Summary
Gene-targeted therapies, such as adeno-associated viral vector (AAV)-mediated gene therapy and cell-mediated therapy using myogenic stem cells, are hopeful molecular strategies for muscular dystrophy. In addition, drug therapies based on the pathophysiology of muscular dystrophy patients are desirable. Multidisciplinary approaches to drug design would offer promising therapeutic strategies. Myostatin, a member of the transforming growth factor-β superfamily, is predominantly produced by skeletal muscle and negatively regulates the growth and differentiation of cells of the skeletal muscle lineage. Myostatin inhibition would increase the skeletal muscle mass and prevent muscle degeneration, regardless of the type of muscular dystrophy. Myostatin inhibitors include myostatin antibodies, myostatin propeptide, follistatin and follistatin-related protein. Although follistatin possesses potent myostatin-inhibiting activity, it works as an efficient inhibitor of activins. Unlike myostatin, activins regulate the growth and differentiation of nearly all cell types, including cells of the gonads, pituitary gland and skeletal muscle. We have developed a myostatin-specific inhibitor derived from follistatin, designated FS I-I. Transgenic mice expressing this myostatin-inhibiting peptide under the control of a skeletal muscle-specific promoter showed increased skeletal muscle mass and strength. mdx mice were crossed with FS I-I transgenic mice and any improvement of the pathological signs was investigated. The resulting mdx/FS I-I mice exhibited increased skeletal muscle mass and reduced cell infiltration in muscles. Muscle strength was also recovered in mdx/FS I-I mice. Our data indicate that myostatin inhibition by this follistatin-derived peptide has therapeutic potential for muscular dystrophy.
Keywords: Myostatin, follistatin, muscular dystrophy
Actions of Myostatin
Skeletal myogenesis is under tight regulation by growth factor signaling. Myostatin is an endogenous negative regulator of muscle growth and plays a major role in determining skeletal muscle mass. Myostatin, also known as growth and differentiation factor-8 (GDF8), belongs to the transforming growth factor (TGF)-β superfamily (1, 2). Similar to other TGF-β superfamily members, myostatin is synthesized as a precursor protein that is biologically inactive. Production of mature myostatin occurs through dimerization of the precursor and subsequent proteolytic processing. Cleavage by furin-like protease is responsible of separating the N-terminal propeptide from the C-terminal mature myostatin, while cleavage of the latent propeptide by the bone morphogenetic protein-1/tolloid (BMP1/TLD) family of metalloproteinases is responsible for activation of latent myostatin (3). The C-terminal dimeric 26-kDa protein acts as mature myostatin. Mice with targeted deletion of the myostatin gene show dramatic and widespread increases in skeletal muscle mass (2). Both muscle fiber hypertrophy and muscle cell hyperplasia are observed.
Myostatin signals through two types of transmembrane serine/threonine kinase receptors, namely activin type II receptors (ACVR2B and ACVR2A) and activin receptor-like kinases 4 and 5 (ALK4 and 5). Its intracellular signaling pathway is similar to those of activin and TGF-β, and mediated by the Smad proteins Smad2 and Smad3 (1, 2, 4). Myostatin negatively regulates G1-to-S progression in the cell cycle and maintains the quiescent status of satellite cells (5). As a result, increased numbers of satellite cells are present in myostatin-deficient mice (5). Involvement of the MAP kinase pathway as well as the Smad pathway is a characteristic of the myostatin-regulated skeletal muscle differentiation program (6). However, the precise mechanism of action and the skeletal-muscle specific signaling of myostatin have not yet been fully elucidated.
Myostatin Inhibition as a Therapeutic Strategy for Muscular Dystrophy
Interestingly, inhibition of myostatin activity is capable of increasing muscle mass and strength in the postnatal period and even in adults. These observations suggest that targeting of myostatin would be a suitable therapy for degenerative muscle diseases, such as muscular dystrophy and cachexia, and may be able to prevent muscle wasting due to aging (1, 2, 7). In fact, antibody-mediated myostatin blockade in mdx mice, a model for Duchenne muscular dystrophy, was found to ameliorate the pathophysiology and muscle weakness (8). Myostatin propeptide-mediated amelioration of the symptoms in mdx mice, limb-girdle muscular dystrophy (LGMD) 1C model mice with caveolin-3 gene mutations and LGMD2A model mice with calpain 3 gene mutations has also been reported (9–11). However, elimination of myostatin did not recover the pathology in laminin-α2-deficient model mice and rather increased their mortality (12). Thus, the effectiveness of myostatin inhibition depends on the disease state (Table 1). In addition to myostatin propeptide and myostatin antibodies, follistatin and follistatin domain-containing proteins can bind to myostatin in vivo and act as effective myostatin inhibitors (1, 13, 14). Small chemical compounds that block the kinase activity of myostatin type I receptor would also serve as myostatin inhibitors (13).
Table 1. Muscular dystrophies and myostatin inhibition.
| Disease | Mode of inheritance | Gene locus | Gene products | Myostatin blockage | Ref [Method of myostatin inhibition] |
| Duchenne | XR | Xp21 | Dystrophin | Effective in mdx mouse | Bogdanovich et al., (8) [1] Wagner et al., (21) [2] Bogdanovich et al., (9) [3] Nakatani et al., (17) [4] |
| LGMD1C (CAV3) | AD | 3p25 | Caveolin-3 | Effective in model mouse | Ohsawa et al., (10) [5] |
| LGMD2A (CAPN3) | AR | 15q15 | Calpain-3 | Gene therapy is effective | Bartoli et al., (11) [6] |
| LGMD2D (SGCA) | AR | 17q12-21 | α-sarcoglycan | Gene therapy is not effective | Bartoli et al., (11) [6] |
| LGMD2F (SGCD) | AR | 5q33-34 | δ-sarcoglycan | Early therapy is effectiveTreat early | Parsons et al., (22) [1, 2] |
| MDC1A(LAMA2) | AR | 6q22 | Lamininα-2 | Not effective in dy mouse Severe fat loss | Li et al., (12) [2] |
The effects of myostatin blockade on various types of muscular dystrophy are summarized. Myostatin inhibition is applicable as a therapy for multiple types of muscular dystrophy. Transgenic approaches, systemic injection and gene therapy have been tried. Myostatin blockade by myostatin antibodies, modified myostatin propeptide or follistatin-derived peptides is effective for ameliorating the pathophysiology in mdx mice. Myostatin inhibition is also effective for ameliorating several types of limb-girdle-type muscular dystrophy caused by mutations of caveolin-3 or calpain-3. Effective therapy would be possible by early treatment. It is noteworthy that elimination of myostatin does not improve the phenotypes of laminin-α2-deficient model mice. Method of myostatin inhibition is shown as brackets.
myostatin antibody treatment;
crossing with myostatin K/O mice;
myostatin propeptide treatment;
crossing with mutated follistatin Tg mice;
crossing with myostatin propeptide Tg mice;
AAV-mediated mutated myostatin propeptide expression. References are shown with parentheses.
Development of Myostatin Inhibitors for Therapies against Muscular Dystrophy
Phage display technology and antibody engineering have been used to develop myostatin-blocking antibodies. The biosafety and effectiveness of humanized myostatin antibodies, designated MYO-029, are being evaluated in phase I/II studies in the United States in 108 patients suffering from muscular dystrophy (3).
Multiple myostatin-binding proteins, such as myostatin propeptide, follistatin and follistatin-related protein, have been characterized. After cleavage of myostatin precursors, myostatin propeptide associates with mature myostatin in sera (14). Proteolytic cleavage of the propeptide at aspartate-76 by the BMP-1/TLD family of metalloproteinases is an important step for activation of the mature disulfide-bonded C-terminal myostatin dimer (2, 3). Mutation of the myostatin propeptide at the BMP-1/TLD cleavage site by replacing aspartate-76 with alanine (D76A) produces a better myostatin inhibitor than the wild-type propeptide in vitro and in vivo (9, 11).
Although the activin type IIB receptor, ACVR2B, is characterized as a receptor for activins and nodal, it is the primary ligand-binding myostatin receptor that transmits myostatin signaling. A soluble form of ACVR2B has potent myostatin-inhibitory activity and causes dramatic increases in muscle mass (15). Only 2 weeks are required for the soluble form of ACVR2B to increase the muscle mass in mice by up to 60% (15). Since the soluble form of ACVR2B even augments muscle mass in myostatin-knockout mice, it has been suggested that it also inhibits other ligands including activins and GDF11 that regulate skeletal muscle growth in addition to myostatin (15).
Myostatin Inhibitor Derived from Follistatin
Follistatin was originally identified as a single-chain polypeptide with a weak inhibitory activity toward follicle-stimulating hormone secretion by anterior pituitary cells. Later, follistatin was found to be an activin-binding protein (1). Gene knockout analyses revealed that follistatin gene ablation causes multiple effects, including skeletal and skin abnormalities, suggesting that follistatin may have additional functions other than activin inhibition (1). Follistatin and follistatin-related gene, FLRG, were shown to bind to myostatin and inhibit its activity (1, 2, 15, 16). Similar to myostatin, activins belong to the TGF-β superfamily and have pleiotrophic effects on numerous tissues. Since activins have a variety of functions in tissues other than skeletal muscles and their inhibition by follistatin is very efficient, follistatin has multiple effects on not only skeletal muscles but also other tissues. In fact, transgenic expression of the follistatin gene has profound effects on reproductive performance and fertility (1).
Recently, we developed a myostatin inhibitor derived from follistatin, designated FS I-I, and characterized its effects on muscle mass and strength in mdx mice (17). Since myostatin blockade is one of the most promising therapies for muscular dystrophy, the results of our study should provide an additional rational therapeutic strategy for intractable muscular diseases, including muscular dystrophy (17).
Follistatin is composed of an N-terminal domain and three cysteine-rich follistatin domains (FS I, FS II and FS III) (1). Recent crystallographic analyses have revealed that the minimal activin-inhibiting fragment of follistatin is comprised of the FS I and FS II domains, and that the individual FS domains may have different activities (18, 19). We created a follistatin mutant containing two FS I domains, and characterized its binding activities toward myostatin and activin A. Interestingly, FS I-I retained its myostatin binding, but showed significantly weaker activin-binding activity. The dissociation constants of follistatin for activin and myostatin are 1.72 and 12.3 pM, respectively. In contrast, the dissociation constants of FS I-I for activin and myostatin are 64.3 nM and 46.8 pM, respectively. FS I-I was capable of inhibiting the actions of myostatin in multiple assays, but hardly affected the activin activity (17). Transgenic mice expressing FS I-I under the control of a skeletal muscle-specific promoter showed increased skeletal muscle mass, especially in the pectoralis major, triceps brachii, gluteus and quadriceps femoris muscles. Muscle strength was also increased. Hyperplasia and hypertrophy were both observed. FS I-I transgenic mice did not show any behavioral abnormalities and reproduced normally. We crossed FS I-I transgenic mice with mdx mice, a model for Duchenne muscular dystrophy. Notably, the skeletal muscles in the resulting mdx/FS I-I mice were enlarged and showed reduced cell infiltration (17). The numbers of infiltrated macrophages in skeletal muscles were dramatically decreased in mdx/FS I-I mice compared with mdx mice (17). Muscle strength was also recovered in mdx/FS I-I mice. These results indicate that myostatin blockade by FS I-I has therapeutic potential for muscular dystrophy and should provide a rational therapeutic strategy for intractable muscular diseases. The possibility that injections of this myostatin inhibitor derived from follistatin may affect the pathophysiology of muscular dystrophy model mice or human patients remains to be determined.
Conclusions
The ability to control the actions of myostatin has great potential for a number of research fields and offers medical applications. Myostatin activity determines the skeletal muscle mass. Myostatin blockade is effective for increasing muscle mass, even in adults (1, 2). Thus, myostatin is considered to be one of the rational drug targets for muscle-wasting diseases, such as muscular dystrophy. There are multiple strategies for inhibiting myostatin activity. Myostatin inhibitors, such as monoclonal myostatin antibodies, myostatin propeptide and follistatin, could be promising lead compounds in drug development for muscular dystrophy and related disorders (1, 2, 17).
There are various types of muscular dystrophy, including Duchenne/Becker muscular dystrophies, congenital muscular dystrophies and limb-girdle muscular dystrophies (20). Myostatin blockade could increase the skeletal muscle mass, regardless of the type of muscular dystrophy. Antibody-mediated or myostatin propeptide-mediated myostatin blockade in mdx mice, a model for Duchenne type muscular dystrophy, ameliorates the pathophysiology and increases muscle strength (8, 9, 18) (Table 1). Crossing of myostatin knockout mice with mdx mice also attenuates severity of muscular dystrophy (21). The pathophysiologies of three models of limb-girdle muscular dystrophy, including δ-sarcoglycan-deficiency, caveolin-3 mutations and calpain-3-deficiency, are also ameliorated by myostatin blockade (10, 11, 22). However, myostatin elimination did not combat laminin-α2-deficiency in mice, but rather increased their postnatal mortality due to fat loss (12). Similarly, myostatin inhibition was not effective for prolonging the survival of LGMD2D model mice with mutations of α-sarcoglycan (11). However, since the expression by AAV-myostatin propeptide used in the study was extremely low, it is still possible that different mode of action, such as the use of neutralizing myostatin antibody could be beneficial for α-sarcoglycan deficiency (11).
Myostatin inhibition would increase the relative ratio of fast myofibers to slow myofibers. Exercise in myostatin-deficient cattle led to early exhaustion, which may have been caused by a decrease in the number of mitochondria (23). However, a decreased number of mitochondria associated with myostatin absence was specific for myostatin-knockout mice and not observed in myostatin-inhibitor-expressing transgenic mice (our unpublished observations). Thus, regulation of the number of mitochondria seems to depend on the way in which myostatin is inhibited. This observation suggests that myostatin inhibition by our follistatin-derived peptide would not decrease the number of mitochondria, although this aspect needs to be clarified in future studies.
Follistatin and FLRG are efficient myostatin blockers, and inhibit not only myostatin but also activins. We have developed a myostatin inhibitor derived from follistatin, designated FS I-I, that does not affect activin activity (17). FS I-I is capable of ameliorating the pathophysiology of mdx mice. It must be determined whether FS I-I affects other TGF-β-like ligands that regulate muscle fiber growth. Since transgenic expression of FS I-I is effective for treatment of mdx mice, FS I-I and related follistatin-derived myostatin inhibitors would join the list of potential therapeutic myostatin inhibitors.
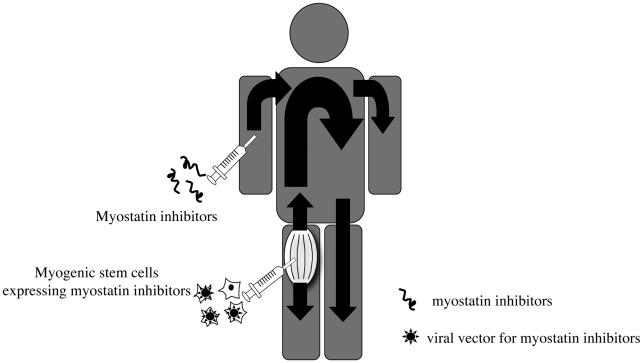
Myostatin inhibitor peptides could be directly infused into muscular dystrophy patients. In addition, a delivery system using myogenic cells is also possible. Furthermore, myostatin inhibition could be combined with other therapeutic approaches. Myostatin inhibition is considered to be most effective when combined with gene correction or other ways of delivering dystrophin (24). In this sense, one advantage of myostatin inhibitor peptides is their application to combined therapy for muscular dystrophy. If cDNAs for myostatin inhibitor peptides can be expressed in myogenic stem cells, cell-mediated therapy with myostatin inhibition would become possible (Fig. 1). By using this method, defective genes such as dystrophin would be amended by myogenic stem cells. Alternatively, viral vectors containing myostatin inhibitor peptides could be combined with other possible therapies for muscular dystrophy, such as exon-skipping reagents or genes (24).
Figure 1.
Potential delivery systems for myostatin inhibitors in vivo.
Studying the role of myostatin in tissues other than skeletal muscle is important to avoid the possible adverse effects of myostatin inhibition. In this respect, it is important to determine whether or not myostatin acts solely on skeletal muscles. Adipose tissues are affected by myostatin signaling. Reduction of adipose tissue mass is observed in myostatin-null mice. Whether myostatin directly acts on adipocytes or factors from hypertrophied skeletal muscle secrete factors affecting adipocyte remains to be determined.
Finally, ethical issues must be considered for use of myostatin inhibition. Athletes are already interested in myostatin for increasement of their muscle strength. There is a discussion that myostatin inhibition would be non-steroidal doping methods that are difficult to identify.
In summary, I have presented an outline of myostatin inhibition therapy for muscular dystrophy with emphasis on a myostatin inhibitor derived from follistatin. I hope that this novel therapeutic strategy will prove useful toward establishing realistic therapies for intractable diseases, such as muscular dystrophy.
Acknowledgments
This research was supported by grants from the Ministry of Health, Labour and Welfare.
References
- 1. Tsuchida K. The role of myostatin and bone morphogenetic proteins in muscular disorders. Expert Opin Biol Ther 2006;6:147-54. [DOI] [PubMed] [Google Scholar]
- 2. Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol 2004;20:61-86. [DOI] [PubMed] [Google Scholar]
- 3. Wolfman NM, McPherron AC, Pappano WN, et al. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA 2003;100:15842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rebbapragada A, Benchabane H, Wrana JL, et al. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol 2003;23:7230-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCroskery S, Thomas M, Maxwell L, et al. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 2003;162:1135-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang W, Chen Y, Zhang Y, et al. Extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res 2006;66:1320-6. [DOI] [PubMed] [Google Scholar]
- 7. Walsh FS, Celeste AJ. Myostatin: a modulator of skeletal-muscle stem cells. Biochem Soc Trans 2005;33:1513-7. [DOI] [PubMed] [Google Scholar]
- 8. Bogdanovich S, Krag TO, Barton ER, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature 2002;420:418-21. [DOI] [PubMed] [Google Scholar]
- 9. Bogdanovich S, Perkins KJ, Krag TO, et al. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. Faseb J 2005;19:543-9. [DOI] [PubMed] [Google Scholar]
- 10. Ohsawa Y, Hagiwara H, Nakatani M, et al. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest 2006;116:2924-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartoli M, Poupiot J, Vulin A, et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Ther 2007;14:733-40. [DOI] [PubMed] [Google Scholar]
- 12. Li ZF, Shelton GD, Engvall E. Elimination of myostatin does not combat muscular dystrophy in dy mice but increases postnatal lethality. Am J Pathol 2005;166:491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsuchida K, Sunada Y, Noji S, et al. Inhibitors of the TGF-beta superfamily and their clinical applications. Mini Rev Med Chem 2006;6:1255-61. [DOI] [PubMed] [Google Scholar]
- 14. Hill JJ, Davies MV, Pearson AA, et al. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 2002;277:40735-41. [DOI] [PubMed] [Google Scholar]
- 15. Lee SJ, Reed LA, Davies MV, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci USA 2005;102:18117-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SJ. Quadrupling Muscle Mass in Mice by Targeting TGF-ss Signaling Pathways. PLoS ONE 2007;2:e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakatani M, Takehara Y, Sugino H, et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. Faseb J 2008;22:477-87. [DOI] [PubMed] [Google Scholar]
- 18. Thompson TB, Lerch TF, Cook RW, et al. The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell 2005;9:535-43. [DOI] [PubMed] [Google Scholar]
- 19. Harrington AE, Morris-Triggs SA, Ruotolo BT, et al. Structural basis for the inhibition of activin signalling by follistatin. Embo J 2006;25:1035-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishino I, Ozawa E. Muscular dystrophies. Curr Opin Neurol 2002;15:539-44. [DOI] [PubMed] [Google Scholar]
- 21. Wagner KR, McPherron AC, Winik N, et al. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 2002;52:832-6. [DOI] [PubMed] [Google Scholar]
- 22. Parsons SA, Millay DP, Sargent MA, et al. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol 2006;168:1975-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amthor H, Macharia R, Navarrete R, et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA 2007;104:1835-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bushby K, Griggs R. 145th ENMC International Workshop: planning for an International Trial of Steroid Dosage Regimes in DMD (FOR DMD), 22-24th October 2006, Naarden, The Netherlands. Neuromuscul Disord 2007;17:423-8. [DOI] [PubMed] [Google Scholar]