. J., Lane T. F., Hankenson K. D., MacDougald O. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rawadi G., Vayssière B., Dunn F., Baron R., Roman-Roman S. (2003) J. Bone Miner. Res. 18, 1842–1853 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.